Abstract
Alcohol withdrawal is associated with affective-behavioral disturbances in both human alcoholics and in animal models. In general, these phenomena are potentiated by increased alcohol exposure duration and by prior withdrawal episodes. Previous studies have also reported locomotor hypoactivity during ethanol withdrawal in rats and mice, but only in novel test environments, not in the home-cage. In the present study, we examined the effects of withdrawal from chronic intermittent ethanol (CIE) vapor exposure on the level and circadian periodicity of wheel-running activity in C57BL/6J mice. CIE treatment resulted in reductions in wheel-running activity relative to plain-air controls that persisted for about one week after withdrawal. Analysis of circadian waveforms indicated that reduced activity occurred throughout the night phase, but that daily activity patterns were otherwise unaltered. CIE failed to alter free-running circadian period or phase in animals maintained under constant darkness. These results show that ethanol withdrawal can result in locomotor hypoactivity even in the habitual, home-cage environment, and suggest that withdrawal-related reductions in wheel-running activity may reflect the specific motivational significance of this behavior.
Keywords: circadian, wheel-running, ethanol, alcohol, withdrawal, inbred mice, C57BL/6J
Introduction
Alcohol withdrawal is associated with severe and persistent affective-behavioral disturbances. In rodents, these disturbances are commonly modeled using measures of locomotor activity, exploration, and anxiety-like behavior. Thus, numerous studies have reported both increased anxiety and reduced locomotor activity during withdrawal from chronic ethanol exposure in rats (Rasmussen et al., 2001; Overstreet et al., 2003; Valdez et al., 2002) and mice (Hutchins et al., 1981; Kliethermes et al., 2004). Since common tests of anxiety-like behaviors such as the elevated-plus maze and light-dark box rely on voluntary movement and exploration within the testing apparatus, it can be challenging to isolate experimental variables reflecting anxiety from those reflecting locomotion (File et al., 1995, 2004). This is a potentially serious confound in studies of ethanol withdrawal, since reduced locomotor activity during withdrawal could result from non-specific factors such as lethargy or malaise (Kliethermes, 2005; Kliethermes et al.,2005).
On the other hand, decreased locomotor activity in a novel test environment has itself been utilized widely as a behavioral marker for anxiety (i.e., in the open-field test; Stanford, 2007), while strain differences in anxiety-like behavior and locomotor activity are strongly correlated among inbred mice, suggesting that these two variables reflect partially overlapping constructs (Milner and Crabbe, 2008). Indeed, withdrawal-induced malaise is unlikely to be a major factor contributing to reduced exploratory locomotion during tests for anxiety-like behavior, since little or no locomotor suppression was seen in ethanol-withdrawn mice when assessed in their home-cage environment (Kliethermes et al., 2005). Thus, the co-occurrence of locomotor suppression and behavioral anxiety during ethanol withdrawal may reveal important biological relationships among different measures of affective behavior, rather than methodological confounding.
In order to better define the generality of ethanol withdrawal-induced hypo-locomotion, we examined the effects of withdrawal from chronic intermittent ethanol vapor (CIE) on home-cage wheel-running activity. Wheel-running activity is thought to reflect a specific form of exploratory motivation, behaviorally distinct from “general activity” (Mather, 1981; Sherwin, 1998). Access to running-wheels is both rewarding and reinforcing (Iversen, 1993; Belke, 1997; Lett et al., 2000; de Visser et al., 2007), can lead to compulsive, addiction-like behavior (Hoffman et al., 1987; Ferreira et al., 2006), and has both antidepressive and anxiolytic effects in a variety of animal models (Brene et al., 2007; Duman et al., 2008; Salam et al., 2009). Further, this behavior is controlled by brain mechanisms similar to those associated with the rewarding properties of drugs of abuse, including the mesolimbic dopamine-opioid system (Lett, 2001, 2002; Werme et al., 2002a; Pietropaolo et al., 2008; Vargas-Perez et al., 2008), and under some conditions may modulate ethanol preference (McMillan et al., 1995; Werme et al., 2002b; Ozburn et al., 2008). We reasoned, therefore, that assessment of wheel-running activity during ethanol withdrawal might reveal effects that were not apparent in simple measures of home-cage ambulation (Kliethermes et al., 2005).
In addition to examining the effects of ethanol withdrawal on the overall level of wheel-running activity, we also investigated possible changes in the circadian aspects of this behavior. While human alcoholics display dramatic disruptions in sleep-wake cycles and other circadian biological rhythms during both acute withdrawal and protracted abstinence, the extent to which these effects reflect alterations in circadian pacemaker function is not know. Such a mechanism seems likely, however, since chronic ethanol intake shortens free-running circadian period (Dwyer and Rosenwasser, 1998; Rosenwasser et al., 2005a; Seggio et al., 2009), and attenuates light-induced circadian phase-shifting (Rosenwasser et al., 2005b; Seggio et al., 2007, 2009; Ruby et al., 2009) in experimental animals. Nevertheless, these studies were all conducted under continuous free-choice access to ethanol, conditions that are unlikely to produce ethanol dependence. Thus the effects of ethanol dependence and withdrawal on circadian rhythms have not been investigated in an animal model.
The current experiments were therefore designed to determine the effects of chronic intermittent ethanol vapor exposure on the level and circadian rhythmicity of wheel-running activity in C57BL/6J mice. We hypothesized that ethanol withdrawal would suppress locomotor activity and disrupt the expression of circadian activity rhythms, similar to the effects reported in withdrawn alcoholics.
Material and methods
Subjects and apparatus
Upon arrival in the laboratory, 6–8 week old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), were weighed (range: 19–22 g) and placed in individual running-wheel cages (wheel diameter: 35 cm). Each running wheel-cage was housed in a separate light- and sound-shielded enclosure equipped with a standard fluorescent bulb. Food (Prolab RMH 3000) and water were freely available throughout the experiment. Wheel-running activity was recorded and analyzed using the ClockLab interface system (Actimetrics Co., Wilmette, IL).
Procedures
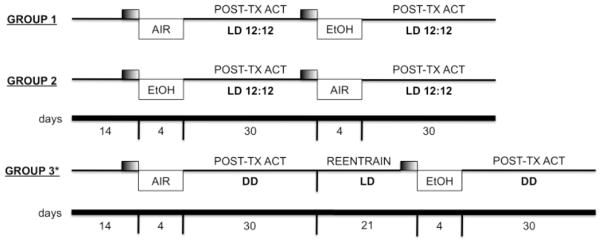
Each animal in this experiment was exposed to both a 4-day chronic intermittent ethanol vapor exposure (CIE) protocol (see below) and a plain-air control procedure, using a repeated-measures cross-over design. All mice were initially maintained under a light-dark (LD) 12:12 cycle for a period of 10–14 days to allow for acclimation to running wheels and stabilization of activity levels. Following this acclimation period, the animals were exposed to either CIE or air treatment. During the 4-day exposure protocol, the running-wheel cages were moved from the normal experimental cabinet and placed within large Plexiglas inhalation chambers (60 × 36 × 60 cm). Thus, while animals had free access to their running wheels throughout the protocol, wheel-running activity was not recorded in the vapor chambers. Following CIE or air treatment, running-wheel cages were returned to the normal recording cabinet, and wheel-turns were recorded for an additional 3–4 week post-treatment period. During the post-treatment phase of the experiment, animals were either maintained under the original LD cycle (Groups 1 and 2), or transferred into constant darkness (DD; Group 3) to evaluate the period and phase of free-running circadian activity rhythms (see below). After four weeks, Group 3 (DD) animals were returned to the original LD cycle until stable re-entrainment was achieved. Finally, all animals were exposed to the second 4-day treatment protocol, either CIE or air, and again studied for a 3–4 week post-treatment period in either LD or DD. A schematic representation of the experimental time-lines for the three groups is presented in Fig. 1.
Fig. 1.
Schematic illustration of repeated-measures cross-over design. Three separate groups of mice were treated with both CIE and plain air exposure and then placed either LD or DD to measure circadian wheel-running activity (Groups 1 and 2 were tested in LD; Group 3 was tested in DD). The duration of each experimental epoch is represented by the timeline at the bottom. Small shaded rectangle represents 5-day period of baseline activity for comparison to post-treatment activity.
Chronic intermittent ethanol (CIE) protocol
The CIE protocol employed in this experiment was based on the work of Becker and coworkers (Becker and Hale, 1993; Becker et al., 1997), and consisted of four daily cycles of 16 hours of ethanol vapor exposure alternating with 8 hours of plain air exposure. Each 16-hour ethanol vapor exposure started at the onset of darkness and ended four hours into the light phase of the LD cycle. Control animals were handled identically but exposed only to plain air. Immediately prior to each ethanol vapor exposure, CIE animals were administered a priming injection containing 1.6 g/kg ethanol and 68.1 mg/kg pyrazole HCl, an alcohol dehydrogenase inhibitor used to rapidly increase and stabilize blood ethanol concentrations (e.g., Becker and Hale,1993). Pyrazole was dissolved in 20% v/v ethanol solution and injected i.p. at a volume of 20 ml/kg, while control animals were administered an identical dose of pyrazole in 0.9% saline solution at the same volume.
Ethanol vapor and air were delivered to the exposure chambers at a rate of 10–12 liters per min, ensuring adequate air flow to meet the animals’ respiratory requirements. Ethanol was vaporized using a pressurized pump to push air through a porous diffusing stone submerged in a 1.0 liter bottle filled with 95% ethanol. Measurement of ethanol concentrations in tail blood and in inhalation chambers
Blood ethanol concentrations (BECs) were measured in all animals at the termination of the CIE treatment protocol, immediately prior to being returned to the activity recording cabinet. Briefly, each mouse was removed from its cage and placed in a plastic restraining tube, and a small (approximately 10 μl) blood sample was collected from the tip of the tail. Blood was collected directly into a heparinized capillary tube and centrifuged for 2 minutes to separate serum from plasma. BECs were determined from 5 μl plasma samples using an AM-1 alcohol analyzer (Analox Instr., Lunenburg, MA).
To ensure ethanol vapor concentrations were within an appropriate range and stable across exposure days, 5.0 ml air samples were extracted from the ethanol chambers using a 60 ml syringe mixed with 55.0 ml of room air. The diluted sample was injected into a breathalyzer (Lifeloc FC-10; Wheat Ridge, CO) and the resultant readings were compared to a standardized calibration curve of known ethanol concentration to determine chamber ethanol concentration. The ethanol concentrations were maintained at 10–12 mg/L throughout the treatment protocol.
Ethical considerations
This experiment was approved by the University of Maine Institutional Animal Care and Use Committee (IACUC).
Data analysis
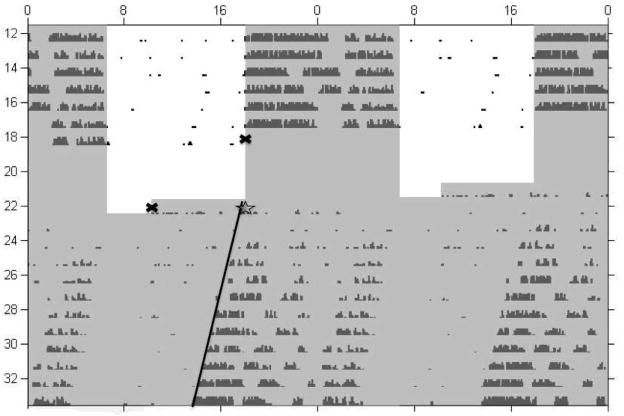
Circadian activity rhythms: Standard raster-style circadian actograms were generated using ClockLab software for visual inspection of activity patterns (cf. Fig. 2). Free-running periods were estimated by the slope of the straight lines fit to activity onsets with assistance of an automated onset-detection feature of the ClockLab software. The initial phase of activity was determined by back-extrapolating the fitted line to the first day following treatment. This method was utilized because a majority, if not all, of the mice exposed to ethanol treatment displayed significantly reduced running-wheel activity on the first post-treatment day and reliable activity onsets could not be determined.
Fig. 2.
Standard raster-style circadian actogram of an animal exposed to CIE-treatment. Lines were fitted to activity onsets on each day and back-extrapolated to determine the onset on day 1 following treatment (represented by star symbol). Shaded region denotes lights off. The x-symbol represents the beginning and end of treatment protocol.
Statistics
Wheel-running activity was compared between groups using repeated-measures ANOVA and standard t-tests for pairwise comparisons. The average wheel-running activity during the final five days prior to treatment was used as the baseline, and each animal’s post-treatment activity was expressed as a percentage of its own baseline in order to reduce the effects of individual differences in activity level. Because of the use of a cross-over design, an initial analysis employed both treatment (ethanol vapor vs. air) and sequence of treatment (vapor-first vs. air-first) as between-groups factors, and post-treatment day as a repeated-measures factor. Since this analysis failed to detect any significant effects or interactions related to sequence, the results reported here are based on a subsequent analysis using only treatment and day as factors.
Results
BECs measured at the time of CIE treatment termination were within the intended target range, averaged 168.86 ± 21.86 (SEM) mg/dl across groups, and did not differ as a function of testing sequence.
Animals averaged 15,683 wheel-turns per 24 hours during the 5-day pretreatment baseline. For reference, this corresponds to a linear distance of 17.24 km, although the energetic demands of wheel-running and locomotion on a flat surface may not be directly comparable. Collapsing across all three treatment groups, CIE treatment produced a significant reduction in wheel-running activity relative to the control air treatment that endured for about one week following treatment (Figs. 2, 3). A two-factor repeated-measures ANOVA revealed a significant main effect of post-treatment day (F 29,754 = 6.628, p = 0.0001) and a significant group by day interaction (F29,754 = 4.36, p = 0.0001). This interaction was explored further by conducting between-groups pairwise comparisons for each post-treatment day using t-tests, which revealed significant group differences for the first six post-treatment days.
Fig. 3.
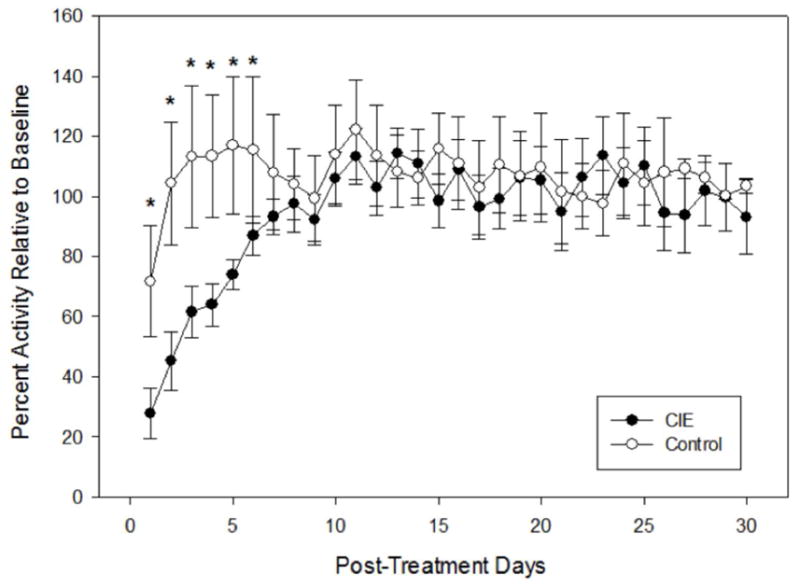
Mean (±SEM) percent activity relative to baseline for days following CIE and plain air treatment conditions, across all groups. A significant reduction in wheel-running activity was found for days 1–6 in CIE-exposed mice (* p < 0.05); see text for details.
Averaged daily waveforms under LD 12:12 were constructed for both CIE and control animals in Groups 1 and 2 using the five pre-treatment days and the first seven post-treatment days. Inspection of these waveforms showed that locomotor activity was reduced throughout the active phase of the circadian day in CIE-exposed animals but not in controls (Fig. 4). Nevertheless, there were no apparent alterations in the overall shape of the circadian activity pattern in either group.
Fig. 4.
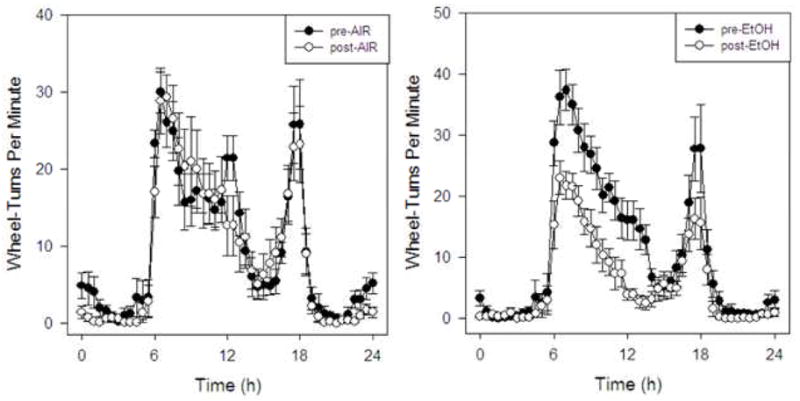
Mean (± SEM) circadian waveforms for CIE and plain air treatment conditions under LD conditions (Groups 1 and 2). Following CIE but not air treatment, reductions in wheel-running activity were exhibited in the active phase (right panel).
Free-running circadian period in DD and the initial phase of activity onset on post-treatment day 1 were determined for both CIE and control animals in Group 3. No significant differences between CIE and control treatments were found for either variable (Fig. 5).
Fig. 5.
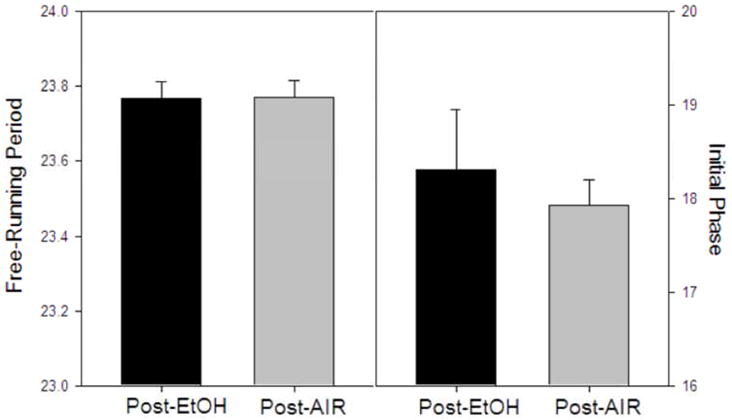
Mean (+ SEM) free-running period and initial phase of activity onset in DD following CIE and plain air treatments (Group 3). No significant differences were found between ethanol- and air-exposed animals for either free-running period (left) or for initial phase of the free-running rhythm (right).
Discussion
The results of this study indicate that ethanol withdrawal following CIE in C57BL/6J mice is associated with marked reductions in wheel-running activity that persist for about one week before returning to pre-treatment levels. While reduced activity was manifest throughout the circadian night phase, the overall circadian waveform was not otherwise altered. Moreover, and contrary to expectations, CIE-withdrawn mice failed to display significant alterations in free-running circadian period or phase when maintained in DD.
While Kliethermes et al. (2004) also reported reduced locomotor activity during withdrawal from ethanol vapor exposure in mice, these observations were made during testing for anxiety-like behavior in novel test environments during the first 48 hours following termination of ethanol administration. In contrast, little or no evidence for withdrawal-induced locomotor hypoactivity was seen in the home-cage environment (Kliethermes et al., 2005b), suggesting that activity suppression may be specific to novel test environments. In contrast, however, ethanol-withdrawn mice in the present study showed persistent locomotor suppression in familiar, home-cage running wheels. Thus, while other procedural differences cannot be excluded (e.g., ethanol vapor protocol, housing conditions, strain differences, etc.), the present results indicate that the type of activity (wheel-running vs. “general” cage activity) is a critical factor determining locomotor suppression during ethanol withdrawal.
More specifically, the pronounced reduction in wheel-running activity seen in this study may reflect the important motivational significance of this behavior, in contrast to simple home-cage ambulation. Specifically, we hypothesize that reduced wheel-running activity during ethanol withdrawal may reflect a generalized deficit in reward-seeking behavior, similar to that posited by Schulties et al. (1995) to underlie reduced responding for brain stimulation reward in ethanol-withdrawn rats. At present, however, we cannot rule out potentially critical roles for other affective or even motoric factors, such as the amount of effort required to turn a wheel relative to other forms of activity.
While previous studies indicate that chronic ethanol intake alters fundamental properties of the circadian pacemaker, this was the first study to examine free-running circadian activity rhythms during ethanol withdrawal, utilizing an experimental protocol shown to produce ethanol dependence. In contrast to our previous results indicating that free-running circadian period is shortened during chronic forced intake of 10% ethanol solution in C57BL/6J mice (Seggio et al., 2009), the present study failed to detect any effect on circadian period or phase following withdrawal from CIE. Since more extended CIE exposures have been shown to lead to more dramatic and enduring effects in other withdrawal-related phenotypes (Becker et al., 1997; Becker and Lopez, 2004; Lopez and Becker, 2005), we are presently conducting additional experiments to determine if CIE effects on the circadian clock may also be revealed by such procedural modifications. In addition, since the C57BL/6J strain used in the present experiment is known for its relative insensitivity to ethanol withdrawal in other phenotypic domains (Metten et al., 1998; Metten and Crabbe, 2005), it is possible that more substantial effects on both the level and the circadian periodicity of wheel-running activity might be revealed in more withdrawal-sensitive strains.
In conclusion, we have shown that ethanol withdrawal following a 4-day CIE protocol leads to a persisting reduction in the level of home-cage wheel-running activity, but fails to alter the circadian periodicity of this behavior, in C57BL/6J mice. Subsequent experiments will further explore relationships between these variables and other withdrawal-related phenotypes, and their possible dependence on genetic background.
Acknowledgments
This work was supported by INIA-Stress, U01 AA13641, PI: K. Grant, OHSU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal otentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Belke TW. Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J Exp Anal Behav. 1997;67:337–351. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer SM, Rosenwasser AM. Neonatal clomipramine treatment, alcohol intake and circadian rhythms in rats. Psychopharmacology (Berl) 1998;138:176–183. doi: 10.1007/s002130050660. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Lamarque S, Boyer P, Perez-Diaz F, Jouvent R, Cohen-Salmon C. Spontaneous appetence for wheel-running: a model of dependency on physical activity in rat. Eur Psychiatry. 2006;21:580–588. doi: 10.1016/j.eurpsy.2005.02.003. [DOI] [PubMed] [Google Scholar]
- File SE. Animal models of different anxiety states. Advances Biochem Psychopharmacol. 1995;48:93–113. [PubMed] [Google Scholar]
- File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8):3. doi: 10.1002/0471142301.ns0803s26. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Thoren P, Ely D. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR) Behav Neural Biol. 1987;47:346–355. doi: 10.1016/s0163-1047(87)90461-4. [DOI] [PubMed] [Google Scholar]
- Hutchins JB, Allen DL, Cole-Harding LS, Wilson JR. Behavioral and physiological measures for studying ethanol dependence in mice. Pharmacol Biochem Behav. 1981;15:55–59. doi: 10.1016/0091-3057(81)90338-5. [DOI] [PubMed] [Google Scholar]
- Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J Exp Anal Behav. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Home cage activity and ingestive behaviors in mice following chronic ethanol vapor inhalation. Physiol Behav. 2005;85:479–488. doi: 10.1016/j.physbeh.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72:355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72:101–105. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- Lopez M, Becker H. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Mather JG. Wheel-running activity: a new interpretation. Mammal Review. 1981;11:41–51. [Google Scholar]
- McMillan DE, McClure GY, Hardwick WC. Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol. Drug Alcohol Depend. 1995;40:1–7. doi: 10.1016/0376-8716(95)01162-5. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2c antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology (Berl) 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Harris RA, Blednov YA. Wheel running, voluntary ethanol consumption, and hedonic substitution. Alcohol. 2008;42:417–424. doi: 10.1016/j.alcohol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192:42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav. 2005a;84:537–542. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiol Internat. 2005b;22:227–236. doi: 10.1081/cbi-200053496. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, Depaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol. 2009;296:R411–418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009 doi: 10.1177/0748730409338449. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Stanford SC. The Open Field Test: reinventing the wheel. J Psychopharmacol. 2007;21:134–135. doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Sellings LH, Paredes RG, Prado-Alcala RA, Diaz JL. Reinforcement of wheel running in BALB/c mice: role of motor activity and endogenous opioids. J Mot Behav. 2008;40:587–593. doi: 10.3200/JMBR.40.6.587-593. [DOI] [PubMed] [Google Scholar]
- de Visser L, van den Bos R, Stoker AK, Kas MJ, Spruijt BM. Effects of genetic background and environmental novelty on wheel running as a rewarding behaviour in mice. Behav Brain Res. 2007;177:290–297. doi: 10.1016/j.bbr.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Werme M, Lindholm S, Thoren P, Franck J, Brene S. Running increases ethanol preference. Behav Brain Res. 2002a;133:301–308. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB regulates wheel running. J Neurosci. 2002b;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]