SUMMARY
Human ES cells are the pluripotent precursor of the three embryonic germ layers. Human ES cells exhibit basal-apical polarity, junctional complexes, integrin-dependent matrix adhesion, and E-cadherin-dependent cell-cell adhesion, all characteristics shared by the epiblast epithelium of the intact mammalian embryo. After disruption of epithelial structures, programmed cell death is commonly observed. If individualized human ES cells are prevented from reattaching and forming colonies, their viability is significantly reduced. Here we show that actin-myosin contraction is a critical effector of the cell death response to human ES cell dissociation. Inhibition of myosin heavy chain ATPase, downregulation of myosin heavy chain, and downregulation of myosin light chain all increase survival and cloning efficiency of individualized human ES cells. ROCK inhibition decreases phosphorylation of myosin light chain, suggesting that inhibition of actin-myosin contraction is also the mechanism through which ROCK inhibitors increase cloning efficiency of human ES cells.
INTRODUCTION
Embryonic stem (ES) cells can proliferate without limit and can differentiate to all cell types of the body (Evans and Kaufman, 1981; Martin, 1981; Thomson et al., 1998). Although human and mouse ES cells share these basic properties, they are distinct in cell surface markers, morphology, and growth factor requirements. These differences now appear to reflect different embryological origins rather than species specific differences, as human ES cells more closely resemble pluripotent cell lines derived from the epithelial cells of the mouse epiblast (EpiSC) (Brons et al., 2007; Tesar et al., 2007). Human ES cells clone at a very poor efficiency under standard culture conditions, likely reflecting their growth in compact, epithelial colonies (Krtolica et al., 2007). Epithelia are tightly coupled by junctions and are separated from stroma by basement membranes, both of which restrict movements between body compartments. Human ES cells are generally grown on matrices that resemble basement membranes, and form colonies with ultrastructural characteristics similar to the epiblast epithelium with tight junctions and apical microvilli (Krtolica et al., 2007; Sathananthan et al., 2002). Cell-cell junctions between human ES cells also include gap junctions and E-cadherin-mediated cell adhesion (Ullmann et al., 2007; Wong et al., 2004). Cell-matrix adhesion is necessary for human ES cell survival, and involves binding through β1 and α integrins (Braam et al., 2008).
The proliferation and survival of cells in epithelial structures are tightly controlled in vivo, and epithelial cells placed out of their normal environment often die through a process termed anoikis (“homeless”), a specialized form of programmed cell death due to tissue disorganization. Anoikis can result from both caspase-dependent and independent pathways (Wang et al., 2003), and is initiated by the disruption of the junctions with the basement membrane and between adjacent epithelial cells (Ellerstrom et al., 2007; Watanabe et al., 2007). Without drug treatment that blocks specific effectors of anoikis, dissociated epithelial cells die in suspension (Watanabe et al., 2007). Anoikis is normally prevented by integrin-mediated extracellular matrix signaling and cadherin-mediated cell-cell signaling (Ndozangue-Touriguine et al., 2008; Simpson et al., 2008). Integrin- and cadherin-mediated signaling pathways influence epithelial cell shape, polarization, and survival through interactions with the cytoskeleton, and disruption of these adhesion pathways dramatically alters cytoskeletal architecture.
The actin-myosin based cytoskeleton is a dynamic system essential for contraction, motility, and tissue organization (Even-Ram et al., 2007; Ivanov, 2008; Ivanov et al., 2007; Montell, 2008). Actin-myosin motors consist of actin filaments and non-muscle myosin II heavy chains (MYH). Actin filaments are often anchored on integrins and E-cadherins through focal-adhesion and α/β catenin complexes, respectively. The hydrolysis of ATP by the MYH ATPase generates energy and causes the MYHs to slide along actin filaments, resulting in contraction. The process is triggered by the binding of myosin light chain (MLC), which is activated by phosphorylation through kinases, such as Rho-associated kinase (ROCK) or MLC kinase (MLCK) (Quintin et al., 2008). In order to maintain normal morphology and function, a balance has to be achieved between actin-myosin contraction forces and opposing anchoring forces (Lecuit and Lenne, 2007; Montell, 2008; Okeyo et al., 2009). If cells are removed from the restraint of their extracellular matrix and cell contacts, the balance is broken and actin-myosin is free to contract, generating altered phenotypes, including excessive membrane blebbing (Fackler and Grosse, 2008; Frey et al., 2006; Ivanov et al., 2005; Sathananthan et al., 2002). Without re-establishing cell adhesion, the excessive actin-myosin contraction can ultimately result in cell death.
Here we report that disruption of actin-myosin contraction in individualized human ES cells dramatically improves cell survival and cloning efficiency. Blebbistatin is a non-muscle myosin II heavy chain inhibitor, and is named after its ability to specifically inhibit membrane blebbing (Limouze et al., 2004; Straight et al., 2003). We find that blebbistatin not only represses cell blebbing of human ES cells, but also dramatically improves cell survival and cloning efficiency in defined conditions. Furthermore, we observe that other approaches to disrupting actin-myosin contraction, including downregulation of myosin heavy chain mRNAs or downregulation of myosin light chain, replicates the effects of blebbistatin both on human ES cell membrane blebbing and survival. Recently, inhibition of Rho-associated kinase (ROCK) was shown to regulate cloning efficiency and epithelial structure of human ES cells. We find that actin-myosin contraction is a downstream target of ROCK regulation in cloning and survival. Our combined results demonstrate a central role of actin-myosin contraction in the death of dissociated human ES cells.
RESULTS
Cell-Cell Contact Promotes Human ES Cell Survival
Most individualized human ES cells die when left in suspension or when plated at clonal cell densities, even when plated on an appropriate matrix that normally supports human ES cells plated in clumps. This cell death could be due to damage associated with cell dissociation, or could be due to a lack of cell-cell survival signals. We first sought to examine the time course of cell death and proliferation of individualized human ES cells (Figure 1A). Most human ES cells attached to the plate in less than 1 hour, and migrated extensively in the first few hours (Video S1A). However, cells started to detach and die at around 6 hours. The number of attached cells that remained reached a low point by about 12 hours, and after 24 hours, cell numbers stabilized or increased, depending on plating density. When the number of surviving, attached cells over time was plotted as a percentage of the cells initially plated, both initial survival and subsequent increase in cell number were higher for cells plated at higher density (Figure 1A). We found that cell survival increases with direct cell-cell contact (Figure S1A), which depends on E-cadherin (Figure S1B and Video S1C).
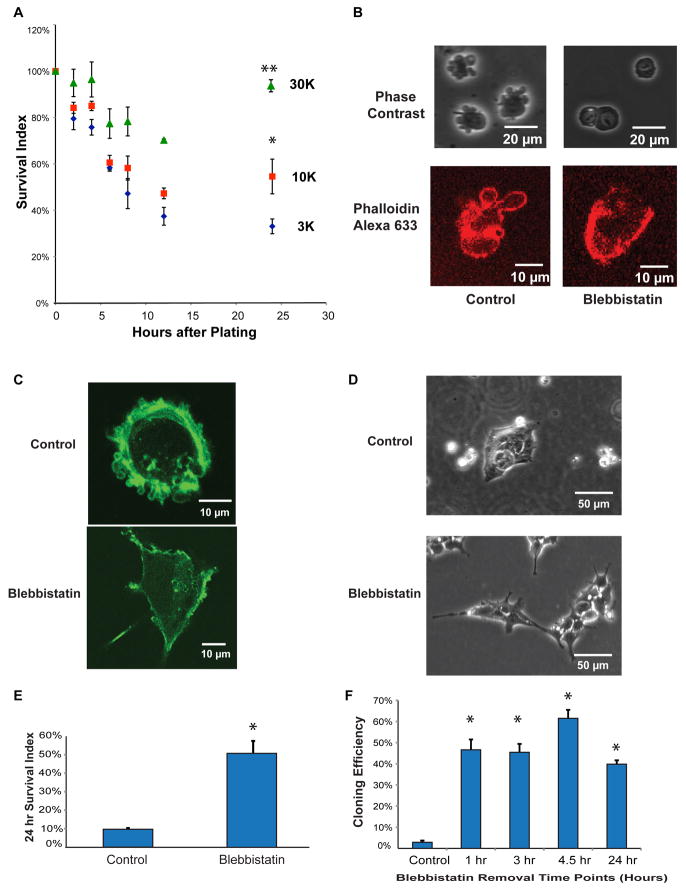
Figure 1. Inhibition of membrane blebbing improves cell viability after dissociation in human ES cells.
(A) A time course of density-dependent survival after dissociation. Different numbers of human ES cells were plated onto each well (3,000/cm2 (3K-blue diamond), 10,000/cm2 (10K-red square) and 30,000/cm2 (30K-green triangle)). The “Survival Index” represents the number of surviving cells divided by the number of input cells. (Note that as cells divide, this index can eventually exceed 100%.) (*) signifies that the 10K density Survival Index differed significantly (p< 0.05) from the 3K density survival at 24 hrs. (**) signifies that the 30K density Survival Index differed significantly (p<0.05) from the 10K survival at 24 hr. For more details, see Experimental Procedures, Statistics section.
(B) Phase contrast images of human ES cells dissociated by TrypLE with or without 10 μM blebbistatin. Blebbistatin changed actin organization immediately after dissociation, as visualized by Alexa-633 conjugated phalloidin (Red).
(C) Blebbistatin inhibited blebbing and improved cell spreading 1 hr after plating as visualized by Alexa- staining.
(D) Colony morphology of human ES cells 24 hours post splitting and treatment with blebbistatin.
(E) The 24-hour survival of individualized human ES cells was significantly improved when 10 μM blebbistatin was added to cells plated at 10,000/cm2 (*p < 0.05). Comparable experiments were repeated more than 10 times.
(F) Treatment of human ES cells with blebbistatin for one hour or longer was sufficient to improve the cloning efficiency (*p <0.05). Individualized cells were plated in the presence and absence of 10 μM Blebbistatin at a density of 150 cells/cm2. Blebbistatin was removed after indicated time points. Cloning efficiency was then calculated after 7 days (*p <0.05). Comparable time series experiments were repeated 2 times. In more than 40 single-time point experiments, blebbistatin consistently increased the cloning efficiency of two human ES cell lines (H1 and H9) and two iPS cell lines (iPS-foreskin and iPS-IMR90) (see Table S1).
Blebbistatin Inhibits Cell Blebbing After Dissociation
Because human ES cells share an epithelial ultrastructure with the epiblast, and because cell contact is important in human ES cell survival, we examined whether cell death of individualized human ES cells shares features of the anoikis observed in other epithelial cell types. Pronounced membrane blebbing, observed after the dissociation of epithelial cells, is an indicator of actin-myosin cytoskeletal contraction and is often a prelude to cell death (Coleman et al., 2001; Croft et al., 2005). We observed bubble-shaped membrane protrusions on the surface of human ES cells just seconds after cells were individualized (Figure 1B). The membrane blebbing was suppressed by blebbistatin, a myosin heavy chain ATPase inhibitor, implicating actin-myosin contraction in human ES cell blebbing (Figure 1B). We next used Phalloidin to visualize the actin filament organization in dissociated cells. Ring-shaped actin structures, characteristic of blebbing in other cell types, were observed only in untreated cells (Figure 1B), and persisted for up to a few hours after plating (Figure 1C). Without drug treatment, blebbing was eventually suppressed at the site of cell-cell junctions of those human ES cells that did successfully form clusters after plating and attachment (Figure S1C). When blebbistatin was added during human ES cell plating, it not only inhibited cell blebbing, but also helped cells attach and spread on the matrigel plates after 30~60 minutes of treatment (Figure 1C). Most individualized human ES cells treated with blebbistatin that survived on matrigel for 24 hours formed loose colonies with distinct cell boundaries (Figure 1D).
Blebbistatin Increases Cell Survival and Cloning Efficiency of Human ES Cells
Membrane blebbing can cause cell death (Croft et al., 2005), so we examined whether inhibition of membrane blebbing improves human ES cell survival. Blebbistatin improved survival of human ES cells examined at 24 hours (Figure 1E), and supported an increased cloning efficiency of cells plated at low density and examined for colony formation after 5 days (Figure 1F). Time-lapse photography confirmed that blebbistatin facilitated colony formation through the proliferation of a single cell, not by aggregation (Video S1B). Comparable effects of blebbistatin on cloning efficiency were observed in two human ES cell lines (H1 and H9), and two human iPS cell lines (iPSimr90C4 and iPSforeskinC6.1) (Yu et al., 2007) (Table S1). After blebbistatin treatment, human ES cells were passaged 5 times and continued to express ES cell markers, including OCT4 (Figure S1D), maintained normal karyotypes (Figure S1E), and formed teratomas. Blebbistatin also helped cell attachment (Figure S1F) and improved human ES cell survival on tissue culture plates not treated with matrigel. Blebbistatin also helped survival of suspended human ES cells examined at 24 hours (Figures S1G and S1H). Combined, our results suggest that inhibition of myosin function by blebbistatin reduces the requirement for cell-cell and cell-matrix associated signaling in the survival of human ES cells.
MYH9 is the Major Human ES Cell Target for Blebbistatin in Survival and Cloning
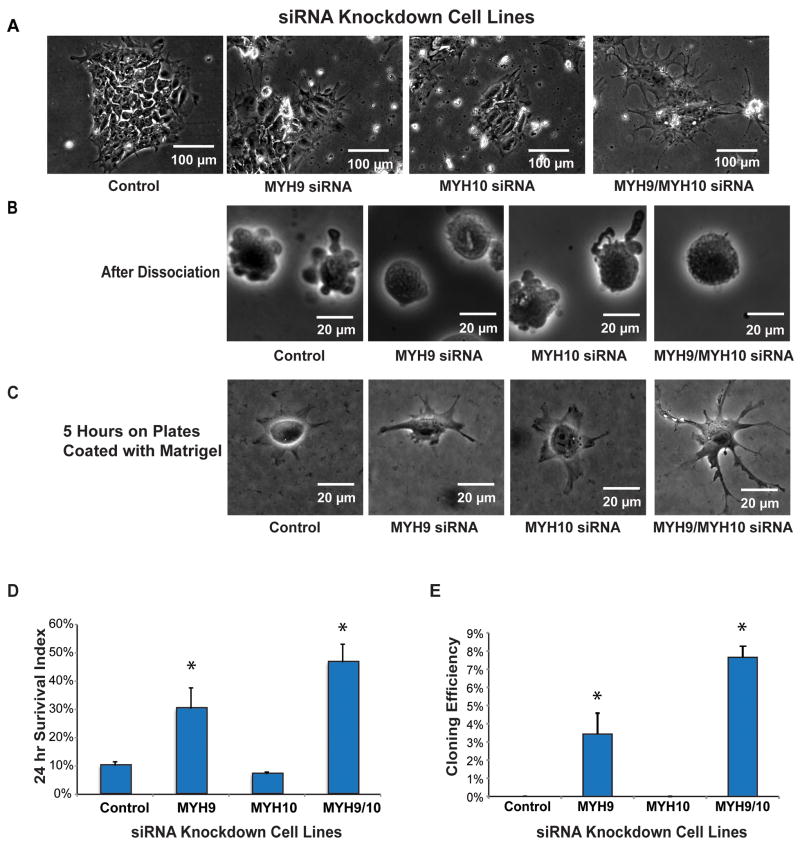
Blebbistatin is a myosin II heavy chain inhibitor whose binding requires four conserved amino acids in the myosin cleft (Allingham et al., 2005; Limouze et al., 2004). Because MYH9 and MYH10 are the most highly expressed MYHs with the conserved sites in human ES cells, we first used SMART Pool siRNAs to knockdown their mRNAs (Table S2). Knockdown efficiency and specificity were examined with quantitative RT-PCR (Table S3 and Figure S2A). Normal morphology and growth were observed after MYH10 siRNA treatment. In a few days, slower cell growth and stretched pseudopods were observed in cells treated with MYH9 siRNA, and the phenotypic changes were most severe when cells were treated with both MYH9 and MYH10 (MYH9/MYH10) siRNAs (Figure 2A). Similar to the effect of blebbistatin, the blebbing phenotype was suppressed after dissociation when MYH9 or MYH9/MYH10 were silenced (Figure 2B). The silencing of MYH9/MYH10 also led to phenotypic spreading changes after plating, comparing to control cells (Figure 2C). Knockdown of MYH9 or MYH9/MYH10 improved both initial cell survival (Figure 2D) and cloning efficiency (Figure 2E). Time-lapse experiments confirmed that colonies were formed from single cells in MYH9 and MYH9/MYH10 siRNA treated cells. Cells treated with MYH10 siRNA alone behaved comparably to control cells (Figure 2). MYH’s role was further confirmed by using individual siRNA duplexes and Select siRNAs that employed a different duplex design (Table S2, Figures S2B-C and S2H-K). All the results demonstrated that MYH9 silencing inhibited blebbing, and improved cloning efficiency, while MYH10 siRNA treated cells behaved comparably to control cells.
Figure 2. Knockdown of Non-Muscle Myosin Heavy Chains (MYH) Reduces Blebbing and Improves Survival and Cloning Efficiency of Human ES Cells.
Knockdown (by siRNA) of MYH9 alone or in combination with MYH10:
(A) changed ES cell morphology in colonies 96 hours after transfection,
(B) reduced blebbing immediately after dissociation,
(C) increased attachment/spreading 5 hr after plating,
(D) increased (*p<0.05) survival 24 hr after plating, and
(E) increased (*p<0.05) cloning efficiency. Comparable results were obtained in multiple experimental repetitions using both H1 (n=15) and H9 human ES cell lines (n=2).
Inhibition of Myosin Light Chain Suppressed Cell Death After Dissociation
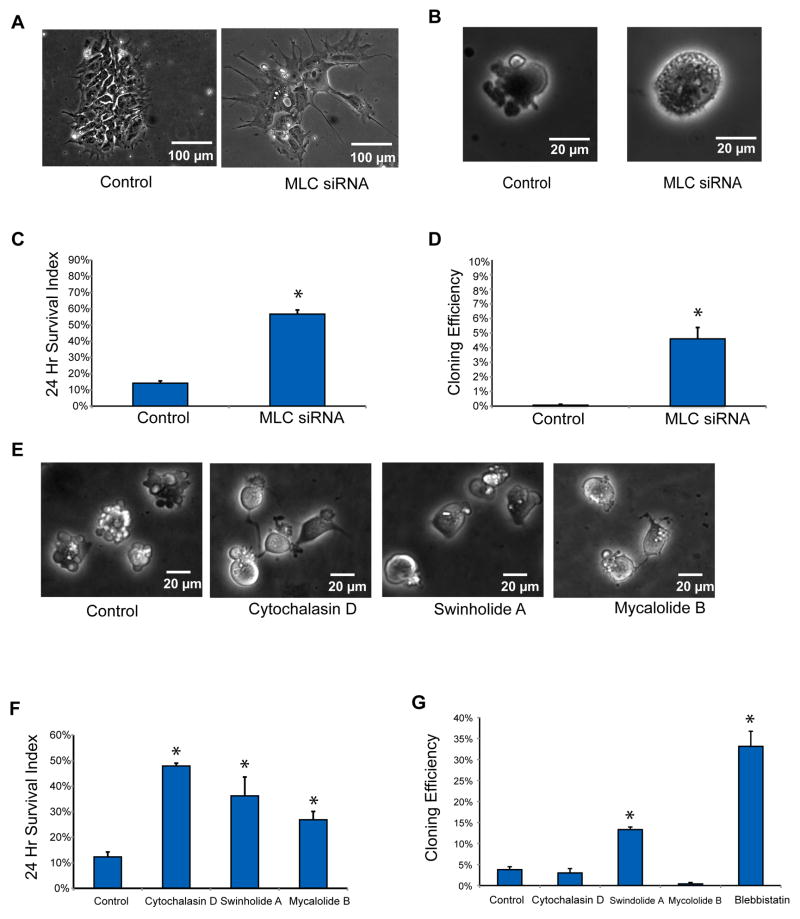
MYH-actin contraction is activated through phosphorylated myosin light chain (MLC). We tested whether reduction of MLC levels could also inhibit cell death. We used SMART pool siRNAs to knock down all three MCL family members (MRLC1, MRLC2 and MRLC3) in human ES cells (Table S2 and Figure S2D). When each gene was individually silenced, membrane blebbing was not repressed and cloning efficiency was not improved (Figures S3A–B). However, when all three genes (MRLC1/2/3) were silenced together, cells spread and stretched widely on plate surfaces, exhibiting the same morphology and reduced growth rate as MYH9/MYH10 knockdown cells (Figure 3A). Blebbing was inhibited after dissociation (Figure 3B), and both initial cell survival and cloning efficiency were improved (Figures 3C–D). These findings were also confirmed with a separate set of siRNAs (Table S2, Figures S2E and S3C–D), and further support that MYH motors are involved in human ES cell blebbing and cell death, and that the motors are regulated through MLC.
Figure 3. Knockdown of Myosin Light Chain (MLC) and Disruption of Actin Filaments Reduce Blebbing and Improve Survival and Cloning Efficiency of Human ES Cells.
Knockdown (by siRNA) of the three MLC gene products (MRLC1/2/3):
(A) changed human ES cell morphology in colonies 96 hours after transfection,
(B) decreased blebbing immediately after dissociation,
(C) increased (*p<0.05) cell survival 24 hr after plating, and
(D) increased (*p<0.05) cloning efficiency. Disruption of actin filaments by small molecules:
(E) reduced membrane blebbing of human ES cells 2 hr after plating in the presence of Cytochalasin D (100 nM), Swinholide A (10 nM) or Mycalolide B (20 μM).
(F) increased human ES cell Survival Index at 24 hr, and
(G) increased cloning efficiency after an initial 2 hr treatment with Swinholide A (10 nM).
Actin Disruption Increased Initial Survival and Cloning Efficiency of Dissociated Human ES Cells
Cell blebbing involves actin-based protrusions (Figures 1B–C), and myosin motors require intact actin filaments to contract. Here we tested whether the disruption of actin filaments could prevent myosin-dependent cell death. Among commonly used actin disruption drugs, Cytochalasin D binds to actin barbed ends to prevent actin-filament polymerization, while Swinholide A and Mycalolide B sever actin filaments. All three actin inhibitors suppressed membrane blebbing (Figure 3E). Twenty-four hours of continuous treatment resulted in significant improvement of cell survival (Figure 3F), but no colony formation at low density after 5 days. However, Swinholide A treatment limited to 3 hours after plating improved cloning efficiency, but not as well as blebbistatin (Figure 3G). These results are not surprising given that actin is involved in other essential cellular functions besides myosin contraction (Grummt, 2006; Zheng et al., 2009). Colonies formed after Swinholide A treatment demonstrated a similar morphology to colonies formed after Blebbistatin treatment (Figure S3E).
MLC Is a Downstream Target of ROCK Kinase in Human ES Cells
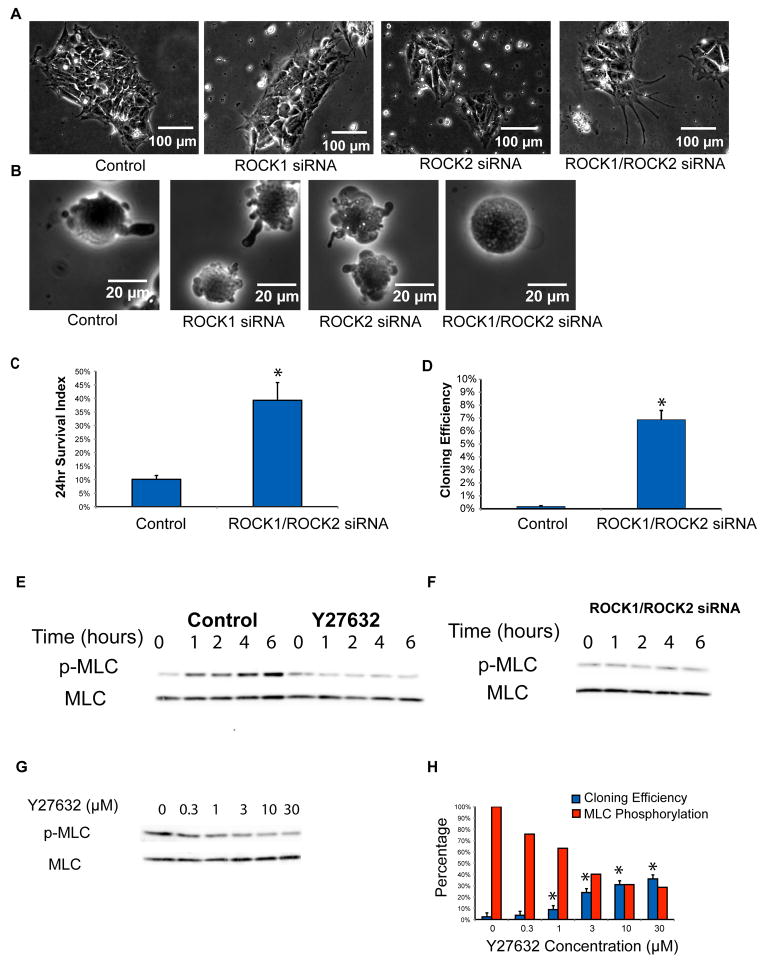
ROCK kinases, either directly or indirectly, regulate MLC phosphorylation (Totsukawa et al., 2000), and it has been shown previously that inhibition of ROCK improves cloning efficiency of human ES cells (Krawetz et al., 2009; Watanabe et al., 2007). Although the ROCK kinase inhibitor Y27632 is fairly specific, contributory off-target effects of the inhibitor were not excluded, and direct evidence that ROCK is the specific target of the inhibitor for the observed increase in human ES cell cloning efficiency has been lacking. Here we knocked down ROCK1 and ROCK2 separately or together with SMART siRNA pools in human ES cells (Table S2 and Figure S2F). We found that single knockdown of either ROCK1 or ROCK2 alone did not inhibit blebbing or improve cloning efficiency (Figure 4B and Figure S4A). However, when both ROCK1 and ROCK2 (ROCK1/ROCK2) were downregulated, cells stretched more than control cells (Figure 4A) and membrane blebbing was suppressed (Figure 4B), similar to the phenotype observed in MYH and MLC knockdown cells. ROCK1/ROCK2 siRNA treatment also improved the survival and cloning of individualized human ES cells (Figures 4C–D). These results were confirmed with a different set of siRNAs against ROCKs (Table S2, Figures S2G and S4B). Colonies formed after ROCK1/ROCK2 siRNA treatment demonstrated morphology similar to normal human ES cells, with high expression of the pluripotency marker OCT4 (Figures S3F–G).
Figure 4. ROCKs Regulate Cell Survival Through MLC Phosphorylation.
Knockdown (by siRNA) of ROCK1 and ROCK2 (ROCK1/ROCK2) together:
(A) changed human ES cell morphology in colonies 96 hours after transfection,
(B) decreased blebbing immediately after dissociation,
(C) increased (*p<0.05) cell survival 24 hr after plating, and
(D) increased (*p<0.05) cloning efficiency. Increased Phosphorylation of MLC (p-MLC) in dissociated human ES cells over time post-plating:
(E) was reduced by the ROCK inhibitor Y27632 (10 μM), and
(F) was reduced by siRNA knockdown of ROCK1/ROCK2. The results were confirmed by repeat experiments (n=2).
(G) Phosphorylation of MLC (p-MLC) was inversely related to the concentration of Y27632. Dissociated human ES cells were treated with the ROCK inhibitor Y27632 at increasing concentrations at time equal to zero. The treated cells were harvested 5 hours after plating and phosphorylation of MLC examined by western blot. The result was confirmed by repeat experiment (n=2).
(H) A decrease in MLC phosphorylation levels correlated with improvements in cloning efficiency (*p<0.05 compared to untreated control). Blue columns represent cloning efficiency, and red columns represent quantified MLC phosphorylation percentage from (G). The result was confirmed by repeat experiment (n=2).
We further tested whether ROCKs regulate MYH function through MLC phosphorylation. Western blot showed that phosphorylation of MLC (Ser-19) was significantly increased after individualized human ES cells were plated, and that the ROCK inhibitor Y27632 (at 10 μM) suppressed the increased phosphorylation (Figure 4E). Similarly, silencing of ROCK1/ROCK2 abolished the increase of MLC phosphorylation (Figure 4F). We then performed a dose response curve to examine whether ROCK-controlled MLC phosphorylation correlated with improved cloning efficiency. With increasing Y27632 concentration, MLC phosphorylation decreased (Figure 4G), which correlated with the improvement of cloning efficiency (Figure 4H). Similar experiments were performed with another ROCK inhibitor H1152 (Figures S4C–D). These results further support that ROCKs regulate MYH function through MLC phosphorylation in human ES cells, which in turn leads to membrane blebbing and cell death.
DISCUSSION
Our results suggest that actin-myosin contraction is a major mechanism promoting death of individualized human ES cells. By disrupting the contraction of the actin-myosin motor, either by chemical inhibitors or by siRNA knockdown of individual motor components, cell survival was dramatically improved. Disruption of actin-myosin contraction by these approaches also inhibited membrane blebbing, a phenotype of individualized human ES cells that correlated with cell death. Cell blebbing is caused by unbalanced stresses resulting from the loss of cell anchorage, and ultimately can lead to cell death if balance is not restored (Charras et al., 2005). Individualized human ES cells plated at a high density restored cell-cell contacts, reduced cell blebbing, and survived at a high frequency, even without exogenous inhibition of actin-myosin contraction. It is noteworthy that immediately after plating individual human ES cells, there was extensive cell migration and subsequent cell aggregation into colonies, so colony formation is not a direct measure of cloning efficiency unless low plating densities are used.
We found that inhibition of actin-myosin contraction during the initial hour after human ES cell dissociation was the critical time period for subsequent survival. For human ES cells plated at clonal densities, this initial inhibition of actin-myosin contraction would give some cells time to attach, divide, and re-establish balanced contractile forces and cytoskeletal architecture through cell-matrix and cell-cell adhesion. Because actin-myosin contraction is also involved in the formation of the cleavage furrow and cytokinesis (von Dassow, 2009) (Kanada et al., 2005), long-term exposure to blebbistatin inhibits cell division, and thus long exposures do not improve human ES cell cloning efficiency. We found that exposure to actin inhibitors, including Cytochalasin D, Swinholide A, and Mycalolide B, improved initial human ES cell survival, and Swinholide A also improved ES cell cloning, but only with limited exposure time, and even then at a lower efficiency than blebbistatin. Actin filaments are a major cytoskeletal component that is involved not only in actin-myosin contraction, but also in other diverse functions, ranging from RNA transcription to chromatin remodeling (Zheng et al., 2009). Blebbistatin should prevent myosin-actin contraction, but leave other actin functions intact; however, the disruption of actin itself would lead to the disruption of these other essential cellular functions.
It has been previously demonstrated that Rho-ROCK-Myosin signaling axis regulates cell-cell integrity in human ES cell colonies (Harb et al., 2008). Our work shows the important role of this pathway in response to cell dissociation. Our results suggest that disruption of actin-myosin contraction is the mechanism by which ROCK inhibitors increase the cloning efficiency of human ES cells, as ROCK inhibition reduces the phosphorylation of MLC, an activator of actin-myosin contraction. Interestingly, in contrast to actin and MYH inhibitors, long-term exposure to ROCK inhibitors support both increased initial survival and cloning efficiency of human ES cells. This suggests that reduced MLC phosphorylation is sufficient to block initial cell blebbing and death, but is not sufficient to block cleavage furrow formation and cell division. It is consistent with our result that ROCK inhibition by Y27362 and H1152 did not inhibit all detectable MLC phosphorylation (Figure 4G and Figure S4C), and the residual MLC phosphorylation is presumably sufficient to support cytokinesis. It is possible that ROCK is incompletely inhibited, but it is also possible that other kinases are responsible for the observed residual MLC phosphorylation, such as MLCK (Kang et al., 2007; Krawetz et al., 2009). We found that MLCK inhibitors failed to improve cell survival and cloning efficiency of human ES cells (Figures S4F–I), and this failure correlated with these inhibitors’ inability to suppress blebbing after dissociation (Figure S4E). One possible explanation for this difference in response to ROCK and MLCK inhibitors is a spatial compartmentalization of the different phosphorylation events (Totsukawa et al., 2004; Totsukawa et al., 2000).
Inhibition of actin-myosin contraction increased human ES cell survival even in suspension or when cultured on uncoated plates. Unattached human ES cells initially survived well in the presence of blebbistatin, but failed to proliferate, not an unexpected result given the effects on cleavage furrow formation and cell division. ROCK inhibition also improved cell survival in suspension (Harb et al., 2008; Watanabe et al., 2007); however, long-term growth was poor compared to cells grown on matrigel-coated surfaces. This suggests that the immediate cell death observed in cells deprived of surface contact is likely related to actin-myosin contraction, but that in non-attached conditions additional signals are still needed to promote robust undifferentiated proliferation.
In summary, our results show that the death of individualized human ES cells is executed by actin-myosin contraction, and that inhibition of actin-myosin contraction by several approaches leads to an increased cloning efficiency of both human ES and iPS cells. These results provide a model for further studies on the role of cytoskeleton organization in human pluripotent stem cells.
EXPERIMENTAL PROCEDURES
Human ES Cell Culture
Human ES cells were maintained in mTeSR medium on matrigel-coated tissue culture plates (Ludwig et al., 2006). Cells were passaged and analyzed with methods described in supplemental experimental procedures.
Cloning Assay
Unless specified, all the experiments were done on 12-well plates. Usually triplicates were prepared for each treatment. Prior to the addition of cells, 500 μl media was loaded in each well. Cells were dissociated with TrypLE for 5 minutes or until fully detached from the plate, neutralized with equal volumes of basic media, counted, washed and then diluted to 5000 cells/ml, and finally 100μl suspension was added into each well. Plates were then placed into 5% O2 and 10% CO2 37°C incubator. Small chemical compounds or proteins were added or washed away according to the specified procedure. Media was changed every 1–2 day(s) if not specified. After 5–6 days, colonies were stained with an APS kit as standard procedure (Vector Lab), and counted.
siRNA Knockdown
Cells were passaged into mTeSR media one day before the transfection at ~5 × 105/well in 6-well plates. Media was changed two hours before transfection. Specific siRNA (200 pmol) was mixed with 4 μl Dharmacon siRNA Transfection Reagent 1 as suggested in the standard protocol. For knockdown efficiency by qRT-PCR, RNA was isolated 24 hours after transfection. For survival and cloning experiment, cells were processed for survival and cloning analysis 72–96 hours posttransfection. If cell density was too high posttransfection, cells were passaged 24 hours after siRNA transfection.
Statistics
In cloning or survival assays, triplicate data were obtained for each condition. A t-test was performed to calculate p-values for the difference between the means of the experimental conditions and control. Each finding was confirmed by three independent biological replicates, unless specified.
Supplementary Material
Acknowledgments
This work was supported by the Charlotte Geyer Foundation, the Morgridge Institute for Research, NIH grant UO1ES017166 (to J.A.T.) and NIH contract RR-05-19 (to J.A.T.). We thank Dr. William Bement and Dr. Ivan Rayment for their discussion and suggestions. We thank Ron Stewart for his help on statistic analysis. We also thank Clay Glennon for assistance with confocal images and videos, and Deborah Faupel for editorial assistance. The authors declare competing financial interests: J.A.T. is a founder, stock owner, consultant and board member of Cellular Dynamics International (CDI). He also serves as scientific advisor to and has financial interests in Tactics II Stem Cell Ventures.
Footnotes
Supplemental data include 3 tables, 4 figures, 1 video and supplemental experimental procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nature Structural & Molecular Biology. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nature Cell Biology. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Croft DR, Coleman ML, Li SX, Robertson D, Sullivan T, Stewart CL, Olson MF. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. Journal of Cell Biology. 2005;168:245–255. doi: 10.1083/jcb.200409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerstrom C, Strehl R, Noaksson K, Hyllner J, Semb H. Facilitated expansion of human embryonic stem cells by single-cell enzymatic dissociation. Stem Cells. 2007;25:1690–1696. doi: 10.1634/stemcells.2006-0607. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin microtubule crosstalk. Nature Cell Biology. 2007;9:299–U104. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey MT, Tsai IY, Russell TP, Hanks SK, Wang YL. Cellular responses to substrate topography: Role of myosin II and focal adhesion kinase. Biophysical Journal. 2006;90:3774–3782. doi: 10.1529/biophysj.105.074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I. Actin and myosin as transcription factors. Curr Opin Genet Dev. 2006;16:191–196. doi: 10.1016/j.gde.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Harb N, Archer TK, Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS One. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci. 2008;13:6662–6681. doi: 10.2741/3180. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One. 2007;2:e658. doi: 10.1371/journal.pone.0000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Molecular Biology of the Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanada M, Nagasaki A, Uyeda TQ. Adhesion-dependent and contractile ring-independent equatorial furrowing during cytokinesis in mammalian cells. Mol Biol Cell. 2005;16:3865–3872. doi: 10.1091/mbc.E05-03-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Jiang Y, Toita R, Oishi J, Kawamura K, Han A, Mori T, Niidome T, Ishida M, Tatematsu K, et al. Phosphorylation of Rho-associated kinase (Rho-kinase/ROCK/ROK) substrates by protein kinases A and C. Biochimie. 2007;89:39–47. doi: 10.1016/j.biochi.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Krawetz RJ, Li X, Rancourt DE. Human embryonic stem cells: caught between a ROCK inhibitor and a hard place. Bioessays. 2009;31:336–343. doi: 10.1002/bies.200800157. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Genbacev O, Escobedo C, Zdravkovic T, Nordstrom A, Vabuena D, Nath A, Simon C, Mostov K, Fisher SJ. Disruption of apical-basal polarity of human embryonic stem cells enhances hematoendothelial differentiation. Stem Cells. 2007;25:2215–2223. doi: 10.1634/stemcells.2007-0230. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Limouze J, Straight AF, Mitchison T, Sellers JE. Specificity of blebbistatin, an inhibitor of myosin II. Journal of Muscle Research and Cell Motility. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Bergendahl V, Levenstein ME, Yu JY, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nature Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- Ndozangue-Touriguine O, Hamelin J, Breard J. Cytoskeleton and apoptosis. Biochemical Pharmacology. 2008;76:11–18. doi: 10.1016/j.bcp.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Okeyo KO, Adachi T, Sunaga J, Hojo M. Actomyosin contractility spatiotemporally regulates actin network dynamics in migrating cells. J Biomech. 2009 doi: 10.1016/j.jbiomech.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Quintin S, Gally C, Labouesse M. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet. 2008;24:221–230. doi: 10.1016/j.tig.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Sathananthan H, Pera M, Trounson A. The fine structure of human embryonic stem cells. Reprod Biomed Online. 2002;4:56–61. doi: 10.1016/s1472-6483(10)61916-5. [DOI] [PubMed] [Google Scholar]
- Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Wu Y, Sasaki Y, Hartshorne DJ, Yamakita Y, Yamashiro S, Matsumura F. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. Journal of Cell Biology. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. Journal of Cell Biology. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann U, In’t Veld P, Gilles C, Sermon K, De Rycke M, Van de Velde H, Van Steirteghem A, Liebaers I. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod. 2007;13:21–32. doi: 10.1093/molehr/gal091. [DOI] [PubMed] [Google Scholar]
- von Dassow G. Concurrent cues for cytokinetic furrow induction in animal cells. Trends Cell Biol. 2009;19:165–173. doi: 10.1016/j.tcb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Wang P, Valentijn AJ, Gilmore AP, Streuli CH. Early events in the anoikis program occur in the absence of caspase activation. J Biol Chem. 2003;278:19917–19925. doi: 10.1074/jbc.M210337200. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnology. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Wong RC, Pebay A, Nguyen LT, Koh KL, Pera MF. Presence of functional gap junctions in human embryonic stem cells. Stem Cells. 2004;22:883–889. doi: 10.1634/stemcells.22-6-883. [DOI] [PubMed] [Google Scholar]
- Yu JY, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zheng B, Han M, Bernier M, Wen JK. Nuclear actin and actin-binding proteins in the regulation of transcription and gene expression. FEBS J. 2009;276:2669–2685. doi: 10.1111/j.1742-4658.2009.06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.