Abstract
Old World monkeys provide naturally-occurring and experimentally-induced phenotypes closely resembling the highly prevalent polycystic ovary syndrome (PCOS) in women. In particular, experimentally-induced fetal androgen excess in female rhesus monkeys produces a comprehensive adult PCOS-like phenotype that includes both reproductive and metabolic dysfunction found in PCOS women. Such a reliable experimental approach enables the use of the prenatally androgenized (PA) female rhesus monkey model to (1) examine fetal, infant and adolescent antecedents of adult pathophysiology, gaining valuable insight into early phenotypic expression of PCOS, and (2) to understand adult pathophysiology from a mechanistic perspective. Elevated circulating luteinizing hormone (LH) levels are the earliest indication of reproductive dysfunction in late gestation nonhuman primate fetuses and infants exposed to androgen excess during early (late first to second trimester) gestation. Such early gestation-exposed PA infants also are hyperandrogenic, with both LH hypersecretion and hyperandrogenism persisting in early gestation-exposed PA adults. Similarly, subtle metabolic abnormalities appearing in young nonhuman primate infants and adolescents precede the abdominal adiposity, hyperliplidemia, and increased incidence of type 2 diabetes that characterize early gestated-exposed PA adults.
These new insights into the developmental origins of PCOS, and progression of the pathophysiology from infancy to adulthood, provide opportunities for clinical intervention to ameliorate the PCOS phenotype thus providing a preventive health care approach to PCOS-related abnormalities. For example, PCOS-like traits in PA monkeys, as in PCOS women, can improve with better insulin-glucose homeostasis, suggesting that lifestyle interventions preventing increased adiposity in adolescent daughters of PCOS mothers also may reduce their risk of acquiring many PCOS-related metabolic abnormalities in adulthood.
Keywords: fetal programming, androgen excess, LH hypersecretion, anovulation, insulin resistance, obesity, PCOS-associated male phenotype
Introduction
Old World macaque species provide unique insight into the pathophysiology of polycystic ovary syndrome (PCOS). Not only do female macaques closely resemble women in terms of genome (Blekhman et al., 2008), reproductive biology (Abbott et al., 2004; Jimenez et al., 2005; Tarantal, 1992; Tarantala and Gargosky 1995; Tarantal et al., 1997), metabolic physiology (Wagner et al., 2006) and aging (Wu et al., 2005; Lee and Tarantal, 1995), they also exhibit PCOS-like traits spontaneously (Arifin et al., 2008) as well as following experimentally-induced androgen excess during early or late gestation (Abbott et al., 1998, 2005), or after acute exposure to androgen excess in adulthood (Vendola et al., 1998; Table 1). Such spontaneous and experimentally-induced PCOS-like traits are unparalleled to date in other species, providing an important model for human disease (Abbott et al, 2006; Rosenfield, 2007).
TABLE 1. The Four Adult Phenotypes For PCOS Women1 Based On The 2003 Rotterdam Consensus Criteria and Their Equivalents In Adult PA Female Rhesus Monkeys.
| Severe PCOS | Hyperandrogenism and chronic anovulation | Ovulatory PCOS | Mild PCOS | |
|---|---|---|---|---|
| Menstrual Periods | Irregular | Irregular | Normal | Irregular |
| Visualization of ovaries | Polycystic | Normal | Polycystic | Polycystic |
| Testosterone Concentrations | High | High | High | Normal |
| Prevalence of each PCOS phenotype in women: | ||||
| Mean of 4 studies: | 59% | 16% | 14% | 11% |
| Mean of the two most severe PCOS phenotypes combined: | ------------------75%----------------- | |||
| Prevalence of metabolic syndrome within each phenotype in PCOS women in two separate studies: | ||||
| Shroff et al., 2007 | 36%*** | 41%*** | 42%*** | 20% |
| Welt et al., 2006 | ………………22%……………… | 11% | 6% | |
| Prevalence of each PCOS phenotype in adult PA female monkeys: | ||||
| Early gestation-exposed (n=14) | 14% | 29% | 0% | 0% |
| Late gestation-exposed (n=9) | 11% | 33% | 11% | 0% |
| Mean of the two most severe PCOS phenotypes combined in both early and late gestation-exposed female monkeys: | -----------------44%----------------- | |||
| Prevalence of traits of metabolic relevance within the two most severe PA monkey phenotypes combined: | ||||
| Early gestation exposed PA | Type 2 diabetes (n=3/3), high insulin (n=2/2, >1SD above control basal mean), high BMI (n=3/5, >1SD above control basal mean) | |||
| Late gestation-exposed PA | High insulin (n=3/3, >1SD above control basal mean) | |||
Definitions of diagnostic criteria in women and adult PA female rhesus monkeys:
Menstrual periods (intermittent or absent): women – cycle duration >35 days or no cycles in the preceding 3 months; PA monkeys – cycle duration >34 days or no cycles during 3-6 month study periods.
Visualization of polycystic ovaries: women - ≥12, 2-9mm follicles in 1 ovary; PA monkeys – multiple ∼1mm diameter follicles in 1 ovary.
Testosterone (T) concentrations (high): women - >2 SD above control basal T mean; PA monkeys - >1 SD above control basal T mean or >1 SD above control T peak values at 24h following recombinant human chorionic gonadotropin (rhCG) administration.
BMI = body mass index (kg/m2)
Four studies of PCOS prevalence used to generate mean incidence of individual phenotypes in women: Dewailly et al., 2006; Welt et al., 2006; Diamanti-Kandarakis and Panidis, 2007; Shroff et al., 2007.
P<0.0002 versus controls (8% incidence of metabolic syndrome, Shroff et al., 2007)
Control incidence of metabolic syndrome in Welt et al., 2006: 11%.
Incidence of PA monkey metabolic traits: n= no. of PA females with the severe PCOS phenotypes/total no. of PA females exhibiting the trait.
Our focus on PCOS comes from the highly prevalent, pervasive, but heterogenous nature of the disease, and our incomplete understanding of its etiology in women. Found in ∼10% of reproductive-aged women, PCOS manifests a complex reproductive and metabolic syndrome that increases lifetime risk of type 2 diabetes, cardiovascular disease and endometrial cancer (Ehrmann, 2005). Considerable insight has been gained, however, into the developmental origins of PCOS through several animal models, particularly those employing fetal androgen excess (Abbott et al, 2006). Fetal androgen excess in genetic females (XX) is an early life endocrine disruption that bestows a myriad of masculinized traits (Abbott et al, 2005). For example, discrete experimental induction of fetal androgen excess produces at least one sign of PCOS in adult female mammals, ranging from rodents (Sullivan and Moenter, 2004; Foecking et al, 2005) and sheep (West et al, 2001), to rhesus monkeys (Abbott et al, 1998). The nonhuman primate model embodies a paradigm shift in clinical consideration from adult to fetal programming origins for PCOS (Dunaif et al., 2008), and provides a developmental trajectory of adult disease by establishing adult PCOS-like phenotypes in prenatally androgenized (PA) female rhesus monkeys that mature from their antecedents in fetal monkeys, as well as infants and adolescents.
PCOS phenotypes in women and their counterparts in adult PA female rhesus monkeys
The diagnostic criteria for PCOS established by the 2003 Rotterdam Consensus conference (Rotterdam, 2004), reflect the heterogeneity of the syndrome in reproductive-aged women. The Rotterdam Consensus criteria require the diagnosis of PCOS from the presence of two out of three characteristic signs and symptoms: (1) clinical and/or biochemical hyperandrogenism, (2) intermittent or absent menstrual cycles, and (3) polycystic ovaries. Related or similar-appearing conditions must be excluded, including classical and non-classical congenital adrenal hyperplasia, Cushing's syndrome, androgen secreting tumors, hyperprolactinemia and hyperthyroidism. Such diagnostic criteria generate four distinct PCOS phenotypes: (1) severe PCOS, (2) hyperandrogenism and chronic anovulation, (3) ovulatory PCOS, and (4) mild PCOS (Table 1; Norman et al., 2007). Despite such diversity of phenotype, most (∼75%) PCOS women manifest one of the two most severe phenotypes. Not surprisingly, as metabolic dysfunction is highly prevalent among PCOS phenotypes, metabolic syndrome is also particularly prevalent among the two most severe phenotypes (Table 1).
As shown in Table 1, PA female rhesus monkeys also exhibit phenotypes analogous to those found in PCOS women, most commonly the two most severe phenotypes. Remarkably, the incidence of these two severe PCOS phenotypes combined is similar in early (43%) and late (44%) gestation-exposed PA female monkeys, being about 60% of the incidence found among PCOS women (mean of 44% versus 75%; Table 1), perhaps because of less strident hyperandrogenism in PA monkeys (basal serum testosterone levels: PA monkeys, 0.3-0.4 ng/ml [∼50-100% elevation above normal] (Abbott et al., 1998; 2006); PCOS women, 0.5-0.7 ng/ml [∼70-200% elevation above normal] (DeVane et al., 1975; Christian et al., 2003; Phy et al., 2004; Foong et al., 2006)), and diminished incidence of polycystic ovaries (PA monkeys: ∼40% (Abbott et al., 1997; 2002); PCOS women: ∼90% (Franks, 1995)).
Another potential explanation for this differential phenotypic expression between PCOS women and PA monkeys is that short-term exogenous fetal androgen excess alone may not fully replicate the life-long androgen excess imparted by the human polycystic ovary. In other words, the androgen-producing theca cells in human PCO ovaries result in hyperandrogenism (Nelson et al, 1999; Jakimuik et al., 2001) throughout a lifetime, whereas androgen administration to PA female rhesus monkeys induces exogenous hyperandrogenism during a discrete time in fetal life, followed by a milder form of endogenous hyperandrogenism, from direct (via androgen receptor) or indirect (via estrogen receptor) fetal programming, that persists in early gestation-exposed PA infants (Abbott et al, 2008a) and in all adult PA female monkeys (Abbott et al, 2005, 2006). Moreover, genetic variability among exposed female monkeys may cause variations in their responsiveness to fetal androgen excess programming, particularly since experimental induction of fetal androgen excess is relatively uniform (Abbott et al, 2008a).
Nevertheless, the association between severe PCOS phenotypes and metabolically relevant traits in both PCOS women and their PA female monkey counterparts is striking. For instance, type 2 diabetes and hyperinsulinemia cluster among the two most severe phenotypes in early gestation-exposed PA monkeys, while hyperinsulinemia also occurs among the most severe PCOS phenotypes in late gestation-exposed PA monkeys (Table 1). In PCOS women, there is a predilection towards an increased incidence of metabolic syndrome among the two most severe PCOS phenotypes (Table 1). Thus, despite a slightly less strident expression of severe PCOS phenotypes in adult female PA female monkeys, there is a strong association of metabolic dysfunction with severe PCOS phenotypes in both women and monkeys.
Table 2 summarizes the major PCOS-like reproductive, endocrine and metabolic traits found in early gestation-exposed PA adult female rhesus monkeys, as well as those found in fetal, infant and adolescent stages, as discussed below. While it remains unclear whether ovarian hyperandrogenism the PA monkey is intrinsic to the ovary (as in PCOS) or depends on LH excess, intermittent menstrual cycles can be normalized in most PA monkeys during six months of insulin sensitizer treatment, similar to findings in PCOS women (Zhou et al., 2007; Figure 1). Therefore, ovarian pathophysiology in PA monkeys, as in PCOS women, appears to involve a component of altered insulin signaling. An additional similarity to PCOS women becomes apparent when insulin sensitizer treatment of PA monkeys ceased: intermittent menstrual cyclicity and anovulation returned (Figure 1). Of equal importance is the impaired oocyte developmental competence noted in early gestation-exposed PA monkeys (Table 2; Dumesic et al., 2002), which implicates transgenerational epigenetic transmission of abnormal cellular function to PA monkey embryos, possibly through abnormal oocyte transcription, as shown in mature PCOS oocytes (Wood et al., 2007). It is intriguing to consider that such epigenetic programming of PCOS-like traits in early gestation-exposed PA monkeys may be imposed by exogenous androgen excess during differentiation of the fetal PA ovary and its subsequent oogenesis and oocyte formation (Abbott et al., 2008b).
TABLE 2. Phenotypic PCOS-like Traits Found in Fetal, Infant, Adolescent and Adult Early Gestation-Exposed PA Female Rhesus Monkeys.
| Developmental stage | Reproductive and endocrine PCOS-like traits | Metabolic PCOS-like traits |
|---|---|---|
| Fetus | LH excess1 | ?? |
| Infant | Androgen excess1 | Relative hypersecretion of insulin2 |
| LH excess1 | Increased body weight2 | |
| Adolescent | Increased intermittent menstrual cycles3 | Increased body weight at menarche3 |
| Luteal phase defects3 | ||
| Adult | Ovarian hyperandrogenism4 | Insulin resistance4,5 |
| Intermittent/absent ovulatory menstrual cycles4,6 | Beta-cell defect5 | |
| Polycystic ovaries6 | Hyperlipidemia7 | |
| LH excess and reduced estradiol/progesterone negative feedback4 | Abdominal adiposity8 | |
| Menstrual cycles normalized by insulin sensitizer treatment7 | Type 2 diabetes9 | |
| Diminished oocyte quality10 | ||
| Adrenal hyperandrogenism11 |
Figure 1.
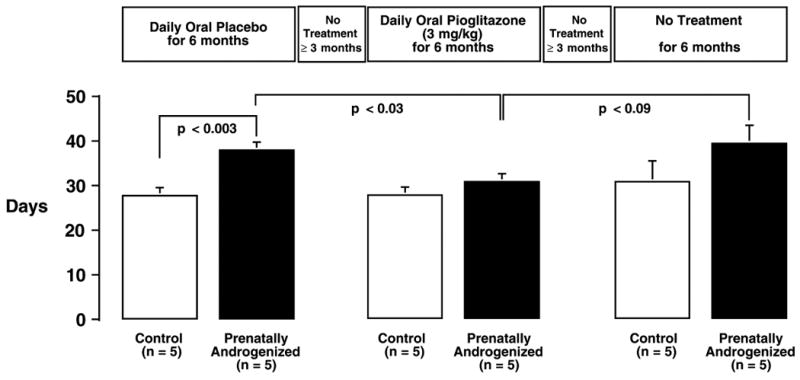
Daily oral administration of 3 mg/kg of the insulin sensitizer, pioglitazone, for six consecutive months normalizes ovulatory menstrual cycle duration in adult PA female rhesus monkeys (solid bars) compared to the previous placebo treatment period. On cessation of pioglitazone treatment, menstrual cycle abnormalities returned in PA females. Regular, ovulatory menstrual cycles were displayed by control female monkeys (open bars) throughout. Differences between individual means (+SEM) were determined from univariate F-tests performed after a significant (P<0.05) overall analysis of variance with repeated measures design. Modified from Zhou et al., 2007.
On this programming note, it is worthwhile mentioning that attempts to induce PCOS-like traits by treating adult female rhesus monkeys with prolonged exogenous androgen excess (many months), including chronic administration of testosterone or androstenedione, have failed to produce PCOS-like traits found in PA monkeys (Billiar et al., 1985, 1987; Faiman et al., 1988).
Metabolic phenotypes in male close relatives of women with PCOS and in adult male PA monkeys
Increased prevalence of impaired glucose tolerance, insulin resistance, type 2 diabetes, dyslipidemia and pancreatic beta-cell defects have all been found in fathers and/or brothers of women with PCOS (Yildiz et al., 2003; Fox, 1999; Sir-Petermann et al., 2002; Sam et al., 2008a,b). Interestingly, adult PA male rhesus monkeys exposed to the same fetal androgen treatment as their female adult PA counterparts exhibit insulin resistance and pancreatic beta-cell defects in much the same manner as found in early gestation-exposed PA females (Bruns et al., 2004). Since exogenous testosterone administration to rhesus monkey fetuses mimics normal fetal male levels of testosterone (Resko et al., 1987; Abbott et al, 2008a), the answer for this highly similar fetal programming of metabolic dysfunction in both sexes may lie in exogenous testosterone pertubation of the maternal metabolic environment during gestation. If testosterone treatment of pregnant monkey dams sufficiently reduces insulin sensitivity (Elbers et al., 2003) resulting in mild-to-moderate glucose intolerance that is otherwise only found in obese pregnant dams (Kemnitz et al., 1988), transfer of excess glucose to the fetus may induce metabolic abnormalities that only become apparent later in postnatal life (Freinkel, 1980) in either sex. The PA monkey model may thus provide novel insight into the developmental origins of metabolic dysfunction for both PCOS women and their close male kin.
Aspects of fetal, infant and adolescent phenotypes in PA monkeys preceding adult expression of PCOS-like signs and symptoms
Experimental induction of PCOS in PA female monkeys in utero allows examination of fetal, infant and adolescent phenotypes that precede expression of mature PCOS signs in adult PA monkeys. Consequently, understanding such antecedents to adult pathophysiology may provide the basis for determining biomarkers that will enable health care professionals to identify young girls at risk for PCOS and implement lifestyle modifications aimed at reducing the risk of developing the adult syndrome. To date, only early gestation-exposed PA female monkeys have been specifically examined at fetal, infant and adolescent stages for PCOS-like traits (Goy and Robinson, 1982; Abbott et al., 2008a).
Fetal phenotype
To date, LH excess is the predominant PCOS-like reproductive endocrine characteristic found in PA monkey fetuses. Following cessation of maternal testosterone propionate treatment for 25-41 consecutive days (commencing on 40-44 days of gestation; ; term 165±10 days), normal circulating androgen levels in the fetus are accompanied by elevated serum bioactive luteinizing hormone (LH) levels (Abbott et al., 2008a; Table 2), perhaps because fetal androgen excess earlier in gestation 1) increased hypothalamic gonadotropin-releasing hormone (GnRH) release (DH Abbott and JE Levine, unpublished results), 2) reduced hypothalamic GnRH sensitivity to steroid negative feedback on LH (Steiner et al., 1976), and/or 3) increased gonadotrope responsiveness to GnRH (Abbott et al., 2005). While normal LH responsiveness in early gestation-exposed PA female fetal monkeys to exogenous GnRH administered in late gestation (Abbott et al., 2008a) does not support the latter mechanism, the concomitant elevation of circulating follicle stimulating hormone (FSH) levels in early gestation-exposed PA fetuses in late gestation (Abbott et al., 2008a) suggests increased pituitary gonadotropin synthesis from enhanced fetal hypothalamic GnRH release, unrestrained by fetal ovarian negative feedback. Whether such gonadotropin findings represent early gestational acquisition of negative feedback regulation of fetal GnRH release, comparable to that acquired by normal fetal male rhesus monkeys at the same gestational age (Ellinwood et al., 1982), remains to be elucidated.
Unlike non-primate models of fetal androgen programming of PCOS-like traits, fetal growth restriction is not a trait of PA monkeys, nor most PCOS pregnancies with the exception of two PCOS populations of Spanish descent (Ibanez et al., 1993; Sir-Petermann et al., 2005). Specifically, the finding that newborn PA monkeys have normal body weights (Abbott et al., 2006, 2008) differs from observations in PA female rats (Slob et al., 1983) and PA ewes (Manikkam et al., 2004), in which both latter species exhibit fetal growth restriction and low birth weight. This species difference in PA female phenotype may represent the greater capacities of the primate liver and placenta to inactivate androgens and rapidly conjugate estrogenic products arising from aromatization (Abbott et al., 2008a), since estrogen elevations are not found in fetal PA monkeys or their dams, even during testosterone treatment. Estrogen toxicity during gestation (Mahendroo et al., 1997) could be a cause of placental impairment and fetal growth restriction in non-primate PA females.
Infant phenotype
Endogenous hyperandrogenism and LH hypersecretion characterize early gestation-exposed PA female infant monkeys (Abbott et al., 2008b; Table 2). While mean circulating levels of testosterone tend to be higher in early gesation-exposed PA versus control female infants, circulating levels of its androgenic precursor, androstenedione, are significantly elevated. With aging, basal levels of androstenedione (Zhou et al., 2005, 2007) and testosterone (Abbott et al., 1998; 2006) are both elevated in adult early gestation-exposed PA monkeys, as they are in PCOS women (DeVane et al., 1975; Dumesic, 1997), perhaps because prolonged LH excess beginning in late fetal life eventually induces a comprehensive ovarian component of androgen excess.
With regard to metabolically relevant traits, while early gestation-exposed PA infants have normal birth weights (Abbott et al., 2006, 2008a), our initial results suggest that they exhibit an approximate 10% greater body weight compared to controls by 2 months of age (Abbott et al., 2007; Table 2). Whether this finding represents relative hypersecretion of insulin, as demonstrated by early gestation-exposed PA infants (Abbott et al., 2007), or results from fetal androgen excess programming of infant weight gain remains unclear. It does, however, provide a potential early indicator of abnormal early gestation-exposed PA adolescent growth (discussed below), which may predispose to increased abdominal adiposity, as previously shown in early gestation-exposed PA adults (Eisner et al., 2003; Table 2).
Adolescent phenotype
considerably less is known about adolescent PA female monkeys than their infant or adult counterparts. The predominant PCOS-like phenotype found to date at this stage of maturation has been ovulatory dysfunction. There is an approximately 6-month delay in the age at menarche in PA adolescents compared to controls (Goy and Robinson, 1982). Following menarche, menstrual cycle differences emerge, with early gestation-exposed PA adolescents initially exhibiting prolonged intervals between ovulatory menstrual cycles, followed by a greater incidence of luteal phase defects (Goy and Robinson, 1982; Table 2). Such luteal phase defects are also found in ovulatory adult early gestation-exposed PA female monkeys (Zhou et al., 2007; R Zhou and DH Abbott, unpublished results) and PCOS women (Fleming et al., 1995; Lunn et al., 2002; Joseph-Horne et al., 2002), and may represent abnormal folliculogenesis (Dodson et al., 1975; Ayabe et al., 1994) as the first direct antecedent of ovulatory dysfunction in adult early gestation-exposedPA female monkeys (Dumesic et al., 1997). In this latter regard, even PA adult females with regular menstrual cycles exhibit diminished elevations in serum estradiol levels across the follicular phase, indicative of abnormal development of the dominant follicle (Dumesic et al., 1997).
Pre-pubertal PA females tend to exhibit ∼5-7% greater gains in body weight than controls, have greater body weight at menarche, and exhibit their maximal pre-pubertal growth velocity before, rather than after, menarche, as normally occurs in controls (Goy and Robinson, 1982; Table 2). Thus, it is interesting to speculate that such altered growth parameters in EPA adolescents could predispose to increased adiposity, which eventually favors a male-type pattern of abdominal distribution that characterizes both early gestation-exposed PA adults and PCOS women (Eisner et al., 2003; Dumesic et al., 1998; Table 2). In this latter regard, approximately 63% of adolescent PCOS girls are obese compared to about 32% obesity in normal adolescent girls (Coviello et al., 2006), and obese PCOS girls had the highest circulating androgen levels. Interestingly, treatment of prepubertal female rhesus monkeys between 5-12 months of age with exogenous androgen excess induces precociously rapid growth and weight gain, and premature menarche at about 12 months of age (van Wagenen, 1949; Figure 2), again repeating the demonstration of inextricable links between androgenic, reproductive and metabolic dysfunction in PCOS (Marshall, 2006).
Figure 2.
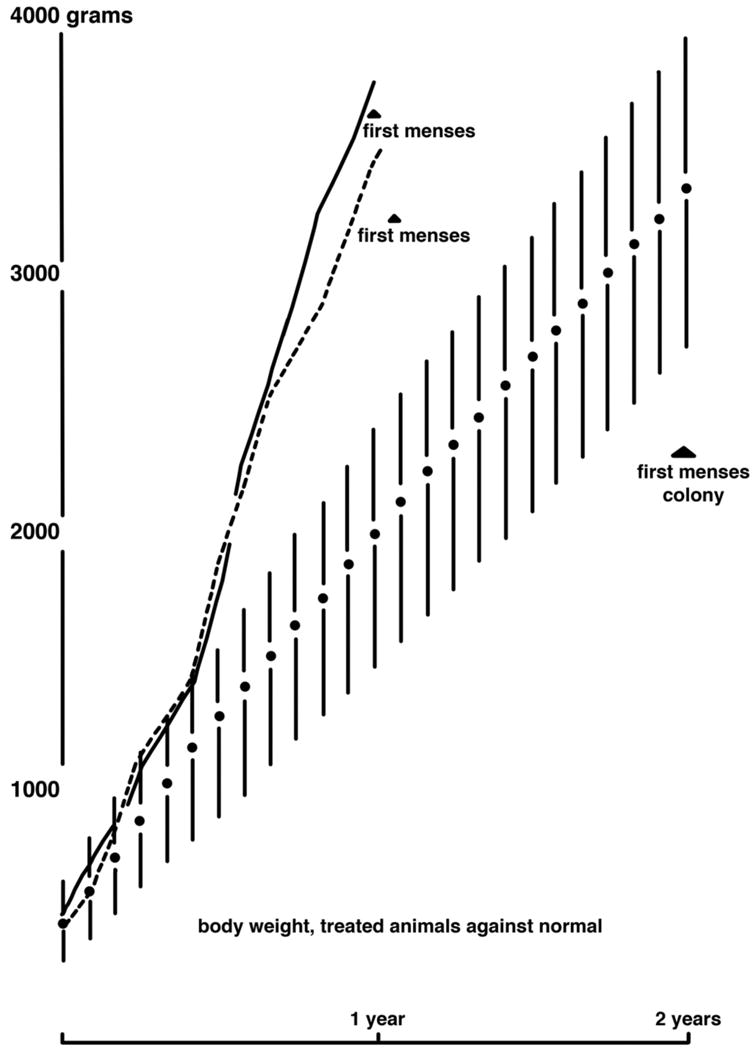
Treatment of two female rhesus monkeys between the ages of 5-12 months with intramuscular testosterone propionate (7.5 mg/kg per week; solid and dashed lines) accelerates growth rate and age at menarche (first menses) compared to untreated female rhesus monkeys (solid circles denote mean values with standard deviations). Reproduced with permission from van Wagenen (1949).
Translation of nonhuman primate findings into human therapies
The PA adult female monkey model for PCOS strongly suggests that an early perturbation from in utero androgen excess resets the reproductive trajectory, while a combination of mild-to-moderate hyperglycemic pregnancy and later-onset metabolic abnormality influences the severity of the adult reproductive phenotype. In support of this so-called “two-hit hypothesis”, the “first-hit” may represent the ability of prenatal androgen excess during a critical time of fetal development to irreversibly alter neuroendocrine function (Steiner et al., 1976; Abbott et al., 2005, 2008b). Ovarian or adrenal fetal hyperandrogenism, mediated through genetic and epigenetic interactions (Dumesic et al., 2005), may stimulate sufficient androgen excess to permanently reduce hypothalamic sensitivity to steroid negative feedback on LH (Steiner et al, 1976; Abbott et al., 2005 2008b). In doing so, it also may perpetuate ovarian hyperandrogenism through persistent LH hypersecretion in a manner characteristic of PCOS women (Pastor, 1998; Eagleson, 2000). As a “second hit”, hyperinsulinemia from adiposity-dependent insulin resistance may further contribute to reproductive dysfunction since excess postnatal weight gain in PA female monkeys (Abbott et al, 1998) and sheep (Steckler et al., in press) amplifies the reproductive disruptions caused by prenatal T excess, and insulin sensitizer-induced reductions in insulin levels improve ovulatory cyclicity for the majority of both PA female monkeys (Zhou et al., 2007) and PA ewes (Veiga-Kioez et al., 2008). Similarly, PCOS women have an increased propensity towards ovulatory dysfunction in the presence of obesity (Welt et al, 2006), with anovulatory PCOS patients having a greater body mass index than their ovulatory sisters, despite both siblings having ovarian hyperandrogenism (Legro, 2002).
That such prenatal reprogramming mechanisms may be profoundly influenced by the postnatal environment, yet go unrecognized until adulthood, raises serious health care concerns about the effect of postnatal obesity on an individual's susceptibility to a wide-range of diseases. With the present epidemic of obesity (Ludwig 2007; James 2008) which will likely induce metabolic abnormalities that may amplify preceding prenatal insults, the need for nonhuman primate models to further understand the developmental reprogramming of reproductive and metabolic function in primates is crucial to the development of new clinical strategies for diagnostics and that target abnormalities of the maternal-fetal and postnatal environments to reduce the risk of long-term adult disease.
Acknowledgments
This work was supported by NIH grants R01 RR013635, P50 HD044405, U01 HD044650, P51 RR000167 (WNPRC base operating grant) and RR00169 (CNPRC base operating grant), and was partly conducted at a facility (WNPRC) constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
References
- Abbott DH, Dumesic DA, Eisner JR, Kemnitz JW, Goy RW. The prenatally androgenized female rhesus monkey as a model for polycystic ovarian syndrome. In: Azziz R, Nestler JE, Dewailly D, editors. Androgen Excess Disorders in Women. Philadelphia: Lippencott-Raven Press; 1997. pp. 369–382. [Google Scholar]
- Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62–67. doi: 10.1016/s1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Eisner JR, Colman RJ, Kemnitz J, Dumesic DA. Prenatal androgen excess programs for PCOS in female rhesus monkeys. In: Chang RJ, Dunaif A, Hiendel J, editors. Polycystic Ovary Syndrome. New York: Marcel Dekker, Inc.; 2002. pp. 119–133. [Google Scholar]
- Abbott DH, Foong SC, Barnett DK, Dumesic DA. Nonhuman primates contribute unique understanding to anovulatory infertility in women. ILAR J. 2004;45:116–131. doi: 10.1093/ilar.45.2.116. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Schramm RD, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental etiology for polycystic ovary syndrome? Human Reproduction Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. Animal models and fetal programming of PCOS. In: Azziz R, Nestler JE, Dewailly D, editors. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. 2nd. Totowa, NJ: Humana Press Inc.; 2006. pp. 259–272. [Google Scholar]
- Abbott DH, Goodfriend TL, Dunaif A, Muller SJ, Dumesic DA. Increased body weight and enhanced insulin sensitivity in infant female rhesus monkeys exposed to androgen excess during early gestation. Abstract P2-348 presented at the 89th Annual Meeting of the Endocrine Society; Toronto, Canada. June 2-5.2007. [Google Scholar]
- Abbott DH, Barnett DK, Levine JE, Padmanbhan V, Dumesic DA, Jacoris S, Tarantal AF. Endocrine antecedents of polycystic ovary syndrome (PCOS) in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008a;79:154–163. doi: 10.1095/biolreprod.108.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Bruns CM, Barnett DK, Tarantal AF, Hoffmann SM, Zhou R, Levine JE, Dumesic DA. Fetal origins of PCOS. In: Dunaif A, Chang RJ, Franks S, Legro RS, editors. Polycystic ovary syndrome: Current controversies from the ovary to the pancreas. Totowa, NJ: Humana Press Inc.; 2008b. pp. 87–106. [Google Scholar]
- Arifin E, Shively CA, Register TC, Cline JM. Polycystic ovary syndrome with endometrial hyperplasia in a cynomolgus monkey (Macaca fascicularis) Veterinary Pathology. 2008;45:512–515. doi: 10.1354/vp.45-4-512. [DOI] [PubMed] [Google Scholar]
- Ayabe T, Tsutsumi O, Momoeda M, Yano T, Mitsuhashi N, Taketani Y. Impaired follicular growth and abnormal luteinizing hormone surge in luteal phase defect. Fertil Steril. 1994;61:652–656. doi: 10.1016/s0015-0282(16)56641-2. [DOI] [PubMed] [Google Scholar]
- Billiar RB, Richardson D, Schwartz R, Posner B, Little B. Effect of chronically elevated androgen or estrogen on the glucose tolerance test and insulin response in female rhesus monkeys. Am J Obstet Gynecol. 1987;157:1297–1302. doi: 10.1016/s0002-9378(87)80319-8. [DOI] [PubMed] [Google Scholar]
- Billiar RB, Richardson D, Anderson E, Mahajan D, Little B. The effect of chronic and acyclic elevation of circulating androstenedione or estrone concentrations on ovarian function in the rhesus monkey. Endocrinology. 1985;116:2209–2220. doi: 10.1210/endo-116-6-2209. [DOI] [PubMed] [Google Scholar]
- Blekhman R, Man O, Herrmann L, Boyko AR, Indap A, Kosiol C, Bustamante CD, Teshima KM, Przeworski M. Natural selection on genes that underlie human disease susceptibility. Curr Biol. 2008;18:883–889. doi: 10.1016/j.cub.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab. 2004;89:6218–6223. doi: 10.1210/jc.2004-0918. [DOI] [PubMed] [Google Scholar]
- Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, 2nd, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562–2568. doi: 10.1210/jc.2003-030334. [DOI] [PubMed] [Google Scholar]
- DeVane GW, Czekala NM, Judd HL, Yen SS. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am J Obstet Gynecol. 1975;121:496–500. doi: 10.1016/0002-9378(75)90081-2. [DOI] [PubMed] [Google Scholar]
- Dewailly D, Catteau-Jonard S, Reyss AC, Leroy M, Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91:3922–3927. doi: 10.1210/jc.2006-1054. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol (Oxf) 2007;67:735–742. doi: 10.1111/j.1365-2265.2007.02954.x. [DOI] [PubMed] [Google Scholar]
- Dodson KS, MacNaughton MC, Coutts JR. Infertility in women with apparently ovulatory cycles. I. Comparison of their plasma sex steroid and gonadotrophin profiles with those in the normal cycle. Br J Obstet Gynaecol. 1975;82:615–624. doi: 10.1111/j.1471-0528.1975.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67:155–163. doi: 10.1016/s0015-0282(97)81873-0. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Eisner JR, Herrmann RR, Reed JE, Welch TJ, Jensen MD. Pituitary desensitization to gonadotropin-releasing hormone increases abdominal adiposity in hyperandrogenic anovulatory women. Fertil Steril. 1998;70:94–101. doi: 10.1016/s0015-0282(98)00098-3. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Abbott DH. Early Origins of Polycystic Ovary Syndrome (PCOS) Reprod Fertil Dev. 2005;17:349–360. doi: 10.1071/rd04092. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Chang RJ, Franks S, Legro RS. Polycystic Ovary Syndrome: Current Controversies, from the Ovary to the Pancreas. Totowa, NJ: Humana Press; 2008. p. vii. [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206–1210. doi: 10.1210/jcem.85.3.6453. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res. 2003;11:279–286. doi: 10.1038/oby.2003.42. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;24352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- Elbers JM, Giltay EJ, Teerlink T, Scheffer PG, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 2003;58:562–571. doi: 10.1046/j.1365-2265.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- Ellinwood WE, Baughman WL, Resko JA. The effects of gonadectomy and testosterone treatment on luteinizing hormone secretion in fetal rhesus monkeys. Endocrinology. 1982;110:183–189. doi: 10.1210/endo-110-1-183. [DOI] [PubMed] [Google Scholar]
- Faiman C, Reyes FI, Dent DW, Fuller GB, Hobson WC, Thliveris JA. Effects of long-term testosterone exposure on ovarian function and morphology in the rhesus monkey. Anat Rec. 1988;222:245–251. doi: 10.1002/ar.1092220305. [DOI] [PubMed] [Google Scholar]
- Fleming R, McQueen D, Yates RW, Coutts JR. Spontaneous follicular and luteal function in infertile women with oligomenorrhoea: role of luteinizing hormone. Clin Endocrinol (Oxf) 1995;43:735–739. doi: 10.1111/j.1365-2265.1995.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72:1475–1483. doi: 10.1095/biolreprod.105.039800. [DOI] [PubMed] [Google Scholar]
- Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–2333. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- Fox R. Prevalence of a positive family history of type 2 diabetes in women with polycystic ovarian disease. Gynecol Endocrinol. 1999;13:390–393. doi: 10.3109/09513599909167585. [DOI] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;28:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- Goy RW, Robinson JA. Prenatal exposure of rhesus monkeys to patent androgens: Morphological, behavioral, and physiological consequences. Banbury Report. 1982;11:355–378. [Google Scholar]
- Ibañez L, Potau N, Virdis R, Zampolli M, Terzi C, Gussinyé M, Carrascosa A, Vicens-Calvet E. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76:1599–1603. doi: 10.1210/jcem.76.6.8501168. [DOI] [PubMed] [Google Scholar]
- Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- Jimenez DF, Leapley AC, Lee CI, Tarantal AF. Fetal CD34+ cells in the maternal circulation and long-term microchimerism in rhesus macaques (Macaca mulatta) Transplantation. 2005;79:142–146. doi: 10.1097/01.tp.0000144468.71962.aa. [DOI] [PubMed] [Google Scholar]
- Joseph-Horne R, Mason H, Batty S, White D, Hillier S, Urquhart M, Franks S. Luteal phase progesterone excretion in ovulatory women with polycystic ovaries. Hum Reprod. 2002;17:1459–1463. doi: 10.1093/humrep/17.6.1459. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Engle MJ, Flitsch TJ, Perelman RH, Farrell PM. Carbohydrate Intolerance and Obesity. New York: Alan R. Liss, Inc.; 1988. Nonhuman primate studies on diabetes; pp. 29–41. [Google Scholar]
- Lee CI, Tarantal AF. Effects of age on the frequency, cell cycle, and lineage maturation of rhesus monkey (Macaca mulatta) CD34+ and hematopoietic progenitor cells. Pediatr Res. 1995;58:315–322. doi: 10.1203/01.PDR.0000169975.30339.32. [DOI] [PubMed] [Google Scholar]
- Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002;87:2128–2133. doi: 10.1210/jcem.87.5.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS. Childhood obesity--the shape of things to come. N Engl J Med. 2007;357:2325–2327. doi: 10.1056/NEJMp0706538. [DOI] [PubMed] [Google Scholar]
- Lunn SF, Fraser HM, Mason HD. Structure of the corpus luteum in the ovulatory polycystic ovary. Hum Reprod. 2002;17:111–117. doi: 10.1093/humrep/17.1.111. [DOI] [PubMed] [Google Scholar]
- Marshall JC. Obesity in adolescent girls: is excess androgen the real bad actor? J Clin Endocrinol Metab. 2006;91:393–395. doi: 10.1210/jc.2005-2665. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Landrum DP, Russell DW. Fetal death in mice lacking 5alpha-reductase type 1 caused by estrogen excess. Mol Endocrinol. 1997;11:917–927. doi: 10.1210/mend.11.7.9933. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Munir I, Yen HW, Geller DH, Torbati D, Bierden RM, Weitsman SR, Agarwal SK, Magoffin DA. Insulin augmentation of 17alpha-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology. 2004;145:175–183. doi: 10.1210/en.2003-0329. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83:582–590. doi: 10.1210/jcem.83.2.4604. [DOI] [PubMed] [Google Scholar]
- Phy JL, Conover CA, Abbott DH, Zschunke MA, Walker DL, Session DR, Tummon IS, Thornhill AR, Lesnick TG, Dumesic DA. Insulin and messenger ribonucleic acid expression of insulin receptor isoforms in ovarian follicles from nonhirsute ovulatory women and polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89:3561–3566. doi: 10.1210/jc.2003-031888. [DOI] [PubMed] [Google Scholar]
- Resko JA, Buhl AE, Phoenix CH. Treatment of pregnant rhesus macaques with testosterone propionate: observations on its fate in the fetus. Biol Reprod. 1987;37:1185–1191. doi: 10.1095/biolreprod37.5.1185. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Sam S, Sung YA, Legro RS, Dunaif A. Evidence for pancreatic beta-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism. 2008;57:84–89. doi: 10.1016/j.metabol.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A. Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care. 2008;31:1237–1241. doi: 10.2337/dc07-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff R, Syrop CH, Davis W, Van Voorhis BJ, Dokras A. Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril. 2007;88:1389–1395. doi: 10.1016/j.fertnstert.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Angel B, Maliqueo M, Carvajal F, Santos JL, Pérez-Bravo F. Prevalence of Type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia. 2002;45:959–964. doi: 10.1007/s00125-002-0836-3. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–2126. doi: 10.1093/humrep/dei009. [DOI] [PubMed] [Google Scholar]
- Slob AK, den Hamer R, Woutersen PJ, van der Werff ten Bosch JJ. Prenatal testosterone propionate and postnatal ovarian activity in the rat. Acta Endocrinol (Copenh) 1983;103:420–427. doi: 10.1530/acta.0.1030420. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental programming: excess weight gain amplifies the effects of prenatal testosterone on reproductive cyclicity- implication to PCOS. Endocrinology. doi: 10.1210/en.2008-1256. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner RA, Clifton DK, Spies HG, Resko JA. Sexual differentiation and feedback control of luteinizing hormone secretion in the rhesus monkey. Biol Reprod. 1976;15:206–212. doi: 10.1095/biolreprod15.2.206. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101:7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantal AF. Sonographic assessment of nongravid female macaques (Macaca mulatta and Macaca fascicularis) J Med Primatol. 1992;21:308–315. [PubMed] [Google Scholar]
- Tarantal AF, Gargosky SE. Characterization of the insulin-like growth factor axis in the fetal macaque (Macaca mulatta and Macaca fascicularis): A cross-sectional study. Growth Reg. 1995;5:190–198. [PubMed] [Google Scholar]
- Tarantal AF, Laughlin LS, Dieter J, Tieu J, Hendrickx AG, Overstreet JW, Lasley BL. Pregnancy detection by ultrasound and chorionic gonadotropin during the peri-implantation period in the macaque (Macaca fascicularis) Early Preg: Biol Med. 1997;4:281–290. [PubMed] [Google Scholar]
- van Wagenen G. Accelerated growth with sexual precocity in female monkeys receiving testosterone propionate. Endocrinology. 1949;45:544–546. doi: 10.1210/endo-45-5-544. [DOI] [PubMed] [Google Scholar]
- Veiga-Kioez A, Herkimer C, Alekseyev A, Padmanabhan V. Developmental programming: rosigilitazone, an insulin sensitizer, improves insulin sensitivity and reproductive cycles in prenatal testosterone-treated female sheep. Abstract P2-495 presented at the 90th Annual Meeting of the Endocrine Society; San Francisco, CA. June15-18.2008. [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, Jr, Kaplan JR. Old World nonhuman primate models of type 2 diabetes mellitus. ILAR J. 2006;47:259–271. doi: 10.1093/ilar.47.3.259. [DOI] [PubMed] [Google Scholar]
- Welt CK, Gudmundsson JA, Arason G, Adams J, Palsdottir H, Gudlaugsdottir G, Ingadottir G, Crowley WF. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab. 2006;91:4842–4848. doi: 10.1210/jc.2006-1327. [DOI] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol. 2001;185:51–59. doi: 10.1016/s0303-7207(01)00632-3. [DOI] [PubMed] [Google Scholar]
- Wood JR, Dumesic DA, Abbott DH, Strauss JF. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med. 2005;230:818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–2036. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:6630–6637. doi: 10.1210/jc.2005-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol. 2007;23:438–448. doi: 10.1016/j.reprotox.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


