Summary
Neural progenitor cells (NPCs) in the adult subventricular zone (SVZ) are associated with ependymal and vasculature niches which regulate stem cell self-renewal and differentiation. Activated Type B stem cells and their progeny, the transit amplifying Type C cells, which express EGFR, are most highly associated with vascular cells, indicating that this niche supports lineage progression. Here we show that proliferative SVZ progenitor cells home to endothelial cells in a stromal-derived factor 1 (SDF1) and CXC chemokine receptor 4 (CXCR4) dependent manner. We show that SDF1 strongly upregulates EGFR and α6 integrin in activated type B and type C cells, enhancing their activated state and their ability to bind laminin in the vascular niche. SDF1 increases the motility of Type A neuroblasts, which migrate from the SVZ towards the olfactory bulb. Thus, differential responses to SDF1 can regulate progenitor cell occupancy of and exit from the adult SVZ vascular niche.
Introduction
The crucial role of the niche in regulating stem cell behavior, the transitions from quiescence, to self-renewal and differentiation, is becoming increasingly appreciated (Alvarez-Buylla and Lim, 2004; Kiel and Morrison, 2008). In the central nervous system (CNS), stem cells reside throughout life in the forebrain, continuing to generate neurons and glia in the subventricular zone (SVZ) surrounding the lateral ventricle and in the dentate gyrus of the hippocampus. Recent studies have highlighted two important niches within the adult SVZ. One is the apical ependymal niche, which consists of ciliated ependymal cells and intercalated GFAP+ astrocyte-like Type B cells that line the lateral ventricle. The other is the basal vasculature niche, which consists of a rich plexus of blood vessels and associated laminin-rich basal lamina. Apical Type B cells associated with the ependymal-lined ventricle send processes to the SVZ plexus blood vessels, suggesting that they can be influenced by both fluid compartments (Mirzadeh et al., 2008; Shen et al., 2008; Tavazoie et al., 2008).
During lineage progression, GFAP+ Type B stem cells become activated, and upregulate EGFR to become GFAP+EGFR+. These cells then produce GFAP−EGFR+ transit amplifying Type C cells (Pastrana et al., 2009). Both actively dividing Type B cells and Type C cells are closely associated with the vascular niche in the SVZ (Shen et al., 2008; Tavazoie et al., 2008). Rapidly dividing Type C cells in turn give rise to Type A neuroblasts, progenitors that divide as they migrate, usually in chains of cells. In the dorsal SVZ, neuroblast chains often run parallel with blood vessels aligned anterior-posterior in the direction of the rostral migratory stream (Shen et al., 2008; Tavazoie et al., 2008), which can help guide neuroblast migration to the olfactory bulb (Snapyan et al., 2009). Secreted factors from endothelial cells increase self renewal and neuron generation from NPCs (Louissaint et al., 2002; Shen et al., 2004) supporting the notion that the vascular niche is a compartment for more activated progenitors progressing through the lineage.
The ability of stem cells to locate and occupy niches is essential for aspects of normal stem cell biology and for regenerative medicine. It has not been established whether NPCs have the capacity to home to their niche, as has been observed for hematopoietic stem cells (HSCs) which home to niches within the bone marrow under physiological conditions and following transplantation. HSCs use a variety of molecules for homing. The chemokine SDF1 and its receptor CXCR4 are important for attracting HSCs out of the blood and into the bone marrow and for retention of cells within the bone marrow niche (Chute, 2006; Kaplan et al., 2007). SDF1 is secreted by the bone marrow stroma, creating a gradient that binds to CXCR4 expressed by HSCs. This causes actin polymerization and upregulation of integrins, resulting in chemotaxis toward the source of SDF1 (Kijowski et al., 2001; Peled et al., 2000; Voermans et al., 2001).
It is tempting to suggest that there might be a parallel function for SDF1/CXCR in attracting CNS stem cells towards the vascular niche. SDF1/CXCR4 signaling has been implicated in various types of CNS cell migration. For instance, during development, SDF1 directs hippocampal dentate granule cells (Bagri et al., 2002) Cajal Retzius cells (Paredes et al., 2006) cerebellar granular neurons (Ma et al., 1998; Zou et al., 1998) and cortical interneurons (Stumm et al., 2003; Tiveron et al., 2006) to their correct locations within the brain. Moreover, neuroblasts in the adult SVZ migrate out of the germinal zone towards sites of ischemic injury after stroke in response to SDF1 release (Arvidsson et al., 2002; Yamashita et al., 2006; Zhang et al., 2004) becoming associated with the vasculature (Ohab et al., 2006; Robin et al., 2006; Thored et al., 2006)
Here we investigated whether adult SVZ stem lineage cells are capable of homing to blood vessels following transplantation and the role of SDF1 in the process. We found that transplanted adult SVZ progenitor cells integrate into the host SVZ and migrate towards blood vessels, both in vitro and in vivo. We show that SVZ cells express CXCR4 and that the vascular plexus and ependymal cells express SDF1. Moreover, we demonstrated that homing to the blood vessels is CXCR4/SDF1 mediated. Importantly, SDF1 has a different effect on different stages of the lineage: SDF1 does not notably stimulate movement of EGFR−Glast+ Type B cells, which include the more primitive, quiescent stem cells. In contrast, SDF1 significantly stimulates chemotaxis of activated Type B and Type C cells and their adhesion to endothelial cells. SDF1 treatment results in upregulation of α6-integrin, which we showed previously enhances binding of NPCs to the vascular niche (Shen et al., 2008), and upregulation of EGFR on cells that are already EGFR+. This suggests a mechanism by which SDF1 could stimulate stem cells to move from ependymal to vascular niches as they progress from quiescence to activation. Furthermore, SDF1 promotes motility of Type A neuroblasts, but has much less effect on their α6-integrin levels, consistent with endothelial-derived SDF1 stimulating their migration to the olfactory bulb.
Results
Transplanted NPCs integrate into the SVZ and associate preferentially with the vasculature
Previously, we and others measured the relationship of normal SVZ lineage cells to the surface of endothelial cells of the SVZ plexus, helping to define and quantify the adult CNS vascular niche (Shen et al., 2008; Tavazoie et al., 2008). Approximately 47% of dividing Type B cells and 46% of Type C cells were found within 5 microns of the vasculature. In contrast Type A cells tended to be further away from the blood vessel surface (only 14% are within 5 microns). Although migrating chains of neuroblasts, especially in the dorsal aspect of the SVZ, appeared to run along with aligned blood vessels, most of the neuroblasts were not as close to the endothelial surface as were dividing Type B and Type C cells.
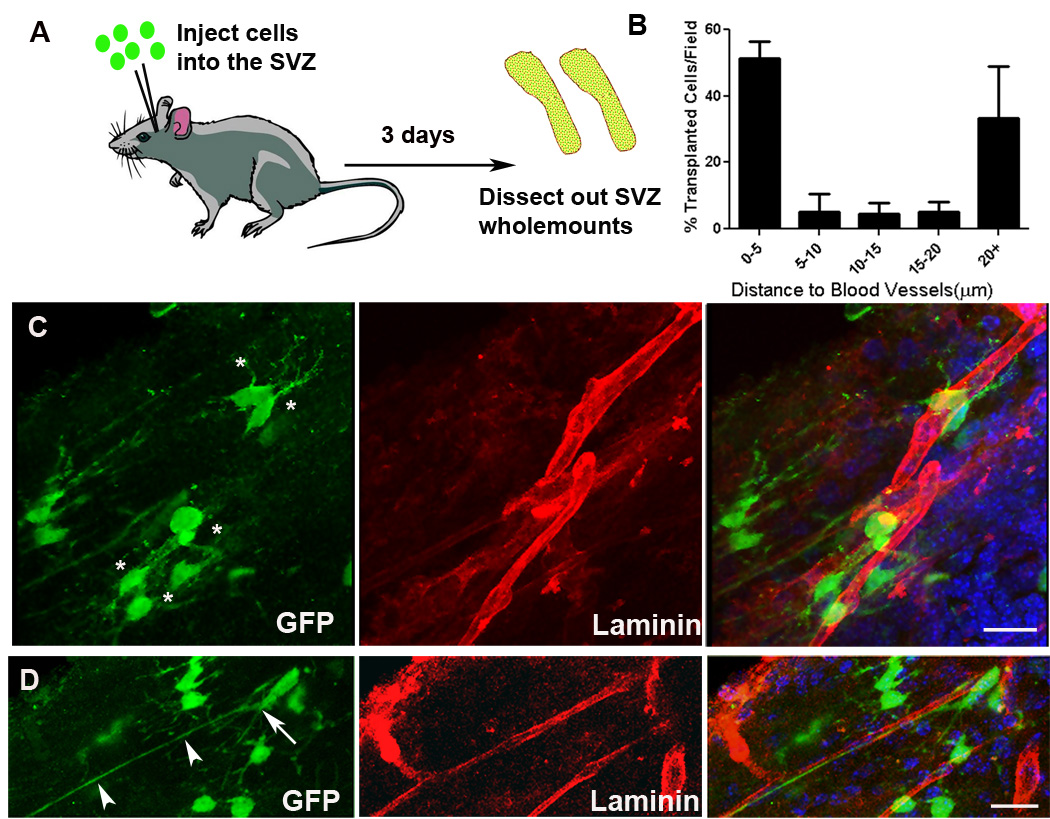
To establish how NPCs integrate into the SVZ following transplantation and specifically whether they home to the vasculature, we stereotaxically injected NPCs derived from green fluorescent protein (GFP) transgenic mice (in which all cells are GFP+) into the SVZ of wildtype mice and measured their relationship to the closest endothelial cell surface. Three days after transplantation, the SVZ region was dissected out as a wholemount, and stained for laminin to visualize the blood vessels (Figure 1A). Donor GFP+ NPC-derived cells were predominantly seen next to the SVZ vasculature, many with prominent processes touching the vessels (Figure 1C). Some transplanted NPCs aligned along vessels, exhibiting an elongated morphology with extended leading processes (Figure 1D). Most (52.5 ± 5.2 %) GFP+ cells were within 5µm of the vasculature, around 17% were 5–20µm away and 33.4 ± 15.5% were over 20µm away (n=3 SVZs) (Figure 1B). Thus, transplanted NPCs find their way to the vasculature in vivo, consistent with them having the ability to home to this niche.
Figure 1.
NPCs associate with the SVZ vasculature following in vivo transplantation. (A) Schematic illustrating experimental design. NPCs were injected into the SVZ and then wholemounts were dissected for analysis by immunohistochemistry and confocal microscopy. (B) Quantification of the distance of transplanted cells to the nearest blood vessel surface. (C) Injected NPCs (green) are found near blood vessels (red); typical short processes of NPCs are indicated with asterisks. (D) NPCs with cell bodies lying directly on blood vessels (arrow) sometimes exhibit long processes extended along the blood vessel (arrowheads). Scale bars 20um.
In order to investigate the process in more detail, we developed an accessible, in vitro, 3-dimensional homing assay. Organotypic SVZ wholemounts were freshly dissected from the adult brain, as described (Doetsch and Alvarez-Buylla, 1996). These were plated on transwell filters in serum-free slice culture medium containing FGF2. GFP+ NPCs were dissociated to single cells and gently overlaid on the wholemount two hours after plating in the transwell. After 16 hours, the wholemounts were fixed and stained for laminin to reveal the SVZ blood vessels (Figure 2A), and the GFP+ cells were located, quantified and their distance from the nearest blood vessel surface was measured. In order to determine whether this was a general phenomenon, or dependent on region or stage, we performed this experiment on a variety of different donor NPCs: a) NPCs derived from the embryonic cerebral cortex and co-cultured with endothelial cells for ten days to enrich for NSCs (Shen et al., 2004) (Figure 2B), b) Adult SVZ NPCs that had been grown for 7 days as neurospheres (Figure 2C&D) to enrich for SVZ progenitor cells or c) acutely dissociated NPCs from the adult SVZ, representing homotopic and homochronic transplantation (Figure 2E). In all cases, the NPCs invaded the wholemount and integrated widely. Interestingly, what appeared to be chains of transplanted NPCs were sometimes observed in the dorsal portion of the SVZ (Figure 2D), a region where endogenous neuroblast chains are prominent. Despite the varied NPC sources, as found in the in vivo experiments, they were consistently located close to blood vessels. Quantification of the distance of GFP+ NPCs from the vasculature revealed that 66 ± 7.6% were located within 5um of blood vessels, significantly more than non-GFP+ cells in the SVZ (24.8±2.5% p<0.001) (Figure 2F). When we flipped the wholemount, and cultured it ependymal side down on the transwell, overlaid NPCs cells were still able to enter from the parenchymal, striatal side and preferentially associate with blood vessels, indicating that they could migrate further and along different routes to this destination (not shown).
Figure 2.
NPCs preferentially associate with blood vessels in an organotypic wholemount assay system. (A) Schematic depicting the organotypic wholemount homing assay. The SVZ wholemount is microdissected from adult mice and cultured in membrane inserts. GFP+ cells are gently laid on the wholemount in a bubble of media and incubated, then processed for immunohistochemistry. Photomicrographs illustrating that NPCs (green) from a variety of sources enter the wholemounts and dispose along blood vessels (red): (B) endothelial cell expanded NPCs (C) Neurosphere-expanded NPCs. (D) Neurosphere expanded NPCs in the dorsal SVZ cluster into chains. Inset shows a higher magnification of (D) with nuclear Dapi stain in blue. (E) Acutely dissociated adult NPCs. (F) Quantification of the distance in microns of NPCs to blood vessels (the last bin represents 25 microns and greater). *p<0.001. Scale bars = 20um.
SDF1 directs homing of NPCs to blood vessels
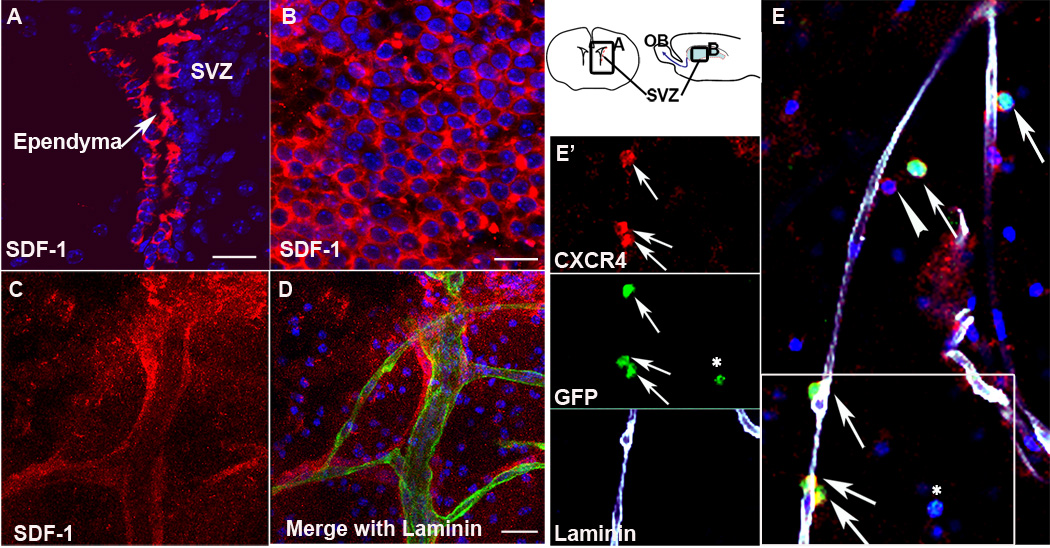
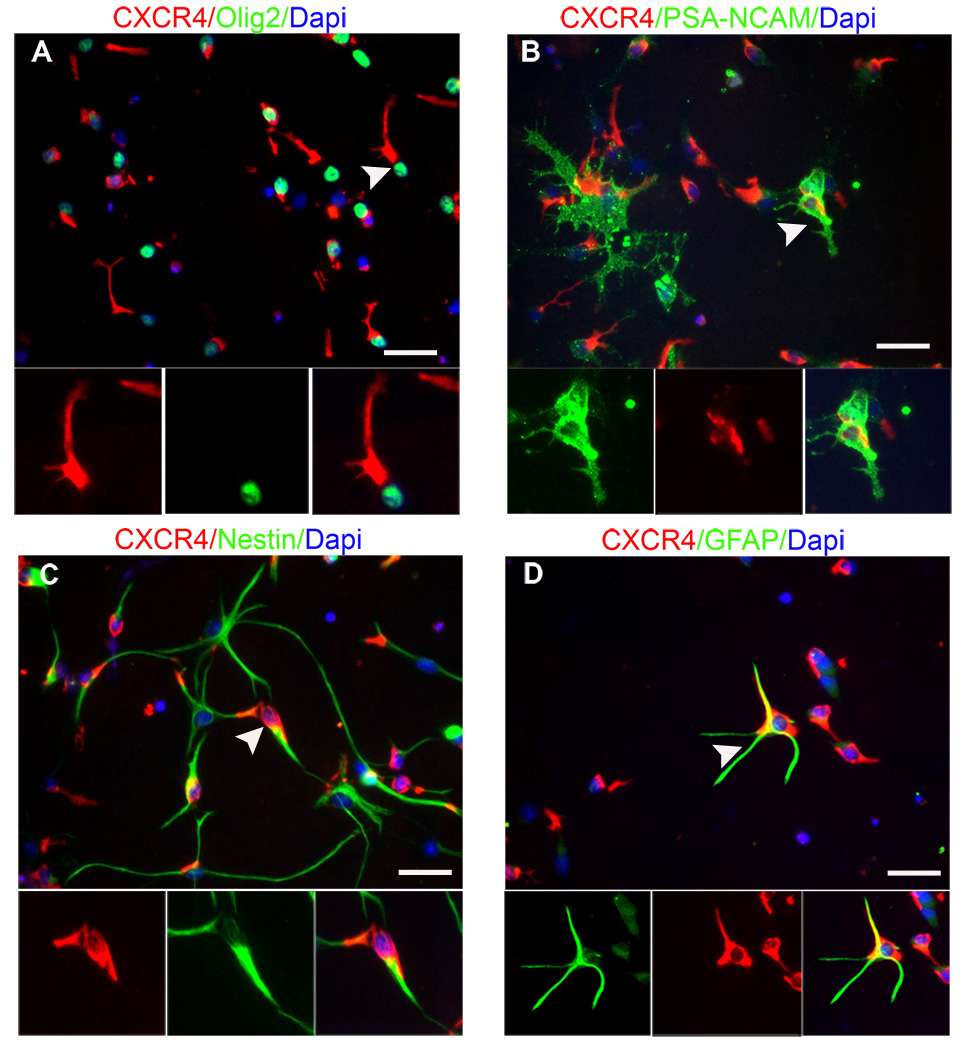
We then went on to examine the molecular mechanism whereby NPCs were able to locate blood vessels. Because the chemokine SDF1 is involved in HSC homing to the bone marrow niche (Chute, 2006; Kaplan et al., 2007) and of adult SVZ cells to areas of CNS injury following ischemia (Ohab et al., 2006; Robin et al., 2006; Thored et al., 2006), we identified SDF1 as a candidate. Immunohistochemistry of SVZ wholemounts and coronal sections revealed that SDF1 was expressed by the ependymal cells (Figure 3A and B) and by the vasculature (Figure 3C and D), two critical SVZ niches. Furthermore, cultured endothelial cells express SDF1 (Suppl. Figure 1). The SDF1 receptor CXCR4 was expressed on all stages of SVZ lineage cells – the Type B, Type C and Type A cells in vivo (Suppl. Figure 2), and in one day in vitro (DIV) cultured SVZ progenitor cells (Figure 4). Importantly, we found that CXCR4 was visible on transplanted NPCs associated with the vasculature following homing into the wholemount (Figure 3E and E’). Thus the ligand is enriched in the niche and adult SVZ NPCs express the receptor, consistent with the hypothesis that this ligand-receptor pair is involved in SVZ NPC homing. SDF1 can bind a second chemokine receptor, CXCR7. In adult rat brain, CXCR7 was not detectable by in situ hybridization (Schonemeier et al., 2008), and we were unable to detect it by immunohistochemistry, (Supple. Fig. 2 D & E), making it unlikely to be involved.
Figure 3.
SDF1 and CXCR4 are expressed in the adult SVZ. SDF1 immunohistochemistry (red) shows strong expression in the ependymal cells of (A) a coronal section of the adult brain and (B) an organotypic wholemount. Schematic (right) indicates where pictures were taken. (C,D) SDF1 is expressed by blood vessels stained for laminin (green), and has a pattern consistent with a secreted gradient. (E) The SDF1 receptor CXCR4 (red) is expressed by transplanted NPCs (green) associated with the vasculature (white). Arrows indicate transplanted cells that express CXCR4. Arrowhead indicates an endogenous CXCR4 positive cell. Asterisk highlights a transplanted cell not associated with the vasculature that is CXCR4 negative. (E’) Individual channels from the boxed area in E. Scale bars = 20um
Figure 4.
NPCs from the adult SVZ express CXCR4. Immunohistochemistry against the chemokine receptor CXCR4 in cultured NPCs after 1DIV reveals expression by a wide range of SVZ subtypes. (A) examples of olig2+ cells, a subpopulation of Type C cells, (B) PSA-NCAM, a Type A neuroblast marker (C) Nestin, a stem cell/progenitor marker and (D) GFAP, a stem/progenitor cell and astrocyte marker. Scale bars = 25um.
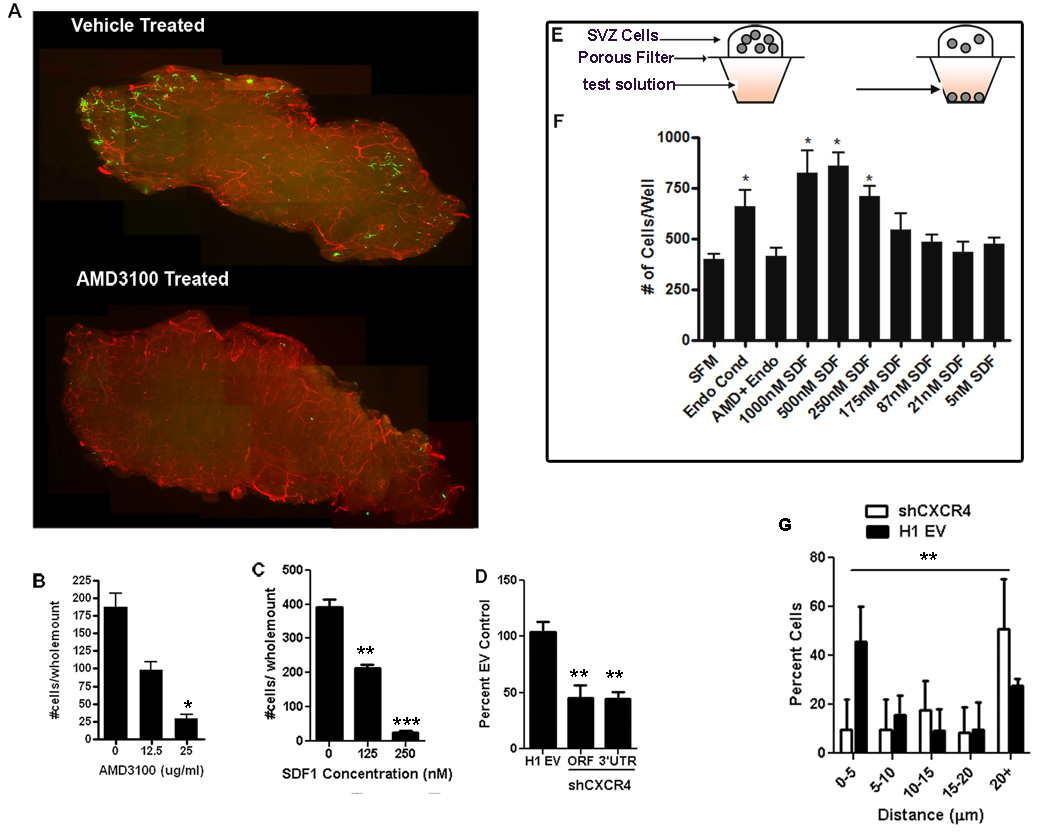
CXCR4 signaling can be blocked by the receptor antagonist AMD3100 (De Clercq, 2000). Adding different concentrations of AMD3100 into the medium gave a dose-dependent reduction in the number of cells that penetrated the wholemounts (p = 0.0048) (Figure 5A&B). We tested whether this was due to AMD3100 treatment causing NPC death or reduced proliferation. After the homing assay, we found activated caspase3 + eGFP+ cells were extremely rare with or without the drug, and there was no significant difference in the proliferative marker Ki67 in infiltrated cells following treatment with AMD3100 or vehicle (29.90 ± 1.20% and 28.65 ±0.35% respectively). AMD3100 did not reduce cultured NPC survival or proliferation (Suppl, Figure 3). Hence, rather than selective survival or proliferation, a more likely explanation is that NPC migration into the wholemount is CXCR4-dependent. To further test this we added various concentrations of SDF1 to the cell suspensions before overlaying them, thus binding available CXCR4 and diminishing the SDF1 gradient in the wholemount. This resulted in significantly fewer cells entering the wholemount (p = 0.0010) (Figure 5C).
Figure 5.
SDF1/CXCR4 signaling is important for NPCs to locate the SVZ vasculature. GFP+ NPCs were overlaid onto SVZ wholemounts, incubated then fixed. The vasculature was stained for laminin (red). The number of cells entering the wholemount was counted (A,B) AMD3100 treated cells show significantly reduced ability to integrate into the wholemount, and this was dose-responsive. (C) SDF1 added to the media overlying the wholemount disrupts the gradient and inhibits cell integration. (D) Quantification of the number of transplanted SVZ cells entering the wholemount following lentiviral knockdown of CXCR4 receptor. (E) Schematic of the chemotaxis chamber assay used to measure attraction of NPCs to various media. (F) The number of cells that migrated towards control serum free media (SFM), endothelial conditioned media (Endo Cond), endothelial conditioned media with 25ug/ml AMD3100 (Endo + AMD3100) or various concentration of the chemokine SDF1 in the media(*p <0.05; **p <0.001; ***p< 0.0001). (G) Quantification of the distance of transplanted cells transduced with shCXCR4 or H1 empty vector to nearest blood vessel surface **p<0.001 on the distribution of shCXCR4 cells versus EV cells.
To confirm this result using another method, we generated shRNA knockdown lentiviral constructs to reduce CXCR4 expression. Transduction of NPCs with shRNAs targeting the open reading frame (ORF) or the 3’ untranslated region (UTR) resulted in 58% and 32% reduction respectively of CXCR4 transcript level compared to cells transduced with the empty vector (EV) control construct, and CXCR4 protein was also significantly reduced (Suppl. Figure 3). NPCs were transduced with CXCR4 shRNA or with EV control lentivirus, then overlaid on separate wholemounts. The percentage of cells homing to the vasculature was significantly reduced after receptor knockdown (45.4±10.8% of control for the ORF shRNA and 44.5±6.2% of control for the 3’UTR shRNA) (Figure 5D), bolstering the evidence that SDF1/CXCR4 is critical for transplanted NPCs to migrate to blood vessels.
NPCs chemotax towards endothelial-released factors
These results show that when NPCs are transplanted into the SVZ in vivo or into isolated SVZ wholemounts they find their way preferentially to blood vessels. The process by which this occurs could be via directed chemotaxis, in which case, the NPCs would move towards the vessels, for example following a chemoattractant gradient. Alternatively, the NPCs could move randomly within the tissue, and simply stop moving once they contact the blood vessel surface and associated extracellular matrix, which is enriched for laminin. To test if NPCs home toward secreted factors from endothelial cells in a directed manner, we used a modified Boyden Chamber assay which measures chemotaxis. In this assay, NPCs suspended in serum-free media are loaded on top of a porous membrane that separates the NPCs from a bottom chamber filled with test or control media. Cells will move into the bottom chamber in response to a chemotactic test agent (Figure 5E). We tested whether NPCs would chemotax towards endothelial conditioned medium or to various concentrations of SDF1.
Endothelial cells were cultured in serum-free medium for 3 days, and the conditioned medium collected, filtered and used in the chemotaxis assay. NPCs were attracted by endothelial conditioned media and this was effectively blocked by applying the CXCR4 antagonist AMD3100 (Figure 5F). This indicates that SDF1/CXCR4 is largely responsible for the ability of SVZ NPCs to chemotax towards blood vessel endothelial cells. Confirming this, NPCs displayed dose dependent chemotaxis toward an SDF1 gradient (Figure 5F). Furthermore when we stereotaxically transplanted adult SVZ neurosphere expanded cells transduced with shCXCR4 into the SVZ in vivo we observed fewer cells closely associated with the vasculature in comparison to EV control transduced cells (p=0.0041) (Figure 5G). We conclude that, like HSCs, adult SVZ progenitors are able to move toward sources of SDF1 in a CXCR4- dependent manner and that this is a key factor in endothelial cell recruitment of NPCs to blood vessels.
Endothelial cells and SDF1 direct preferential homing of activated Type B cells and downstream SVZ progenitors
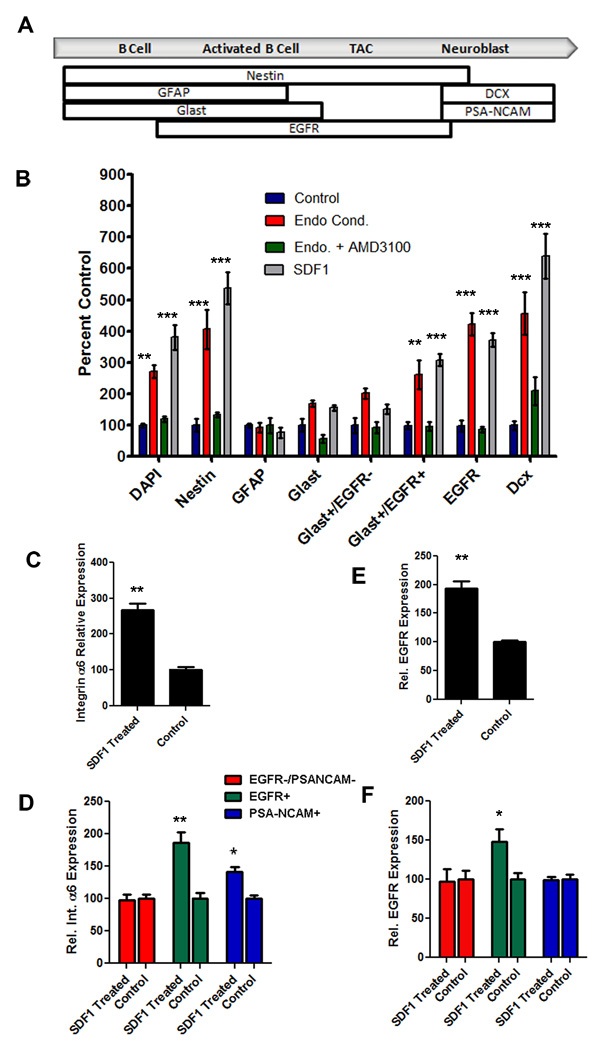
Prior studies showed that activated Type B and Type C cells, which are characterized by high EGFR expression (Pastrana et al., 2009) are found close to endothelial cell surfaces (Shen et al., 2008; Tavazoie et al., 2008). Hence, we wanted to distinguish whether different types of progenitors are more or less attracted towards SDF1 released from endothelial cells. After performing the in vitro chemotaxis assay, we characterized, based on marker expression (Figure 6A), which cells had been attracted towards the SDF1 or the endothelial cell conditioned medium.
Figure 6.
Endothelial factors and SDF1 have differential effects on NPC populations. (A) Schematic showing the markers used to define the different populations of NPCs (modified from Pastrana et al., 2009). (B) Quantification of the types of NPCs that respond to endothelial conditioned media (red bars), endothelial conditioned media with AMD3100 (green bars) or 500nM SDF1 (grey bars) normalized to control (blue bars). (C) Expression levels of α6 integrin mRNA in acutely dissociated SVZ cells following treatment with 500nM SDF1 normalized to vehicle (D) a6 integrin expression levels in subpopulations of SVZ cells following SDF1 treatment. (E) EGFR expression in acutely dissociated SVZ cell population following treatment with SDF1 normalized to controls. (F) EGFR expression levels in subpopulations of SVZ cells following treatment with SDF1. *p<0.05, **p<0.001, ***p<0.0001
The total population of cells that labeled with GFAP or GLAST antibodies (which includes astrocytes and both quiescent and activated Type B stem cells) (Bolteus and Bordey, 2004; Liu et al., 2006; Platel et al., 2009) did not move significantly toward endothelial conditioned medium or SDF1. However, when the GLAST-positive population was divided based on EGFR expression, an indication of activation, we saw that activated Type B cells (GLAST+EGFR+) (Platel et al., 2009) were attracted significantly more strongly to endothelial cell conditioned medium or SDF1 than non-activated Type B cells and astrocytes (GLAST+EGFR−) (Figure 6B). Although the GLAST+EGFR− cells showed a tendency to respond to the test gradients compared to controls, this difference did not reach significance.
Later stages in the lineage, the transit amplifying Type C cells and their progeny the Type A neuroblasts, also showed significant chemotaxis towards endothelial conditioned medium and SDF1 compared to controls (Figure 6B). Interestingly, while DCX+ neuroblasts are attracted to endothelial conditioned medium and treatment with AMD3100 blocks most of this effect, the inhibition is not as complete as for activated Type B and Type C cells (Figure 6): 47.73% of DCX+ cells were blocked from migrating toward the endothelial conditioned media by AMD3100 compared to control whereas 96.95% and 87.5% of Glast+ EGFR+ cells and EGFR+ cells respectively were blocked.
SDF1 has differential effects on integrin and EGFR expression in SVZ subpopulations
Alpha 6 integrin is expressed by SVZ stem cells, and is a marker of a variety of stem cell types (Ramalho-Santos et al., 2002). It is expressed by Type B and Type C cells that also express β1 integrin, forming a α6β1 laminin receptor. We showed previously that α6 and β1 integrin blockers prevent SVZ stem cells from effectively adhering and spreading on endothelial cell surfaces in vitro, and that infusing an α6 blocking antibody in vivo makes SVZ stem cells separate from the vascular niche (Shen et al., 2008). Hence we examined whether SDF1 treatment impacts expression of α6 integrin.
We acutely dissociated NPCs from the adult SVZ and treated them with 500nM SDF1 or with vehicle for two hours, then measured α6 integrin expression via qPCR. SDF1 produced a significant increase in α6 integrin expression in the total NPC population compared to vehicle treated controls (Figure 6C) (266 ± 19.2% normalized to control p=0.007). Previously we found that α6 integrin was expressed strongly by a subpopulation of Type B and Type C progenitor cells but to a much lesser extent by PSA-NCAM+ neuroblasts (Shen et al., 2008). To test whether SDF1 has a differential effect on α6 integrin expression in these NPC sub-populations we used fluorescent activated cell sorting to separate activated Type B and C cells (EGFR+), PSA-NCAM+ Type A neuroblasts and the remaining SVZ cell types (negative fraction). We then treated these with 500nM SDF1 or vehicle followed by qPCR for α6 integrin. Interestingly, SDF1 had the largest effect on α6 integrin expression levels in the activated (EGFR+) subpopulation of SVZ cells when normalized to vehicle control (186 ± 15.9% p= 0.0084). Type A neuroblasts, which express lower levels of α6 integrin, also showed upregulation, although to a lesser extent (141±7.5% p=.011). In contrast, the negative cell fraction, which includes quiescent Type B cells and ependymal cells, did not upregulate α6 integrin expression (Figure 6D).
In addition to its effect on α6 integrin expression, SDF1 treatment resulted in increased EGFR expression in acutely dissociated SVZ cells when compared to vehicle (Figure 6E). To test whether this was due to activation of NPCs (converting EGFR− to EGFR+ cells) or to upregulation of EGFR on already expressing, activated cells, we performed FACs as described above, then examined the response of SVZ subpopulations. SDF1 enhanced EGFR expression in the activated, EGFR+ population compared to vehicle treated cells (151±9.6% p=0.037). However, SDF1 treatment did not result in EGFR expression in the type A neuroblasts or the non-activated, EGFR− SVZ cells (Figure 6F), suggesting that SDF1 acts on already activated cells to further increase EGFR expression. Thus, SDF1 treatment alters expression of key receptors associated with proliferation, adhesion and migration of SVZ progenitor cell sub-types, enabling pleitropic effects on the SVZ lineage, as in the HSC lineage (Lapidot et al., 2005).
Discussion
Stem cell activity is governed by environmental signals from the surrounding niche. Increasingly, we perceive the stem cell-niche relationship as dynamic, with cells moving in and out of different niches as they move between states of quiescence, activation and differentiation. Thus, how stem cells occupy and leave niches is an important aspect of stem cell biology. Moreover, for stem cell based repair strategies to be effective, understanding whether or not homing to endogenous niches occurs has implications for repopulating depleted niches. In particular, demonstrating whether or not stem cells home to vascular cells is significant, as this interaction could allow NSCs to integrate into vascularized parenchymal regions of the CNS that are not endogenous niches, but could be co-opted for this purpose to enhance tissue repair. Here we show that NSCs home to the vasculature, chemotax towards endothelial cells and that the molecular mechanism is SDF-1/CXCR4 dependent.
Prior transplantation studies revealed that NPCs transplanted into the adult SVZ or hippocampus, integrate and generate neurons destined for the olfactory bulb and dentate gyrus, respectively (Gage et al., 1995; Lois and Alvarez-Buylla, 1994). While the end product of the transplanted cells was described in these studies, (i.e. neurons or glia) how the donor cells integrated into the host niche remained to be elucidated. Here we show that implanted NPCs preferentially bind to blood vessels in vivo and in isolated wholemounts. We found after 16 hours in the wholemount assay negligible cell death and fewer than 30% of the cells express the proliferation marker Ki67. Hence it is unlikely that cells preferentially survive or proliferate near blood vessels although these factors could contribute to the build-up of neural lineage cells around blood vessels in the longer term. Pursuing the hypothesis that homing was responsible, we demonstrated that blood vessel endothelial cells secrete factors that can elicit NPC chemotaxis when provided as a gradient. We did not test other blood vessel associated cells such as smooth muscle or other pericytes, so it is possible that these also impact the chemotaxic response.
Our first candidate for the homing response was SDF-1, given that endothelial cells express significant levels and that it is required for HSC homing to the bone marrow. Wholemounts of the SVZ have a staining pattern consistent with SDF-1 being made by blood vessels and released into the nearby environment, and we found the receptor CXCR4 is expressed widely on SVZ progenitor cells. Knockdown of the CXCR4 receptor decreased NPC association with blood vessels following transplantation. Importantly, AMD3100 effectively blocked the homing of activated Type B and Type C cells to blood vessels and to isolated endothelial factors, demonstrating that SDF1/CXCR4 is a critical component of the homing mechanism, and likely the major factor in this response. Interestingly, while Type A cells chemotax towards endothelial factors this was less effectively blocked by AMD3100, implying additional chemoattractive factors. The fact that we found SDF1 is key for NSC homing to endothelial cells provides a satisfying parallel with the homing mechanism in HSCs, and it will be worth pursuing other molecular parallels in the future.
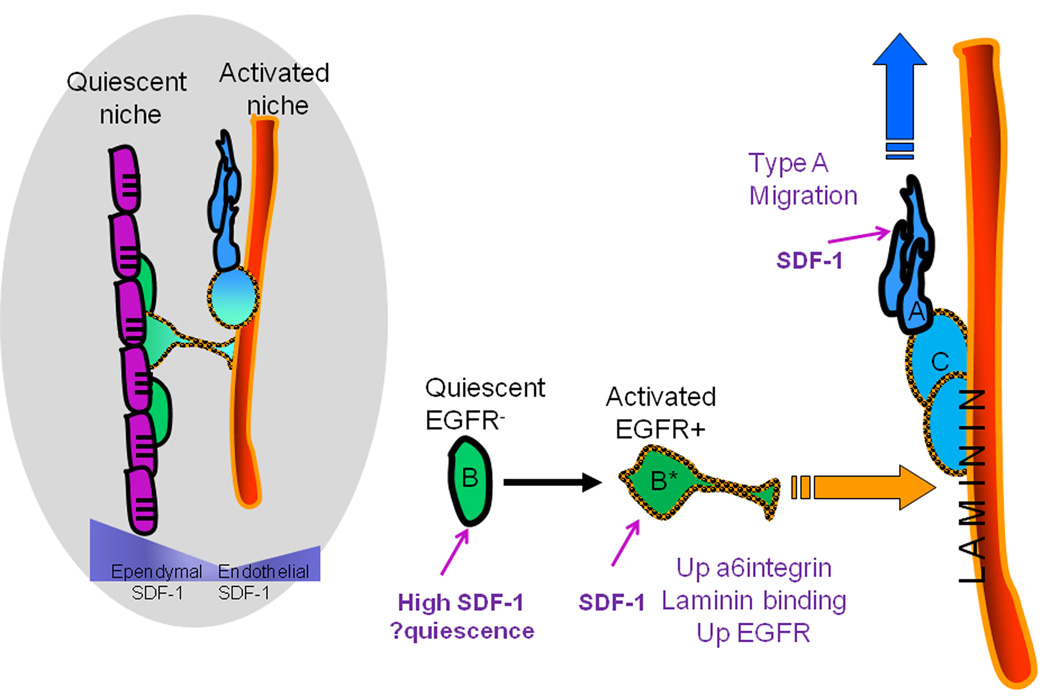
In the adult SVZ, evidence is accruing for multiple niches. Some apical Type B stem cells are distributed close to and even intercalated within the ependymal layer, and others lie close to the vasculature, with some cells spanning between. Both ependymal and vascular cells provide essential factors for NSC regulation. For example, Noggin expressed by ependymal cells promotes neurogenesis (Lim et al., 2000) and PEDF secreted by both ependymal and blood vessels supports self-renewal (Ramirez-Castillejo et al., 2006). Interestingly, few of the apical Type B cells within the ependymal zone are proliferating (Nam and Benezra, 2009) while activated Type B and Type C cells that are dividing are enriched near blood vessel surfaces (Tavazoie et al., 2008). This leads to the idea that the ependymal niche harbors largely quiescent stem cells, and the vascular niche, activated stem cells that are proliferating and producing transit amplifying Type C cells and Type A neuroblasts (Figure 7). Consistent with this, we find that activated Type B cells, Type C cells and Type A cells, all of which are proliferating compartments, show significantly more attraction to endothelial-derived factors than do quiescent stem cells. Interestingly, in the bone marrow, a sub-population of hematopoietic progenitors is found close to the vascular niche, and these may represent an activated progenitor cell while more quiescent cells lie close to osteoblasts (Kaplan et al., 2007).
Figure 7.
Summary model to describe the movement of SVZ NPCs to the vascular niche during lineage progression. SVZ blood vessels and ependymal cells (purple) represent two niches for SVZ stem cells - quiescent and activated, respectively. Both ependymal cells and endothelial cells secrete SDF1 creating a u-shaped gradient.CXCR4 is expressed on all stages of the SVZ lineage, but SDF1 has differential effects on the progenitor stages. High levels of SDF1 from ependymal cells stimulate quiescence of non-activated EGFR Type B cells (green). Upon activation and expression of EGFR (orange checked outline), SDF1 in the niche stimulates further upregulation of EGFR and α6 integrin that favor movement of the activated stem cells (green cell B*) towards the blood vessel surface, proliferation and generation of Type C cells (light blue cell). SDF1 also enhances migration of Type A cells (dark blue), which express lower levels of α6 integrin, thus promoting egress from the niche.
In the SVZ, there are clearly different levels of SDF1 expression. The ependymal zone has stronger expression than vascular cells, creating a u-shaped gradient across the germinal zone. We speculate that the high levels of SDF1 in the ependymal layer could help promote quiescence, given that in HSCs high SDF1 levels can result in receptor internalization, desensitization and quiescence, while lower concentrations result in proliferation and differentiation (Lapidot et al., 2005). Once SVZ Type B stem cells become activated and express EGFR, they chemoattract towards the blood vessel surface. Why they leave the ependymal environment for the vascular environment could be explained, in part, by our observation that SDF1 up-regulates α6 integrin on the surface of activated Type B cells, which could increase the effective binding to laminin concentrated on blood vessel surfaces. In addition, SDF1 upregulates EGFR on activated Type B cells, perhaps achieving levels needed for chemotaxis (Aguirre et al., 2005; Ayuso-Sacido et al., 2009) (Figure 7). Cross-talk between EGFR and CXCR4 signaling can augment cell proliferation and growth (Guo et al., 2007; Porcile et al., 2005). It will be important to explore further how the SDF1-upregulation of EGFR might affect migration and proliferation of SVZ NPCs.
We found that Type A cells were similarly driven towards endothelial factors, in fact even more than activated Type B and Type C cells. However in the SVZ, Type A cells are not found as close to the vascular surface as activated Type B and Type C cells. SDF1 treatment strongly upregulates α6 integrin expression on activated Type B and Type C cells, but less so on Type A cells. These observations are consistent with a model in which SDF1 stimulates Type A cells to move towards blood vessels, but not to bind to their surface as tightly as Type B and Type C cells. This could help promote Type A egress from the vascular niche and their migration towards the olfactory bulbs. Vascular cells help guide Type A neuroblasts, which run in chains parallel to blood vessels in the dorsal SVZ and occasionally move to directly touch the blood vessel surface (Bovetti et al., 2007; Snapyan et al., 2009); perhaps SDF1 signaling and low levels of α6 integrin expression helps guide these migratory movements. In summary, SDF1 signaling has differential effects on different stages of SVZ progenitor cells, which we suggest, as shown in a summary model (Figure 7), contributes to the dynamic changes of cell niche relationships as they progress down the lineage pathway.
The multiple effects of SDF1 in the SVZ is consistent with its known pleiotropism. CXCR4 is a G-protein coupled receptor, and can elicit a variety of downstream effects depending on its concentration, the state of the responding cell and other factors in the environment. SDF1 treatment increases proliferation of cultured rat or human NPCs in a dose dependent manner (Imitola et al., 2004; Liu et al., 2008), although others have reported SDF1 has no effect on proliferation but increases differentiation of adult mouse NPCs (Barkho et al., 2008) or maintains human fetal NPCs in a quiescent state (Kijowski et al., 2001). Overexpression of CXCR4 decreases proliferation of adult rat SVZ NPCs, however this effect was abolished if SDF1 was added at high concentrations (Liu et al., 2008), suggesting that the balance between availability of SDF1 and receptor level are important regulators of NPC proliferation, as for HSCs (Lapidot et al., 2005). SDF1 is involved in quiescence, activation, migration and homing via different signaling pathways (Wong and Korz, 2008). Hence, we anticipate that the molecular mechanisms underlying SDF1 signaling on different SVZ cell compartments will be a fertile area for future study.
These studies have focused on the interaction of normal NSCs and the vascular niche. Brain cancer cells also use blood vessels as a substrate for migration and spread within the CNS (Farin et al., 2006), and it is important to determine the factors involved in attraction of glioma cells to the vasculature. Following transplant into the SVZ we observed NPCs lying closely to blood vessels with a unipolar morphology and a process extending along the blood vessel, reminiscent of transplanted glioma cells (Farin et al., 2006). Given that brain cancer cells may arise from NPCs (Jackson and Alvarez-Buylla, 2008), perhaps spread of cancer cells in the brain might recapitulate the homing and migration of normal NPCs within the vascular niche. Glioma cells can express CXCR4 (Aboody et al., 2000; Benedetti et al., 2000; Glass et al., 2005), so it will be important to test whether they use the SDF1/CXCR4 mechanism to home to and migrate along blood vessels to spread within the CNS, and whether blockade of the receptor could disrupt this interaction and malignancy.
In conclusion, we have shown for the first time that activated adult SVZ stem cells home to the vasculature and reveal the crucial role of SDF1/CXCR4 in the mechanism. Uncovering how homing occurs has important implications for understanding the dynamic nature of stem cell niches, the process of stem cell activation and the events occurring after stem cell transplantation, which is necessary to fulfill the promise of neuroregenerative stem cell applications.
Experimental Procedures
Tissue Culture
Neurosphere generation
SVZ cells from adult GFP mice (C57BL/6-Tg(UBC-GFP) 30Scha/J) or wildtype mice were dissociated with 0.25% papain and 12ug/ml DNase at 37°C for 45 minutes followed by titration with a pipette. Cells were washed 3X in DMEM by centrifugation and 10,000 cells per well were plated in ultra low attachment 6 well plates (Costar) to generate neurospheres in serum free medium: DMEM containing 1mM L-glutamine, 1mM sodium pyruvate, B-27, (Stem Cells Inc.,) N2 (Gibco), 1mM N-acetyl-cysteine, supplemented with 20ng/ml epidermal growth factor (EGF) and 20ng/ml basic fibroblast growth factor (FGF2), . Neurospheres were maintained by addition of EGF and FGF2, B27 and N2 every three days and harvested after 7–10 days.
Adherent culture
E11cortical cells or adult SVZ cells were expanded with endothelial cells as previously reported (Shen et al., 2008). After 10 DIV, cells were harvested with 0.25% trypsin, rinsed by centrifugation three times and resuspended in HBSS.
SVZ Whole-Mount Organotypic Culture and Homing Assay
Adult Swiss Webster mice (Taconic) SVZ wholemounts were microdissected from the corpus collosum to the ventral tip of the lateral ventricle with minimal white matter. The wholemount was placed ventricular side up on a 1.0uM pore size tissue culture membrane insert (Corning) in a 6-well plate in serum free slice culture (SFSC) medium (see supplemental methods) Dissociated eGFP+ donor cells from adherent culture, neurospheres or acutely dissociated SVZ were suspended in SFSC medium (2000 cells in 5ul medium) were gently pipetted onto SVZ wholemounts and incubated for 16 hours. For quanitification, we used the neurosphere expanded eGFP cells overlaid onto the wholemounts which were fixed in 4%PFA and the position of the cells analyzed. Five z-stacks (0.5um) per SVZ (n=4) were captured using a Zeiss LSM510Meta-NLO confocal microscope with LSM510 software. Z-stacks were taken along the dorsal side of the SVZ at random intervals from the ependymal surface to the striatal white matter using a 40× objective. Distance of cell nuclei (eGFP+ and eGFP−) from blood vessels was quantified using an automated system, described previously (Shen et al., 2008). To test the effect of AMD3100 on survival and proliferation, neurosphere expanded eGFP+ cells were pipetted onto wholemounts and incubated overnight then treated with 25ug/ml AMD3100 or vehicle overnight before being fixed and stained for activated caspase 3 and Ki67
Chemotaxis Assay
Chemotaxis assays were performed on acutely dissociated adult SVZ cells using a poly-l-ornithine coated 96 well chemotaxis chamber with 10µm pore size (Neuroprobe Inc). 25,000 cells in 25µl of serum free media without growth factors was deposited in the upper chamber and the lower chamber was filled with 28µl of either serum free medium, endothelial conditioned medium, endothelial conditioned medium with 25µg/ml AMD3100, or serum free medium with various concentrations of mouse recombinant SDF1 (Peprotech). After incubation at 37°C for 16 hours, cells in the lower chambers were fixed with 4% PFA for 20 minutes and incubated with the nuclear dye DAPI (1:2000) for 5 minutes. Chambers were rinsed and the total number of cells that migrated through the membrane was quantified by counting the number of DAPI positive cells per well (n=5) for each condition. To characterize the phenotype of cells that responded to the gradients, immunocytochemistry was performed as described in supplemental methods.
AMD3100 Blockade and SDF1 Dose Response
AMD3100
GFP+ cells were dissociated with papain, rinsed and resuspended at 4000 cells/10 µl in serum free medium containing 50, 25 or 12.5 µg/ml or no AMD3100 (Sigma). Cells were then pipetted onto cultured SVZ wholemounts and incubated at 37°C for 16 hours.
SDF1 dose response experiment
cells were suspended at 8000cells/10ul in serum free media with 250, 125nM or no SDF1 then overlaid on the wholemounts as above. Wholemounts were then rinsed, fixed in 4% PFA and coverslipped. Cells in the wholemount were counted using a Zeiss Axiovert D1 and a FITC filter.
Transplantation of Cells into the SVZ
eGFP+ endothelial expanded cells (n=3), or adult SVZ neurosphere expanded cells transduced with H1 empty vector (n=4) or shCXCR4 (n=4) were suspended in HBSS at 10,000 cells/ul. 0.5ul of cells were injected into the SVZ of adult mice at 0.5mm anterior, 0.85mm lateral to bregma at a depth of 3.4mm from the pial surface. Mice were sacrificed after 3 days and SVZs microdissected and prepared for immunostaining. Distance of transplanted cells to blood vessels was measured using Ziess LSM 510 Software using the orthogonal section distance function from the edge of the transplanted cell to the surface of the blood vessel.
Statistical Analysis
Values are expressed as mean ± SEM. One-way ANOVAs followed by a Tukey or Two-way ANOVA followed by a Bonferonni post test were used if more than two groups were compared and Students t-tests or Mann Whitney tests were performed for two group comparison using GraphPad Prizm 4.03 software.
Supplementary Material
Acknowledgements
We thank Chris Fasano and Tim Phoenix for assistance in generating lentiviral shRNA contructs and Patty Lederman for invaluable technical support. This project was supported by NIH grant R01NS051531, the Regenerative Research Foundation, and EK was supported in part by NIDA grant 5T32DA007307.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Rizvi TA, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J Neurosci. 2005;25:11092–11106. doi: 10.1523/JNEUROSCI.2981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Ayuso-Sacido A, Moliterno JA, Kratovac S, Kapoor GS, O'Rourke DM, Holland EC, Garcia-Verdugo JM, Roy NS, Boockvar JA. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J Neurooncol. 2009 doi: 10.1007/s11060-009-0035-x. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–3149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T, Puche AC. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute JP. Stem cell homing. Curr Opin Hematol. 2006;13:399–406. doi: 10.1097/01.moh.0000245698.62511.3d. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Inhibition of HIV infection by bicyclams, highly potent and specific CXCR4 antagonists. Mol Pharmacol. 2000;57:833–839. [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R, Synowitz M, Kronenberg G, Walzlein JH, Markovic DS, Wang LP, Gast D, Kiwit J, Kempermann G, Kettenmann H. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cai S, Fang R, Chen H, Du J, Tan Y, Ma W, Hu H, Cai S, Liu Y. The synergistic effects of CXCR4 and EGFR on promoting EGF-mediated metastasis in ovarian cancer cells. Colloids Surf B Biointerfaces. 2007;60:1–6. doi: 10.1016/j.colsurfb.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Alvarez-Buylla A. Characterization of adult neural stem cells and their relation to brain tumors. Cells Tissues Organs. 2008;188:212–224. doi: 10.1159/000114541. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, Janowska-Wieczorek A, Ratajczak MZ. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19:453–466. doi: 10.1634/stemcells.19-5-453. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Bolteus AJ, Balkin DM, Henschel O, Bordey A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Santra M, Hozeska-Solgot A, Zhang RL, Wang L, Teng H, Lu M, Zhang ZG. Functional response to SDF1 alpha through overexpression of CXCR4 on adult subventricular zone progenitor cells. Brain Res. 2008;1226:18–26. doi: 10.1016/j.brainres.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell. 2009;5:515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes MF, Li G, Berger O, Baraban SC, Pleasure SJ. Stromal-derived factor-1 (CXCL12) regulates laminar position of Cajal-Retzius cells in normal and dysplastic brains. J Neurosci. 2006;26:9404–9412. doi: 10.1523/JNEUROSCI.2575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- Platel JC, Gordon V, Heintz T, Bordey A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia. 2009;57:66–78. doi: 10.1002/glia.20735. [DOI] [PubMed] [Google Scholar]
- Porcile C, Bajetto A, Barbieri F, Barbero S, Bonavia R, Biglieri M, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308:241–253. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. "Stemness": transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, Mira H, Escribano J, Farinas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Schonemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol. 2008;510:207–220. doi: 10.1002/cne.21780. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J, Konig N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voermans C, Anthony EC, Mul E, van der Schoot E, Hordijk P. SDF-1-induced actin polymerization and migration in human hematopoietic progenitor cells. Exp Hematol. 2001;29:1456–1464. doi: 10.1016/s0301-472x(01)00740-8. [DOI] [PubMed] [Google Scholar]
- Wong D, Korz W. Translating an Antagonist of Chemokine Receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14:7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.