Abstract
Purpose
Proof-of-principle in vitro experiments evaluated a prototype ultrasound technology to size kidney stone fragments.
Materials and Methods
Nineteen human stones were measured using manual calipers. A 10-MHz, 1/8″ (10F) ultrasound transducer probe pinged each stone on a kidney tissue phantom submerged in water using two methods. In Method 1, the instrument was aligned such that the ultrasound pulse traveled through the stone. In Method 2, the instrument was aligned partially over the stone such that the ultrasound pulse traveled through water.
Results
For Method 1, the correlation between caliper- and ultrasound-determined stone size was r2 = 0.71 (P < 0.0001). All but two stone measurements were accurate and precise to within 1 mm. For Method 2, the correlation was r2 = 0.99 (P < 0.0001), and measurements were accurate and precise to within 0.25 mm.
Conclusions
The prototype technology and either method measured stone size with good accuracy and precision. This technology may be possible to incorporate into ureteroscopy.
Introduction
Ureteroscopy often involves basket extraction of stone fragments. Fragments must be small enough, however, to fit through the ureter.1 Basket extraction may be faster than continued lithotripsy, provided there are no delays in attempting to extract fragments too large to remove either through narrow portions of the ureter or the ureteral access sheath. Injury to the ureter or loss of operative time may result if the surgeon attempts to extract fragments too large to remove, risking impaction of the stone and basket.2
Currently, estimating stone and stone fragment size during ureteroscopy is subjective. This report describes a prototype ultrasound technology to size stones or stone fragments that may be adapted for ureteroscopy.
Materials and Methods
Single stones from 19 separate patients were obtained from a stone reference laboratory. All stones were more than 95% pure composition and included three different stone types (seven calcium oxalate monohydrate, six cystine, six calcium hydrogen phosphate dihydrate) with a variety of shapes and sizes. Stones were rehydrated for 24 hours in deionized water (Fig. 1).
Fig. 1.
Experimental setup of the ultrasound probe measuring stone size on the kidney tissue phantom with a penny for size reference. Actual measurements were performed with the experimental setup submerged in 20°C water.
The height of each stone was determined using manual calipers. The stone was then placed in the same orientation on a planar kidney tissue phantom (Sylgard silicone elastomer, Dow Corning Corporation, Midland, MI; plastic microspheres, The PQ Corporation, Valley Forge PA; and nickel powder, Alfa Aesar, Ward Hill, MA) and submerged in 20°C water.
A 10 MHz, 1/8″ diameter (10F) transducer probe (model M112, Panametrics NDT (now Olympus NDT), Waltham, MA) sent and received ultrasound pulses through a pulse receiver (Model 5072PR, Olympus NDT, Waltham, MA) at 100 Hz and with signals displayed in real time on a digital oscilloscope. Ultrasound measurements were then obtained using two different methods (Fig. 2).
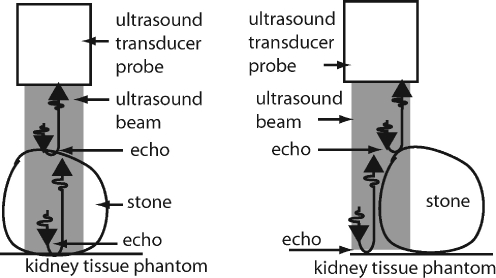
Fig. 2.
Measurement of stones using Method 1 (left) and Method 2 (right).
In Method 1, the ultrasound transducer was aligned directly over the stone such that the ultrasound wave traveled through the full thickness of the stone. We determined when the ultrasound waves reflected from the proximal surface of the stone and after traveling through the stone and reflecting off the back surface of the stone. In Method 2, the ultrasound transducer was aligned partially over the stone such that some of the ultrasound pulse reflected from the stone's proximal surface and some from the kidney tissue phantom on which the stone rested.
In Method 1, there was a continuous signal as the sound waves reflected from the internal structure of the stone. The time of interest was determined from the duration of the signal (Fig. 3). In Method 2, the time of interest was determined from the start of the reflection from the proximal stone surface and the start of the signal from the kidney tissue phantom where the stone rested. There was negligible detected echo between the two signals because of few scattering objects in the water. Stone thickness was calculated by
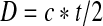 |
(1) |
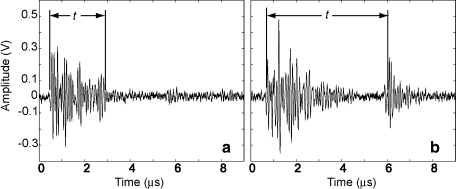
Fig. 3.
Representative signal for Method 1 (a) and Method 2 (b) of the same stone. Time (t) of interest was measured for the duration of Method 1 spike and the difference between the two spikes for Method 2. Note the t measured with Method 1 is roughly half that measured with Method 2 because the sound speed in stone (Method 1) is roughly twice that of the sound speed in water (Method 2). Reprinted with permission from Proceedings of the 2nd International Urolithiasis Research Symposium.10
where D = thickness of the stone; c = speed of sound; t = time; “2” is included because the ultrasound pulse must pass and return through the stone (Method 1) or water (Method 2). Despite some known variability by stone type, when determining stone thickness using Method 1 we used 3000 m/s as the stone sound speed for all stone types because the individual stone type was unlikely to be known at the time of a procedure.3,4 In Method 2, the sound speed of water (or urine in practice) is predictable within a few percent, and for 20°C water, the sound speed is 1482 m/s.5 Measurements were all repeated three times, and mean and standard deviations were reported.
The operator aligned the transducer by hand and recorded three signals for each of the two methods. The operator aligned the transducer probe visually but made final decisions by watching the oscilloscope and aligning to capture a clear signal. The signals were then analyzed by a blinded investigator to determine the time of interest used to calculate stone size. Regression analyses were performed to evaluate the correlation between mean caliper measurements and ultrasound based measurements.
Results
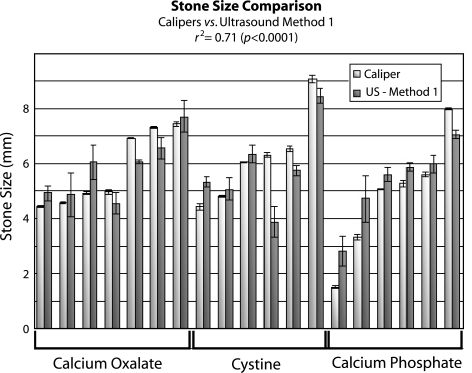
Both Method 1 and Method 2 yielded a measurement for each stone (Figs. 4 and 5). Caliper measurements and the two ultrasound methods were statistically similar, although Method 2 was more precise. Correlation between the caliper measurements and Method 1 measurements was r2 = 0.71 (P < 0.0001). Overall, Method 1 tended to overestimate stone size by 7%. This method, however, overestimated some stones and underestimated others with no observed differential bias by stone type. Accuracy was good for all stone types with 15 of 19 (79%) stones measured to within 1 mm of caliper measurements. Measurements were also precise with an average standard deviation of 0.38 mm and all measurements having a standard deviation of less than 0.8 mm.
Fig. 4.
Comparison of stone size measurements using calipers (white) vs ultrasound Method 1 (grey). Accuracy and precision are good with a correlation r2 = 0.71 (P < 0.0001). Ultrasound measurements do not appear to depend on stone composition.
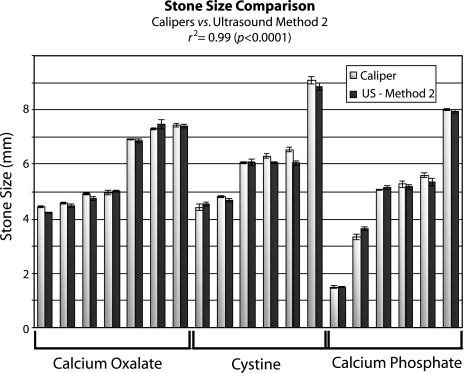
Fig. 5.
Comparison of stone size measurements using calipers (white) vs ultrasound Method 2 (black). Correlation is excellent (r2 = 0.99, P < 0.0001) with 95% of stones measured by ultrasound to within 0.5 mm of caliper measurements.
Correlation between the caliper measurements and Method 2 was r2 = 0.99 (P < 0.0001). Overall, Method 2 tended to slightly underestimate stone size by less than 1%, again with no differential bias by stone type. Compared with caliper measurement, accuracy was excellent for all stones using Method 2 with 19 of 19 (100%) stones being measured to within 1 mm of caliper measurements and all but one stone (95%) being measured to within 0.5 mm of caliper measurements. Precision was also excellent using Method 2, with an average standard deviation of 0.07 mm and all measurements having a standard deviation of less than 0.2 mm.
Role in Endourology
This ultrasound-based technology accurately and precisely measured stone fragment size by two methods. The methods rely on obtaining reflections from the stone and, for Method 2, the surface on which the stone rests. Measurements were made using a hand-held transducer, as would be done in surgery. Stones with a flat surface gave the strongest and clearest reflections; however, even irregularly shaped stones produced adequate reflections. Both methods by this measure were sensitive. Future work with smaller ultrasound probes will be important to determine if there is any loss of sensitivity. Although measurements were statistically more accurate and precise using Method 2, both methods would likely perform well clinically. We selected a conservative sound speed in stone (3000 m/s). We assume it would be better for this technology to overestimate rather than underestimate stone size. Previous studies have demonstrated stone sound speeds ranging from approximately 2700 to 4700 m/s, and accuracy in this study would have been improved by selecting a faster sound speed.3,4
This technology has applications to urologic clinical practice. Real-time measurements of stone fragment size could help to make ureteroscopy safer, giving the clinician an objective and reliable measure of stone size as opposed to a subjective approximation. The technology is scalable and has been commonly used in other medical “non-destructive evaluation.”6 The equipment is simple, consisting of a small ceramic element, wires, and a voltage source and receiver.
This potentially inexpensive technology could help to avoid potential ureteral injury and lost operative time if basket removal of a stone fragment is attempted before adequate fragmentation. This technology could also be applied to impacted ureteral stones where the operator cannot see beyond the stone. Also, a clinician could use this technology to confirm an accurate accounting of the presence of all the stones visualized on preoperative CT scan and their location within the calices, renal pelvis, and/or ureter.
This technology could be passed as a small caliber instrument through the ureteroscopic working channel. A small 2.5F ultrasound transducer of similar technology already exists for intracranial targeted treatment of clot in acute ischemic stroke; however, this transducer generates 360-degree circumferential ultrasound pulses and is not configured to create directed ultrasound waves for our application.6,7 Perhaps more conveniently, a 0.2 mm ceramic ultrasound element could be incorporated into the very distal tip of a 7.5F or 8.5F flexible ureteroscope. In either case, the calculations are simple, and the signals are typically strong, which would facilitate automation and rapid or even continuous feedback on stone size.
The use of endoluminal ultrasound in the urinary tract was first described in 1991.8 This technology has been used primarily to evaluate structures outside the ureter, such as imaging for crossing vessel in ureteropelvic junction obstruction and to diagnose and stage upper tract transitional-cell carcinoma.8,9 Although the concept and principle of the technology are similar, endoluminal ultrasound requires interpretation of an ultrasound image and is typically performed with fluoroscopic guidance rather than direct vision. These factors have limited the widespread use of endoluminal ultrasound. We hope that our ultrasound technology could be incorporated into flexible or semirigid ureteroscopy to allow direct vision and an automated assessment of stone size in the ureter, renal pelvis, and/or renal calices.
This technology offers promise for real-time fragmentation size measurements during ureteroscopy. Further experiments are necessary to evaluate smaller ultrasound transducer probes and the sensitivity of this technology to detect smaller stones, multiple stone fragments, and vascular structures, such as crossing vessels, with measurements deep within the collecting system of a real kidney.
Acknowledgments
This work was supported by grants NIH DK43881 and NSBRI SMST01601. Preliminary data regarding this project was presented and can be found in the Proceedings from the 2nd International Urolithiasis Research Symposium.
Disclosure Statement
Dr. Sorensen and Dr. Bailey: No competing financial interests exist.
Dr. Teichman: Consultant/Advisor for Ortho-McNeil, unrelated to this project.
References
- 1.Teichman JM. Vassar GJ. Bishoff JT. Bellman GC. Holmium:YAG lithotripsy yields smaller fragments than lithoclast, pulsed dye laser or electrohydraulic lithotripsy. J Urol. 1998;159:17–23. doi: 10.1016/s0022-5347(01)63998-3. [DOI] [PubMed] [Google Scholar]
- 2.Teichman JM. Kamerer AD. Use of the holmium:YAG laser for the impacted stone basket. J Urol. 2000;164:1602–1603. [PubMed] [Google Scholar]
- 3.Chuong CJ. Zhong P. Preminger GM. Acoustic and mechanical properties of renal calculi: Implications in shock wave lithotripsy. J Endourol. 1993;7:437–444. doi: 10.1089/end.1993.7.437. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach D. Munver R. Zhong P. Jacobs J. Hesse A. Müller SC. Preminger GM. Acoustic and mechanical properties of artificial stones in comparison to natural kidney stones. J Urol. 2000;164:537. :544. [PubMed] [Google Scholar]
- 5.Kinsler LE. Frey AR. Coppens AB. Sanders JV. Fundamentals of Acoustics. 3rd. New York: John Wiley and Sons; 1982. pp. 107–112. [Google Scholar]
- 6.Tsivgoulis G. Culp WC. Alexandrov AV. Ultrasound enhanced thrombolysis in acute arterial ischemia. Ultrasonics. 2008;48:303–311. doi: 10.1016/j.ultras.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Mahon BR. Nesbit GM. Barnwell SL. Clark W. Marotta TR. Weill A. Teal PA. Qureshi AI. North American clinical experience with the EKOS MicroLysUS infusion catheter for the treatment of embolic stroke. AJNR Am J Neuroradiol. 2003;24:534–538. [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg BB. Bagley D. Liu JB. Merton DA. Alexander A. Kurtz AB. Endoluminal sonography of the urinary tract: Preliminary observations. AJR Am J Roentgenol. 1991;156:99–103. doi: 10.2214/ajr.156.1.1898578. [DOI] [PubMed] [Google Scholar]
- 9.Lee DI. Bagley DH. Liu JB. Experience with endoluminal ultrasonography in the urinary tract. J Endourol. 2001;15:67–74. doi: 10.1089/08927790150500980. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen MD. Teichman JM. Bailey MR. A prototype ultrasound instrument to size stone fragments during ureteroscopy. Renal Stone Disease 2. 2nd International Urolithiasis Research Symposium. American Institute of Physics Conference Proceedings; 2008. pp. 348–352. [Google Scholar]