Abstract
Leptin regulates energy homeostasis and reproductive, neuroendocrine, immune, and metabolic functions. In this review, we describe the role of leptin in human physiology and review evidence from recent “proof of concept” clinical trials using recombinant human leptin in subjects with congenital leptin deficiency, hypoleptinemia associated with energy-deficient states, and hyperleptinemia associated with garden-variety obesity. Since most obese individuals are largely leptin-tolerant or -resistant, therapeutic uses of leptin are currently limited to patients with complete or partial leptin deficiency, including hypothalamic amenorrhea and lipoatrophy. Leptin administration in these energy-deficient states may help restore associated neuroendocrine, metabolic, and immune function and bone metabolism. Leptin treatment is currently available for individuals with congenital leptin deficiency and congenital lipoatrophy. The long-term efficacy and safety of leptin treatment in hypothalamic amenorrhea and acquired lipoatrophy are currently under investigation. Whether combination therapy with leptin and potential leptin sensitizers will prove effective in the treatment of garden-variety obesity and whether leptin may have a role in weight loss maintenance is being greatly anticipated.
Keywords: leptin, leptin deficiency, obesity, leptin resistance, energy homeostasis, insulin resistance, adipokines, amenorrhea, lipoatrophy
INTRODUCTION
During the second half of the twentieth century, scientists studied extensively two phenotypes of obese mice. It was later discovered that their phenotypes derive from homozygous mutations of the mouse obese (ob) and diabetic (db) genes, respectively [104; 148; 103]. Using the parabiosis paradigm (i.e. connecting the vascular systems of two animals to permit the exchange of circulating hormones), scientists noted that ob/ob mice continued to be obese when joined with wild-type mice and lost weight when joined with db/db mice [39]. In contrast, db/db mice did not exhibit any change in weight when joined with either wild-type or ob/ob mice [39]. Furthermore, wild-type and ob/ob mice joined to db/db mice died of starvation [39]. From these findings, it was postulated that a circulating factor present in the wild-type mice was absent in ob/ob mice, and that this factor was produced in excess in db/db mice, which were resistant or tolerant to its effects [39; 229].
In 1994, Zhang et al. at Rockefeller University discovered through positional cloning that the ob/ob mouse model has an inactivating mutation of the ob/ob gene and that its phenotype results from complete deficiency of the ob gene product [257]. This product became known as leptin, which is derived from the Greek root leptos, meaning thin [86]. The discovery that the mouse db gene codes for the leptin receptor followed soon after [119]. It shortly became evident that exogenous leptin administration reduces weight and reverses the metabolic, endocrine, and immune disturbances in ob/ob mice; however, it has no obvious effect in db/db mice [257; 90; 101].
The discovery that most obese humans are resistant or tolerant to leptin quickly dispelled the idea of leptin as a wonder drug for obesity, and leptin proved to be extremely effective only in the exceptionally rare cases of humans with congenital leptin deficiency [59]. Despite leptin's inability to induce weight loss in the majority of obese individuals [93], ongoing exploratory clinical trials are investigating whether combination therapy with leptin and potential leptin sensitizers will prove effective in the treatment of garden-variety obesity [191]. Furthermore, recent studies suggest that leptin could potentially have a role in weight loss maintenance [195]. Emerging research also suggests that leptin plays a more important role in acute (e.g. fasting) and chronic energy-deficient states (e.g. diet- or exercise-induced hypothalamic amenorrhea and lipoatrophy) than in energy-replete states (e.g. obesity) [31]. These energy-deficient states are associated with relative leptin deficiency, which, in turn, is associated with infertility and other neuroendocrine abnormalities, metabolic dysfunction, depressed immune function, and bone loss. Human recombinant leptin may serve as a treatment option in these conditions.
In this review, we offer a description of leptin physiology; an explanation of its role in energy homeostasis, reward processing, brain development, neuroendocrine function, metabolism, immune function, and bone metabolism; and insights into emerging clinical applications and therapeutic uses of recombinant leptin in humans.
LEPTIN BIOLOGY
Leptin, known as the prototypical adipokine, is a 167-amino acid peptide with a four-helix bundle motif similar to that of a cytokine [256; 24]. It is produced primarily in adipose tissue but is expressed in a variety of tissues including the placenta, ovaries, mammary epithelium, bone marrow [143], and lymphoid tissues [145].
Leptin levels are pulsatile and follow a circadian rhythm, with highest levels between midnight and early morning and lowest levels in the early- to mid- afternoon [214; 129; 18]. Specifically, the concentration of circulating leptin may be up to 75.6% higher during the night as compared to afternoon trough levels [214]. The pulsatile characteristics of leptin secretion are similar in obese and lean individuals, except the obese have higher pulse amplitudes [214; 129; 18].
Leptin concentration reflects the amount of energy stored in body fat. Circulating leptin levels are directly proportional to the amount of body fat [41] and fluctuate with acute changes in caloric intake [19; 30]. This system is especially sensitive to energy deprivation. In our initial study of six healthy, lean men, we measured leptin levels both in the baseline fed state and in the fasting state [30]. We noted that after only 2 or 3 days of fasting, leptin levels dropped to ~ 40 or 10% of baseline, respectively [30]. Women tend to have higher leptin levels than men, although women experience a significant decline in the amount of circulating leptin after menopause [112]. This sexual dimorphism is largely independent of body mass index (BMI), and is due in part to differences in sex hormones, fat mass, and body fat distribution [196; 199; 112]. Women tend to accumulate body fat peripherally whereas men are prone to an abdominal or android distribution of fat. Subcutaneous fat expresses more leptin mRNA than omental fat, and this may partially explain the higher leptin concentrations in women compared to men [154; 199]. Hormones and cytokines other than sex steroids also affect leptin secretion but to a smaller degree (Table 1).
Table 1.
Factors that regulate circulating leptin levels1
| Factors promoting leptin secretion |
| *Excess energy stored as fat (obesity) |
| *Overfeeding |
| Glucose |
| Insulin |
| Glucocorticoids |
| Estrogens |
| Inflammatory cytokines, including Tumor Necrosis Factor-α and Interleukin-6 (acute effect) |
| Factors inhibiting leptin secretion |
| *Low energy states with decreased fat stores (leanness) |
| *Fasting |
| Catecholamines and adrenergic agonists |
| Thyroid hormones |
| Androgens |
| Peroxisome Proliferator-activated Receptor-γ (PPARγ) agonists† |
| Inflammatory cytokines, including Tumor Necrosis Factor-α (prolonged effect) |
Adapted from reference [24]
Denotes major factor influencing leptin levels.
Unlike animals, in humans PPARγ agonists decrease leptin gene expression but increase subcutaneous fat mass. Thus, the net effect is null.
Leptin binds to leptin receptors (ObRs) located throughout the central nervous system and several peripheral tissues [66]. At least six variations or isoforms of the leptin receptor have been identified (ObRa, ObRb, ObRc, ObRd, ObRe, and ObRf) [119]. These isoforms have homologous extracellular domains but distinct intracellular domains, which vary by length and sequence due to alternative mRNA splicing [119; 226]. The short isoforms ObRa and ObRc are thought to play important roles in transporting leptin across the blood–brain barrier (BBB) [17; 94]. The long leptin receptor isoform ObRb is primarily responsible for leptin signaling [119; 226; 17]. This functional leptin receptor ObRb, expressed in several organs, is strongly expressed throughout the central nervous system but particularly in the hypothalamus, where it regulates energy homeostasis and neuroendocrine function described further below [66; 55]. In the db/db mouse model, the ObRb is dysfunctional, resulting in obesity and the metabolic syndrome [245].
LEPTIN SIGNALING
Leptin and STAT3 Signaling
Activation of ObRb sets off a cascade of several signal transduction pathways (Table 2), of which the best studied pathway is the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) pathway [72]. STAT3 has been shown to mediate the transcription of several genes that affect a number of cellular processes [124].
Table 2.
Leptin Signaling
| Signaling Pathway | Primary Site of Action | Known Mechanisms of Action | Clinical Results |
|---|---|---|---|
| JAK-STAT3 | Hypothalamus | Stimulates transcription of POMC [14; 80] and suppresses transcription of NPY [14; 80]. | Regulates appetite and, thus, body weight [14]. May also contribute to neuroendocrine function as neural-specific STAT3 deletion results in decreased linear growth and infertility [74]. |
| P13K | Hypothalamus | Stimulates POMC neurons [157] [185; 240]. Inhibits FOXO1, an inhibitor of POMC transcription, to increase POMC expression [114]. |
Regulates appetite and body weight [169; 114; 157]. May contribute to leptin resistance in obesity, given the overlapping pathway with insulin [174]. May mediate the stimulation of sympathetic outflow [189]. |
| MAPK | Hypothalamus, liver, pancreas, adipose tissue, and myocytes | Stimulates POMC neurons [190] and inhibits AgRP/NPY neurons [240]. | Regulates appetite and body weight [190]. Increases sympathetic activity to brown adipose tissue [190]. Increases fatty acid oxidation in peripheral tissues [211]. Promotes cardiomyocyte hypertrophy [255]. |
| AMPK | Hypothalamus, muscle | Stimulates ACC activity in the hypothalamus to regulate food intake and weight [151; 157]. Inhibits ACC activity in muscle [152; 220]. |
Regulates appetite and weight [151; 157]. Stimulates fatty-acid oxidation in muscle [152; 220] and may sensitize muscle to insulin [53]. |
| mTOR | Hypothalamus | Induces phosphorylation of S6K1 to regulate protein synthesis [43]. | Regulates appetite and weight [44]. |
Abbreviations: ACC, acetyl coenzyme A carboxylase; AMPK, 5'adenosine monophosphate-activated protein kinase; ATP, adenosine triphosphate; FOXO1, forkhead box O1; JAK-STAT3, janus kinase-signal transducers and activator of transcription 3; K+, potassium; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; P13K, phosphatidylinositol 3-kinase; S6K1, S6 Kinase 1.
The JAK2/STAT3 pathway plays crucial roles in energy homeostasis and possibly neuroendocrine function. Activation of STAT3 by leptin induces transcription of proopiomelanocortin (POMC), an anorexigenic neuropeptide, in the arcuate nucleus (ARC) of the hypothalamus [56]. S/s mice with a targeted mutation at the key intracellular tyrosine residue Tyr1138 of the ObRb, which disrupts STAT3 signaling, are hyperphagic and obese, similar to db/db mice [14]. However, unlike db/db mice, these mice are fertile and of normal length, suggesting that STAT3-independent signals may be responsible for neuroendocrine regulation by leptin [14]. Gao et al. have studied mice with neural-specific STAT3 deletion [74]. These mice had decreased POMC expression in the ARC and also developed hyperphagia, obesity, and diabetes [74]. However, Gao et al. also observed neuroendocrine defects including decreased linear growth and infertility [74]. The discrepancies between the s/s mice and the mice with neural-specific STAT3 deletion were felt to be due to the fact that the mutation in the Tyr1138 residue of s/s mice may allow for a low level of STAT3 activation by leptin [74].
In addition to activating POMC neurons, the JAK2/STAT3 pathway likely also suppresses AgRP/NPY neurons in the ARC, which produce the orexigenic neuropeptides agouti-related peptide (AgRP) and neuropeptide Y (NPY), but to a lesser degree [14; 80]. Gong et al. specifically deleted STAT3 from AgRP/NPY neurons in mice, which resulted in hyperleptinemia, hyperphagia, modest weight gain, and high-fat diet-induced hyperinsulinemia [14; 80]. These mice were found to have increased NPY mRNA, although AgRP mRNA levels were unaffected [14; 80]. It is likely that other pathways contribute to leptin's action on AgRP/NPY neurons as Munzberg et al. found that AgRP/NPY neurons were appropriately suppressed by leptin in s/s mice [161]. However, as mentioned above, a mutation in Tyr1138 residue may allow for a low level of STAT3-induced activation by leptin.
Finally, Xu et al. bred mice lacking functional STAT3 in only POMC-expressing neurons [249; 163]. These mice were found to have diminished expression of POMC but were responsive to the anorexigenic effects of leptin and were not hypersensitive to a high-fat diet [249; 163].
These elegant experiments in mice have indicated that leptin binding to its ObRb receptor leads to STAT3 phosphorylation and activation and thus in turn upregulates POMC and downregulates NPY and AgRP, which mediate leptin's effect on energy homeostasis and neuroendocrine function. Moreover, leptin administration has been shown to phosphorylate STAT3 in several cell lines in vitro and a variety of animals ex vivo and in vivo [116]. For example, leptin has been shown to activate STAT3 in vivo not only in rat hypothalamic tissue but also in rat adipose tissue and liver [116; 127]. Leptin has also been shown to activate STAT3 phosphorylation in human tissues and cells in vitro and ex vivo, but similar effects of leptin in humans in vivo remain to be explored and elucidated. Further studies of leptin signaling in humans with the ultimate goal of developing novel therapies for the treatment of human obesity are expected with great anticipation.
Leptin and PI3K Signaling
In vitro and in vivo studies have suggested that leptin stimulates the phosphatidylinositol 3-kinase (PI3K) pathway in a variety of murine cells and tissues, including myocytes [16], hepatocytes [242], and hypothalamic tissue [157]. Similarly, leptin may stimulate this pathway in human cancer cells [91; 71] and in human adipose, liver, and muscle tissues [116]. The activation of PI3K occurs downstream from the insulin receptor substrate (IRS), particularly IRS-2 [157].
Activation of PI3K in the hypothalamus contributes to leptin's anoretic and weight loss effects. Similar to the STAT3 pathway, the PI3K pathway activates POMC neurons [157] by activating ATP-sensitive potassium channels [185] and voltage-gated calcium channels [240]. Similar to insulin [168], the appetite-suppressing effects of leptin [169] are attenuated by intracerebroventricular administration of PI3K inhibitors. Interestingly, the insulin-PI3K pathway hyperpolarizes POMC neurons, making them less sensitive to leptin [185], and this may be one common mechanism for concomitant leptin and insulin resistance in obesity [174]. More recently, forkhead box O1 (FOXO1), a transcriptional factor that stimulates the expression of AgRP and NPY and inhibits the expression of POMC, has been found to be an important downstream mediator of the PI3K pathway [157]. Expression and activity of hypothalamic FOXO1 is inhibited by leptin through the PI3K pathway [114]. Finally, PI3K may also be involved in the regulation of sympathetic outflow [189] but the exact mechanisms remain unclear.
Thus, activation of the P13K signaling pathway in the hypothalamus may influence energy homeostasis and neuroendocrine function whereas activation of this pathway in the periphery may mediate leptin's effect on insulin resistance.
Leptin and MAPK Signaling
Mitogen-activated protein kinases (MAPK) are a group of kinases that control a large number of cellular processes, including differentiation, proliferation, and apoptosis, and comprise of a number of signaling molecules including extracellular signal-related kinases 1 and 2 (ERK1/2), c-Jun amino-terminal kinases (JNK), and p38 [208; 211]. MAPK can be activated by leptin [71], and leptin receptor-deficient obese rats have decreased or no activation of MAPK [190].
ERK1/2 is the major MAPK involved in leptin's central effects [116] and plays a key role in the regulation of food intake, body weight, and energy expenditure. ERK1/2 is stimulated by leptin downstream of src homology-2 containing protein tyrosine phosphatase 2 (SHP2) and Tyr985 of the ObRb after JAK2 activation [164; 71; 157]. When leptin is administered in vivo, both centrally and systemically, a response in ERK1/2 is seen in the POMC neurons of the ARC [190]. Inhibition of ERK1/2 reversed the decrease in food intake and body weight seen after leptin administration [190]. Furthermore, inhibition of ERK1/2 also prevented leptin-induced increase in sympathetic activity to brown adipose tissue [190].
The other kinases in the MAPK family, including p38 and JNK, have mainly peripheral actions. Unlike ERK1/2, p38 does not respond to leptin stimulation in neuronal cells, including the hypothalamus [190; 241]. In rat cardiomyocytes, administration of leptin activates p38, which corresponds to an increase in fatty acid oxidation, and inhibition of p38 activation prevents leptin-induced fatty acid oxidation [211]. Activation of p38 by leptin is also seen in skeletal muscle of rats after intraperitoneal administration [144].
Similar to p38, JNK has not been shown to be activated in response to leptin treatment in neuronal cells [241], but leptin has been postulated to promote cancer via activation of the JNK pathway. Leptin has been demonstrated to activate JNK in human breast cancer cells in a both time- and dose-dependent manner, with greater levels of phosphorylated JNK after long-term leptin exposure [170; 149]. In breast cancer, activation of the JNK pathway by leptin results in up-regulation of matrix metalloproteinase-2 (MMP-2) activity, promoting cancer cell invasion [149]. In addition, leptin stimulates proliferation and inhibits apoptosis, both of which are mediated in part by JNK activation, in human colon cancer cells [170].
Leptin and AMPK Signaling
Leptin's actions in peripheral tissues are in part due to its effects on 5'- adenosine monophosphate-activated protein kinase (AMPK), which, in addition to p38, contributes to leptin's effects on fatty acid oxidation and glucose uptake [152; 2; 130]. In mouse skeletal muscle, leptin activates AMPK, which leads to phosphorylation and inhibition of acetyl co-enzyme A carboxylase (ACC) and subsequent stimulation of fatty acid oxidation [152; 219]. There appears to be two mechanisms by which leptin activates AMPK in muscle. One pathway involves the hypothalamus and the α-adrenergic pathway and results in a prolonged effect [152]. After intrahypothalamic or intravenous injection of supra-physiological and near-physiological doses of leptin in mice, increased AMPK activity was observed for up to 6 hours [152]. The second pathway is a direct effect on skeletal muscle, as evidenced in ex vivo experiments [152]. The ability of leptin to stimulate fatty acid oxidation in skeletal muscles may prevent lipotoxicity and subsequent insulin resistance [152] and may underlie leptin's ability to improve insulin resistance and metabolic syndrome in hypoleptinemic humans (see below). Interestingly, it has been demonstrated that suppressor of cytokine signaling 3 (SOCS3) inhibits leptin activation of AMPK in skeletal muscle in obese humans but not in lean humans, and this may also contribute to the dysregulation of fatty acid metabolism observed in obesity [219].
In contrast to its effects in muscle, leptin has been found to have an inhibitory effect on AMPK in the hypothalamus, including the ARC and PVH. This results in stimulation of hypothalamic ACC and subsequent reduction of food intake and weight gain [151; 157]. It appears that leptin's effects on AMPK in the hypothalamus are at least partially mediated by the melanocortin-4 receptor (MC4R) pathway, which promotes anorexia [151].
In summary, leptin controls energy homeostasis and body weight primarily by activating ObRb in the hypothalamus [72]. The ObRb activate numerous JAK2/STAT3-dependent and -independent signaling pathways that act in coordination as a network to fully mediate leptin's action. The activation of individual pathways in the leptin signaling network appears to be differentially regulated in discrete subpopulations of ObRb-expressing neurons. These pathways are also likely to be regulated by various other hormonal, neuronal, and metabolic signals that cross-talk with leptin. Hence, it is important to fully determine whether and how positive and negative regulators of ObRb signaling, metabolic state, and/or neuronal activity regulate leptin signaling networks in a cell/tissue type-specific manner and how activation of these signaling pathways mediates leptin's effects in humans.
THE ROLE OF LEPTIN IN HUMAN PHYSIOLOGY
The Role of Leptin in Energy Homeostasis
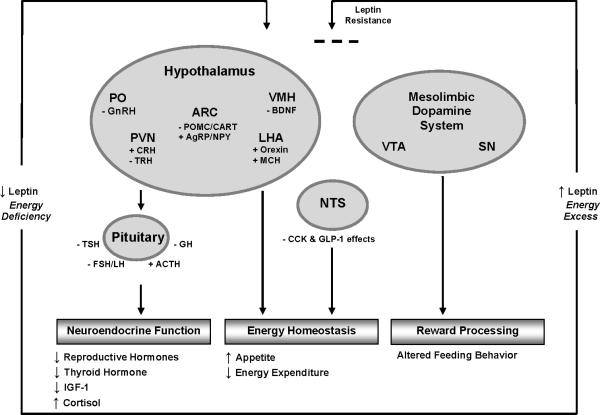
Leptin maintains energy homeostasis, primarily in the energy-deficient state, by adjusting appetite/food intake and energy expenditure. Leptin interacts with several neuronal pathways in and outside of the hypothalamus to regulate energy intake via orexigenic and anorexigenic neuropeptides (Figure 1).
Figure 1.
Leptin's action in the brain during states of energy excess and energy deficiency. During states of leptin and energy excess, leptin's access to the hypothalamus and other brain areas is impaired and leptin's action is blunted. In states of leptin and energy deficiency, neuropeptides that are normally inhibited by leptin are elevated (+) and neuropeptides stimulated by leptin are suppressed (-). A change in the concentrations of these neuropeptides leads to alterations in neuroendocrine function and energy homeostasis. Alterations in leptin levels may also affect the hedonic aspects of feeding behavior. The role of leptin in the brain is discussed in greater detail in the text. Adapted from references [31; 24; 18].
Abbreviations: ACTH, adrenocorticotropic hormone; AgRP, agouti-related peptide; ARC, arcuate nucleus; BDNF, brain-derived neurotrophic factor; CART, cocaine- and amphetamine- regulated transcript; CCK, cholecystokinin; CRH, corticotropin-releasing hormone; FSH, follicle-stimulating hormone; GH, growth hormone; GLP-1, glucagon-like peptide 1; GnRH, gonadotropin-releasing hormone; IGF-1, insulin-like growth factor 1; LH, luteinizing hormone; LHA, lateral hypothalamic area; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; NST, nucleus of the solitary tract; PO, preoptic area; POMC, proopiomelanocortin; PVN, paraventricular nucleus; SN, substantia nigra; TRH, thyrotropin-releasing hormone; TSH, thyrotropin-stimulating hormone; VMH, ventromedial hypothalamus; VTA, ventral tegmental area.
The ARC of the hypothalamus is a critical site of leptin action. It lies adjacent to the third ventricle and immediately above the median eminence, a region where the blood brain barrier is specially modified to allow peripheral peptides (e.g. leptin and insulin) access to receptors [5]. Leptin acts on two populations of neurons in the ARC. Via ObRb-receptor binding, leptin directly stimulates neurons to secrete POMC, a precursor protein that is cleaved into α-melanocyte-stimulating hormone (α-MSH) [158]. α-MSH is an anorexigenic neuropeptide that decreases food intake by activating melanocortin-4 (MC4R) and melanocortin-3 receptors (MC3R) [218; 46; 163]. Leptin also stimulates POMC neurons to secrete cocaine- and amphetamine- regulated transcript (CART), which also suppresses appetite [194]. Both CART and POMC expression are decreased in states of leptin deficiency [51]. Although ablation of central POMC receptors results in profound obesity, the selected deletion of ObRb from POMC neurons results in only mild hyperphagia and obesity in mice [9; 163]. Similarly, although mutation of the STAT3 binding site on ObRb leads to profound obesity [14], the selected deletion of STAT3 specifically from POMC neurons in the ARC only modestly impacts body weight [249; 163].
Thus, it has been found that in conjunction with the stimulation of POMC neurons in the ARC, leptin also inhibits AgRP/NPY neurons that coexpress the orexigenic neuropeptides AgRP and NPY [46]. AgRP antagonizes α-MSH/MC4R signaling and inhibits endogenous MC4R activity [171; 163], and NPY effectively increases appetite and reduces energy expenditure [217; 73]. Leptin inhibits the orexigenic effect of these neurons by hyperpolarizing ATP-sensitive potassium channels, thus decreasing the action potential firing rate [45].
The ARC is not the only area of the hypothalamus involved in the regulation of energy homeostasis by leptin. ObRb is well-dispersed throughout the CNS with the ARC accounting for only 15-20% of ObRb-expressing neurons [126; 163]. The ventromedial hypothalamus (VMH) is another key site of leptin action. Using laser scanning photostimulation of brain slices from transgenic mice, Sternson et al. found that ObRb-expressing neurons in the VMH project excitatory impulses onto POMC neurons in the ARC [223]. Leptin has also been shown to stimulate the secretion of two anorexigenic neuropeptides expressed in the VMH, steroidogenic factor-1 (SF-1) and brain-derived neurotrophic factor (BDNF). SF-1 is a transcription factor necessary for the development of the VMH [50; 73]. Dhillon et al. found that mice lacking ObRb on SF-1 neurons are markedly hyperphagic and obese, and mice lacking ObRb on both SF-1 neurons and POMC neurons are more obese than either mutant alone [50]. BDNF is a neurotrophin known to promote brain development and has also been shown to regulate food intake [1]. Deletion of the BDNF gene results in mice that are obese and hyperleptinemic [192], and mice with partial deficiency of the BDNF receptor tyrosine kinase B (TrkB) exhibit hyperphagia and obesity when fed a high-fat diet [250].
The paraventricular nucleus (PVN) also contains neurons that express ObRb and have numerous projections from neurons in the ARC that contribute to the regulation of food intake and weight [5; 216; 231]. Compared to mice that do not express MC4R, mice with selective MC4R expression in the PVN are significantly less hyperphagic and obese [10]. Leptin has also been shown to upregulate expression of proTRH [121] and corticotropin-releasing hormone (CRH) [42] in PVN neurons. Furthermore, injection of a CRH agonist into the third ventricle of fasted rats attenuates the anorectic effect of leptin [234]. Leptin may also indirectly regulate energy homeostasis via its influences on the thyroidal and adrenal axes through these mechanisms, and the role of leptin in mediating the thyroidal and adrenal axes is discussed further below.
The lateral hypothalamus (LHA), which has been described as a hunger center, contains neurons that express the orexigenic neuropeptides melanin-concentrating hormone (MCH) and orexin, both of which are influenced by leptin [1]. Intracerebroventricular administration of MCH leads to increased food intake in rats [187], and mice with homozygous deletion of the MCH gene are excessively hypophagic and lean [212]. Leptin decreases MCH gene expression in vitro [15], and intracerebroventricular administration of leptin prior to injection of MCH prevents an increase in food consumption in rats [200]. Leptin also inhibits orexin expression by hyperpolarizing and decreasing the firing rate of orexin neurons [252]. In rats, central administration of orexin stimulates food intake and fasting increases expression of orexin mRNA [201]. Leptin administration to ob/ob mice blunts the fasting-induced increase in orexin expression [252]. Recently, another population of neurons in the LHA that express ObRb has been identified and these neurons have a γ-aminobutyric acid (GABA)-releasing/inhibitory effect on food intake [123]. Finally, neurons in the LHA densely innervate the ventral tegmental area (VTA), part of the mesolimbic dopamine system which is involved in reward processing [123]. The role of leptin in reward processing is discussed further below.
In addition to the hypothalamus, ObRb is widely expressed throughout the dorsal vagal complex and other structures of the caudal brainstem [73]. The dorsal vagal complex includes the area postrema, nucleus of the solitary tract (NTS), and dorsal motor nucleus of the vagus nerve (DMV) and has been implicated as a crucial site of meal size control [83; 73]. In mice, peripheral leptin administration has been shown to induce STAT3 phosphorylation in neurons of the NTS and DMV [98]. Another mice study showed that leptin administered directly into the dorsal vagal complex significantly reduces food intake and body weight [83]. At the NTS, leptin may work synergistically with peripheral satiety signals including glucagon-like peptide 1 (GLP-1) and cholecystokinin (CCK) to create a sense of fullness in response to food intake [235]. Williams et al. demonstrated that intraperitoneal leptin treatment strongly enhances the anorexic effects of GLP-1 in rats [246]. Leptin also enhances the effect of CCK on the activation of neurons in the NTS and area postrema, and this effect requires leptin signaling in the ARC [159]. Finally, leptin has also been proposed to act synergistically with amylin, a hormone secreted by pancreatic β-cells in response to caloric intake and contributes to the satiety signal short-term [35]. When co-administered in rodents, amylin increased leptin binding within the VMH and ARC, and the mice experienced an additive decrease in food intake and greater than additive reduction in body weight and epididymal fat [232]. The role of amylin as a potential leptin sensitizer in weight loss is discussed further below.
In addition to controlling energy intake via appetite and satiety, leptin also regulates energy expenditure via activation of the adrenergic system [141] and the melanocortin system [183]. In rodents, leptin increases sympathetic outflow to brown adipose tissue, which may contribute to the increased metabolic rate and core temperature observed after chronic leptin administration in animal studies [40]. In fact, leptin has been shown to increase oxygen consumption and expression of uncoupling protein 1 (UCP1), which is necessary for thermogenesis in brown adipose tissue, in rats [204]. It has also been proposed that these effects are due to the suppression of MCH by leptin [206]. Segal-Liberman et al. generated ob/ob mice that also lacked the MCH-1 receptor [206]. These “double null” mice had dramatically less body fat than ob/ob mice but were still hyperphagic [206]. The weight control appeared to result from increased energy expenditure, including increased locomotor activity and increased resting energy expenditure, compared to ob/ob mice [206].
Although these effects have not been confirmed in humans [33], recent evidence suggests that adult humans have functionally active brown adipose tissue [48; 239]. More importantly, the presence of brown adipose tissue inversely correlated with BMI and fasting plasma glucose activity [48], and, furthermore, its activity is reduced in men who are overweight or obese [237]. It remains to be seen if leptin affects brown adipose tissue in humans and if it plays a role in the inverse association between brown fat and obesity.
The Role of Leptin in Reward Processing
Leptin also influences the hedonic aspects of feeding and thus contributes to the maintenance of energy homeostasis. Leptin interacts with the mesolimbic dopaminergic system, which is known to regulate arousal, mood, and reward [73]. Mesolimbic areas include the VTA, substantia nigra, amygdala, prefrontal cortex, anteromedial ventral striatum (nucleus accumbens and caudate nucleus), and posterolateral ventral striatum (globus pallidus and putamen) [58; 124]. ObRb has been found in both the VTA and substantia nigra [67]. In rats, direct administration of leptin into the VTA reduces the excitability of dopamine neurons there and results in decreased food intake [97]. As previously mentioned, ObRb-expressing neurons in the LHA innervate the VTA and leptin administration directly into the LHA promotes expression of tyrosine hydroxylase, a rate-limiting enzyme involved in the production of dopamine, in the VTA [123]. Collectively, this suggests that leptin influences the mesolimbic dopamine system to decrease the incentive for food intake.
Consistent with the notion that leptin plays an important role in the mesolimbic system, research using functional magnetic resonance imaging (fMRI) is beginning to elucidate leptin's role in both the homeostatic and hedonic regulation of food intake. fMRI serves as an indirect marker of blood oxygen supply in the brain by measuring the hemodynamic response to brain activity as blood oxygen level-dependent (BOLD) signals [132]. Only three studies have directly studied the effect of leptin administration on brain activity using fMRI, two of which studied leptin administration in patients with congenital leptin deficiency and one of which studied leptin administration in subjects with garden-variety obesity who had recently lost 10% of their body weight [58; 197].
Farooqi et al. [58] conducted an fMRI study of congenitally leptin-deficient subjects (n=2) who underwent fMRI testing before and after seven days of recombinant human leptin treatment. The anteromedial and posterolateral ventral striatum were markedly active in response to food images in the baseline leptin-deficient state relative the leptin-replaced state, but after seven days of leptin treatment, no differential activation of mesolimbic areas was noted [58]. Leptin treatment was also associated with lower food intake, decreased sense of hunger in the fasting state, increased postprandial satiety, and decreased liking of food images in the fed state [58]. Furthermore, prior to leptin treatment, activation of the accumbens and caudate nuclei correlated with how much the subjects liked food images in the both the fed and fasting state, whereas after leptin treatment, the ‘liking’ ratings only correlated with activation in the accumbens-caudate in the fasting state. The authors thus suggest that leptin, via effects on the mesolimbic system, enhances the ability to discriminate the rewarding properties of food and may diminish the perception of food-related reward post-food consumption [58].
Similarly, Baicy et al. [7] studied three adults with congenital leptin deficiency and performed fMRI scanning following 57 months of leptin replacement, 33 days after discontinuing leptin replacement, and again 14 days after leptin replacement therapy was reinstated. In response to viewing high-calorie food images, leptin-replaced subjects showed decreased activation of the insula and increased activation of the prefrontal cortex [7]. The authors proposed that in states of leptin deficiency, the insular cortex, which has interoceptive function [47], may mediate enhanced feelings of hunger and also suggested that greater activation of the prefrontal cortex in the leptin-replaced state may mediate behavioral inhibition (i.e., the inhibition of consuming high-calorie foods) as well as satiety [7]. Consistent with the patterns of brain activation observed, subjects’ hunger declined after leptin replacement [7].
Rosenbaum et al. [197] examined patterns of brain activation in response to food and nonfood images in a randomized, placebo-controlled, cross-over study of obese individuals who lost 10% of their body weight. fMRI imaging was performed pre-weight loss, after stabilization of a 10% weight loss with the administration of leptin or placebo, after crossing over from leptin to placebo and vice versa. Weight loss was associated with changes in neural activity in brain areas associated with the regulatory, emotional, and cognitive control of food intake [197]. Importantly, administration of leptin in weight-reduced subjects produced a pattern of brain activation that was similar to the pattern observed at baseline (pre-weight loss). The behavioral implications of this finding are unknown but may lead to decreased food intake, as decreased feelings of hunger in association with leptin administration were reported [117]. The authors propose that the weight-reduced state is one of leptin insufficiency and that administration of leptin decreases one's responsiveness to the emotional and sensory aspects of food and increases the cognitive control of food intake [197].
While these studies certainly support the idea that leptin influences both the hedonic and homeostatic regulation of food intake, we are only beginning to understand how leptin is involved in the complex interaction between the hedonic and homeostatic systems. Generally, evidence from fMRI studies suggests that in addition to its homeostatic actions (which primarily involve the hypothalamus and ARC), leptin activates brain regions which could improve cognitive control and behavioral inhibition as well as diminish the perceived rewarding value of food, as evidenced by leptin's effects on activity levels in limbic and cortical structures. However, each of the three fMRI studies involving leptin administration has identified distinct regions differentially activated by leptin administration, making it difficult to draw concrete conclusions. This, however, can be explained by many factors, including methodological discrepancies. For example, while all three studies utilized whole brain analyses and functional region of interest analyses, they differ with respect to whether the reported results focus on the whole brain versus region of interest analyses and further differ regarding the specific regions of interest chosen [7; 58; 197]. These studies also differ with respect to the control (or lack thereof) of dietary intake, presentation and nature of the visual stimuli, duration of leptin administration, and the inclusion or exclusion of behavioural tasks (e.g., hunger ratings) performed by subjects while in the scanner.
Another possibility to consider is that weight-reduced subjects and congenitally leptin-deficient subjects may not react similarly to leptin (and therefore show different patterns of brain activation in response to leptin administration) because of metabolic, physiological, and developmental differences between such individuals. The severity of leptin deficiency (in both duration and degree) [181] may have important implications for the efficacy and/or nature of the brain's response to leptin administration. Additionally, the effects of leptin deficiency on brain development during prenatal, perinatal, and early development may influence brain physiology long-term. Researchers have found that children with congenital leptin deficiency exhibit cognitive improvements [181] and adults exhibit increases in gray matter concentration in the anterior cingulate gyrus, cerebellum, and the inferior parietal lobule in association with leptin administration [146], suggesting that leptin plays a role in brain development over the lifespan. Changes in brain volume over the course of these fMRI studies as a result of leptin treatment may influence results and explain inconsistencies in findings. Additional fMRI studies with variable degrees of leptin deficiency are needed to better understand the effects of leptin on the brain. Larger sample sizes, although difficult to achieve when studying rare diseases such as congenital leptin deficiency, are also needed to draw more substantial conclusions about leptin and brain activity. Nevertheless, the brain's response to leptin in states of relative leptin deficiency is currently under investigation and remains an exciting area of research. Hopefully, fMRI technology will be able to shed light on how the hedonic aspects of eating interact with, and can even override homeostatic satiety signals in states of energy excess, such as overweight and obesity [58].
The Role of Leptin in Brain Development and Synaptic Connection Plasticity
Ob/ob and db/db mice have decreased brain weight, decreased cortical volume, decreased total brain DNA content, impaired myelination, and an immature pattern of expression of synaptic and glial proteins as compared to wild-type mice [3; 222; 233]. Leptin replacement in embryonic and juvenile ob/ob mice has been shown to increase brain weight and total brain DNA content [3; 222; 233], normalize expression of synaptic and glial markers [3; 233], and promote migration of neurons to the cortical plate [233]. Leptin treatment has also been found to promote neurite outgrowth from the ARC in neonatal ob/ob mice [21]. Collectively, these studies signify the importance of leptin in early development of neurons. The effects of leptin on the brain may not be limited to the development stages. Using volumetric magnetic resonance imaging, Matochik et al. found that leptin treatment in human adults with congenital leptin deficiency results in increased gray matter tissue in the anterior cingulated gyrus, the inferior parietal lobule, and the cerebellum [147].
Leptin may also rearrange synaptic connections, or stimulate synaptic plasticity, in the hypothalamus, and this may contribute to leptin's regulation of energy homeostasis long-term [1]. Using electron microscopic stereology, Pinto et al. analyzed the synaptic density on POMC and AgRP/NPY neurons from the ARC of ob/ob and wild-type mice [184]. Compared to their wild-type littermates, ob/ob mice had significantly more inhibitory synapses and less excitatory synapses onto POMC neurons and more excitatory synapses and less inhibitory synapses onto AgRP/NPY neurons [184]. After 12 days of leptin treatment, the number of excitatory and inhibitory synapses onto POMC and NPY neurons in ob/ob mice were restored to the levels of their wild-type littermates [184]. Further studies are needed to determine whether or not these findings also apply to humans.
In addition to providing insight into the role of leptin in reward processing, fMRI can be used to assess synaptic plasticity in humans. Utilizing diffusion tensor imaging (DTI), potential changes in the anatomical connectivity between brain areas can be detected. Current studies are using DTI by fMRI to look for differences in white matter tracts in humans with relative leptin deficiency and for changes after leptin treatment.
The Role of Leptin in Neuroendocrine Physiology and Pathophysiology
Fasting results in rapid decline of leptin levels which occurs before and out of proportion to loss of fat mass [19], and this decline triggers an adaptive mechanism to conserve energy [30]. In mice and humans, this response includes decreasing reproductive hormone levels (which decreases fertility and prevents pregnancy), decreasing thyroid hormone levels that slow metabolic rate, increasing growth hormone (GH) level that may mobilize energy stores, and decreasing insulin-like growth factor-1 (IGF-1) level that may slow growth-related processes [4; 150]. The interaction between leptin and the GH axis is likely less important in humans than in animal models since patients with congenital leptin deficiency have normal linear growth, unlike mice [59; 61]. Similarly, humans, but not mice, with congenital leptin deficiency have normal adrenal function [59; 61].
We originally observed a fall in leptin concentration and associated neuroendocrine abnormalities in acutely energy-deprived lean men (i.e. fasted for 3 days), and most of these abnormalities were reversed with physiologic replacement doses of leptin [30]. We also found similar neuroendocrine abnormalities in normal-weight women after caloric deprivation; however, replacement doses of leptin only modestly restored the changes in LH pulsatility and did not affect any other neuroendocrine axes [32].
We felt that the discrepancies between these two studies were due to leptin's permissive role in regulating neuroendocrine function. Only when the circulating leptin level falls below a certain threshold does it exert a physiologically important effect. In the former study on lean men, leptin levels fell to a mean of 0.34 ng/ml in the placebo group [30]. In the latter study on normal-weight women, leptin concentrations fell by approximately 80% from mid-physiologic (14.7 ± 2.6 ng/ml) to low-physiologic (2.8 ± 0.3 ng/ml) levels with fasting [32]. From these studies, we believe that a leptin threshold of ~3 ng/mL is necessary for normal reproductive function, perhaps signaling the brain that energy stores in adipose tissue are adequate to bring pregnancy to term, as well as other neuroendocrine functions.
We then extrapolated the concept that falling leptin levels may mediate the neuroendocrine response to energy deprivation to women who are chronically energy-deprived (i.e. women with diet- and exercise-induced amenorrhea). We first hypothesized that these conditions are associated with hypoleptinemia, which was confirmed by observational studies [136; 6; 150; 110]. We then hypothesized that long-standing hypoleptinemia leads to the neuroendocrine dysfunction associated with hypothalamic amenorrhea. After performing and publishing the first pharmacodynamic studies of leptin administration in the fed [120] and fasting states [30; 32], we conducted a proof-of-concept trial of leptin treatment in replacement doses in women with hypothalamic amenorrhea [244]. We also studied neuroendocrine function [23] and insulin sensitivity/metabolism in hypoleptinemic participants with lipoatrophy [120; 138; 139]. (see below).
Leptin in relation to the hypothalamic pituitary gonadal axis
Although leptin has been shown to stimulate luteinizing hormone (LH) secretion in rodents in in vitro and in vivo studies [254], hypothalamic gonadotropin-releasing hormone (GnRH) neurons do not express ObRb [68; 85; 188]. Therefore, leptin must regulate the reproductive axis via an indirect mechanism. Recent studies have proposed that leptin mediates reproductive function by activating neurons which project afferent input to GnRH neurons in the preoptic area (PO) and other hypothalamic areas [95]. Several neurons involved in energy homeostasis are anatomically associated with GnRH neurons (e.g. AgRP/NPY and POMC neurons) and may be the link between changes in energy balance and subsequent alterations in reproductive function [95].
In addition, recent evidence suggests that leptin may mediate the reproductive axis through regulation of kisspeptins, products of the Kiss1 gene, and neurokinin B. Mutations causing dysfunction of the kisspeptin receptor gene GPR54 result in hypogonadotrophic hypogonadism in both mice and humans [49; 207], and in animals various kisspeptins have been shown to stimulate the release of GnRH and increase plasma levels of LH, follicule stimulating hormone (FSH), and total testosterone in vivo [82; 105; 228; 209]. Approximately 40% of Kiss1 mRNA-expressing cells in the ARC co-express ObRb mRNA [215]. Moreover, ob/ob mice have lower levels of Kiss1 mRNA than wild-type mice, and when ob/ob mice were treated with leptin, levels of Kiss1 mRNA increased, suggesting that the reproductive dysfunction associated with leptin deficiency may be partially due to diminished expression of kisspeptins [215]. Similarly, human mutations in the gene encoding neurokinin B or its receptor leads to defective GnRH release and subsequent hypogonadism [122]. Furthermore, a subpopulation of neurons in the ARC co-express kisspeptin, neurokinin B, and dynorphin, another peptide that is implicated in the feedback regulation of GnRH neurons [122]. These neurons also contain leptin receptors [215], and, thus, may mediate the effects of nutritional status and stress on the hypothalamic-pituitary-gonadal axis [122]. Although the exact brain areas and molecular mechanisms involved remain to be elucidated [95], compelling evidence from basic and clinical studies indicate that leptin is an important mediator of the hypothalamic-pituitary-gonadal axis.
Ob/ob mice are infertile, and their fertility can be restored with exogenous leptin administration, independent of weight loss alone [36]. In humans, congenital leptin deficiency is associated with inadequate secretion of GnRH, manifesting in hypogonadotrophic hypogonadism and, in most of the subjects, resultant failure to reach puberty, including lack of growth spurt, secondary sex characteristics, and menarche [224]. Leptin replacement can permit the appropriate onset of puberty in individuals with congenital leptin deficiency [59; 61; 128]. An uncontrolled study observed the effects of leptin replacement in three children with congenital leptin deficiency [61]. One child, who was of appropriate pubertal age, exhibited a gradual increase in gonadotropins and estradiol during leptin therapy and exhibited normal pulsatile secretion of LH and FSH after 24 months of treatment [61]. However, the other two children, both of whom were prepubertal, did not respond to leptin treatment [61]. Another uncontrolled study of adults with congenital leptin deficiency demonstrated that leptin replacement in males increased testosterone levels; increased facial, pubic, and axillary hair; promoted of penal and testicular growth; and permitted normal ejaculatory patterns [128]. These findings are consistent with our longitudinal analysis that rising leptin levels herald the onset of puberty in normal boys [137] and indicate a crucial role for leptin in mediating reproductive function.
Fasting results in decreased gonadotropin pulsatility and secretion and blunts reproductive function [4]. In otherwise healthy but acutely energy-deprived mice, leptin administration normalizes LH levels and the estrous cycle in females and restores testosterone levels in males [4]. We have shown that caloric deprivation blunts LH pulsatility and testosterone secretion in healthy, lean male participants, and that leptin replacement can restore these hormones to pre-fasting baseline levels [30]. In normal-weight women, caloric deprivation decreases LH peak frequency, which is also fully corrected with leptin [32].
Leptin replacement has also been promising as a treatment for infertility due to chronic relative leptin deficiency. In our proof-of-concept trial of leptin replacement in women with hypothalamic amenorrhea, we found that leptin can normalize LH concentration and pulse frequency within weeks of treatment and can restore ovulatory function after only months of treatment [244]. These results are remarkable as many participants were amenorrheic for years [244]. Furthermore, these improvements were independent of any weight gain or decrease in exercise activity [244]. Similar results have been seen in patients with congenital lipoatrophy [162]. An open-label study of individuals with congenital lipoatrophy found that leptin replacement increased LH response to GnRH in women and increased testosterone levels in men [162]. In addition, all eight women who had been amenorrheic prior to treatment resumed normal menses after approximately twelve months of leptin replacement [162].
As mentioned above, leptin's role in regulating reproductive function seems to be permissive with a leptin threshold of ~3 ng/mL. There are no studies using a dose-dependent design to study the effects of leptin on reproduction function in states of relative leptin deficiency. However, we have recently completed a randomized, placebo-controlled, double-blinded trial of leptin replacement in women with hypothalamic amenorrhea, and we increased the administered dose of leptin in women who had not begun menstruating with good effects (Mantzoros, unpublished data).
Leptin in relation to the hypothalamic pituitary thyroid axis
Leptin mediates its influences on the thyroidal axis by regulating thyrotropin-releasing hormone (TRH) expression [202]. In vitro and in vivo rodent studies have shown that leptin directly stimulates PVN neurons that express TRH to upregulate proTRH gene expression [121]. Leptin also indirectly influences TRH neurons in the PVN through signals from the ARC, as melanocortins can stimulate the thyroid axis and AgRP can inhibit it [115; 180]. Leptin upregulates prohormone convertases 1 and 2 (PC1 and PC2), which cleave TRH from proTRH, in the PVN [202]. In states of fasting, PC1 and PC2 levels are decreased, and leptin has been shown to restore PC1 and PC2 to pre-fasting levels [202]. Rodent studies have shown that caloric deprivation rapidly suppresses TRH expression in the PVN, leading to decreased levels of T4 and T3 [69], and leptin has been shown to reverse these changes [4].
Both complete and relative leptin deficiencies are associated with hypothalamic hypothyroidism. Ob/ob mice are hypothyroid from birth [236], and normal mice experience a drop in T4 levels with fasting [4]. In healthy individuals, TSH is secreted in a pulsatile fashion similar to that of leptin, reaching a peak in the early morning hours and a nadir in late morning [140]. Individuals with congenital leptin deficiency have highly disorganized secretion of TSH, suggesting that leptin may regulate TSH pulsatility and circadian rhythm [140]. Despite the altered circadian rhythm of TSH, the thyroid phenotype varies among humans with congenital leptin deficiency. A small, uncontrolled interventional study showed no change in TSH after leptin replacement in children with congenital leptin deficiency, though participants did exhibit increased levels of T3 and T4 [61]. Another uncontrolled study of three adults and one boy with congenital leptin deficiency observed normal thyroid function and no changes with leptin replacement [121; 180].
Humans with acute, relative leptin deficiency also have abnormalities in their hypothalamic-pituitary-thyroid axis. Our group has shown that leptin in replacement doses prevents the fasting-induced changes in TSH pulsatility and increases free T4 levels but does not affect the fasting-induced fall in T3 levels in lean males with starvation-induced leptin deficiency [30]. However, in our trial in which we studied seven normal-weight women, replacement leptin had no effect on the fasting-induced changes in TSH pulsatility or thyroid hormone levels [32]. We propose that the fall in serum leptin levels in the women did not reach the aforementioned threshold [32].
In women with hypothalamic amenorrhea, for which the average baseline leptin level was 3.4 ng/ml, leptin treatment significantly increased T3 and free T4 levels but did not affect TSH levels [244]. Rosenbaum et al. studied both lean and obese participants with relative hypoleptinemia from 10% weight loss over an average of eight weeks. Although all participants experienced a fall in leptin levels, the obese participants still had leptin levels ranging from 10 to 60 ng/ml [195]. They found that circulating concentrations of T3, T4, and TSH were reduced compared to baseline and that leptin replacement increased both T3 and T4 levels but did not affect TSH levels [195].
The lack of a significant change in TSH levels in most studies may be due to the pulsatile nature of TSH. It has also been proposed that leptin may directly stimulate T4 release from the thyroid gland and/or increase the bioactivity of TSH [195].
Leptin in relation to the hypothalamic pituitary growth hormone axis
Both ob/ob and db/db mice are known to have stunted growth curves and fragile growth plates [75; 76]. Leptin has been shown to enhance growth hormone-releasing hormone (GHRH)-induced GH secretion in rat anterior pituitary cells in vitro [186]. Furthermore, leptin has been shown to blunt the fasting-induced decrease in GH and to increase longitudinal bone growth in normal rodents [76].
The GH axis in humans, however, is not affected by leptin in the same manner or to the same extent as in mice. Children with congenital leptin deficiency have normal linear growth [59; 61], but children with the leptin receptor mutation can experience an early growth delay with inadequate concentrations of GH, IGF-I, and insulin growth factor-binding protein 3 (IGF-BP3) [38]. In healthy men, leptin replacement attenuated the starvation-induced fall in IGF-I but had no detectable short-term effect on circulating GH levels [30]. In normal-weight women, leptin replacement did not prevent the starvation-induced fall in IGF-1 and IGF-BP3 levels [32]. In women with hypothalamic amenorrhea and chronic hypoleptinemia, we have observed a significant increase in IGF-I and IGF-BP3 levels after leptin administration [244].
Leptin in relation to the hypothalamic pituitary adrenal axis
Corticotropin-releasing hormone (CRH) is synthesized in the PVN, and leptin has been found to cause a dose-dependent stimulation of CRH release in vitro [42]. Ob/ob mice have evidence of increased adrenal stimulation by adrenocorticotropic hormone (ACTH) [22], and leptin administration blunts the stress-mediated increase in ACTH and cortisol in normal mice [92]. From our analysis of leptin's pulse parameters, we have identified an inverse relationship between circulating leptin and ACTH in healthy men [129]. However, studies in humans with mutations in the leptin or leptin receptor genes reveal that these subjects still maintain normal adrenal function [38; 64]. Our studies of relative leptin deficiency in men with acute energy deprivation (i.e. starvation) [30] and women with chronic energy deprivation (i.e. hypothalamic amenorrhea) [30; 244] showed no major abnormalities in the adrenal axis, and we observed no significant change in ACTH or cortisol levels after leptin replacement. Others found similar results in women with lipoatrophy and hypoleptinemia [172]. However, all these studies were open-label. We have recently conducted a larger, randomized, placebo-controlled trial in women with hypothalamic amenorrhea and found a small but statistically significant decrease in cortisol levels with leptin treatment (Mantzoros, unpublished observations).
The Role of Leptin in Metabolism
Several leptin-deficient states, including complete leptin deficiency and lipoatrophy, are associated with insulin resistance and diabetes, and studies in both rodents and humans have shown that administration of leptin may improve glucose metabolism either directly or indirectly via reduction of fat deposited ectopically, i.e. in tissues other than adipose tissue such as the liver [24]. After being injected with leptin, ob/ob mice exhibited significantly greater reductions in serum glucose levels as compared to pair-fed ob/ob controls, even before any significant reduction in body weight [205]. Schwartz et al. calculated that 42% of leptin's hypoglycemic action was independent of weight reduction [205]. Like ob/ob mice, humans with congenital leptin deficiency are profoundly obese and can exhibit hyperinsulinemia and dyslipidemia. Leptin replacement can improve insulin, cholesterol, triglyceride, and LDL cholesterol levels in these individuals [61; 79].
Mouse models of lipoatrophy also present with a similar array of metabolic disturbances. Serving as a model of lipoatrophy, AZIP/F-1 mice completely lack white adipose tissue and are, thus, unable to produce adequate amounts of leptin, resulting in a 20-fold reduction in leptin concentration [153; 24]. Transplantation of white adipose tissue in physiologic quantities [78; 113] as well as exogenous leptin administration [213] improves insulin sensitivity in these mice. Other studies have shown that insulin sensitivity is fully restored with administration of the combination of leptin and adiponectin to lipoatrophic mice, and only partially restored with either agent alone [253]. Studies on humans with congenital and acquired lipoatrophy have noted similar improvements after leptin administration and are described further below [173; 182; 54; 108; 109; 120; 160].
Leptin improves insulin resistance by not only decreasing body weight and fat mass, especially ectopic fat or intraabdominal fat [155], but also by activating insulin-sensitive tissues, including adipose tissue and liver tissue [116]. Furthermore, leptin activates signaling pathways that overlap with those of insulin, including STAT3, MAPK, and PI3K [116]. This overlap suggests a common pathogenesis of leptin and insulin resistance in obesity and needs to be explored in human studies as it may prove significant in emerging therapies [174].
The Role of Leptin in Immune Function
Hypoleptinemic states are associated with increased risk of infection [24]. It is likely that leptin directly affects immune function as a variety of immune cells have been found to express ObRb [133; 24; 178]. In ex vivo studies, leptin has been shown to enhance phagocytic activity in macrophages [135]; to promote production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-12 (IL-12) [131]; and to stimulate chemotaxis in polymorphonuclear cells [26; 24]. In addition, we have shown that leptin promotes lymphocyte survival in vitro by suppressing Fas-mediated apoptosis [178]. Overall, leptin promotes Th1 cell differentiation and cytokine production [145]. An in vivo study showed that leptin administration improved the immune dysfunction of ob/ob mice and protected against immune dysfunction in acutely starved wild-type mice [100].
Individuals with congenital leptin deficiency have a higher incidence of infection than the general population due to decreased proliferation and function of CD4+ T cells, which normalize with exogenous leptin administration [61]. Our group has studied the effect of leptin replacement on immune function in chronically energy-deprived individuals. Leptin replacement in women with hypothalamic amenorrhea and chronic relative leptin deficiency leads to greater activation of the TNF-α system [34].
Leptin's role in immune function also seems to be a permissive one [18]. In normal-weight women with a starvation-induced decrease in leptin to 2.8 ng/ml, leptin replacement had no major effect on fasting-induced changes on most immunophenotypes and did not affect in vitro proliferative and cytokine-producing capacity of T cells, suggesting that this degree of acute leptin deficiency only minimally disrupts immune function [32]. Our group also showed that raising circulating leptin levels to between high physiologic (as seen in obesity) and low pharmacologic (as seen in extreme obesity) in lean men resulted in no significant increase in cytokines and inflammatory markers, including those known to be important in insulin resistance and cardiovascular disease such as interferon-γ (IFN-γ), IL-10, and TNF-α [29]. We found similar results in obese diabetic subjects after 4 to 16 weeks of leptin administration [29]. As such, our data does not suggest a role for leptin in the pathogenesis of the obesity-related pro-inflammatory state.
The Role of Leptin in Bone Metabolism
Early research on the effect of leptin on bone metabolism in mice suggested that leptin may decrease bone mass. Initial radiographic and histological analyses suggested that ob/ob mice have a high bone mass phenotype, and intracerebroventricular injection of leptin appeared to reduce bone mass independent of weight loss [52]. However, it now appears that leptin's action in murine bone is not consistent throughout the skeleton, but varies among skeletal regions [37]. As compared to normal mice, ob/ob mice have significantly increased bone mineral density and trabecular bone volume in the lumbar area but lower bone mineral density, trabecular bone volume, and cortical thickness in the femora [37]. Since the appendicular skeleton constitutes 80% of total bone volume and is comprised primarily of cortical bone, ob/ob mice have lower bone mass than lean mice with regard to overall bone volume [37]. In the ob/ob and db/db mice models, there is a 20-25% decrease in total bone mass, primarily at the site of cortical bone [134; 221; 88]. Leptin treatment has been shown to increase bone mineral content and bone density in ob/ob mice [221; 88].
Leptin affects bone metabolism via central and peripheral means. In mice, leptin appears to regulate cortical bone formation via sympathetic activation [87]. Ob/ob mice have diminished sympathetic tone [225], and leptin action in the VMH can increase sympathetic activity [87]. Furthermore, compared to normal mice, β1- and β2- adrenergic receptor knockout mice exhibit decreased femoral mass and cortical thickness [87] and low leptin levels [141]. Data from mice studies also indicate that leptin may mediate cortical bone formation by regulating the expression of several neuropeptides in the hypothalamus. One study found that the femora of mice lacking the NPY receptor Y2 have increased cortical bone mass and density [8], indicating that NPY inhibits cortical bone formation [87]. However, leptin injection into the PVN has been shown to increase expression of neuromedin U [248], which may decrease both cortical and trabecular bone [203]. Moreover, Yadav et al. have shown that brainstem-derived serotonin binds to Htr2c receptors on VMH neurons, favoring bone mass accrual, and that leptin inhibits this effect by reducing serotonin synthesis and firing of serotonergic neurons [251]. Thus, it appears that leptin affects the expression of many neuropeptides that affect bone metabolism either positively or negatively and more research is needed to fully elucidate the role of leptin in central regulation of bone metabolism.
Peripherally, leptin interacts with bone marrow stromal cells, osteoblasts, and osteoclasts to increase bone mass. Leptin increases expression of osteogenic genes in stromal cells and directs them to the osteogenic instead of the adipogenic pathway [227; 88; 89]. In in vitro and in vivo mice studies, leptin has also been shown to increase osteoblast proliferation, de novo collagen synthesis, and mineralization [81]. Finally, leptin stimulates stromal cells to increase osteoprotegerin expression and decrease RANK ligand, leading to decreased osteoclastogenesis [96].
To date, it is unclear if the data derived from rodent studies will also apply to humans. Indeed, the contrasting effects of leptin in the axial and appendicular skeletons of ob/ob mice do not appear to occur in humans, as individuals with leptin deficiency have decreased bone mass and density throughout the skeleton [87]. Using dual-energy x-ray absorptiometry, we assessed bone mineral content, density, and apparent density in normal weight children and adolescents. After adjusting for age, fat mass, bone-free fat mass, and serum IGF-1 and estradiol levels, we found no relationship between leptin and bone mineral density or content for either total body or body regions [193]. It has also been observed that leptin levels do not correlate with bone mineral density or bone mineral content in healthy postmenopausal women after adjustment for body composition [111]. We interpret these data as indicating that leptin's effect on bone metabolism reflects that of fat mass and is possibly mediated through changes in estradiol and IGF-1 levels.
Interventional studies suggest that leptin may play a more important role in patients with leptin deficiency. Despite the fact that patients with congenital leptin deficiency have age- and gender-appropriate bone mineral content and bone mineral density, leptin treatment increases their skeletal maturation [61]. In women with hypothalamic amenorrhea, we reported a significant increase in bone-formation markers after leptin administration [244]. However, we were unable to determine whether this increase in bone-formation markers is a direct effect of leptin and/or mediated by restoration of estradiol and/or IGF-1 levels. In addition, the duration of the study was too short to evaluate whether the increase in bone markers would translate into increased bone density or decreased risk of fractures; therefore, long-term studies are needed to fully elucidate leptin's role in bone metabolism in these subjects.
LEPTIN RESISTANCE
In contrast to the obese subjects with congenital leptin deficiency, most obese individuals have higher leptin levels than lean individuals and are resistant or tolerant to the effects of leptin [41]. In a small, proof-of-concept, phase II clinical trial, leptin was administered in escalating doses to obese participants for up to 24 weeks [93]. Although a dose-dependent response was noted, participants exhibited only modest weight loss despite supra-physiologic doses of leptin [93]. With the highest dose of leptin at 0.30 mg/kg/day, obese participants who completed the study lost a mean of 7.1 kg; however, when participants who withdrew from the study were included in the analysis, there was only a mean weight loss of 3.3 kg [93]. Furthermore, there was significant variability among the participants [93]. We have observed a similar lack of weight-reducing effects of leptin in the context of two randomized, placebo-controlled trials—one in obese, otherwise healthy participants and the other in obese, diabetic participants (Mantzoros, unpublished observations). In accordance with leptin resistance or tolerance in obesity, the effects of leptin appear to be most pronounced during states of relative leptin deficiency, e.g. fasting and hypothalamic amenorrhea or lipoatrophy.
Leptin resistance was first thought to be due to mutations of the leptin receptor and other rare monogenic obesity syndromes (Table 3). Mutations of other genes downstream of leptin, including POMC and MC4R, also result in an obese phenotype with associated neuroendocrine dysfunction [65]. However, only a few cases of human obesity are due to monogenic syndromes; instead, most instances appear to be multifactorial [64].
Table 3.
States of Leptin Deficiency and Leptin Resistance1
| Syndrome | Estimated Prevalence | Associated Features |
|---|---|---|
| Obesity associated with leptin deficiency | ||
| Complete congenital leptin deficiency | Rare | Early-onset morbid obesity, profound hyperphagia, hyperinsulinemia and type II diabetes, hypogonadotrophic hypogonadism, advanced bone age, immune dysfunction [62] |
| Heterozygous leptin deficiency | Unknown | Obesity with significantly lower than expected serum leptin levels with respect to % body fat, normal neuroendocrine function [62] |
| Fat loss associated with leptin deficiency | ||
| Congenital generalized lipoatrophy | Rare | Generalized fat wasting, impaired glucose tolerance, insulin resistance/type II diabetes, hepatic steatosis, acanthosis nigricans [138] |
| HAART-induced lipoatrophy | 15-36% of HIV-infected patients [107] | Fat wasting of the face, arms, legs, and buttocks; hypertriglyceridemia, impaired glucose tolerance, insulin resistance/type II diabetes, hepatic steatosis [138] |
| Hypothalamic amenorrhea (functional) | Up to 69% of trained female athletes [166]; 7.6% in women aged 15-24, 3.0% in women aged 25-34, and 3.7% in women aged 35-44 years [243] | Psychogenic stress, strenuous exercise, reduced fat mass, osteoporosis, amenorrhea with decreased GnRH pulsatility and estradiol levels, decreased thyroid hormone levels, increased growth hormone levels, and decreased IGF-1 [31] |
| Obesity associated with leptin resistance | ||
| Leptin receptor deficiency | Rare | Phenotype similar to congenital leptin deficiency with early-onset obesity, hyperphagia but less remarkable hyperinsulinemia. In addition, exhibit hypogonadotrophic hypogonadism, mild growth retardation, hypothalamic hypothyroidism [38] |
| POMC deficiency | Rare | Early-onset obesity, hyperphagia, ACTH deficiency with adrenal insufficiency/crisis, pale skin and red hair from lack of MSH function at MC1Rs [118] |
| MC4R deficiency | 0.5% of obese adults [57], 6% of early-onset, severe obesity [60] | Early-onset obesity, hyperphagia, increased lean mass, increased linear growth, and severe hyperinsulinemia [60] |
| Prohormone convertase 1 deficiency | Rare | Early-onset severe obesity, abnormal glucose homeostasis, hypoinsulinemia, hypogonadotropic hypogonadism, hypocortisolism [106] |
| Neurotrophin receptor tropomyosin-related kinase B mutations | Rare | BDNF deficiency resulting in severe obesity, hyperphagia, developmental delay, and cognitive dysfunction [57] |
First of all, leptin transport across the blood-brain barrier is impaired in obesity. This is partially due to saturation of the transporter by hyperleptinemia, which is associated with obesity, and subsequent decrease in transport activity [238; 25; 11]. Moreover, different brain regions may saturate at different concentrations. Banks et al. used a brain perfusion method in mice to deliver leptin at certain concentrations [12]. At a concentration of 1 ng/ml, a value seen in lean humans, the hypothalamus contained a higher concentration of leptin than any other brain region [12]. At 30 ng/mL, which represents a level in the obese range, the hypothalamus contained less leptin than other regions [12]. By selectively activating brain regions at certain concentrations, there can be different thresholds for different actions of leptin [12]. In addition to hyperleptinemia, hypertriglyceridemia also impairs the transport of leptin [13; 230]. Finally, the soluble leptin receptor isoform ObRe has also been shown to antagonize leptin transport by inhibiting surface binding and endocytosis of leptin [230].
Leptin signaling is subject to negative feedback regulation, which may be more pronounced in the obese state and associated hyperleptinemia [174]. SOCS3 is a major inhibitor of leptin signaling. SOCS3 expression is induced by the JAK2/STAT3 pathway, and SOCS3 acts to inhibit the phosphorylation and activation of JAK2 and ObRb tyrosine residue Tyr985 [163]. SOCS3 knock-out mice have been shown to have increased STAT3 expression and greater weight reduction in response to leptin administration [99; 156]. SHP2, which is also activated by leptin, is another negative regulator of STAT3-mediated gene induction [28]. Finally, protein-tyrosine phosphatase 1B (PTP1B) is another molecule that may inhibit leptin signaling by dephosphorylating JAK2, and PTP1B knock-out mice also have increased leptin sensitivity and leanness [174]. These feedback mechanisms may explain why excessive leptin can actually result in the same disturbances as leptin deficiency (e.g. insulin resistance and infertility) [24].
Endoplasmic reticulum (ER) stress has recently been shown to play a role in the development of leptin resistance. Obese, diabetic animals and humans have been shown to have higher levels of ER stress in the liver, adipose tissue, and pancreatic β-cells [177; 142; 20; 210]. Increased ER stress in obese mice has been shown to inhibit leptin receptor signaling in the hypothalamus, and chemical chaperones that decrease ER stress have been shown to increase leptin sensitivity [176]. Furthermore, intracerebrovetricular administration of thapsigargin, which induces ER stress, results in increased food intake and body weight [247]. Thus, the negative effect of ER stress on leptin sensitivity may also contribute to insulin resistance.
Targeting these mechanisms of leptin resistance will be important in the treatment for obesity and has led to development of insulin sensitizers, such as the chemical chaperones mentioned above. Roth et al. has also proposed that amylin may act synergistically with leptin to induce fat-specific weight loss [198], and the amylin analog pramlintide has been tried in conjunction with leptin in clinical trials for weight loss with modest effects [191]. However, given the complexity of leptin signaling and regulation, it is unlikely that any one target will be the key.
EMERGING CLINICAL APPLICATIONS
States of Leptin Deficiency
Congenital leptin deficiency
Complete congenital leptin deficiency from a homozygous mutation of the leptin gene is extremely rare and is often associated with consanguineous marriage [63]. Children with complete congenital leptin deficiency are born with normal weight but rapidly gain weight during the first year of life mainly due to hyperphagia [59; 61]. Both children and adults exhibit profound obesity, hyperphagia, hyperinsulinemia, and hyperlipidemia as well as reproductive and immune dysfunction [61], and anecdotal evidence indicates that a number of children with congenital leptin deficiency may die early in life due to infections [175]. Leptin in replacement doses decreases appetite, food intake, weight, and serum insulin and cholesterol levels [61]. It also facilitates appropriate pubertal development [61; 63]. Finally, leptin therapy also restores immune function by increasing the number of CD4+ T cells and memory T cells and the production of the proinflammatory cytokines IFN-γ, IL-4, and IL-10 [61]. Leptin is available for individuals with congenital leptin deficiency through an Amylin-sponsored compassionate use program.
Hypothalamic amenorrhea
Hypothalamic amenorrhea is characterized by the cessation of menstrual cycles due to dysfunction of the hypothalamic-pituitary-gonadal axis in the absence of organic disease. It occurs with excessive exercise, psychological stress, or decreased food intake and includes patients with anorexia nervosa, female athletes with the well-recognized triad (low energy availability with or without disordered eating, amenorrhea/neuroendocrine dysfunction, and osteoporosis), and normal-weight patients with ovulatory dysfunction.
Our proof-of-concept study demonstrated that replacement with human recombinant leptin in women with hypothalamic amenorrhea and chronic hypoleptinemia restores ovulatory menses [244]. After two weeks of leptin administration, mean LH concentration and pulse frequency were restored, and after three months of therapy, increases in endometrial thickness, ovarian volume, maximal follicular diameter, number of dominant follicles, and estradiol concentrations were observed [244]. Furthermore, leptin replacement normalized thyroid hormones and IGF-1 levels, but did not affect cortisol or ACTH levels [244]. Leptin replacement also increased bone-formation markers (e.g. bone alkaline phosphatase and osteocalcin) [244], and current trials are studying the effect of leptin replacement on bone mineral density. We have recently confirmed these findings in the context of a randomized, placebo-controlled trial and have found a small but statistically significant decrease in cortisol levels in response to leptin treatment (Mantzoros, unpublished observations). Thus, leptin may play a crucial role in neuroendocrine function and bone metabolism in these women and may prove to be a physiological replacement treatment for this and related conditions sharing the phenotype of “female triad.”
Lipoatrophy
Humans with congenital or HIV-associated lipoatrophy present with profound lack of subcutaneous fat, insulin resistance, fatty infiltration of the liver, and increased cardiovascular risk [27; 84] and are also found to have relative leptin deficiency [179; 165].
Congenital lipoatrophy is a rare autosomal recessive condition often associated with consanguineous marriage [179; 167]. Approximately 100 subjects have been studied in the context of small, uncontrolled interventional studies, which have shown that leptin replacement dramatically improves dyslipidemia and insulin sensitivity in these individuals and reduces glycosylated hemoglobin, hepatic gluconeogenesis, and hepatic fat content [173; 182; 108; 109]. The reduction of HbA1c by approximately 3% in these subjects who are resistant to other antihyperglycemic medications and/or high doses of insulin is impressive. Furthermore, leptin also normalizes testosterone levels and restores menstrual function in women with lipoatrophy who have polycystic ovarian syndrome [162]. Leptin is currently available through Amylin Pharmaceuticals, Inc., through an expanded access program for individuals with congenital lipoatrophy [139].
HIV-associated lipoatrophy is a much more prevalent condition, affecting an estimated 15-36% of HIV-infected patients [107]. In our initial randomized, placebo-controlled, interventional study, we found that leptin in physiologic doses improves insulin resistance, hyperlipidemia, and central fat mass in these patients [120]. A longer independent study of 6 months duration confirmed our results [160]. Studies performed to date indicate that leptin's ability to improve lipid levels and truncal fat mass is comparable to or better than that of insulin-sensitizers [138].
Current therapeutic options for patients with HIV-associated lipoatrophy and the metabolic syndrome include metformin, thiazolidinediones, GH, and synthetic GHRH [138]. We recently demonstrated that leptin acts independently of changes in the GH-IGF-1 pathway, inviting the possibility that combination therapy may produce an additive effect [23; 138]. Pioglitazone, a thiazolidinedione that increases adiponectin levels, may have beneficial effects in HIV-associated lipoatrophy and the metabolic syndrome [77] and may be another option for combination therapy. Currently, there are no published studies using combination therapy with leptin for HIV-associated lipoatrophy. Larger, randomized, placebo-controlled clinical trials of leptin alone and in combination with other therapeutics are needed to elucidate the indications, optimal dosing, and duration of leptin therapy [139], which seems to represent a viable therapeutic option for these conditions.
States of Leptin Excess
Garden-variety obesity
Initial attempts to utilize leptin as a monotherapy for obesity included using supra-physiologic doses of leptin [93; 70; 125]; however, data from these trials revealed modest, if any, weight loss and with significant variability among the participants. Hukshorn et al. have also tried pegylated leptin, which has a much longer half-life [102]. In their randomized, double-blinded trial of weekly injections of pegylated leptin in obese men for 12 weeks, there were no significant differences in weight loss or percent body fat [102].
Most recently, human recombinant leptin has been combined with potential leptin sensitizers in an attempt to overcome the effects of leptin resistance. As mentioned above, a recent phase II clinical trial confirmed that the combination of pramlintide, an amylin analog, and human recombinant leptin in obese and overweight participants produces significantly more weight loss than either treatment alone [191]. The results appear additive, however, which suggests that amylin may not improve sensitivity to leptin. These clinical data are consistent with our ex vivo and in vitro signaling data (Mantzoros, unpublished data).
Emerging evidence suggests that leptin may play a more important role in weight loss maintenance as it regulates the body's adaptation to weight loss. Reduction in fat mass results in decreased leptin concentrations, and the weight-reduced state can be interpreted by the hypothalamus as one of relative leptin deficiency. As leptin levels fall, energy expenditure, sympathetic nervous system tone, and thyroid hormones decrease to collectively drive patients to regain weight [195]. In addition, as discussed above, fMRI data also suggests that leptin treatment can prevent the changes in brain activity involved in the regulatory, emotional, and cognitive control of food intake following weight loss [197]. Leptin replacement in patients actively losing weight may allow for weight loss maintenance and is currently under investigation.
CONCLUSION
Observational and interventional studies have shown that leptin contributes to the regulation of energy homeostasis, reward processing, brain development, neuroendocrine function, metabolism, immune function, and bone metabolism. Both complete (e.g. congenital leptin deficiency) and partial leptin deficiency states (e.g. female triad / exercise induced hypothalamic amenorrhea and lipoatrophy) present with dysfunction in these systems that can be reversed with leptin treatment. Thus, we propose that leptin deficiency should be considered (and treated as) as a clinical syndrome similar to other hormone deficiency states. Although the vast majority of obese individuals are resistant or tolerant to leptin, the use of leptin in a subset of garden-variety obesity, i.e. subjects who are heterozygotes for leptin gene mutations and are thus relatively hypoleptinemic, has been suggested as a promising treatment. Further research is needed to elucidate whether leptin sensitizers will be useful and if leptin has a role in weight loss maintenance. Hopefully, more randomized, placebo-controlled clinical trials will establish leptin's efficacy and safety in states of energy deficiency and obesity, so that leptin may become part of our therapeutic armamentarium for these conditions in the not so distant future.
Acknowledgements
This work was supported by grants DK58785, DK079929, and DK081913 from the National Institute of Health to Christos Mantzoros.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abizaid A, Gao Q, et al. Thoughts for food: brain mechanisms and peripheral energy balance. Neuron. 2006;51(6):691–702. doi: 10.1016/j.neuron.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(Suppl 5):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 3.Ahima RS, Bjorbaek C, et al. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140(6):2755–62. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 4.Ahima RS, Prabakaran D, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 5.Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity--a review. Neuropeptides. 2006;40(6):375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Audi L, Mantzoros CS, et al. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry. 1998;3(6):544–7. doi: 10.1038/sj.mp.4000418. [DOI] [PubMed] [Google Scholar]
- 7.Baicy K, London ED, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104(46):18276–9. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldock PA, Allison S, et al. Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res. 2006;21(10):1600–7. doi: 10.1359/jbmr.060705. [DOI] [PubMed] [Google Scholar]
- 9.Balthasar N, Coppari R, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–91. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Balthasar N, Dalgaard LT, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des. 2001;7(2):125–33. doi: 10.2174/1381612013398310. [DOI] [PubMed] [Google Scholar]
- 12.Banks WA, Clever CM, et al. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab. 2000;278(6):E1158–65. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA, Niehoff ML, et al. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res. 2002;950(1-2):130–6. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- 14.Bates SH, Stearns WH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 15.Bayer L, Jacquemard C, et al. Survival of rat MCH (melanin-concentrating hormone) neurons in hypothalamus slice culture: histological, pharmacological and molecular studies. Cell Tissue Res. 1999;297(1):23–33. doi: 10.1007/s004410051330. [DOI] [PubMed] [Google Scholar]
- 16.Berti L, Kellerer M, et al. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a P13-kinase mediated effect. Diabetologia. 1997;40(5):606–9. doi: 10.1007/s001250050722. [DOI] [PubMed] [Google Scholar]
- 17.Bjorbaek C, Elmquist JK, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139(8):3485–91. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 18.Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89(3):991S–997S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden G, Chen X, et al. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81(9):3419–23. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 20.Boden G, Duan X, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57(9):2438–44. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouret SG, Draper SJ, et al. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 22.Bray GA, York DA. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979;59(3):719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- 23.Brennan AM, Lee JH, et al. r-metHuLeptin improves highly active antiretroviral therapy-induced lipoatrophy and the metabolic syndrome, but not through altering circulating IGF and IGF-binding protein levels: observational and interventional studies in humans. Eur J Endocrinol. 2009;160(2):173–6. doi: 10.1530/EJE-08-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology--emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2(6):318–27. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- 25.Burguera B, Couce ME, et al. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes. 2000;49(7):1219–23. doi: 10.2337/diabetes.49.7.1219. [DOI] [PubMed] [Google Scholar]
- 26.Caldefie-Chezet F, Poulin A, et al. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res. 2003;37(8):809–14. doi: 10.1080/1071576031000097526. [DOI] [PubMed] [Google Scholar]
- 27.Capeau J, Magre J, et al. Diseases of adipose tissue: genetic and acquired lipodystrophies. Biochem Soc Trans. 2005;33(Pt 5):1073–7. doi: 10.1042/BST0331073. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter LR, Farruggella TJ, et al. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci U S A. 1998;95(11):6061–6. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan JL, Bullen J, et al. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab. 2005;90(3):1618–24. doi: 10.1210/jc.2004-1921. [DOI] [PubMed] [Google Scholar]
- 30.Chan JL, Heist K, et al. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111(9):1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366(9479):74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 32.Chan JL, Matarese G, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103(22):8481–6. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan JL, Mietus JE, et al. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin Endocrinol (Oxf) 2007;66(1):49–57. doi: 10.1111/j.1365-2265.2006.02684.x. [DOI] [PubMed] [Google Scholar]
- 34.Chan JL, Moschos SJ, et al. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-alpha receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab. 2005;90(3):1625–31. doi: 10.1210/jc.2004-1823. [DOI] [PubMed] [Google Scholar]
- 35.Chan JL, Roth JD, et al. It takes two to tango: combined amylin/leptin agonism as a potential approach to obesity drug development. J Investig Med. 2009;57(7):777–83. doi: 10.2310/JIM.0b013e3181b91911. [DOI] [PubMed] [Google Scholar]
- 36.Chehab FF, Lim ME, et al. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12(3):318–20. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 37.Cirmanova V, Bayer M, et al. The effect of leptin on bone: an evolving concept of action. Physiol Res. 2008;57(Suppl 1):S143–51. doi: 10.33549/physiolres.931499. [DOI] [PubMed] [Google Scholar]
- 38.Clement K, Vaisse C, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 39.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9(4):294–8. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 40.Collins S, Kuhn CM, et al. Role of leptin in fat regulation. Nature. 1996;380(6576):677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 41.Considine RV, Sinha MK, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 42.Costa A, Poma A, et al. Stimulation of corticotrophin-releasing hormone release by the obese (ob) gene product, leptin, from hypothalamic explants. Neuroreport. 1997;8(5):1131–4. doi: 10.1097/00001756-199703240-00014. [DOI] [PubMed] [Google Scholar]
- 43.Cota D, Matter EK, et al. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28(28):7202–8. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cota D, Proulx K, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 45.Cowley MA. Hypothalamic melanocortin neurons integrate signals of energy state. Eur J Pharmacol. 2003;480(1-3):3–11. doi: 10.1016/j.ejphar.2003.08.087. [DOI] [PubMed] [Google Scholar]
- 46.Cowley MA, Smart JL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 47.Critchley HD, Wiens S, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 48.Cypess AM, Lehman S, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Roux N, Genin E, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhillon H, Zigman JM, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 51.Duan J, Choi YH, et al. Effects of subcutaneous leptin injections on hypothalamic gene profiles in lean and ob/ob mice. Obesity (Silver Spring) 2007;15(11):2624–33. doi: 10.1038/oby.2007.314. [DOI] [PubMed] [Google Scholar]
- 52.Ducy P, Amling M, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 53.Dyck DJ. Adipokines as regulators of muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab. 2009;34(3):396–402. doi: 10.1139/H09-037. [DOI] [PubMed] [Google Scholar]
- 54.Ebihara K, Masuzaki H, et al. Long-term leptin-replacement therapy for lipoatrophic diabetes. N Engl J Med. 2004;351(6):615–6. doi: 10.1056/NEJM200408053510623. [DOI] [PubMed] [Google Scholar]
- 55.Elmquist JK, Bjorbaek C, et al. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–47. [PubMed] [Google Scholar]
- 56.Ernst MB, Wunderlich CM, et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29(37):11582–93. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farooqi IS. Monogenic human obesity. Front Horm Res. 2008;36:1–11. doi: 10.1159/000115333. [DOI] [PubMed] [Google Scholar]
- 58.Farooqi IS, Bullmore E, et al. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farooqi IS, Jebb SA, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 60.Farooqi IS, Keogh JM, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 61.Farooqi IS, Matarese G, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farooqi IS, O'Rahilly S. Monogenic human obesity syndromes. Recent Prog Horm Res. 2004;59:409–24. doi: 10.1210/rp.59.1.409. [DOI] [PubMed] [Google Scholar]
- 63.Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med. 2005;56:443–58. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- 64.Farooqi IS, Wangensteen T, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237–47. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farooqi S. Insights from the genetics of severe childhood obesity. Horm Res. 2007;68(Suppl 5):5–7. doi: 10.1159/000110462. [DOI] [PubMed] [Google Scholar]
- 66.Fei H, Okano HJ, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94(13):7001–5. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Figlewicz DP, Evans SB, et al. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964(1):107–15. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 68.Finn PD, Cunningham MJ, et al. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139(11):4652–62. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- 69.Flier JS, Harris M, et al. Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring. J Clin Invest. 2000;105(7):859–61. doi: 10.1172/JCI9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fogteloo AJ, Pijl H, et al. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr Metab. 2003;16(2):109–14. [PubMed] [Google Scholar]
- 71.Gao J, Tian J, et al. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 2009;100(3):389–95. doi: 10.1111/j.1349-7006.2008.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab. 2008;294(5):E817–26. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- 73.Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS Lett. 2008;582(1):132–41. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao Q, Wolfgang MJ, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101(13):4661–6. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garris DR, Burkemper KM, et al. Influences of diabetes (db/db), obese (ob/ob) and dystrophic (dy/dy) genotype mutations on hind limb bone maturation: a morphometric, radiological and cytochemical indices analysis. Diabetes Obes Metab. 2007;9(3):311–22. doi: 10.1111/j.1463-1326.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 76.Gat-Yablonski G, Phillip M. Leptin and regulation of linear growth. Curr Opin Clin Nutr Metab Care. 2008;11(3):303–8. doi: 10.1097/MCO.0b013e3282f795cf. [DOI] [PubMed] [Google Scholar]
- 77.Gavrila A, Hsu W, et al. Improvement in highly active antiretroviral therapy-induced metabolic syndrome by treatment with pioglitazone but not with fenofibrate: a 2 × 2 factorial, randomized, double-blinded, placebo-controlled trial. Clin Infect Dis. 2005;40(5):745–9. doi: 10.1086/427697. [DOI] [PubMed] [Google Scholar]
- 78.Gavrilova O, Marcus-Samuels B, et al. Leptin and diabetes in lipoatrophic mice. Nature. 2000;403(6772):850. doi: 10.1038/35002663. discussion 850-1. [DOI] [PubMed] [Google Scholar]
- 79.Gibson WT, Farooqi IS, et al. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab. 2004;89(10):4821–6. doi: 10.1210/jc.2004-0376. [DOI] [PubMed] [Google Scholar]
- 80.Gong L, Yao F, et al. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology. 2008;149(7):3346–54. doi: 10.1210/en.2007-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gordeladze JO, Drevon CA, et al. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85(4):825–36. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 82.Gottsch ML, Cunningham MJ, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 83.Grill HJ, Schwartz MW, et al. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143(1):239–46. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 84.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 85.Hakansson ML, Brown H, et al. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18(1):559–72. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halaas JL, Gajiwala KS, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 87.Hamrick MW. Leptin and Bone: A Consensus Emerging? BoneKEy-Osteovision. 2007;4(3):99–107. [Google Scholar]
- 88.Hamrick MW, Della-Fera MA, et al. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20(6):994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 89.Hamrick MW, Della Fera MA, et al. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res. 2007;327(1):133–41. doi: 10.1007/s00441-006-0312-3. [DOI] [PubMed] [Google Scholar]
- 90.Harris RB, Zhou J, et al. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139(1):8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 91.Harvey J, McKay NG, et al. Essential role of phosphoinositide 3-kinase in leptin-induced K(ATP) channel activation in the rat CRI-G1 insulinoma cell line. J Biol Chem. 2000;275(7):4660–9. doi: 10.1074/jbc.275.7.4660. [DOI] [PubMed] [Google Scholar]
- 92.Heiman ML, Ahima RS, et al. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138(9):3859–63. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 93.Heymsfield SB, Greenberg AS, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282(16):1568–75. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 94.Hileman SM, Pierroz DD, et al. Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143(3):775–83. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- 95.Hill JW, Elmquist JK, et al. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294(5):E827–32. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holloway WR, Collier FM, et al. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17(2):200–9. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 97.Hommel JD, Trinko R, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 98.Hosoi T, Kawagishi T, et al. Brain stem is a direct target for leptin's action in the central nervous system. Endocrinology. 2002;143(9):3498–504. doi: 10.1210/en.2002-220077. [DOI] [PubMed] [Google Scholar]
- 99.Howard JK, Cave BJ, et al. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10(7):734–8. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 100.Howard JK, Lord GM, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104(8):1051–9. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsu A, Aronoff DM, et al. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. 2007;150(2):332–9. doi: 10.1111/j.1365-2249.2007.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hukshorn CJ, Saris WH, et al. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85(11):4003–9. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 103.Hummel KP, Dickie MM, et al. Diabetes, a new mutation in the mouse. Science. 1966;153(740):1127–8. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 104.Ingalls AM, Dickie MM, et al. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–8. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 105.Irwig MS, Fraley GS, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 106.Jackson RS, Creemers JW, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16(3):303–6. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 107.Jacobson DL, Knox T, et al. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis. 2005;40(12):1837–45. doi: 10.1086/430379. [DOI] [PubMed] [Google Scholar]
- 108.Javor ED, Cochran EK, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(7):1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 109.Javor ED, Ghany MG, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41(4):753–60. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 110.Jimerson DC, Mantzoros C, et al. Decreased serum leptin in bulimia nervosa. J Clin Endocrinol Metab. 2000;85(12):4511–4. doi: 10.1210/jcem.85.12.7051. [DOI] [PubMed] [Google Scholar]
- 111.Jurimae J, Jurimae T, et al. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26(6):618–23. doi: 10.1007/s00774-008-0861-5. [DOI] [PubMed] [Google Scholar]
- 112.Kiess W, Petzold S, et al. Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab. 2008;22(1):135–53. doi: 10.1016/j.beem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Kim JK, Gavrilova O, et al. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275(12):8456–60. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 114.Kim MS, Pak YK, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9(7):901–6. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- 115.Kim MS, Small CJ, et al. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest. 2000;105(7):1005–11. doi: 10.1172/JCI8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim YB, Uotani S, et al. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology. 2000;141(7):2328–39. doi: 10.1210/endo.141.7.7536. [DOI] [PubMed] [Google Scholar]
- 117.Kissileff HR, Thornton JC, et al. Maintenance of reduced body weight in humans is associated with leptin-reversible changes in appetite-related ratings.. Society for Neuroscience Annual Meeting; San Diego, CA, USA. 2007. [Google Scholar]
- 118.Krude H, Biebermann H, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 119.Lee GH, Proenca R, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 120.Lee JH, Chan JL, et al. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91(7):2605–11. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 121.Legradi G, Emerson CH, et al. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138(6):2569–76. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 122.Lehman MN, Coolen LM, et al. Minireview: Kisspeptin/Neurokinin B/Dynorphin (KNDy) Cells of the Arcuate Nucleus: A Central Node in the Control of Gonadotropin-Releasing Hormone Secretion. Endocrinology. 2010 doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leinninger GM, Jo YH, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10(2):89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leinninger GM, Myers MG., Jr. LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiol (Oxf) 2008;192(1):49–59. doi: 10.1111/j.1748-1716.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- 125.Lejeune MP, Hukshorn CJ, et al. Effect of dietary restraint during and following pegylated recombinant leptin (PEG-OB) treatment of overweight men. Int J Obes Relat Metab Disord. 2003;27(12):1494–9. doi: 10.1038/sj.ijo.0802431. [DOI] [PubMed] [Google Scholar]
- 126.Leshan RL, Bjornholm M, et al. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(Suppl 5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- 127.Levin BE, Dunn-Meynell AA, et al. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R143–50. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 128.Licinio J, Caglayan S, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101(13):4531–6. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Licinio J, Mantzoros C, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3(5):575–9. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 130.Lim CT, Kola B, et al. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2009;44(2):87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- 131.Loffreda S, Yang SQ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12(1):57–65. [PubMed] [Google Scholar]
- 132.Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357(1424):1003–37. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lord GM, Matarese G, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 134.Lorentzon R, Alehagen U, et al. Osteopenia in mice with genetic diabetes. Diabetes Res Clin Pract. 1986;2(3):157–63. doi: 10.1016/s0168-8227(86)80017-1. [DOI] [PubMed] [Google Scholar]
- 135.Mancuso P, Gottschalk A, et al. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168(8):4018–24. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 136.Mantzoros C, Flier JS, et al. Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab. 1997;82(6):1845–51. doi: 10.1210/jcem.82.6.4006. [DOI] [PubMed] [Google Scholar]
- 137.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130(8):671–80. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 138.Mantzoros CS. Whither recombinant human leptin treatment for HIV-associated lipoatrophy and the metabolic syndrome? J Clin Endocrinol Metab. 2009;94(4):1089–91. doi: 10.1210/jc.2009-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mantzoros CS. W(h)ither metreleptin for lipodystrophy and the metabolic syndrome? Endocr Pract. 2010:1–18. doi: 10.4158/EP10038.ED. [DOI] [PubMed] [Google Scholar]
- 140.Mantzoros CS, Ozata M, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86(7):3284–91. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- 141.Mantzoros CS, Qu D, et al. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45(7):909–14. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- 142.Marchetti P, Bugliani M, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50(12):2486–94. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 143.Margetic S, Gazzola C, et al. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26(11):1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 144.Maroni P, Bendinelli P, et al. Early intracellular events induced by in vivo leptin treatment in mouse skeletal muscle. Mol Cell Endocrinol. 2003;201(1-2):109–21. doi: 10.1016/s0303-7207(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 145.Matarese G, Moschos S, et al. Leptin in immunology. J Immunol. 2005;174(6):3137–42. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 146.Matochik JA, London ED, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. Journal of Clinical Endocrinology & Metabolism. 2005;90(5):2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 147.Matochik JA, London ED, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90(5):2851–4. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 148.Mayer J, Bates MW, et al. Hereditary diabetes in genetically obese mice. Science. 1951;113(2948):746–7. doi: 10.1126/science.113.2948.746. [DOI] [PubMed] [Google Scholar]
- 149.McMurtry V, Simeone AM, et al. Leptin utilizes Jun N-terminal kinases to stimulate the invasion of MCF-7 breast cancer cells. Clin Exp Metastasis. 2009;26(3):197–204. doi: 10.1007/s10585-008-9231-x. [DOI] [PubMed] [Google Scholar]
- 150.Miller KK, Parulekar MS, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab. 1998;83(7):2309–12. doi: 10.1210/jcem.83.7.4975. [DOI] [PubMed] [Google Scholar]
- 151.Minokoshi Y, Alquier T, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428(6982):569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 152.Minokoshi Y, Kim YB, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 153.Moitra J, Mason MM, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12(20):3168–81. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Montague CT, Prins JB, et al. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46(3):342–7. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- 155.Moran SA, Patten N, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism. 2004;53(4):513–9. doi: 10.1016/j.metabol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 156.Mori H, Hanada R, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10(7):739–43. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 157.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297(6):E1247–59. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta. 2009;1792(5):401–8. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morton GJ, Blevins JE, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115(3):703–10. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mulligan K, Khatami H, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94(4):1137–44. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Munzberg H, Jobst EE, et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27(1):69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Musso C, Cochran E, et al. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism. 2005;54(2):255–63. doi: 10.1016/j.metabol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 163.Myers MG, Cowley MA, et al. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–56. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 164.Myers MG., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 165.Nagy GS, Tsiodras S, et al. Human immunodeficiency virus type 1-related lipoatrophy and lipohypertrophy are associated with serum concentrations of leptin. Clin Infect Dis. 2003;36(6):795–802. doi: 10.1086/367859. [DOI] [PubMed] [Google Scholar]
- 166.Nattiv A, Loucks AB, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–82. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 167.Nishiyama A, Yagi M, et al. Two Japanese infants with congenital generalized lipodystrophy due to BSCL2 mutations. Pediatr Int. 2009 doi: 10.1111/j.1442-200X.2009.02863.x. [DOI] [PubMed] [Google Scholar]
- 168.Niswender KD, Morrison CD, et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52(2):227–31. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 169.Niswender KD, Morton GJ, et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–5. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 170.Ogunwobi OO, Beales IL. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int J Colorectal Dis. 2007;22(4):401–9. doi: 10.1007/s00384-006-0181-y. [DOI] [PubMed] [Google Scholar]
- 171.Ollmann MM, Wilson BD, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 172.Oral EA, Ruiz E, et al. Effect of leptin replacement on pituitary hormone regulation in patients with severe lipodystrophy. J Clin Endocrinol Metab. 2002;87(7):3110–7. doi: 10.1210/jcem.87.7.8591. [DOI] [PubMed] [Google Scholar]
- 173.Oral EA, Simha V, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–8. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 174.Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity (Silver Spring) 2009;18(2):221–9. doi: 10.1038/oby.2009.228. [DOI] [PubMed] [Google Scholar]
- 175.Ozata M, Ozdemir IC, et al. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 176.Ozcan L, Ergin AS, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 177.Ozcan U, Cao Q, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 178.Papathanassoglou E, El-Haschimi K, et al. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176(12):7745–52. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 179.Pardini VC, Victoria IM, et al. Leptin levels, beta-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatropic diabetes. J Clin Endocrinol Metab. 1998;83(2):503–8. doi: 10.1210/jcem.83.2.4567. [DOI] [PubMed] [Google Scholar]
- 180.Paz-Filho G, Delibasi T, et al. Congenital leptin deficiency and thyroid function. Thyroid Res. 2009;2(1):11. doi: 10.1186/1756-6614-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Paz GJ, Babikian T, et al. Leptin Replacement Improves Cognitive Development. PLoS One. 2008;3(8):7. doi: 10.1371/journal.pone.0003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Petersen KF, Oral EA, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345–50. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pierroz DD, Ziotopoulou M, et al. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51(5):1337–45. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- 184.Pinto S, Roseberry AG, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 185.Plum L, Ma X, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116(7):1886–901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pombo M, Pombo CM, et al. Hormonal control of growth hormone secretion. Horm Res. 2001;55(Suppl 1):11–6. doi: 10.1159/000063456. [DOI] [PubMed] [Google Scholar]
- 187.Qu D, Ludwig DS, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 188.Quennell JH, Mulligan AC, et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150(6):2805–12. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rahmouni K, Haynes WG, et al. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41(3 Pt 2):763–7. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 190.Rahmouni K, Sigmund CD, et al. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58(3):536–42. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ravussin E, Smith SR, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17(9):1736–43. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rios M, Fan G, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15(10):1748–57. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 193.Roemmich JN, Clark PA, et al. Relationship of leptin to bone mineralization in children and adolescents. J Clin Endocrinol Metab. 2003;88(2):599–604. doi: 10.1210/jc.2001-012025. [DOI] [PubMed] [Google Scholar]
- 194.Rogge G, Jones D, et al. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9(10):747–58. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rosenbaum M, Goldsmith R, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115(12):3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rosenbaum M, Nicolson M, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81(9):3424–7. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 197.Rosenbaum M, Sy M, et al. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118(7):2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Roth JD, Roland BL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A. 2008;105(20):7257–62. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Saad MF, Damani S, et al. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82(2):579–84. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 200.Sahu A. Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology. 1998;139(11):4739–42. doi: 10.1210/endo.139.11.6432. [DOI] [PubMed] [Google Scholar]
- 201.Sakurai T, Amemiya A, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5):1, 696. doi: 10.1016/s0092-8674(02)09256-5. page following. [DOI] [PubMed] [Google Scholar]
- 202.Sanchez VC, Goldstein J, et al. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest. 2004;114(3):357–69. doi: 10.1172/JCI21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sato S, Hanada R, et al. Central control of bone remodeling by neuromedin U. Nat Med. 2007;13(10):1234–40. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- 204.Scarpace PJ, Matheny M, et al. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273(1 Pt 1):E226–30. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- 205.Schwartz MW, Baskin DG, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45(4):531–5. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 206.Segal-Lieberman G, Bradley RL, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci U S A. 2003;100(17):10085–90. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Seminara SB, Messager S, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 208.Severin S, Ghevaert C, et al. The mitogen-activated protein kinase signalling pathways: role in megakaryocyte differentiation. J Thromb Haemost. 2009 doi: 10.1111/j.1538-7836.2009.03658.x. [DOI] [PubMed] [Google Scholar]
- 209.Shahab M, Mastronardi C, et al. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sharma NK, Das SK, et al. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93(11):4532–41. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sharma V, Mustafa S, et al. Stimulation of cardiac fatty acid oxidation by leptin is mediated by a nitric oxide-p38 MAPK-dependent mechanism. Eur J Pharmacol. 2009;617(1-3):113–7. doi: 10.1016/j.ejphar.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 212.Shimada M, Tritos NA, et al. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396(6712):670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 213.Shimomura I, Hammer RE, et al. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401(6748):73–6. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 214.Sinha MK, Ohannesian JP, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344–7. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Smith JT, Acohido BV, et al. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18(4):298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 216.Smith PM, Ferguson AV. Neurophysiology of hunger and satiety. Dev Disabil Res Rev. 2008;14(2):96–104. doi: 10.1002/ddrr.13. [DOI] [PubMed] [Google Scholar]
- 217.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35(26):2635–42. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 218.Ste Marie L, Miura GI, et al. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97(22):12339–44. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Steinberg GR, McAinch AJ, et al. The suppressor of cytokine signaling 3 inhibits leptin activation of AMP-kinase in cultured skeletal muscle of obese humans. J Clin Endocrinol Metab. 2006;91(9):3592–7. doi: 10.1210/jc.2006-0638. [DOI] [PubMed] [Google Scholar]
- 220.Steinberg GR, Rush JW, et al. AMPK expression and phosphorylation are increased in rodent muscle after chronic leptin treatment. Am J Physiol Endocrinol Metab. 2003;284(3):E648–54. doi: 10.1152/ajpendo.00318.2002. [DOI] [PubMed] [Google Scholar]
- 221.Steppan CM, Crawford DT, et al. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92(1-3):73–8. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 222.Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256(3):600–2. doi: 10.1006/bbrc.1999.0382. [DOI] [PubMed] [Google Scholar]
- 223.Sternson SM, Shepherd GM, et al. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8(10):1356–63. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 224.Strobel A, Issad T, et al. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18(3):213–5. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 225.Takeda S, Elefteriou F, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 226.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272(10):6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 227.Thomas T, Gori F, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–8. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 228.Thompson EL, Patterson M, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16(10):850–8. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 229.Tritos NA, Mantzoros CS. Leptin: its role in obesity and beyond. Diabetologia. 1997;40(12):1371–9. doi: 10.1007/s001250050838. [DOI] [PubMed] [Google Scholar]
- 230.Tu H, Kastin AJ, et al. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214(2):301–5. doi: 10.1002/jcp.21195. [DOI] [PubMed] [Google Scholar]
- 231.Tung YC, Ma M, et al. Novel leptin-regulated genes revealed by transcriptional profiling of the hypothalamic paraventricular nucleus. J Neurosci. 2008;28(47):12419–26. doi: 10.1523/JNEUROSCI.3412-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Turek VF, Trevaskis JL, et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology. 2010;151(1):143–52. doi: 10.1210/en.2009-0546. [DOI] [PubMed] [Google Scholar]
- 233.Udagawa J, Hashimoto R, et al. The role of leptin in the development of the cortical neuron in mouse embryos. Brain Res. 2006;1120(1):74–82. doi: 10.1016/j.brainres.2006.08.116. [DOI] [PubMed] [Google Scholar]
- 234.Uehara Y, Shimizu H, et al. Hypothalamic corticotropin-releasing hormone is a mediator of the anorexigenic effect of leptin. Diabetes. 1998;47(6):890–3. doi: 10.2337/diabetes.47.6.890. [DOI] [PubMed] [Google Scholar]
- 235.Valassi E, Scacchi M, et al. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18(2):158–68. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 236.van der Kroon PH, Boldewijn H, et al. Congenital hypothyroidism in latent obese (ob/ob) mice. Int J Obes. 1982;6(1):83–90. [PubMed] [Google Scholar]
- 237.van Marken Lichtenbelt WD, Vanhommerig JW, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 238.Vila R, Adan C, et al. Plasma leptin turnover rates in lean and obese Zucker rats. Endocrinology. 1998;139(11):4466–9. doi: 10.1210/endo.139.11.6296. [DOI] [PubMed] [Google Scholar]
- 239.Virtanen KA, Lidell ME, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 240.Wang JH, Wang F, et al. Leptin regulated calcium channels of neuropeptide Y and proopiomelanocortin neurons by activation of different signal pathways. Neuroscience. 2008;156(1):89–98. doi: 10.1016/j.neuroscience.2008.04.079. [DOI] [PubMed] [Google Scholar]
- 241.Wang R, Swick AG. Identification and characterization of a leptin-responsive neuroblastoma cell line. Biochem Biophys Res Commun. 2009;379(4):835–9. doi: 10.1016/j.bbrc.2008.12.157. [DOI] [PubMed] [Google Scholar]
- 242.Wang Y, Kuropatwinski KK, et al. Leptin receptor action in hepatic cells. J Biol Chem. 1997;272(26):16216–23. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- 243.Warren MP. Clinical review 77: evaluation of secondary amenorrhea. J Clin Endocrinol Metab. 1996;81(2):437–42. doi: 10.1210/jcem.81.2.8636244. [DOI] [PubMed] [Google Scholar]
- 244.Welt CK, Chan JL, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 245.White DW, Tartaglia LA. Leptin and OB-R: body weight regulation by a cytokine receptor. Cytokine Growth Factor Rev. 1996;7(4):303–9. doi: 10.1016/s1359-6101(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 246.Williams DL, Baskin DG, et al. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55(12):3387–93. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 247.Won JC, Jang PG, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17(10):1861–5. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 248.Wren AM, Small CJ, et al. Hypothalamic actions of neuromedin U. Endocrinology. 2002;143(11):4227–34. doi: 10.1210/en.2002-220308. [DOI] [PubMed] [Google Scholar]
- 249.Xu AW, Ste-Marie L, et al. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148(1):72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 250.Xu B, Goulding EH, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yadav VK, Oury F, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–89. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yamanaka A, Beuckmann CT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 253.Yamauchi T, Kamon J, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 254.Yu WH, Kimura M, et al. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A. 1997;94(3):1023–8. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yue TL, Gu JL, et al. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275(48):37895–901. doi: 10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]
- 256.Zhang F, Basinski MB, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387(6629):206–9. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 257.Zhang Y, Proenca R, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]