Abstract
Rett syndrome (RTT) is a neurodevelopmental disorder caused by mutations in the methyl CpG binding protein 2 (MECP2) gene. MECP2 protein is primarily expressed in neurons, and mutations in the gene lead to the clinical features of RTT in human patients and neurological deficits in murine models. Visual function is relatively preserved in RTT patients, but the cause for this is unknown. We analyzed the eyes of two RTT patients who died of the disease, and found no gross or microscopic changes. MECP2 expression was examined using immunohistochemistry, and nuclear protein expression was largely limited to ganglion cells and the portion of the inner nuclear layer populated by amacrine cells. No significant differences in MECP2 protein level or distribution were identified in the two eyes from the RTT patients as compared to eleven controls. The findings were compared to MECP2 expression in the brain of these two subjects and in MeCP2 deficient mice. Our findings suggest that the normally limited expression of MECP2 in visual pathway neurons may underlie intact vision in RTT.
Introduction
In the present study, we examined the eyes of two patients who died from complications of Rett syndrome (RTT), and used immunohistochemistry to characterize expression of MECP2 protein in the retina of these RTT cases as well as in normal controls.
Rett syndrome, a severe X-linked neurodevelopmental disorder, primarily targets the central nervous system pathologically and clinically [1]. Children with RTT develop normally until 6–12 months of life. However, they subsequently develop microcephaly, growth retardation, weight loss, and stereotyped hand movements [2]. Of great clinical interest is the observation that between 12 months to 3 years of age they lose learned skills like language and purposeful hand use, and develop irregular respirations in the wake period, gait abnormalities, stereotyped hand movements, seizures, and poor eye contact that are often mistaken for autism [2]. Subsequently, they remain cognitively impaired but with improved eye contact even using eye gaze as a preferred means of communication.
The genetic basis of RTT is due to mutations in the methyl CpG binding protein 2 (MECP2) gene that are identified in more than 95% of RTT cases[3,4]. The gene encodes MECP2, a methyl CpG binding protein that associates with DNA and regulates chromatin structure, thereby affecting transcription of a wide variety of genes [5, 6]. Several groups have examined the spatiotemporal and cellular expression of MECP2 mRNA and protein in the brain, and have found that it is primarily expressed in neurons, with some protein also detected in astrocytes [1, 4, 7–11]. Neurons of the brainstem and some neurons of the cortex express MECP2 during early gestation [1, 8]. By mid gestation, neurons of basal ganglia start expressing MECP2 and, in late gestation, the most mature cortical neurons are positive. The postnatal cortex continues to increase its expression of neuronal MECP2 [7, 8]. In some brain regions of patients with RTT, MECP2 protein expression is reduced, with the brainstem and thalamus reported to show the largest change [8]. Therefore, MECP2 expression in brain is not uniform and changes with age.
The neural retina develops as an extension of the CNS, thus, one might expect RTT to affect neuronal function in this tissue as well. However, although a high incidence of large refractive errors have been recorded in female subjects with RTT, no significant visual evoked potential abnormalities have been seen [12–14], suggesting that retinal neurons may be relatively resistant to the effects of MECP2 mutation. The selective preservation of occipital cortex in patients with RTT is also consistent with preserved visual function [15]. The basis for this resistance is unknown, as prior studies did not analyze MECP2 expression in the retina. To our knowledge, general histopathological findings in the eyes of RTT patients have also not yet been reported.
Materials and Methods
Clinical and Animal Material
The eyes and brains of two patients with RTT were removed at autopsy and sent at the request of the families to the University of Maryland Brain and Tissue Bank for Developmental Disorders to be utilized for research by one of the authors (SN). Control globes and brain tissues from fetal, pediatric, and adult autopsies were obtained from the Pathology Laboratory of Johns Hopkins Hospital. Clinical and demographic details are listed in Table 1. These studies were performed with Johns Hopkins University Institutional Review Board approval. The Bird Model, Mecp2-null mice (KO; n=4) as well as wild-type controls (n=2; WT) were sacrificed at postnatal day 8. The eyes were removed and processed in the same fashion as the human tissues.
Table 1.
Clinical and demographic details of two cases of Rett syndrome and eleven controls
| Age | Sex | Diagnosis | PMI |
|---|---|---|---|
| 22 years (Case 1) | F | Rett syndrome | 5 hours |
| 19 years (Case 2) | F | Rett syndrome | 13 hours |
| 24 week | F | Prematurity hyaline membrane disease | 13 hours |
| Newborn (38 weeks) | F | Preeclampsia | 15 hours |
| 4 months | M | Spinal muscular atrophy | 16 hours |
| 16 years | M | Congenital heart disease | 46 hours |
| 33 years | M | Cardiac failure | 47 hours |
| 49 years | M | Metastatic carcinoma | 40 hours |
| 50 years | M | Acute liver disease | 33 hours |
| 55 years | M | Acute bacterial endocarditis | 23 hours |
| 62 years | M | Cholangiocarcinoma | 6 hours |
| 63 years | F | Hypertension | 16 hours |
| 74 years | F | Myocardial infarction | 43 hours |
Abbreviations:
M = Male
F = Female
PMI = Post mortem interval
Immunohistochemical Analysis
For immunohistochemical studies, formalin-fixed, paraffin-embedded 4 micron thick sections were deparaffinized, rehydrated, washed, and treated for antigen retrieval in a citrate buffer. Sections were then immersed in 3% H2O2 to block endogenous peroxidase activity and incubated for 45 min with rabbit polyclonal antibodies (1:300 dilution) directed toward MECP2 (cat# 07-013, Upstate/Millipore, Billerica, MA). Antibody binding was detected using the avidin-biotin complex (ABC) method with diaminobenzidine serving as the chromogen (VECTASTAIN Elite, Vector Laboratories, Burlingame, CA). Positive immunolabeling was defined as uniform intense nuclear staining, with normal autopsy cortex used as positive controls. Immunohistochemical staining of eyes from both RTT and control cases, as well as KO and WT mice, were performed at least twice, with similar results.
Results
Case Presentations
Case 1
The patient had a normal prenatal and perinatal history. Her development was unremarkable until 13 months of age. Following this, her milestones were significantly delayed, with severe receptive and expressive language impairment. In the first two years of life she had four episodes of seizure associated with aspiration pneumonia requiring hospitalization. Testing at 25 months revealed a 16.1 month equivalent on the Bayley Motor Scale, and a 14.2 month equivalent on the Bayley Mental Scale. She displayed stereotyped hand movements along with hand twirling, lack of coordination, episodic high pitched screaming, and bruxism. Genetic testing revealed a D151Y missense mutation in the MECP2 gene. On routine physical examination at 18 years of age, she was normocephalic (head circumference in the 50th percentile), obese, tremulous, and inattentive but used her hands to tap at picture cards for communication, or occasionally used single words. Her pupils were equal, round, and reactive to light. She had no respiratory irregularity but had mild lumbar lordosis and scoliosis. Neurological examination showed intact cranial nerves, increased muscle tone in the lower extremities, unsustained clonus, downgoing plantars, delayed response to pain, and vasomotor instability of the lower limbs. She was placed on antiepileptic drugs and methylphenidate. The patient died unexpectedly in sleep at 22- years of age. The eyes were removed following a post-mortem interval of 5 hours.
Case 2
The patient had a history of developmental delay, unprovoked crying and laughing spells, and poor sleep. She was home schooled and received physical and speech therapies. Her mutation analysis consisted of a single nucleotide A>G transition in intron 3 of the MECP2 gene, two bases upstream of exon 4 that is predicted to cause abnormal splicing resulting in aberrant MECP2 protein. On routine clinical examination at 18 years of age, she was well nourished, nonverbal, with hand wringing, and was wheelchair bound. Her medical history was significant for severe constipation, multiple upper respiratory tract infections, and complex partial seizures treated with carbamazepine and gabapentin. Systemic examination of heart, lungs, and abdomen was normal, except for a gastrostomy and tracheostomy tubes placed at approximately 16 years of age. Despite these procedures, she had frequent pneumonias. Pupils were equal, round, and reactive to light. Fundoscopic examination was normal. Muscle bulk was decreased throughout with increased tone in both upper and lower limbs, associated with ankle and knee contractures, and brisk reflexes. Scoliosis was present. She died unexpectedly in sleep at 19 years of age following a year of frequent hospitalizations for pneumonia. An autopsy was performed and globes were removed following a post mortem interval of 13 hours.
Histopathological features and MECP2 Expression in Normal and RTT Globes
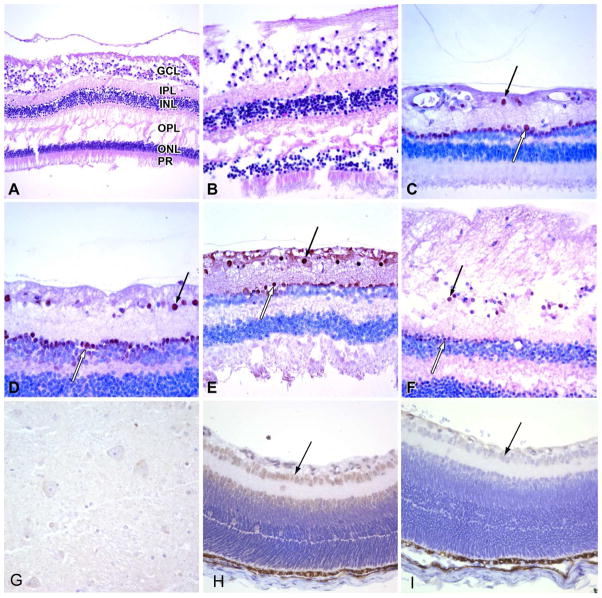
Globes from both cases (one from each patient) were grossly and microscopically unremarkable. Specifically, the corneas were unremarkable with normal-appearing Bowman and Descemet membranes and intact endothelium. The lenses were in place and free of cataractous changes. The iris and ciliary body was normal in each case. The vitreous was attached. The retina, including sections through the macula, showed no abnormality in ganglion cells or other neuronal layers (Figure 1A, B). The retinal pigment epithelium, choroid, optic nerve and sclera were also normal. Longitudinal and cross sections of the optic nerve did not disclose any abnormality.
Figure 1.
(A, B) The retina in both cases of Rett syndrome shows normal ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL) outer plexiform later (OPL), outer nuclear layer (ONL) and photoreceptor outer segment (PR) morphology (H&E stains, original magnifications 200x and 400x, respectively). (C, D) Immunostaining for MeCP2 protein in a normal adult control eye shows brown nuclear positivity in the ganglion cell layer (black arrow) and in a few innermost cells of inner nuclear layer (white arrow; original magnification 400x). (E, F) MeCP2 protein immunoreactivity in ganglion cells (black arrows) and neurons of the inner nuclear layer (white arrows) was the same in Rett cases 1 (E) and 2 (F) as in the controls (original magnification 400x). (G) Case 1 with the D151Y mutation shows MeCP2 expression in the deep grey matter of the brain which was diffuse but weak and was similar in both cases. (H, I) In mouse eyes MeCP2 was also restricted to the ganglion cell (black arrow) and inner nuclear layers (H), and immunoreactivity was lost in knock-out animals lacking the gene (I).
We studied the expression of MECP2 protein using immunohistochemical staining in normal eyes removed at autopsy from 1 fetus, 1 newborn term infant, 2 older children, and 7 adults. All showed variably positive nuclear staining in ganglion cells, as well as cells in the inner nuclear layer of the retina (Figure 1C, D). In the ganglion cell layer, more cells were positive in the periphery than in the central region, including the macula. The inner nuclear layer positivity was not present in all neurons rather; immunoreactivity was restricted to the innermost aspects of the layer where amacrine cells reside. Deeper retinal locations containing bipolar and horizontal neurons, as well as photoreceptor cells, also had non-reactive nuclei. We did observe some staining in the photoreceptor outer segments, but no nuclear positivity. Radial glial cells in the retina (Muller glia) as well as optic nerve oligodendroglia and astrocytes were also negative for MECP2 protein. While some minor variations in staining were observed between cases, we did not identify any clear difference in MECP2 expression in terms of its spatial distribution or intensity, among fetal, pediatric, or adult retinal neurons.
No pronounced difference was observed in immunoexpression of MECP2 in the globes from RTT patients when compared to the control eyes (Fig 1E, F). Specimens were taken with a similar post-mortem interval (Table 1). We also evaluated MECP2 expression in sections from the brains of the same two patients, as well as several age-matched controls. Deep grey matter structures were examined, as these have previously been reported to show a reduction in MECP2 in some RTT patients 37. Variable, sometimes weak, expression was noted in neurons of both case 1 (Fig. 1G) and case 2, but the levels of MECP2 appeared at most slightly decreased compared to controls (data not shown).
To further address the localization of MECP2 in the retina, we examined expression in the eyes of mice. WT mice showed robust expression of MeCP2 protein in the ganglion cell and inner nuclear layers, a pattern essentially identical to that seen in humans (Fig. 1H). When transgenic animals in which MeCP2 is deleted were stained, protein was no longer detected, confirming the specificity of the antibody (Fig. 1I).
Discussion
RTT is a severe neurodevelopmental disorder predominantly affecting females. The disease consists of developmental arrest followed by stages of clinical changes [2, 16]. The clinical spectrum of the RTT phenotype is broad, but is associated with mutations in a single gene, MECP2 [4, 17–19]. To date, more than 200 different changes in the MECP2 gene have been identified in patients with RTT [4]. These include missense, frameshift, and nonsense mutations, as well as intragenic deletions [4, 20–23]. It has been hypothesized that most known MECP2 mutations cause partial or total loss of function of the protein, which may lead to an abnormal transcriptionally active state in a wide variety of other genes [24].
Neuropathological studies in RTT patients identified a reduction in brain volume without clear evidence of atrophy or degeneration [15, 26]. In most RTT subjects, head circumference is reduced [27]. The overall morphology suggests an arrest of development during infancy [30] or a late prenatal development disorder [27]. It is thought that reduced neuronal size and dendritic branching of neurons is responsible for the small brains in RTT patients [29, 31]. However, the effects on brain volume are not uniform, as volumetric analyses by neuroimaging confirm the preservation of the occipital regions relative to other brain regions [15].
Interestingly, neurons in different brain locations are affected to varying degrees in RTT. MECP2 protein, identified by immunohistochemical methods, increases in the brain as the neurons mature [1, 32]. There is a temporal correlation between the time at which the clinical deficits are observed in the RTT patients, and the time of the normal maturation of cortical layers [33]. Indeed, MECP2 protein expression is closely related to the process of brain development, as increases in expression follow the developmental maturation of the CNS that coincides with synaptogenesis in the postnatal brain [1, 34, 35]. In RTT subjects, neurons of frontal, motor, hippocampus-entorrhinal, speech related cortex, and limbic system are affected most severely, resulting in speech, gait, and motor problems. Neurons of the superior temporal and occipital cortex do not show the same reduction in size of neurons and dendritic branching [29]. In RTT patients, MECP2 expression decreases, with a reduction in levels most apparent in brainstem and thalamic neurons [8]. Therefore, not all neurons appear to be affected equally.
Although substantial refractive errors are common in RTT patients, afferent visual pathways are not affected, and they have normal visual acuity [12, 14]. This retained visual function could be due either to a lack of expression of MECP2 in the eye, or a reduced requirement for the protein in retinal neurons. In this study, we examined MECP2 expression in retinal neurons of normal individuals, as well as patients with RTT having mutations in the MECP2 gene. We found that MECP2 nuclear immunoreactivity was predominantly present in ganglion cells, whose long axons form the optic nerve and carry all visual information leaving the eye, and in the portion of the retinal inner nuclear layer populated by amacrine cell nuclei. Amacrine cells are a diverse class of inhibitory retinal interneurons, which help to modulate bipolar and ganglion cell function [36]. Interestingly, the rods and cones which initiate vision in the retina failed to express MECP2, as did retinal glia and inner nuclear layer interneurons other than amacrine cells. No expression of MECP2 was identified in the optic nerve. An identical MeCP2 pattern was identified in the eyes of mice, suggesting the selective expression observed in humans is conserved in other mammals.
The two cases we analyzed showed no significant differences between affected globes and controls in terms of MECP2 immunoreactivity. In case 1, the patient was known to have a D151Y missense mutation in the MECP2 gene, whereas case 2 had an A>G transition at nucleotide 378-2 in intron 3 near exon 4 of MECP2 resulting in a splice site mutation. Both of these molecular changes are thought to result in loss of function, but could result in retained protein expression. Indeed, we did not identify significant reductions in MECP2 protein in deep grey matter neurons of the brain, in either individual.
In summary, we have shown that in the retina only ganglion and amacrine cells express detectable levels of MECP2. No major changes were detected in MECP2 expression in the eyes of two young females who died from this disease, a finding consistent with the relatively stable brain expression observed in the two cases. More cases with other mutations will need to be analyzed before firm conclusions can be drawn regarding how MECP2 protein levels might be altered in the full genetic spectrum of RTT patients. However, our data do indicate that the potential role for MECP2 protein in ocular neurons is limited to only a few cell types, and that no major anatomical disruptions accompany the two mutations examined in humans, or complete excision of the gene in mice. It is possible that while MECP2 is expressed in ganglion and amacrine cells, its function is not absolutely required, resulting in retained vision.
Acknowledgments
We thank the families for their generous gift. These studies are supported by PO HD24448 and Institute for Clinical and Translational Research-UL1RR025005 (SN), and Research to Prevent Blindness (CGE). We also thank Dr Ronald Zielke from the University of Maryland Brain and Tissue Bank for Developmental Disorders, which is supported by NO1-HD-4-3368 and NO1-HD-4-3383.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shahbazian MD, Antalffy B, Armstrong DL, et al. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet. 2002;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- 2.Trevathan E, Naidu S. The clinical recognition and differential diagnosis of Rett syndrome. J Child Neurol. 1988;3 (Suppl):S6–16. doi: 10.1177/0883073888003001s03. [DOI] [PubMed] [Google Scholar]
- 3.Amir RE, Van den Veyver IB, Wan M, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 4.RETTBASE. http://mecp2.chw.edu.au.
- 5.Chahrour M, Jung SY, Shaw C, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meehan RR, Lewis JD, Bird AP. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992;20:5085–5092. doi: 10.1093/nar/20.19.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balmer D, Goldstine J, Rao YM, et al. Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J Mol Med. 2003;81:61–68. doi: 10.1007/s00109-002-0396-5. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DD, Deguchi K, Antallfy B. Survey of MeCP2 in the Rett syndrome and the non-Rett syndrome brain. J Child Neurol. 2003;18:683–687. doi: 10.1177/08830738030180100601. [DOI] [PubMed] [Google Scholar]
- 9.Cassel S, Revel MO, Kelche C, et al. Expression of the methyl-CpG-binding protein MeCP2 in rat brain. An ontogenetic study. Neurobiol Dis. 2004;15:206–211. doi: 10.1016/j.nbd.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Maezawa I, Swanberg S, Harvey D, et al. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballas N, Lioy DT, Grunseich C, et al. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders KJ, McCulloch DL, Kerr AM. Visual function in Rett syndrome. Dev Med Child Neurol. 1995;37:496–504. doi: 10.1111/j.1469-8749.1995.tb12037.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalmanchey R. Evoked potentials in the Rett syndrome. Brain Dev. 1990;12:73–76. doi: 10.1016/s0387-7604(12)80181-1. [DOI] [PubMed] [Google Scholar]
- 14.Glaze DG. Neurophysiology of Rett syndrome. J Child Neurol. 2005;20:740–746. doi: 10.1177/08830738050200090801. [DOI] [PubMed] [Google Scholar]
- 15.Carter JC, Lanham DC, Pham D, et al. Selective cerebral volume reduction in Rett syndrome: a multiple-approach MR imaging study. AJNR Am J Neuroradiol. 2008;29:436–441. doi: 10.3174/ajnr.A0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagberg B, Witt-Engerstrom I. Rett syndrome: a suggested staging system for describing impairment profile with increasing age towards adolescence. Am J Med Genet Suppl. 1986;1:47–59. doi: 10.1002/ajmg.1320250506. [DOI] [PubMed] [Google Scholar]
- 17.Renieri A, Meloni I, Longo I, et al. Rett syndrome: the complex nature of a monogenic disease. J Mol Med. 2003;81:346–354. doi: 10.1007/s00109-003-0444-9. [DOI] [PubMed] [Google Scholar]
- 18.Naidu S, Bibat G, Kratz L, et al. Clinical variability in Rett syndrome. J Child Neurol. 2003;18:662–668. doi: 10.1177/08830738030180100801. [DOI] [PubMed] [Google Scholar]
- 19.Hoffbuhr KC, Moses LM, Jerdonek MA, et al. Associations between MeCP2 mutations, X-chromosome inactivation, and phenotype. Ment Retard Dev Disabil Res Rev. 2002;8:99–105. doi: 10.1002/mrdd.10026. [DOI] [PubMed] [Google Scholar]
- 20.Schollen E, Smeets E, Deflem E, et al. Gross rearrangements in the MECP2 gene in three patients with Rett syndrome: implications for routine diagnosis of Rett syndrome. Hum Mutat. 2003;22:116–120. doi: 10.1002/humu.10242. [DOI] [PubMed] [Google Scholar]
- 21.Laccone F, Junemann I, Whatley S, et al. Large deletions of the MECP2 gene detected by gene dosage analysis in patients with Rett syndrome. Hum Mutat. 2004;23:234–244. doi: 10.1002/humu.20004. [DOI] [PubMed] [Google Scholar]
- 22.Mnatzakanian GN, Lohi H, Munteanu I, et al. A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nat Genet. 2004;36:339–341. doi: 10.1038/ng1327. [DOI] [PubMed] [Google Scholar]
- 23.Hoffbuhr K, Devaney JM, LaFleur B, et al. MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology. 2001;56:1486–1495. doi: 10.1212/wnl.56.11.1486. [DOI] [PubMed] [Google Scholar]
- 24.Ballestar E, Yusufzai TM, Wolffe AP. Effects of Rett syndrome mutations of the methyl-CpG binding domain of the transcriptional repressor MeCP2 on selectivity for association with methylated DNA. Biochemistry. 2000;39:7100–7106. doi: 10.1021/bi0001271. [DOI] [PubMed] [Google Scholar]
- 25.Wenk GL, O’Leary M, Nemeroff CB, et al. Neurochemical alterations in Rett syndrome. Brain Res Dev Brain Res. 1993;74:67–72. doi: 10.1016/0165-3806(93)90084-n. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong DD. The neuropathology of Rett syndrome. Brain Dev. 1992;14 (Suppl):S89–98. [PubMed] [Google Scholar]
- 27.Naidu S. Rett syndrome: a disorder affecting early brain growth. Ann Neurol. 1997;42:3–10. doi: 10.1002/ana.410420104. [DOI] [PubMed] [Google Scholar]
- 28.Belichenko PV, Hagberg B, Dahlstrom A. Morphological study of neocortical areas in Rett syndrome. Acta Neuropathol. 1997;93:50–61. doi: 10.1007/s004010050582. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong D, Dunn JK, Antalffy B, et al. Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol. 1995;54:195–201. doi: 10.1097/00005072-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong DD, Dunn JK, Schultz RJ, et al. Organ growth in Rett syndrome: a postmortem examination analysis. Pediatr Neurol. 1999;20:125–129. doi: 10.1016/s0887-8994(98)00124-6. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong DD, Dunn K, Antalffy B. Decreased dendritic branching in frontal, motor and limbic cortex in Rett syndrome compared with trisomy 21. J Neuropathol Exp Neurol. 1998;57:1013–1017. doi: 10.1097/00005072-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Cohen DR, Matarazzo V, Palmer AM, et al. Expression of MeCP2 in olfactory receptor neurons is developmentally regulated and occurs before synaptogenesis. Mol Cell Neurosci. 2003;22:417–429. doi: 10.1016/s1044-7431(03)00026-5. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong DD. Neuropathology of Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:72–76. doi: 10.1002/mrdd.10027. [DOI] [PubMed] [Google Scholar]
- 34.Akbarian S, Chen RZ, Gribnau J, et al. Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol Dis. 2001;8:784–791. doi: 10.1006/nbdi.2001.0420. [DOI] [PubMed] [Google Scholar]
- 35.Johnston MV, Blue ME, Naidu S. Rett syndrome and neuronal development. J Child Neurol. 2005;20:759–763. doi: 10.1177/08830738050200091101. [DOI] [PubMed] [Google Scholar]
- 36.Baccus SA, Olveczky BP, Manu M, et al. A retinal circuit that computes object motion. J Neurosci. 2008;28:6807–6817. doi: 10.1523/JNEUROSCI.4206-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong DD. Rett syndrome: neuropathology review. Brain Dev. 2001;(Suppl 1):S72–6. doi: 10.1016/s0387-7604(01)00332-1. [DOI] [PubMed] [Google Scholar]