Abstract
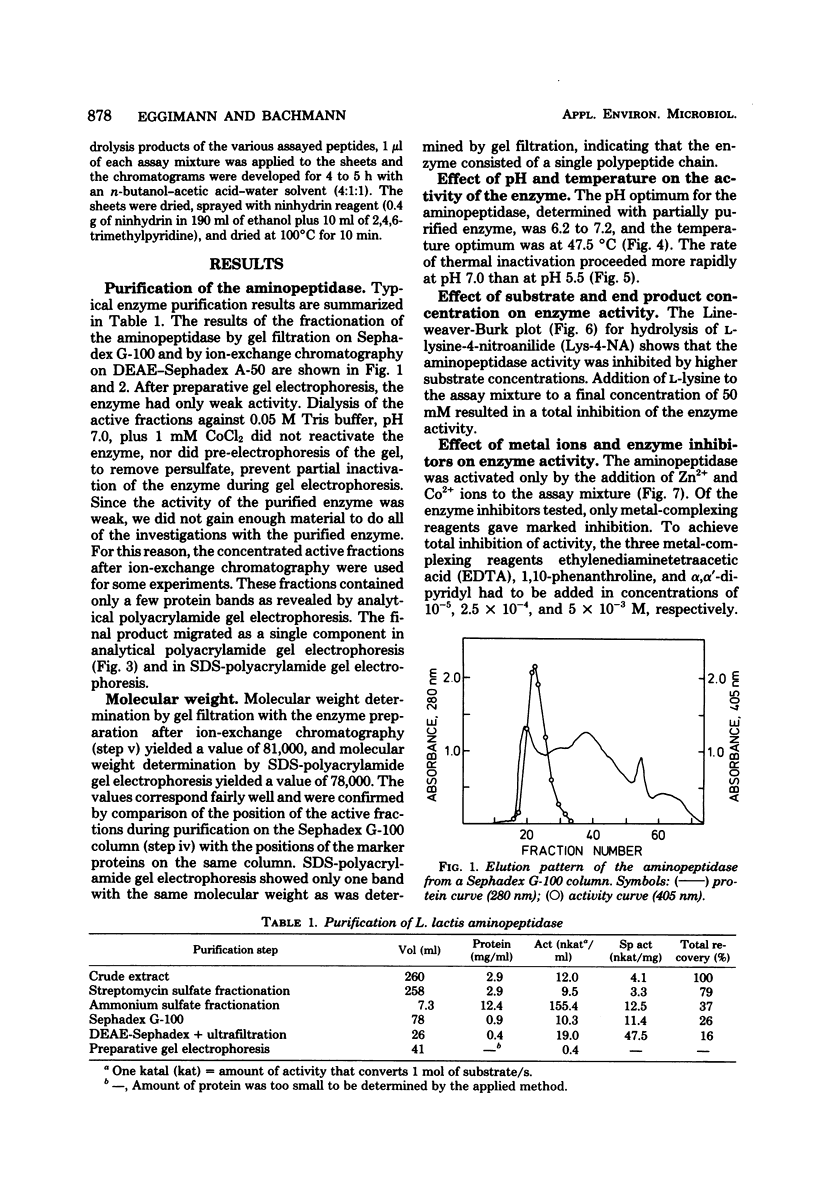
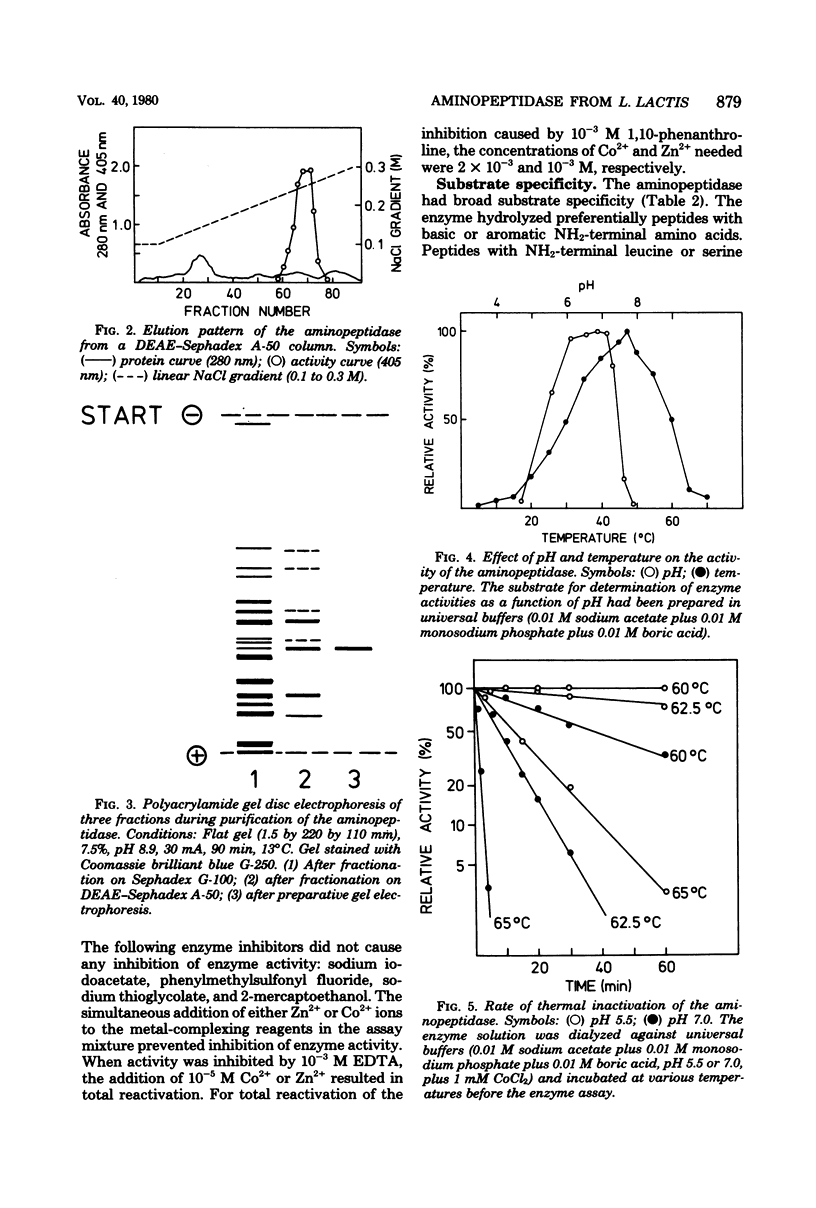
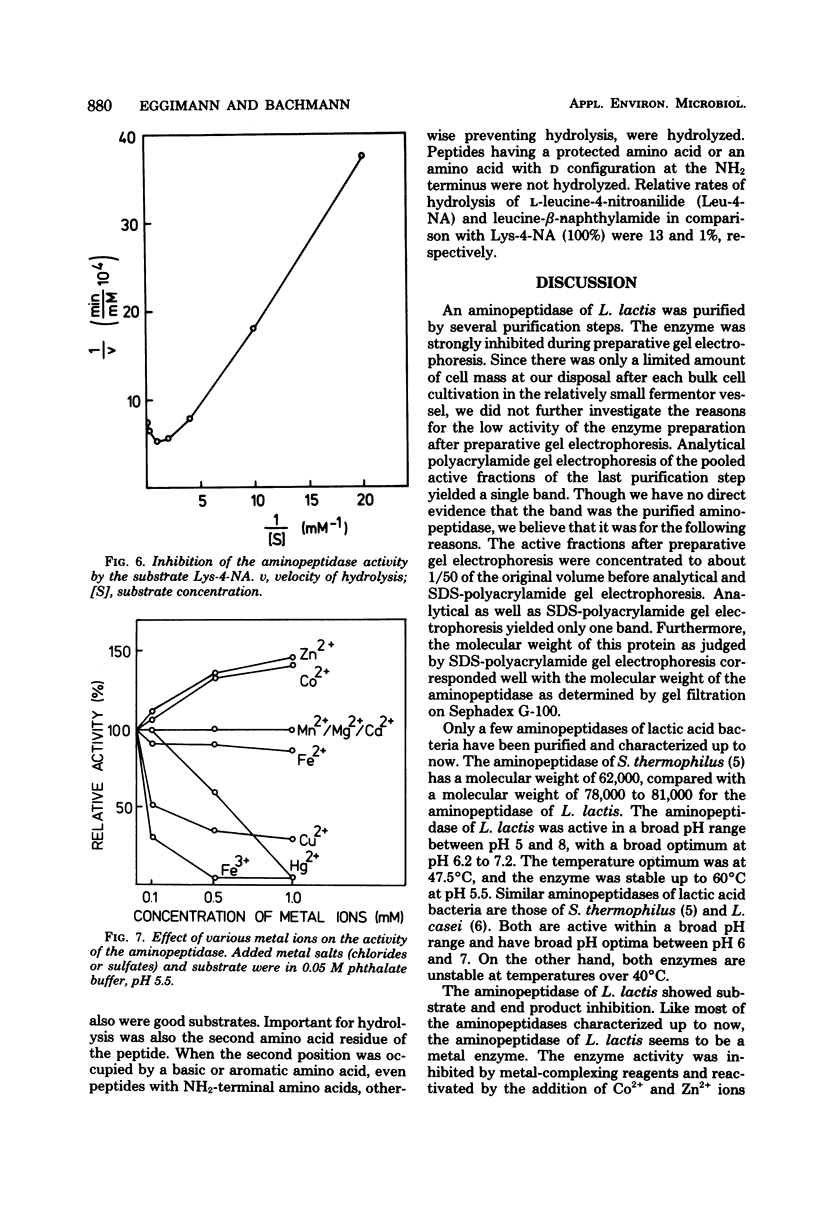
A surface-bound aminopeptidase of Lactobacillus lactis cells was solubilized with lysozyme, and the extract was subjected to streptomycin sulfate precipitation, ammonium sulfate fractionation, chromatography on Sephadex G-100 and diethylaminoethyl-Sephadex A-50, and preparative polyacrylamide gel electrophoresis. The purified enzyme was homogeneous in disc electrophoretic analysis and consisted of a single polypeptide chain with a molecular weight of 78,000 to 81,000. The optimal pH and optimal temperature for enzyme activity were 6.2 to 7.2 and 47.5°C, respectively, for l-lysine-4-nitroanilide as the substrate. The enzyme was activated by Co2+ and Zn2+ ions and inhibited by Cu2+, Hg2+, and Fe3+ ions and by the metal-complexing reagents ethylenediaminetetraacetic acid, 1,10-phenanthroline, and α,α′-dipyridyl. Higher concentrations of substrate and hydrolysis products also inhibited the activity of the enzyme. The aminopeptidase had broad substrate specificity and hydrolyzed many amino acid arylamides and many peptides with unsubstituted NH2-terminal amino acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyle P. J., Mathison G. E., Chandan R. C. Production of cell-bound proteinase by Lactobacillus bulgaricus and its location in the bacterial cell. J Appl Bacteriol. 1976 Aug;41(1):175–184. doi: 10.1111/j.1365-2672.1976.tb00616.x. [DOI] [PubMed] [Google Scholar]
- El Soda M., Bergère J. L., Desmazeaud M. J. Detection and localization of peptide hydrolases in Lactobacillus casei. J Dairy Res. 1978 Oct;45(3):519–524. doi: 10.1017/s0022029900016757. [DOI] [PubMed] [Google Scholar]
- El Soda M., Desmazeaud M. J., Bergère J. L. Peptide hydrolases of Lactobacillus casei: isolation and general properties of various peptidase activities. J Dairy Res. 1978 Oct;45(3):445–455. doi: 10.1017/s0022029900016666. [DOI] [PubMed] [Google Scholar]
- Holbrook I. B., Leaver A. G. A procedure to increase the sensitivity of staining by Coomassie brilliant blue G250-perchloric acid solution. Anal Biochem. 1976 Oct;75(2):634–636. doi: 10.1016/0003-2697(76)90118-4. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Ohmiya K., Sato Y. Purification and Properties of Intracellular Proteinase from Streptococcus cremoris. Appl Microbiol. 1975 Nov;30(5):738–745. doi: 10.1128/am.30.5.738-745.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi S. T., Barr J. K. A simplified method of quantitating protein using the biuret and phenol reagents. Anal Biochem. 1978 May;86(1):193–200. doi: 10.1016/0003-2697(78)90334-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]



