Abstract
Many of the most dangerous human diseases are transmitted by insect vectors. After decades of repeated insecticide use, all of these vector species have demonstrated the capacity to evolve resistance to insecticides. Insecticide resistance is generally considered to undermine control of vector-transmitted diseases because it increases the number of vectors that survive the insecticide treatment. Disease control failure, however, need not follow from vector control failure. Here, we review evidence that insecticide resistance may have an impact on the quality of vectors and, specifically, on three key determinants of parasite transmission: vector longevity, competence, and behaviour. We argue that, in some instances, insecticide resistance is likely to result in a decrease in vector longevity, a decrease in infectiousness, or in a change in behaviour, all of which will reduce the vectorial capacity of the insect. If this effect is sufficiently large, the impact of insecticide resistance on disease management may not be as detrimental as previously thought. In other instances, however, insecticide resistance may have the opposite effect, increasing the insect's vectorial capacity, which may lead to a dramatic increase in the transmission of the disease and even to a higher prevalence than in the absence of insecticides. Either way—and there may be no simple generality—the consequence of the evolution of insecticide resistance for disease ecology deserves additional attention.
Introduction
Vector-borne diseases are among the major causes of illness and death, particularly in tropical and subtropical countries. Vector control, through the use of insecticides, plays a key role in the prevention and control of infectious diseases such as malaria, dengue, and filariasis [1]. The widespread use of insecticides can, however, lead to the development of insecticide resistance, making insecticide use ineffective and limiting the available options for disease control [2]. Insecticide resistance, including resistance to multiple types of insecticides, has arisen in all the insect species that are the major vectors of human diseases (Table 1). Consequently, insecticide resistance is considered a serious public health challenge.
Table 1. Insecticide resistance mechanisms reported to date in natural populations of the main insect vectors of human diseases.
| Vector | Pathogen (Disease) | Insecticide Resistance | |
| Metabolic | Target Site | ||
| Diptera (mosquitoes, flies) | |||
| Aedes sp. | Brugia, Wuchereria (lymphatic filariasis), yellow fever virus, dengue virus, encephalitis virus | EST [37] GST [37] | SCH [37] GABA[37] |
| Anopheles sp. | Plasmodium sp. (malaria), Wuchereria (filariasis) | EST [37] GST [37], [118] MOX [37] | SCH [37], [118] AChE [37], [118] GABA [114] |
| Culex sp. | Wuchereria (filariasis), West Nile virus, encephalitis virus | EST [37] GST [114] MOX [37] | SCH [114] AChE [37] GABA [114] |
| Phlebotomus sp. | Leishmania sp. (leishmaniasis) | EST [119] | AChE [119] |
| Simulium sp. | Onchocerca sp. (river blindness) | EST [120], [121] | - |
| Haemiptera (true bugs) | |||
| Rhodnius sp. | Trypanosoma sp. (Chagas disease) | ? [122] | ? [122] |
| Triatoma sp. | Trypanosoma sp. (Chagas disease) | EST [123], [124] MOX [123], [125] | - |
| Phiraptera (body lice) | |||
| Pediculus sp. | Rickettsia sp. (epidemic thyphus) | ? [33] | ? [33] |
| Siphonaptera (fleas) | |||
| Xenopsylla sp. | Pasturella (bubonic plague) | ? [126], [127] | ? [126], [127] |
Metabolic resistance: EST, enhanced esterase activity; GST, enhanced glutatione-S-transferase activity; MOX, enhanced p450 monoxygenase activity. Target site resistance: AChE, modification of the acetylcholinesterase; GAB, modification of the GABA receptors; SCH, modification of the sodium channels. ?, Insecticide resistance present but mechanism unknown or unconfirmed to the best of our knowledge.
What is the impact of insecticide resistance on the transmission of vector-borne diseases? This can be best explored using a fundamental concept describing the transmissibility of infectious diseases: the parasite's basic reproductive number, or 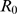 (see Box 1). This quantity plays a central role in epidemiology because it provides a synthetic index of transmission intensity and establishes threshold criteria for disease establishment or eradication. In particular, the prevalence of a disease is expected to increase in a naive population only when
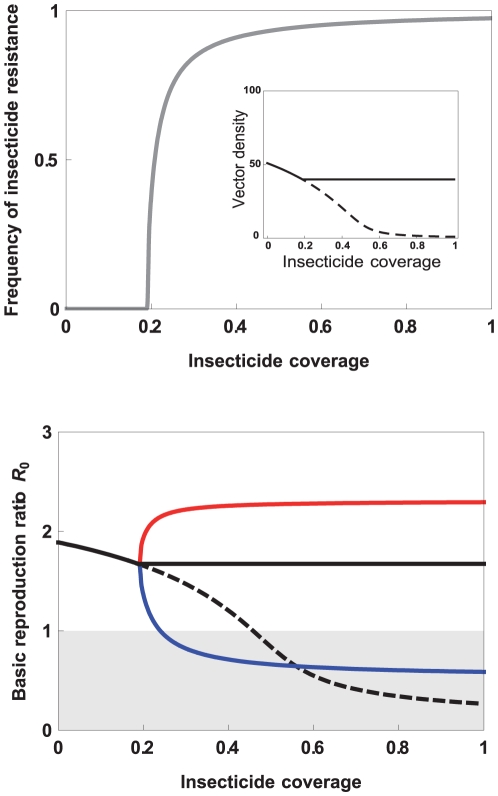
(see Box 1). This quantity plays a central role in epidemiology because it provides a synthetic index of transmission intensity and establishes threshold criteria for disease establishment or eradication. In particular, the prevalence of a disease is expected to increase in a naive population only when 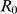 is greater than one. A major aim of insecticide spraying is to reduce the number of vectors (and thus m in Box 1). The emergence of insecticide resistance, however, counters this control method by increasing the number of mosquitoes that survive the insecticide treatment (Figure 1, top). This can result in substantial increases in vector numbers, possibly to pre-treatment densities (or nearly so, if there are costs associated with insecticide resistance [3]–[9]). Concerns about rebounding vector populations have been sufficient to motivate the search for novel insecticides [10], [11], the development of continent-wide resistance surveillance networks [12], [13], and work on resistance management strategies aimed at retarding or preventing the spread of resistance [14]–[18].
is greater than one. A major aim of insecticide spraying is to reduce the number of vectors (and thus m in Box 1). The emergence of insecticide resistance, however, counters this control method by increasing the number of mosquitoes that survive the insecticide treatment (Figure 1, top). This can result in substantial increases in vector numbers, possibly to pre-treatment densities (or nearly so, if there are costs associated with insecticide resistance [3]–[9]). Concerns about rebounding vector populations have been sufficient to motivate the search for novel insecticides [10], [11], the development of continent-wide resistance surveillance networks [12], [13], and work on resistance management strategies aimed at retarding or preventing the spread of resistance [14]–[18].
Box 1. Basic Reproductive Number of Vector-Borne Diseases
In the following, we distinguish the vector (e.g., mosquito in malaria) from the host (e.g., mammalian host in malaria). A general expression for R 0 can be readily derived for simple vector-borne diseases [41], [117], [118]. We present the expression of R 0 when the vector population is heterogeneous, consisting of both susceptible and resistant (prime symbol) individuals:
Where 1/r is the expected duration of the infection in the host (r is the rate of clearance of the infection in the host), m is the number of adult vectors per host, and IC is the individual vectorial capacity of the vector (modified from [119], [120]):
The other parameters are defined as follows:
aU and aI: number of (uninfected and infected bites) on the focal host, per vector and per day, which depends on fU and fI, the vector's feeding rates, and on QU and QI, the proportion of those bites on the focal host (i.e., human versus other mammals in human malaria) such that aU = fUQU and aI = fIQI.
b: probability that a host becomes infected from a bite of an infected vector (i.e., host susceptibility and vector infectiousness).
c: probability that a vector becomes infected from a bite on an infected host (i.e., vector susceptibility).
g: death rate of the vector. In other words, 1/g is the expected lifespan of a vector, and e −g is the probability a mosquito survives one day.
n: incubation time of the parasite in the vector (i.e., number of days required for the vector to become infectious after biting an infected host).
Insecticide resistance can have an effect on vector abundance (m′) but may also alter the vector's individual vectorial capacity (IC′) by modifying the vector's longevity (1/g′), competence (b′, c′, n′), and behaviour (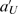 and
and 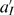 ).
).
Figure 1. Effect of increasing insecticide coverage on (top) the frequency of insecticide resistance (IR, gray line), and, in the inset, the vector density with (full line) or without (dashed line) IR evolution; (bottom) the basic reproductive ratio of the infectious disease transmitted by the vector (see Box 1).
(Bottom) We consider different scenarios: in the absence of IR evolution in the vector (dashed black line), and after IR evolution when the IR insects are equally good vectors as the susceptible ones (full black line), better (red line), or worse (blue line). The gray area delimits the area where the parasite goes to extinction (R 0<1). See Appendix S1 for the details of the model and parameter values.
One factor that has been largely overlooked is the potential effects of insecticide resistance on the ability of the vectors themselves to transmit disease (the individual vectorial capacity, Box 1): are insecticide-resistant insects better or worse vectors of diseases than susceptible ones? Far from being mere flying syringes, vectors provide a very specific environment in which the parasites differentiate, proliferate, and migrate to the correct tissues to ensure transmission to the next host. Recent work suggests that this environment is drastically modified when insects become resistant to insecticides [19], [20]. McCarroll et al. [21], [22] have shown that insecticide-resistant Culex quinquefasciatus mosquitoes are less likely to transmit the filaria parasite Wuchereria bancrofti than their insecticide-susceptible counterparts, and insecticide resistance in Culex pipiens seems to interact in a complex way with microsporidian and bacterial organisms [23]–[25]. Thus, increasing numbers of resistant insects need not lead to proportionate increases in disease transmission: it depends on whether those insects are more or less permissive transmitters than their susceptible ancestors. In this review, we survey a range of possibilities. We conclude that if the few data that exist extend to other combinations of vector species, insecticide resistance mechanisms, and parasites, it is currently not possible to evaluate the public health significance of insecticide resistance.
Insecticide Resistance Mechanisms
Four classes of chemical insecticides are the mainstay of vector control programmes: organochlorines, organophosphates, carbamates, and pyrethroids [1]. More recently, two alternative insecticide types have been introduced, largely for the control of mosquito larvae: biopesticides (e.g., Bacillus thuringiensis, Bacillus sphaericus) and insect growth regulators, such as the juvenile hormone mimic, methoprene [1]. Cases of resistance to these alternative insecticides are still limited (but see [26]–[29]) and the underlying mechanisms are only beginning to be identified [30]–[32].
To date, four types of resistance mechanisms against the chemical insecticides mentioned above have been described: metabolic resistance, target site resistance, penetration resistance, and behavioural resistance. To illustrate our arguments, we focus on metabolic and target site resistance because they have been extensively investigated at both the genetic and molecular levels [33].
Metabolic resistance involves the sequestration, metabolism, and/or detoxification of the insecticide, largely through the overproduction of specific enzymes [34], [35]. Three main groups of enzymes have been identified (Table 1): carboxylesterases (efficient against organophosphate and carbamate insecticides), glutathione-S-transferases or GSTs (efficient against organophosphates, organochlorine, and pyrethroid insecticides) and cytochrome P450-dependent monoxygenases (efficient against most insecticide types, frequently in conjunction with other enzymes). The overproduction of these enzymes may be achieved via two non-exclusive mechanisms: gene amplification increasing the gene's copy number [35] and gene expression via modifications in the promoter region or mutations in trans-acting regulatory genes [35], [36]. In addition, in some mosquito species, carboxylesterase resistance to the insecticide malathion has been associated with a qualitative change in the enzyme (a few amino acid substitutions can increase the rate of hydrolysis of the enzyme [37]).
In contrast, target site resistance is achieved by point mutations that render the actual targets of an insecticide less sensitive to the active ingredient [33], [38]. Most insecticides developed to date are neurotoxic and aim for one of the following three targets: the acetylcholinesterase (whose role is the hydrolysis of the neurotransmitter acetylcholine), the γ-aminobutyric acid (GABA) receptors (chloride-ion neurotransmission channels in the insect's nervous system), or the sodium channels (responsible for raising the action potential in the neurons during the nerve impulses). The acetylcholinesterase is the target of organophosphorous and carbamate insecticides, the GABA receptors are the main targets of cyclodiene (organochlorine) insecticides, and the sodium channels are the targets of pyrethroid and organochlorine insecticides. Mutations in all three of these can confer resistance (Table 1).
What Effects on Parasite Transmission?
The evolution of insecticide resistance entails a battery of correlated life history changes in the insect, which are widely thought to be the result of pleiotropic effects of the insecticide resistance genes themselves, or of genes closely linked with them as a result of hitchhiking. These life history changes are often, though not always [39], [40], associated with fitness costs [3]–[9], that is, reduced fitness in the absence of insecticides. The question with which we are concerned here is how these changes interfere with the infection, development, and transmission of the parasite. Aside from the effect on mosquito population density, insecticide resistance can impact all of the main mosquito-related parameters in 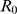 (given in Box 1). These include vector longevity, vector competence, and vector feeding behaviour. Below, we analyse each of them separately (see Table 2 for a summary).
(given in Box 1). These include vector longevity, vector competence, and vector feeding behaviour. Below, we analyse each of them separately (see Table 2 for a summary).
Table 2. Potential effects of the different mechanisms of insecticide resistance (IR) on vector longevity, competence and behaviour, and expected effects on the parasite's R 0.
| Pleiotropic Effects of Insecticide Resistance | Mechanisms Concerned | Traits Affected | Effect on R 0 |
| Vector longevity | |||
| IR trades off with resources needed to insure longevity | EST, GST, MOX | Decreased longevity (1/g) | Negative |
| IR increases oxidative stress | MOX, EST | Decreased longevity (1/g) | Negative |
| IR protects against oxidative stress | GST | Increased longevity (1/g) | Positive |
| Vector competence | |||
| IR renders the vector toxic for the parasite | EST, MOX | Decreased probability of infection (c), decreased parasite growth and development (b) | Negative |
| IR blocks the immune response | GST | Increased probability of infection (c), increased parasite growth and development (b) | Positive |
| IR stimulates the immune response | EST | Decreased probability of infection (c), decreased parasite growth and development (b) | Negative |
| IR trades off with resources needed to insure immunity | EST, GST, MOX | Increased probability of infection (c), increased parasite growth and development (b) | Positive |
| IR trades off with resources needed for parasite development | EST, GST, MOX | Decreased parasite growth and development (b), increased parasite incubation time (n) | Negative |
| Vector behaviour | |||
| IR alters the functioning of the nervous system | AChE, GABA, SCH | Hyperactive or sluggish vector: decreased or increased biting rate of the focal host (a) | Positive/Negative |
| IR trades off with resources needed for vector mobility | EST, GST, MOX | Sluggish vector: decreased biting rate of the focal host (a) | Negative |
| IR switches feeding preferences away from blood | EST, GST, MOX | Decreased biting rate of the focal host (a) | Negative |
See Table 1 for acronyms.
Insecticide Resistance and Vector Longevity
Vector longevity is an essential parameter in disease transmission, as it increases the potential for infective bites to hosts. As pointed out by MacDonald [41], the effect of longevity on disease transmission is particularly poignant for parasites like Plasmodium that need a minimum incubation period in the vector before being transmitted to a new host (Box 1). Yet, to our knowledge, there have been no thorough analyses on the effects of insecticide resistance on the longevity of Anopheles or indeed of any other vector of human disease, with one exception. In C. pipiens, insecticide resistance has been associated with a reduced longevity in the laboratory [24] and overwintering survival in the field [42], [43]. Similar effects of insecticide resistance on longevity have been obtained in other (non-vector) insect species [44]–[46]. Two main mechanisms may underlie this reduction in longevity: resource-based trade-offs and oxidative stress.
A well-known paradigm in evolutionary ecology is that diverting resources to one trait will, directly or indirectly, diminish the resources available for other traits [47]. This has been often put to the test using insect models, where it has been shown that, when resources are limited, an increased investment in certain fitness-associated traits such as fecundity is often coupled with a significant reduction in longevity [48], [49]. The deployment of insecticide resistance mechanisms, and in particular the overproduction of the detoxifying enzymes, likely requires substantial investment of resources. In the mosquito C. pipiens, for example, certain resistant genotypes can produce up to 50 times more esterases than their susceptible counterparts [50]. In other insects, these overproduced esterases can represent up to 3% of the total body proteins [51]. Lipids are likely victims of this large overinvestment in proteins, as they are an important source of the acetyl groups needed to synthesise the enzyme's constitutive amino acids [52]. Lipids are also the main fuel for insect survival [53], [54]. Unfortunately, so far as we are aware, no studies have quantified the level of lipids—or, indeed, any other energetic resource—in insecticide-resistant and -susceptible vectors.
Oxidative stress results from a mismatch between the production of damaging reactive oxygen species (ROS) and the production of protective antioxidants [55]. All organisms produce ROS as a result of the normal metabolic functioning of their cells [55]. The unwanted ROS produced in such reactions exert irreversible deleterious effects in the body [56] and have been widely proposed as a mechanism for ageing [55]–[57]. Blood feeding insects, in particular, face a considerable challenge from oxidative stress, because the digestion of haemoglobin results in a large production of ROS [58], [59]. In Anopheles, excess ROS production, though unrelated to insecticide resistance, has been recently shown to lead to a significant increase in mortality [60]. Two insecticide resistance mechanisms, in particular, may drastically alter ROS levels in insects, albeit in radically opposite ways: the p450 monoxygenases and the GSTs. The increased activity of p450 monoxygenases results in an excess production of harmful ROS because the stoichiometric demands of the enzymatic reaction are often not met [61]. This fact, previously known only from vertebrates [62], has been recently demonstrated in the house fly [63], and is thus likely to extend to other insect species. In contrast, GSTs have been shown to protect tissues against oxidative damage by increasing their solubility and aiding the excretion of free radicals [64]–[66]. A recent comparative study has found a clear association between GST expression and extended lifespan in fruit flies, nematodes, and mice [67]. Moreover, transgenic lines of Caenorhabditis elegans that produce 2.4 times more GSTs than controls show a 22% extension in their longevity [68]. The overexpression of GSTs in these transgenic worms is within the range found in insecticide-resistant vectors [69], [70]. Again, however, we are unaware of any studies addressing the longevity of vectors that are resistant to insecticides through the overproduction of GSTs.
Insecticide Resistance and Vector Competence
Vector competence, the successful invasion and subsequent development of the parasite in the vector, depends on the plethora of physiological and immunological factors that determine the insect's internal environment for the parasite. Insecticide resistance could interfere with parasite development in at least two ways. First, the physiological modifications that accompany the deployment of insecticide resistance mechanisms may render the vector toxic to parasites. In one of the few studies to have explicitly investigated the connection between insecticide resistance and disease transmission, McCarroll and collaborators showed that the development of the filaria W. bancrofti larvae was arrested in insecticide-resistant C. quinquefasciatus mosquitoes [21], [22] (but see [71]). Exactly what rendered the insecticide-resistant mosquito toxic to the parasite is not known, but it was hypothesised that the overproduction of carboxylesterases (see Table 1) in these mosquitoes resulted in a change in the redox potential of the tissues hosting the parasite, which led to the death of the larvae. Pending confirmation of a correlation between carboxylesterase and ROS production, these results could extend to other parasites whose vector stages have been shown to be highly susceptible to oxidative stress (such as Plasmodium [72] and Trypanosoma [73]), as well as to other insecticide resistance mechanisms (such as the p450 monoxygenases and GSTs) with a proven link with oxidative stress (see above).
Second, insecticide resistance could affect vector immunity. The combined complexity of the mode of action and the multiple substrate specificities of the enzymes involved in metabolic insecticide resistance (see Box 1) is such that these enzymes may have pleiotropic effects on one of the many steps of the immune cascade, from the recognition of the parasite as foreign, to the transduction of the signal and the deployment of the killing mechanism [74]. Yet, aside from a microarray study that showed upregulation of certain immune-related genes in insecticide-resistant strains of Anopheles gambiae [20], there are no studies that explicitly investigate the potential effects of insecticide resistance on insect immunity. Here we suggest two as yet unexplored possibilities.
The first concerns the protective role of GSTs (Table 1) against the effects of ROS on the parasites. Inducible ROS are a key component of the immune defence of Anopheles mosquitoes against Plasmodium [60], [75]. By neutralising the oxidative response of the mosquito to the parasite, overproduced GSTs could potentially increase the susceptibility of mosquitoes to the parasite.
The second concerns carboxylesterases (Table 1). Due to the overlapping substrate specificities existing between these enzymes and the serine proteases implicated in the melanization cascade, it has been suggested that carboxylesterases could have a positive effect on the formation of a melanin capsule around the parasite [76]. Two decades ago, an interesting association was found between an allele in an esterase locus and resistance by encapsulation in the G3 strain of A. gambiae infected with the B strain of Plasmodium cynomolgi [77]. The product of this gene was found to be a carboxylesterase with considerable sequence similarity to the carboxylesterase overproduced by insecticide-resistant Culex mosquitoes [78]. Subsequent (unpublished) studies, however, did not find any pattern of association between the carboxylesterase phenotype and Plasmodium susceptibility [79], but, to our knowledge, the question has not been investigated any further. More recently, carboxylesterases have been shown to be inducibly produced after bacterial [80] and viral [81] infections, suggesting that they may play a direct role in the invertebrate immune system. Thus, it is possible that upregulation of carboxylesterases as an adaptation against insecticides could, as an incidental side effect, make mosquitoes more resistant to pathogens.
Immunocompetence could also be affected through resource-based trade-offs. There is plenty of evidence that there are significant resource costs involved in the deployment and maintenance of the insect immune system [82]. Proteins seem to be the limiting resource for the encapsulation and antimicrobial responses in caterpillars [82], [83], and lipid metabolism has been shown to be implicated in the immune response of Aedes aegypti mosquitoes to a Plasmodium and a bacterial infection [84]. The production of large amounts of detoxifying enzymes, such as esterases or GSTs, is likely to deplete the resource pool, limiting the vector's ability to mount an immune response, therefore favouring the development of the parasite. It is worth noting, however, that resource limitation could also have the opposite effect if redirection of resources to insecticide resistance puts those resources beyond the reach of parasites: it could limit the development of parasites that depend on the host's energetic reserves to fulfil their own metabolic needs [85]. In vitro studies have, for example, shown that the mosquito gut stages of Plasmodium are greedy consumers of amino acids [86], lipids [87], and glucose [88]. There is also evidence that parasite production is positively correlated with resource availability in several invertebrates [89]–[92]. In these systems, the redirection of resources towards insecticide resistance is likely to impair the ability of the parasites to develop inside the vectors.
Insecticide Resistance and Vector Behaviour
Vector behaviour, particularly host choice, and biting rate have key effects on parasite transmission (Box 1). Mosquitoes with transmissible stages of Plasmodium persist at biting for longer than uninfected mosquitoes or mosquitoes infected with non-infectious stages [93]. Similar results have been obtained with Leishmania-infected sandflies [94]. In addition, recent work shows that uninfected mosquitoes are preferentially attracted to humans infected with transmissible gametocytes [95]. Because of its direct effect on the vector's neural system, target site resistance in particular has the potential for modifying the biting behaviour of uninfected and infected vectors alike.
Target-site resistance mutates key components of the vector's neural network, drastically modifying their performance and, thus, potentially also their response to external stimuli. In C. pipiens, for example, the single point mutation that renders the acetylcholinesterase insensitive to insecticides reduces the activity of the enzyme by up to 60% [96], which is likely to result in an excess of acetylcholine in the synapses and in a hyperactivity of the nervous system [6]. The most compelling examples of the effect of target site resistance on insect behaviour have not been carried out in vectors of diseases but on aphids and flies. In these insects, the kdr mutations alter the normal functioning of the sodium channels, causing a reduction in the excitability of the nervous system [97], [98]. Consequently, kdr-resistant aphids are less responsive to the presence of pheromone released by conspecifics [98], [99], increasing their vulnerability to parasitoid attack [100]. Furthemore, kdr-resistant flies are also less responsive to changes in temperature gradient than their insecticide-susceptible counterparts [98]. In mosquitoes, sodium channels are implicated in the transduction of the olfactory signal from the olfactory receptors to the central nervous system [101]. Target-site modifications, such as the kdr mutation, may render mosquitoes less responsive to the olfactory cues, such as lactic acid or ammonia [102], [103], that allow them to locate their hosts, thus reducing their efficiency as vectors. Rowland [104], [105] found that target-site resistance to organochlorine insecticides rendered A. gambiae and Anopheles stephensi mosquitoes less responsive to oviposition and predation-risk stimuli, but the effects on blood feeding behaviour have, to our knowledge, never been investigated.
Perhaps less intuitively, however, the behavioural side effects of insecticide resistance also extend to metabolic resistance. Foster et al. [98] showed that, in aphids, insecticide resistance through increased carboxylesterase titres were associated with a reduced ability to move away from senescing leaves. Berticat et al. [4] found that adults of C. pipiens that are resistant to insecticides through the overproduction of carboxylesterases suffered higher predation rates than susceptible ones, probably due to a decreased locomotive performance. This seemingly decreased mobility of insecticide-resistant insects is likely to be the result of resource depletion associated with the overproduction of carboxylesterases [98]. When applied to a blood-feeding vector, reduced motility may translate into reduced host-seeking efficiency and biting rates, although this has never been tested. A decrease in the energetic reserves may also switch the feeding preference of vectors away from hosts. In Ae. aegypti and Culex nigripalpus mosquitoes, resource deprivation, which is directly correlated with low energetic reserves, renders mosquitoes more responsive to sugar-rich odours like honey and less responsive to host odours [106].
Discussion
Whether a particular insect is a good vector, an occasional vector, or whether it presents an infection barrier for the parasite depends on a plethora of physiological, immunological, and behavioural variables. In this review, we have argued that any of these factors may potentially be altered by the evolution of insecticide resistance, with potentially drastic consequences for the epidemiology of disease (Figure 1). If insecticide resistance decreases the individual vectorial capacity of the vector (blue line in Figure 1), the transmission of the disease can decrease below the level attained in the absence of insecticide resistance evolution. In this case, insecticide resistance evolution may thus decrease the level of insecticide coverage needed to drive the parasite to extinction. An increase in the individual vectorial capacity (red line in Figure 1), on the other hand, may lead to a dramatic increase in the transmission of the disease and even to a higher prevalence than in the absence of insecticides. Moreover, even when local eradication does not occur (perhaps because initial 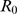 is very high), the extent to which the very impressive disease control often achieved by insecticides is eroded as resistance spreads will depend not only on how vector densities recover but also on the vectorial capacity of individual vectors, which, as we have argued, can be dramatically altered by resistance. As is clear from our discussion above, surprisingly little work directly addresses this important issue. Below, we summarise what we consider to be the three main questions to be answered, and we outline some predictions that arise from the mode of action of the different insecticide resistance mechanisms.
is very high), the extent to which the very impressive disease control often achieved by insecticides is eroded as resistance spreads will depend not only on how vector densities recover but also on the vectorial capacity of individual vectors, which, as we have argued, can be dramatically altered by resistance. As is clear from our discussion above, surprisingly little work directly addresses this important issue. Below, we summarise what we consider to be the three main questions to be answered, and we outline some predictions that arise from the mode of action of the different insecticide resistance mechanisms.
The first question is whether insecticide-resistant vectors have a different lifespan than their susceptible counterparts. We expect that, in most cases, the effect of insecticide resistance will be to reduce vector longevity. This has been already shown in insects of agricultural interest as well as in Culex mosquitoes [24], [42]–[46], but it needs to be tested in the other vectors of diseases, most particularly those that transmit parasites with long incubation periods (e.g., the mosquitoes Anopheles and Aedes, and the kissing bugs Rhodnius and Triatoma) (Table 1). We further expect this longevity reduction to be especially drastic in insects with metabolic insecticide resistance as a result of resource-based trade-offs and/or increased oxidative stress. The one exception to this rule may be vectors overexpressing the GST, which has been shown to increase lifespan in organisms as diverse as Drosophila and nematodes [67], [68]. The longevity reduction in insecticide-resistant insects may, however, be offset by the parasite's influence on longevity. In C. pipiens mosquitoes infected with the microsporidia Vavraia culicis, the decrease in longevity associated with insecticide resistance is much larger for uninfected than for infected mosquitoes [24]. Indeed, parasites often have an effect on the longevity of their vectors, both positive and negative [107]. Thus, whenever possible, the potential interaction between insecticide resistance and parasite-mediated effects on the vector's lifespan needs to be investigated, ideally, using natural vector–parasite combinations [107], [108].
The second question is whether insecticide resistance alters the probability an insect becomes infected and/or the subsequent intensity of infection and production of transmission stages (or vector competence). McCarroll and co-workers [21], [22] have shown that insecticide-resistant mosquitoes have lower burdens of filaria parasites, possibly due to an increase in oxidative stress. Vontas et al. [109] failed to show differences in parasite burden between insecticide-resistant and -susceptible An. stephensi mosquitoes infected with Plasmodium yoellii, although the different geographic origin of the resistant and susceptible strains and the unnatural combination of an Asian vector with an African rodent parasite make these results difficult to interpret (see below). We expect the effects on parasite burden to be more drastic in vectors with metabolic resistance, as the production of large amounts of detoxification enzymes will likely render the physiological environment of the vector less than ideal for parasite development. Unfortunately, a mere reduction in parasite burden in insecticide-resistant insects is unlikely to have a drastic effect on disease transmission because, in most cases, a few parasites suffice to initiate a new infection in the host. As few as ten Plasmodium parasites are sufficient to establish a malaria infection [110]. One way in which parasite burden may influence transmission, however, is if it correlates with vector survival. There indeed is evidence, again from Plasmodium, that more heavily infected mosquitoes die faster [108], [111].
The third question is whether insecticide resistance modifies the biting rate or host choice of the uninfected and/or infected vector. We expect this effect to appear particularly in vectors that are resistant through modifications in the neural targets of the insecticide, because of the obvious connections between behaviour and the nervous system. Depending on the underlying mechanism, these modifications may result in either a hyperactive or a sluggish nervous system, but how this translates into feeding and host-choice behaviour remains to be investigated. Finally, of particular interest is whether insecticide resistance may be able to alter the parasite-mediated manipulation of vector feeding behaviour, even though, in most cases, this manipulation takes place through a physical interference with blood ingestion [107], without the involvement of the nervous system.
As illustrated above, the physiological mechanisms underlying insecticide resistance yield clear predictions as to how insecticide resistance may affect the different components of the parasite's 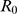 (vector longevity, competence, and behaviour, Table 2). However, the same insecticide resistance mechanism may have opposite effects on each of these components by, for instance, increasing the vector's lifespan but interfering with the parasite's development (see GST, Table 2). It is therefore difficult to predict the overall effect of insecticide resistance on a parasite's
(vector longevity, competence, and behaviour, Table 2). However, the same insecticide resistance mechanism may have opposite effects on each of these components by, for instance, increasing the vector's lifespan but interfering with the parasite's development (see GST, Table 2). It is therefore difficult to predict the overall effect of insecticide resistance on a parasite's 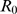 . In addition, our predictions in Table 2 probably do not encompass all possible effects of insecticide resistance on disease transmission. The enzymes involved in the detoxification of insecticides belong to particularly complex families of enzymes whose substrate specificities and biological functions are not yet fully known [112]. Similarly, target-site mutations seem to have pleiotropic effects that go beyond the nervous system [113]. The problem of prediction gains additional intricacy from the fact that many insect vectors are now resistant to multiple insecticide types through a combination of different metabolic and target-site modification mechanisms [114], [115]. These different insecticide resistance mechanisms have been shown to interact with each other [6], but what consequences these interactions may have for parasite transmission will have to be resolved on a case-by-case basis.
. In addition, our predictions in Table 2 probably do not encompass all possible effects of insecticide resistance on disease transmission. The enzymes involved in the detoxification of insecticides belong to particularly complex families of enzymes whose substrate specificities and biological functions are not yet fully known [112]. Similarly, target-site mutations seem to have pleiotropic effects that go beyond the nervous system [113]. The problem of prediction gains additional intricacy from the fact that many insect vectors are now resistant to multiple insecticide types through a combination of different metabolic and target-site modification mechanisms [114], [115]. These different insecticide resistance mechanisms have been shown to interact with each other [6], but what consequences these interactions may have for parasite transmission will have to be resolved on a case-by-case basis.
Studies investigating the vectorial capacity of insecticide-resistant and -susceptible vectors are, in our view, urgently needed, but we note three experimental challenges that need to be overcome in order to reach strong conclusions. The first is that single comparisons of allopatric-resistant and -susceptible vector strains [19], [20], [109], [116] cannot disentangle the effects of insecticide resistance genes from other differences that inevitably arise during divergent evolutionary history. Much stronger inferences can be made if sympatric-resistant and -susceptible mosquitoes are compared, but in areas with a long and complex history of insecticide use, fully susceptible individuals can be very hard to find. If obtaining matched sympatric lines is not feasible, many unmatched resistant and sensitive lines are required. Another way forward is the comparison of laboratory-selected lines. But this raises a second experimental difficulty: the conclusions from laboratory-selected, insecticide-resistant strains may not be directly applicable to the conditions in the field. Curtis [21], [71] and McCarroll et al. [21] pointed out that McCarroll's [22] results with Culex and Wuchereria may have been the result of unnaturally high esterase levels in the laboratory-selected strains of the mosquito. In addition, while selecting for insecticide resistance in the laboratory, one may inadvertently select for other traits, such as developmental time, body size, immunocompetence, or longevity, which may have consequences for parasite transmission. The final experimental issue is that, whenever possible, studies should be carried out on natural vector–parasite combinations. Lessons from Plasmodium studies have taught us that results obtained using laboratory models, most notably concerning mosquito longevity [108] and immunity [117], are not necessarily applicable to natural vector–parasite combinations. We agree that overcoming all three of these pitfalls is not easy, but the logistic difficulties do not mean the problems can be ignored.
Thus far, we have concentrated our discussion on the short-term effects of insecticide resistance on parasite transmission through its impact on the parasite's 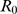 (epidemiological time scale). However, the interaction between the parasite and the insecticide-resistant vector can also have long-term (evolutionary time scale) consequences. Insecticide resistance could exert a selective pressure for the evolution of the parasites by selecting for parasites with, for example, shorter incubation times (to compensate for the reduction in longevity), or faster multiplication rates (to compensate for higher parasite mortality). Conversely, if parasite burden is reduced in insecticide-resistant vectors, as McCarroll et al. [22] showed, this could facilitate the spread of insecticide resistance in vector populations submitted to a significant parasite pressure. Exploring these two evolutionary consequences is beyond the scope of this paper, but the interaction between insecticide resistance and parasitism clearly deserves further investigation.
(epidemiological time scale). However, the interaction between the parasite and the insecticide-resistant vector can also have long-term (evolutionary time scale) consequences. Insecticide resistance could exert a selective pressure for the evolution of the parasites by selecting for parasites with, for example, shorter incubation times (to compensate for the reduction in longevity), or faster multiplication rates (to compensate for higher parasite mortality). Conversely, if parasite burden is reduced in insecticide-resistant vectors, as McCarroll et al. [22] showed, this could facilitate the spread of insecticide resistance in vector populations submitted to a significant parasite pressure. Exploring these two evolutionary consequences is beyond the scope of this paper, but the interaction between insecticide resistance and parasitism clearly deserves further investigation.
Insecticide resistance is generally thought to undermine the control of vector-transmitted diseases. Consequently, there are ongoing efforts to develop resistance-breaking compounds [10], [11] and evolution-proof insecticidal strategies [14], [15], as well as improved resistance surveillance in the field [12], [13]. We suggest that another research problem be added to this agenda: the disease transmission capacity of resistant insects. In some instances, insecticide resistance may impair the ability of the vector to transmit diseases. If this effect is sufficiently large, the impact of insecticide resistance on disease management may not be as detrimental as previously thought. If so, current paradigms might be leading to a misallocation of research and control resources. We contend that there are surprisingly few well-documented cases of disease outbreaks in response to the evolution of insecticide resistance (in marked contrast to the well-documented public health problems caused by the evolution of drug resistance). Alternatively, insecticide resistance could improve the individual vectorial capacity of insects, further emphasising the urgent need for novel insecticides and resistance management strategies. Either way—and there may be no simple generality—the consequence of the evolution of insecticide resistance for disease ecology deserves additional attention.
Supporting Information
Acknowledgments
We thank Christine Chevillon, Katey Glunt, Jacob Koella, Penny Lynch, Antoine Nicot, Nicole Pasteur, Matt Thomas and the attendees of the European Science Foundation (ESF) conference “The Impact of the Environment on Innate Immunity: The Threat of Diseases” (Obergurgl, Austria, May 2009) for useful discussions on this subject.
Footnotes
The authors have declared that no competing interests exist.
This work is financed by an ANR SEST to A. Rivero, a GABBA grant to J. Vézilier, the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, the Fogarty International Center and the National Institutes of Health to A. Read, and by an ANR Jeune Chercheur and an ERC Starting Grant to S. Gandon. The funders had no role in the study design, data collection and analysis, decision to publish, or in preparation of the manuscript.
References
- 1.World Health Organisation. Pesticides and their application for the control of vectors and pests of public health importance. 2006. WHO/CDS/NTD/WHOPES/GCDPP/2006.1.
- 2.World Health Organisation. Test procedures for insecticide resistance monitoring in malaria vectors; bio-efficacy and persistence of insecticides on treated surfaces. 1998. WHO/CDS/CPC/MAL/9812.
- 3.Hardstone MC, Lazzaro BP, Scott JG. The effect of three environmental conditions on the fitness of cytochrome P450 monooxygenase-mediated permethrin resistance in Culex pipiens quinquefasciatus. BMC Evol Biol. 2009;9:42. doi: 10.1186/1471-2148-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berticat C, Duron O, Heyse D, Raymond M. Insecticide resistance genes confer a predation cost on mosquitoes, Culex pipiens. Genet Res. 2004;83:189–196. doi: 10.1017/s0016672304006792. [DOI] [PubMed] [Google Scholar]
- 5.Bourguet D, Guillemaud T, Chevillon C, Raymond M. Fitness costs of insecticide resistance in natural breeding sites of the mosquito Culex pipiens. Evolution. 2004;58:128–135. doi: 10.1111/j.0014-3820.2004.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 6.Berticat C, Bonnet J, Duchon S, Agnew P, Weill M, et al. Costs and benefits of multiple resistance to insecticides for Culex quinquefasciatus mosquitoes. BMC Evol Biol. 2008;8:104. doi: 10.1186/1471-2148-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berticat C, Boquien G, Raymond M, Chevillon C. Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet Res. 2002;79:41–47. doi: 10.1017/s001667230100547x. [DOI] [PubMed] [Google Scholar]
- 8.Roush RT, McKenzie JA. Ecological genetics of insecticide and acaricide resistance. Annu Rev Entomol. 1987;32:361–380. doi: 10.1146/annurev.en.32.010187.002045. [DOI] [PubMed] [Google Scholar]
- 9.Sakyi KY, Sarfo B, Brown CA, Wilson MD, Boakye DA. Investigation into the fitness cost of kdr insecticide resistance in Anopheles gambiae malaria vectors. Am J Trop Med Hyg. 2005;73:155–155. [Google Scholar]
- 10.Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The innovative vector control consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22:308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Zaim M, Guillet P. Alternative insecticides: an urgent need. Trends Parasitol. 2002;18:161–163. doi: 10.1016/s1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]
- 12.Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis. 2008;8:387–389. doi: 10.1016/S1473-3099(08)70045-8. [DOI] [PubMed] [Google Scholar]
- 13.Coleman M, Sharp B, Seocharan I, Hemingway J. Developing an evidence-based decision support system for rational insecticide choice in the control of African malaria vectors. J Med Entomol. 2006;43:663–668. doi: 10.1603/0022-2585(2006)43[663:daedss]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Read AF, Lynch PA, Thomas MB. How to make evolution-proof insecticides for malaria control. PLoS Biol. 2009;7:e1000058. doi: 10.1371/journal.pbio.1000058. doi: 10.1371/journal.pbio.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koella JC, Lynch PA, Thomas MB, Read AF. Towards evolution-proof malaria control with insecticides. Evolutionary Applications. 2009;2:469–480. doi: 10.1111/j.1752-4571.2009.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenormand T, Raymond M. Resistance management: the stable zone strategy. Proc R Soc Lond B Biol Sci. 1998;265:1985–1990. [Google Scholar]
- 17.Curtis CF, Miller JE, Hodjati MH, Kolaczinski JH, Kasumba I. Can anything be done to maintain the effectiveness of pyrethroid-impregnated bednets against malaria vectors? Philos Trans R Soc Lond B Biol Sci. 1998;353:1769–1775. doi: 10.1098/rstb.1998.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penilla RP, Rodriguez AD, Hemingway J, Trejo A, Lopez AD, et al. Cytochrome P-450-based resistance mechanism and pyrethroid resistance in the field Anopheles albimanus resistance management trial. Pestic Biochem Physiol. 2007;89:111–117. [Google Scholar]
- 19.Vontas J, David JP, Nikou D, Hemingway J, Christophides GK, et al. Transcriptional analysis of insecticide resistance in Anopheles stephensi using cross-species microarray hybridization. Insect Mol Biol. 2007;16:315–324. doi: 10.1111/j.1365-2583.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 20.Vontas J, Blass C, Koutsos AC, David JP, Kafatos FC, et al. Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Mol Biol. 2005;14:509–521. doi: 10.1111/j.1365-2583.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 21.McCarroll L, Hemingway J. Can insecticide resistance status affect parasite transmission in mosquitoes? Insect Biochem Mol Biol. 2002;32:1345–1351. doi: 10.1016/s0965-1748(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 22.McCarroll L, Paton MG, Karunaratne S, Jayasuryia HTR, Kalpage KSP, et al. Insecticides and mosquito-borne disease. Nature. 2000;407:961–962. doi: 10.1038/35039671. [DOI] [PubMed] [Google Scholar]
- 23.Duron O, Labbe P, Berticat C, Rousset F, Guillot S, et al. High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution. 2006;60:303–314. [PubMed] [Google Scholar]
- 24.Agnew P, Berticat C, Bedhomme S, Sidobre C, Michalakis Y. Parasitism increases and decreases the costs of insecticide resistance in mosquitoes. Evolution. 2004;58:579–586. [PubMed] [Google Scholar]
- 25.Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M. High Wolbachia density in insecticide-resistant mosquitoes. Proc R Soc Lond B Biol Sci. 2002;269:1413–1416. doi: 10.1098/rspb.2002.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornel AJ, Stanich MA, McAbee RD, Mulligan FS. High level methoprene resistance in the mosquito Ochlerotatus nigromaculis (Ludlow) in Central California. Pest Manag Sci. 2002;58:791–798. doi: 10.1002/ps.521. [DOI] [PubMed] [Google Scholar]
- 27.Dame DA, Wichterman GJ, Hornby JA. Mosquito (Aedes taeniorhynchus) resistance to methoprene in an isolated habitat. J Am Mosq Control Assoc. 1998;14:200–203. [PubMed] [Google Scholar]
- 28.Paul A, Harrington LC, Zhang L, Scott JG. Insecticide resistance in Culex pipiens from New York. J Am Mosq Control Assoc. 2005;21:305–309. doi: 10.2987/8756-971X(2005)21[305:IRICPF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Chevillon C, Bernard C, Marquine M, Pasteur N. Resistance to Bacillus sphaericus in Culex pipiens (Diptera : Culicidae) interaction between recessive mutants and evolution in southern France. J Med Entomol. 2001;38:657–664. doi: 10.1603/0022-2585-38.5.657. [DOI] [PubMed] [Google Scholar]
- 30.Chalegre KDD, Romao TP, Amorim LB, Anastacio DB, de Barros RA, et al. Detection of an allele conferring resistance to Bacillus sphaericus binary toxin in Culex quinquefasciatus populations by molecular screening. Appl Environ Microbiol. 2009;75:1044–1049. doi: 10.1128/AEM.02032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darboux I, Charles JF, Pauchet Y, Warot S, Pauron D. Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera : Culicidae). Cell Microbiol. 2007;9:2022–2029. doi: 10.1111/j.1462-5822.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 32.Opota O, Charles JF, Warot S, Pauron D, Darboux I. Identification and characterization of the receptor for the Bacillus sphaericus binary toxin in the malaria vector mosquito, Anopheles gambiae. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:419–427. doi: 10.1016/j.cbpb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 34.Hemingway J, Karunaratne S. Mosquito carboxylesterases: a review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Med Vet Entomol. 1998;12:1–12. doi: 10.1046/j.1365-2915.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- 35.Hemingway J, Hawkes N, Prapanthadara LA, Jayawardenal KGI, Ranson H. The role of gene splicing, gene amplification and regulation in mosquito insecticide resistance. Philos Trans R Soc Lond B Biol Sci. 1998;353:1695–1699. doi: 10.1098/rstb.1998.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rooker S, Guillemaud T, Berge J, Pasteur N, Raymond M. Coamplification of esterase A and B genes as a single unit in Culex pipiens mosquitoes. Heredity. 1996;77:555–561. [PubMed] [Google Scholar]
- 37.Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, et al. Insecticide resistance in mosquito vectors. Nature. 2003;423:136–137. doi: 10.1038/423136b. [DOI] [PubMed] [Google Scholar]
- 39.Arnaud L, Haubruge E, Gage MJG. The malathion-specific resistance gene confers a sperm competition advantage in Tribolium castaneum. Funct Ecol. 2005;19:1032–1039. [Google Scholar]
- 40.McCart C, Buckling A, ffrench-Constant RH. DDT resistance in flies carries no cost. Curr Biol. 2005;15:R587–R589. doi: 10.1016/j.cub.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957. [Google Scholar]
- 42.Gazave E, Chevillon C, Lenormand T, Marquine M, Raymond M. Dissecting the cost of insecticide resistance genes during the overwintering period of the mosquito Culex pipiens. Heredity. 2001;87:441–448. doi: 10.1046/j.1365-2540.2001.00926.x. [DOI] [PubMed] [Google Scholar]
- 43.Chevillon C, Bourguet D, Rousset F, Pasteur N, Raymond M. Pleiotropy of adaptive changes in populations: comparisons among insecticide resistance genes in Culex pipiens. Genet Res. 1997;70:195–203. doi: 10.1017/s0016672397003029. [DOI] [PubMed] [Google Scholar]
- 44.Boivin T, Chabert d'Hieres C, Bouvier JC, Beslay D, Sauphanor B. Pleiotropy of insecticide resistance in the codling moth, Cydia pomonella. Entomologia Experimentalis et Applicata. 2001;99:381–386. [Google Scholar]
- 45.Konno RH, Omoto C. Fitness cost associated with carbosulfan resistance in Aphis gossypii Glover (Hemiptera : aphididae). Neotrop Entomol. 2006;35:246–250. doi: 10.1590/s1519-566x2006000200014. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto A, Yoneda H, Hatano R, Asada M. Influence of hexythiazox resistance on life history parameters in the citrus red mite Panonychus citri (McGregor). J Pesticide Science. 1995;20:521–527. [Google Scholar]
- 47.Stearns SC. The evolution of life histories. Oxford: Oxford University Press; 1992. 249 [Google Scholar]
- 48.Reznick D. Measuring the costs of reproduction. Trends Ecol Evol. 1992;7:42–45. doi: 10.1016/0169-5347(92)90104-J. [DOI] [PubMed] [Google Scholar]
- 49.Kirkwood TLB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- 50.Raymond M, Berticat C, Weill M, Pasteur N, Chevillon C. Insecticide resistance in the mosquito Culex pipiens: what have we learned about adaptation? Genetica. 2001;112:287–296. [PubMed] [Google Scholar]
- 51.Devonshire AL, Moores GD. A carboxylesterase with broad substrate specificity causes organophosphorous, carbamate and pyrethroid resistance in peach potato aphids (Myzus persicae). Pestic Biochem Physiol. 1982;18:235–246. [Google Scholar]
- 52.Nijhout HF. Insect hormones. Princeton (New Jersey): Princeton University Press; 1994. 267 [Google Scholar]
- 53.Rivero A, Casas J. Incorporating physiology into parasitoid behavioral ecology: the allocation of nutritional resources. Res Popul Ecol (Kyoto) 1999;41:39–45. [Google Scholar]
- 54.Clements AN. The biology of mosquitoes: development, nutrition and reproduction. London: Chapman & Hall; 1992. 509 [Google Scholar]
- 55.Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett. 2009;12:75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- 56.Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc R Soc Lond B Biol Sci. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricklefs RE. The evolution of senescence from a comparative perspective. Funct Ecol. 2008;22:379–392. [Google Scholar]
- 58.Ranson H, Hemingway J. Mosquito glutathione transferases. Methods Enzymol. 2005;401:226–241. doi: 10.1016/S0076-6879(05)01014-1. [DOI] [PubMed] [Google Scholar]
- 59.Graca-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GRC, Paes MC, et al. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 2006;36:322–335. doi: 10.1016/j.ibmb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Molina-Cruz A, Dejong RJ, Charles B, Gupta L, Kumar S, et al. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- 61.Ortiz de Montellano PR, de Voss JJ. Substrate oxidation by cytochrome p450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome p450: Structure, mechanism and biochemistry. New York: Kluwer Academic; 2005. pp. 183–245. [Google Scholar]
- 62.Bast A. Is formation of reactive oxygen by cytochrome p-450 perilous and predictable? Trends Pharmacol Sci. 1986;7:266–270. [Google Scholar]
- 63.Murataliev MB, Guzov VM, Walker FA, Feyereisen R. P450 reductase and cytochrome b(5) interactions with cytochrome P450: effects on house fly CYP6A1 catalysis. Insect Biochem Mol Biol. 2008;38:1008–1015. doi: 10.1016/j.ibmb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 65.Vontas JG, Small GJ, Hemingway J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem J. 2001;357:65–72. doi: 10.1042/0264-6021:3570065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parkes TL, Hilliker AJ, Phillips JP. Genetic and biochemical analysis of gluthatione-S-transferase in the oxigen defense system of Drosophila melanogaster. Genome. 1993;36:1007–1014. doi: 10.1139/g93-134. [DOI] [PubMed] [Google Scholar]
- 67.McElwee JJ, Schuster E, Blanc E, Piper MD, Thomas JH, et al. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8:R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, et al. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005;4:257–271. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- 69.Ding YC, Hawkes N, Meredith J, Eggleston P, Hemingway J, et al. Characterization of the promoters of Epsilon glutathione transferases in the mosquito Anopheles gambiae and their response to oxidative stress. Biochem J. 2005;387:879–888. doi: 10.1042/BJ20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ranson H, Rossiter L, Ortelli F, Jensen B, Wang XL, et al. Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem J. 2001;359:295–304. doi: 10.1042/0264-6021:3590295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curtis CF. Insecticide resistance and mosquito-borne disease. Lancet. 2001;357:656–656. doi: 10.1016/s0140-6736(00)04152-0. [DOI] [PubMed] [Google Scholar]
- 72.Vega-Rodriguez J, Franke-Fayard B, Dinglasan RR, Janse CJ, Pastrana-Mena R, et al. The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog. 2009;5:1000302. doi: 10.1371/journal.ppat.1000302. doi: 10.1371/journal.ppat.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacLeod ET, Maudlin I, Darby AC, Welburn SC. Antioxidants promote establishment of trypanosome infections in tsetse. Parasitology. 2007;134:827–831. doi: 10.1017/S0031182007002247. [DOI] [PubMed] [Google Scholar]
- 74.Dimopoulos G. Insect immunity and its implication in mosquito-malaria interactions. Cell Microbiol. 2003;5:3–14. doi: 10.1046/j.1462-5822.2003.00252.x. [DOI] [PubMed] [Google Scholar]
- 75.Kumar S, Christophides GK, Cantera R, Charles B, Han YS, et al. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci U S A. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myers M, Richmond RC, Oakeshott JG. On the origins of esterases. Mol Biol Evol. 1988;5:113–119. doi: 10.1093/oxfordjournals.molbev.a040485. [DOI] [PubMed] [Google Scholar]
- 77.Vernick KD, Collins FH. Association of a Plasmodium-refractory phenotype with an esterase locus in Anopheles gambiae. Am J Trop Med Hyg. 1989;40:593–597. doi: 10.4269/ajtmh.1989.40.593. [DOI] [PubMed] [Google Scholar]
- 78.Collins FH, Paskewitz SM, Crewsoyen AE. A genetic study of Plasmodium susceptibility in the African malaria vector Anopheles gambiae. Annales De La Societe Belge De Medecine Tropicale. 1991;71:225–232. [PubMed] [Google Scholar]
- 79.Crews-Oyen AE, Kumar V, Collins FH. Association of two esterase genes, a chromosomal inversion, and susceptibility to Plasmodium cynomolgi in the African malaria vector Anopheles gambiae. Am J Trop Med Hyg. 1993;49:341–347. doi: 10.4269/ajtmh.1993.49.341. [DOI] [PubMed] [Google Scholar]
- 80.Shiotsuki T, Kato Y. Induction of carboxylesterase isozymes in Bombyx mori by E. coli infection. Insect Biochem Mol Biol. 1999;29:731–736. doi: 10.1016/s0965-1748(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 81.Chongsatja PO, Bourchookarn A, Lo CF, Thongboonkerd V, Krittanai C. Proteomic analysis of differentially expressed proteins in Penaeus vannamei hemocytes upon Taura syndrome virus infection. Proteomics. 2007;7:3592–3601. doi: 10.1002/pmic.200700281. [DOI] [PubMed] [Google Scholar]
- 82.Povey S, Cotter SC, Simpson SJ, Lee KP, Wilson K. Can the protein costs of bacterial resistance be offset by altered feeding behaviour? J Anim Ecol. 2009;78:437–446. doi: 10.1111/j.1365-2656.2008.01499.x. [DOI] [PubMed] [Google Scholar]
- 83.Lee KP, Cory JS, Wilson K, Raubenheimer D, Simpson SJ. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc R Soc Lond B Biol Sci. 2006;273:823–829. doi: 10.1098/rspb.2005.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheon HM, Shin SW, Bian GW, Park JH, Raikhel AS. Regulation of lipid metabolism genes, lipid carrier protein lipophorin, and its receptor during immune challenge in the mosquito Aedes aegypti. J Biol Chem. 2006;281:8426–8435. doi: 10.1074/jbc.M510957200. [DOI] [PubMed] [Google Scholar]
- 85.Hall SR, Simonis JL, Nisbet RM, Tessier AJ, Cáceres CE. Resouce ecology of virulence in a planktonic host-parasite system: an explanation using dynamic energy budgets. Am Nat. 2009;174:149–162. doi: 10.1086/600086. [DOI] [PubMed] [Google Scholar]
- 86.Ball GH, Chao J. Use of amino acids by Plasmodium relictum oocysts in vitro. Exp Parasitol. 1976;39:115–118. doi: 10.1016/0014-4894(76)90018-7. [DOI] [PubMed] [Google Scholar]
- 87.Atella GC, Bittencourt-Cunha PR, Nunes RD, Shahabuddin M, Silva-Neto MAC. The major insect lipoprotein is a lipid source to mosquito stages of malaria parasite. Acta Trop. 2009;109:159–162. doi: 10.1016/j.actatropica.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Schiefer BA, Ward RA, Eldridge BF. Plasmodium cynomolgi: effects of malaria infection on laboratory flight performance of Anopheles stephensi mosquitoes. Exp Parasitol. 1977;41:397–404. doi: 10.1016/0014-4894(77)90111-4. [DOI] [PubMed] [Google Scholar]
- 89.Bedhomme S, Agnew P, Sidobre C, Michalakis Y. Virulence reaction norms across a food gradient. Proc R Soc Lond B Biol Sci. 2004;271:739–744. doi: 10.1098/rspb.2003.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tseng M. Interactions between the parasite's previous and current environment mediate the outcome of parasite infection. Am Nat. 2006;168:565–571. doi: 10.1086/507997. [DOI] [PubMed] [Google Scholar]
- 91.Hall SR, Knight CJ, Becker CR, Duffy MA, Tessier AJ, et al. Quality matters: resource quality for hosts and the timing of epidemics. Ecol Lett. 2009;12:118–128. doi: 10.1111/j.1461-0248.2008.01264.x. [DOI] [PubMed] [Google Scholar]
- 92.Pulkkinen K, Ebert D. Host starvation decreases parasite load and mean host size in experimental populations. Ecology. 2004;85:823–833. [Google Scholar]
- 93.Anderson RA, Koella JC, Hurd H. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc Biol Sci. 1999;266:1729–1733. doi: 10.1098/rspb.1999.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rogers ME, Bates PA. Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog. 2007;3:e91. doi: 10.1371/journal.ppat.0030091. doi: 10.1371/journal.ppat.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3:e298. doi: 10.1371/journal.pbio.0030298. doi: 10.1371/journal.pbio.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bourguet D, Lenormand T, Guillemaud T, Marcel V, Fournier D, et al. Variation of dominance of newly arisen adaptive genes. Genetics. 1997;147:1225–1234. doi: 10.1093/genetics/147.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee SH, Smith TJ, Knipple DC, Soderlund DM. Mutations in the house fly Vssc1 sodium channel gene associated with super-kdr resistance abolish the pyrethroid sensitivity of Vssc1/tipE sodium channels expressed in Xenopus oocytes. Insect Biochem Mol Biol. 1999;29:185–194. doi: 10.1016/s0965-1748(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 98.Foster SP, Young S, Williamson MS, Duce I, Denholm I, et al. Analogous pleiotropic effects of insecticide resistance genotypes in peach-potato aphids and houseflies. Heredity. 2003;91:98–106. doi: 10.1038/sj.hdy.6800285. [DOI] [PubMed] [Google Scholar]
- 99.Foster SP, Woodcock CM, Williamson MS, Devonshire AL, Denholm I, et al. Reduced alarm response by peach-potato aphids, Myzus persicae (Hemiptera : Aphididae), with knock-down resistance to insecticides (kdr) may impose a fitness cost through increased vulnerability to natural enemies. Bull Entomol Res. 1999;89:133–138. [Google Scholar]
- 100.Foster SP, Tomiczek M, Thompson R, Denholm I, Poppy G, et al. Behavioural side-effects of insecticide resistance in aphids increase their vulnerability to parasitoid attack. Anim Behav. 2007;74:621–632. [Google Scholar]
- 101.Zwiebel LJ, Takken W. Olfactory regulation of mosquito-host interactions. Insect Biochem Mol Biol. 2004;34:645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Braks MAH, Meijerink J, Takken W. The response of the malaria mosquito, Anopheles gambiae, to two components of human sweat, ammonia and L-lactic acid, in an olfactometer. Physiol Entomol. 2001;26:142–148. [Google Scholar]
- 103.Steib BM, Geier M, Boeckh J. The effect of lactic acid on odour-related host preference of yellow fever mosquitoes. Chem Senses. 2001;26:523–528. doi: 10.1093/chemse/26.5.523. [DOI] [PubMed] [Google Scholar]
- 104.Rowland M. Activity and mating competitiveness of gamma-HCH/dieldrin resistant and susceptible male and virgin female Anopheles gambiae and An. stephensi mosquitos, with assessment of an insecticide rotation strategy. Med Vet Entomol. 1991;5:207–222. doi: 10.1111/j.1365-2915.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 105.Rowland M. Behavior and fitness of gamma-HCH/dieldrin resistant and susceptible female Anopheles gambiae and An. stephensi mosquitos in the absence of insecticide. Med Vet Entomol. 1991;5:193–206. doi: 10.1111/j.1365-2915.1991.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 106.Clements AN. The biology of mosquitoes: sensory reception and behaviour. Wallingford: CABI Publishing; 1999. 740 [Google Scholar]
- 107.Lefevre T, Thomas F. Behind the scene, something else is pulling the strings: Emphasizing parasitic manipulation in vector-borne diseases. Infect Genet Evol. 2008;8:504–519. doi: 10.1016/j.meegid.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Ferguson HM, Read AF. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18:256–261. doi: 10.1016/s1471-4922(02)02281-x. [DOI] [PubMed] [Google Scholar]
- 109.Vontas JG, McCarroll L, Karunaratne S, Louis C, Hurd H, et al. Does environmental stress affect insect-vectored parasite transmission? Physiol Entomol. 2004;29:210–213. doi: 10.1111/j.0307-6962.2004.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sinden RE, Dawes EJ, Alavi Y, Waldock J, Finney O, et al. Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathogens. 2007;3:e195. doi: 10.1371/journal.ppat.0030195. doi: 10.1371/journal.ppat.0030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dawes EJ, Churcher TS, Zhuang S, Sinden RE, Basáñez MG. Anopheles mortality is both age- and Plasmodium-density dependent: implications for malaria transmission. Malaria J. 2009;8:228. doi: 10.1186/1475-2875-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ranson H, Claudianos C, Ortelli F, Abgrall C, Hemingway J, et al. Evolution of supergene families associated with insecticide resistance. Science. 2002;298:179–181. doi: 10.1126/science.1076781. [DOI] [PubMed] [Google Scholar]
- 113.Labbe P, Berticat C, Berthomieu A, Unal S, Bernard C, et al. Forty years of erratic insecticide resistance evolution in the mosquito Culex pipiens. PLoS Genet. 2007;3:e205. doi: 10.1371/journal.pgen.0030205. doi: 10.1371/journal.pgen.0030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Corbel V, N'Guessan R, Brengues C, Chandre F, Djogbenou L, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin (West Africa) and operational challenge for malaria vector control. Am J Trop Med Hyg. 2007;77:230. doi: 10.1016/j.actatropica.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 115.Perera MDB, Hemingway J, Karunaratne SHPP. Multiple insecticide resistance mechanisms involving metabolic changes and insensitive target sites selected in anopheline vectors of malaria in Sri Lanka. Malaria J. 2008;7:168. doi: 10.1186/1475-2875-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Okoye PN, Brooke BD, Hunt RH, Coetzee M. Relative developmental and reproductive fitness associated with pyrethroid resistance in the major southern African malaria vector, Anopheles funestus. Bull Entomol Res. 2007;97:599–605. doi: 10.1017/S0007485307005317. [DOI] [PubMed] [Google Scholar]
- 117.Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, et al. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.VectorBase. AnoBase: the Anopheles database. Insecticide resistance database search. 2010. Available: http://anobase.vectorbase.org/ir/. Accessed 6 July 2010.
- 119.Surendran SN, Karunaratne SHPP, Adams Z, Hemingway J, Hawkes NJ. Molecular and biochemical characterization of a sand fly population from Sri Lanka: evidence for insecticide resistance due to altered esterases and insensitive acetylcholinesterase. Bull Entomol Res. 2005;95:371–380. doi: 10.1079/ber2005368. [DOI] [PubMed] [Google Scholar]
- 120.Hemingway J, Callaghan A, Kurtak DC. Temephos resistance in Simulium damnosum Theobald (Diptera, Simuliidae): A comparative study between larvae and adults of the forest and savanna strains of this species complex. Bull Entomol Res. 1989;79:659–669. [Google Scholar]
- 121.Hemingway J, Callaghan A, Kurtak DC. Biochemical characterization of chlorphoxim resistance in adults and larvae of the Simulium damnosum complex (Diptera, Simulidae). Bull Entomol Res. 1991;81:401–406. [Google Scholar]
- 122.Vassena CV, Picollo MI, Zerba EN. Insecticide resistance in Brazilian Triatoma infestans and Venezuelan Rhodnius prolixus. Med Vet Entomol. 2000;14:51–55. doi: 10.1046/j.1365-2915.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 123.Audino PG, Vassena C, Barrios S, Zerba E, Picollo MI. Role of enhanced detoxication in a deltamethrin-resistant population of Triatoma infestans (Hemiptera, reduviidae) from Argentina. Mem Inst Oswaldo Cruz. 2004;99:335–339. doi: 10.1590/s0074-02762004000300018. [DOI] [PubMed] [Google Scholar]
- 124.Orihuela PLS, Vassena CV, Zerba EN, Picollo MI. Relative contribution of monooxygenase and esterase to pyrethroid resistance in Triatoma infestans (Hemiptera : Reduviidae) from Argentina and Bolivia. J Med Entomol. 2008;45:298–306. doi: 10.1603/0022-2585(2008)45[298:rcomae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 125.Picollo MI, Vassena C, Orihuela PS, Barrios S, Zaidemberg M, et al. High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera : Reduviidae) from northern Argentina. J Med Entomol. 2005;42:637–642. doi: 10.1093/jmedent/42.4.637. [DOI] [PubMed] [Google Scholar]
- 126.Kumar K, Jamil-Ur-Rahman S, Sharma SK, Gill KS, Katyal R, et al. Entomological and rodent surveillance in plague-suspected areas during September 1994 and thereafter. Jpn J Med Sci Biol. 1997;50:97–111. doi: 10.7883/yoken1952.50.97. [DOI] [PubMed] [Google Scholar]
- 127.Kilonzo BS. DDT resistance in Xenopsylla brasiliensis the common plague vector in Tanzania. Insect Science and Its Application. 1985;6:111–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.