Abstract
OBJECTIVES
Data on temporal changes in alcoholic liver disease (ALD)-related mortality in the United States are lacking. This longitudinal assessment is important, given the divergent data on trends in worldwide ALD-related mortality, concerns for underestimation of mortality attributed to ALD in previous investigations, and shifting attention to hepatitis C virus (HCV)-related mortality.
METHODS
We analyzed mortality data compiled in the multiple cause-of-death public-use data file from the National Vital Statistics System from 1980 to 2003 using categorization by both International Classification of Diseases (ICD)-9 and ICD-10 systems. The main outcome measure was age- and sex-adjusted death rates attributable to ALD, HCV, or both (ALD/HCV) listed as immediate or underlying cause of death.
RESULTS
A total of 287,365 deaths were observed over the 24-year period. Age- and sex- adjusted incidence rates of ALD-related deaths decreased from 6.9/100,000 persons in 1980 to 4.4/100,000 persons by 2003. After introduction of HCV diagnostic testing, HCV-related liver mortality increased to 2.9/100,000 persons by 2003. Death rates for subjects with concomitant ALD/HCV rose to 0.2/100,000 persons by 1999 and then remained unchanged through 2003. Age-specific mortality related to ALD was highest in the ages of 45–64 years. Between 1980 and 2003, the age- and sex-adjusted ALD-related mortality (per 100,000 persons) decreased from 6.3 to 4.5 among Caucasians, 11.6 to 4.1 among African Americans, and 8.0 to 3.7 among the “other” race group.
CONCLUSIONS
Despite a decline in ALD-related mortality, the proportion of alcohol-related liver deaths is still considerably large and comparable in scope to that of HCV.
INTRODUCTION
Alcohol-related liver disease (ALD) is a significant burden on health, with alcohol consumption accounting for an estimated 3.8% of global mortality (1). In 1997, ALD was responsible for 40% of all deaths from cirrhosis and 28% of all deaths from liver disease (2). However, data on temporal changes in ALD-related death rate in the United States are lacking.
An accurate characterization of these trends is important for many reasons. First, although mortality from alcohol-related cirrhosis shows favorable trends in most countries of the world, an alarming increase in mortality has been observed in the United Kingdom and certain European countries(3).Whether-ALD related mortality continues to decline in the United States in the context of high-risk drinking patterns and uniformly for all racial groups is unknown. For example, an estimated 28% of adults engage in binge drinking, which has been associated with alcoholic hepatitis (4,5). Whether such maladaptive consumption patterns lead to excess ALD-related deaths is not known. In addition, whether racial disparities still exist in trends in ALD-related mortality for minority race groups is not well described (6). This information is important to focus preventive efforts in subgroups of the population that are at the highest risk for ALD. Second, current data may underestimate the mortality attributed to ALD (6). Analysis of death rate attributed to liver cirrhosis by the NIAAA (National Institute on Alcohol Abuse and Alcoholism) only takes into account a single underlying cause of death and does not account for significant conditions that may still be related to alcohol (e.g., portal hypertension). Third, there are limited data comparing trends in ALD-related mortality and hepatitis C virus infection (HCV)-related mortality. As alcohol and hepatitis C are the two most common causes of liver disease, comparing mortality trends in these two groups would be helpful to gauge the relative contribution of ALD-related mortality (7). Finally, longitudinal assessment of the effect of concurrent ALD and HCV (ALD/HCV) is lacking.
To address these concerns, we examined the temporal changes in mortality related to ALD and HCV from 1980 to 2003, using publicly available mortality data with the hypothesis that alcohol continues to be a significant cause of liver-related mortality when compared with HCV. We examined the death rate stratified by diagnosis (ALD, HCV, or ALD/HCV) over the time period and assessed the effect of age and race on mortality trends.
METHODS
Data source
Mortality data were collected from the multiple cause-of-death public-use data file from the National Vital Statistics System from 1980 to 2003. This data file includes individual death certificates for all resident deaths from the 50 states and the District of Columbia (8). Information regarding age (5-year increments), sex, race (African American, Caucasian, and “other”), place of birth, and death is available. The standard death certificates were extracted for information in part I of the certificate, which records the “immediate” or “underlying” causes of death. Information from part II, which includes information on other “significant conditions contributing to death but not resulting in the underlying cause given in Part I,” was not extracted, given that ALD or HCV may have been incidentally present in a person who died from an unrelated cause (e.g., myocardial infarction).
Case ascertainment
We identified all death certificates with ALD, HCV, or both as potential causes of death. Medical conditions recorded in multiple cause-of-death data were coded from 1980 to 1998 in accordance with the International Classification of Diseases, ninth revision (ICD-9), and from 1999 to 2003 in accordance with the International Classification of Diseases, tenth revision (ICD-10). Differences in the terms referenced reflect changes in nomenclature from ICD-9 to ICD-10 for ALD and HCV.
We only included cases in which any of the diagnoses of interest were mentioned in part I of the certificate (immediate or underlying). All patients with either alcoholic hepatitis (ICD-9 code 571.1 or ICD-10 code K70.1) or alcoholic cirrhosis (ICD-9 code 571.2 or ICD-10 code K70.3) were retrieved. However, it is possible that death related to ALD may be incorrectly coded. To increase ascertainment, we identified all death certificates with alcohol indicated as a cause (e.g., unspecified alcoholic liver damage, ICD-9 code 571.3) and a liver diagnosis of interest (e.g., portal hypertension, ICD-9 code 572.3). Diagnostic testing for HCV was available in 1991 and therefore HCV-related deaths were obtained from 1991 to 2003. Data regarding age, gender, and race (African American, Caucasian, and “other”) were also extracted.
Data analysis
First, we compared the baseline characteristics at the time of death based on diagnoses at death: ALD alone, HCV alone, or both ALD and HCV (ALD/HCV). Only these deaths for subjects of ≥ 15 years were included in the analyses. We also calculated the percentage of deaths due to alcoholic hepatitis (4). Second, mortality (incidence rates of death) attributable to ALD or HCV or both diagnoses (ALD/HCV) was calculated considering the entire population of the United States at risk. We examined trends in death rates over the 24-year study period by adjusting denominator age- and sex-specific person-years to the year 2000 US census data. Finally, we calculated age-specific trends (by decade starting at age 15) and age- and sex-adjusted rates by race (African American, Caucasian, and “other”) for ALD-related mortality.
RESULTS
Decedent characteristics
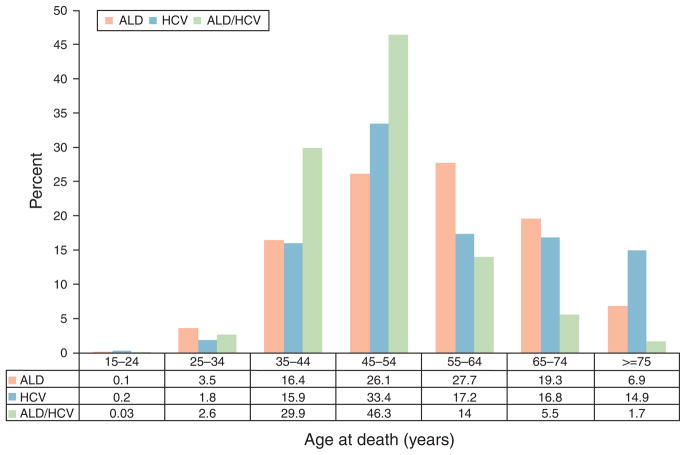
From 1980 to 2003 (Table 1), a total of 287,365 deaths were attributable to ALD (n=234,357), HCV (n=49,801), and concomitant ALD/HCV (n = 3,207). In total, the median age at death was 55 years and the majority of decedents were male (71.2 %) and Caucasian (82.8%). A higher percentage of African Americans died because of HCV when compared with ALD or concomitant ALD/HCV (16.4 % vs. 13.7 % vs. 15.4 %). When compared with 1980–1998, the overall median age at death for all decedents decreased from 55 years (interquartile range 45–65) to 50 years (interquartile range 45–60) between 1999 and 2003. Next, we compared the age at death among the three groups (Figure 1). The median age at death was lower in patients in the combined ALD/HCV group when compared with either ALD or HCV alone (45 vs. 55 vs. 50 years), respectively. A higher percentage of ALD/HCV-related deaths occurred between ages 45 and 54 years when compared with either ALD or HCV alone (46.3% vs. 26.1 % vs. 33.4 %), respectively.
Table 1.
Patient characteristics at the time of death
| ALD only (n =234,357) | HCV only (n = 49,801) | ALD/HCV (n =3,207) | Total (n =287,365) | |
|---|---|---|---|---|
| Age at death median (IQR) | 55.0 (45.0–65.0) | 50.0 (45.0–65.0) | 45.0 (40.0–50.0) | 55.0 (45.0–65.0) |
| Male (%) | 170,004 (72.5%) | 32,021 (64.1%) | 2,558 (79.7%) | 204,583 (71.2%) |
| Race (%) | ||||
| Caucasian | 195,610 (83.5%) | 39,762 (79.6%) | 2,618 (81.6%) | 237,990 (82.6%) |
| African American | 32,103 (13.7%) | 8,199 (16.4%) | 493 (15.4%) | 40,795 (14.2%) |
| Other | 6,644 (2.8%) | 1,973 (4.0%) | 99 (3.1%) | 8,716 (3.0%) |
| Total number of deaths (percentage of deaths due to AH) | ||||
| 1980–1984 | 57,890 (8.5%) | n/a | n/a | 57,890 |
| 1985–1989 | 47,483 (8.9%) | n/a | n/a | 47,483 |
| 1990–1994 | 39,198 (9.5%) | 4,419 | 232 (13.8%) | 43,849 |
| 1995–1998 | 39,221 (9.0%) | 13,539 | 1,022 (8.2%) | 55,782 |
| 1999–2003 | 50,565 (8.7%) | 31,843 | 1,953 (9.3%) | 84,361 |
AH, alcoholic hepatitis; ALD, alcoholic liver disease; HCV, hepatitis C virus infection; IQR, interquartile range; n/a: not applicable.
Figure 1.
Age at death due to alcoholic liver disease (ALD), hepatitis C virus infection (HCV)-related liver disease, or both ALD and HCV (ALD/HCV).
Temporal changes in ALD and HCV mortality
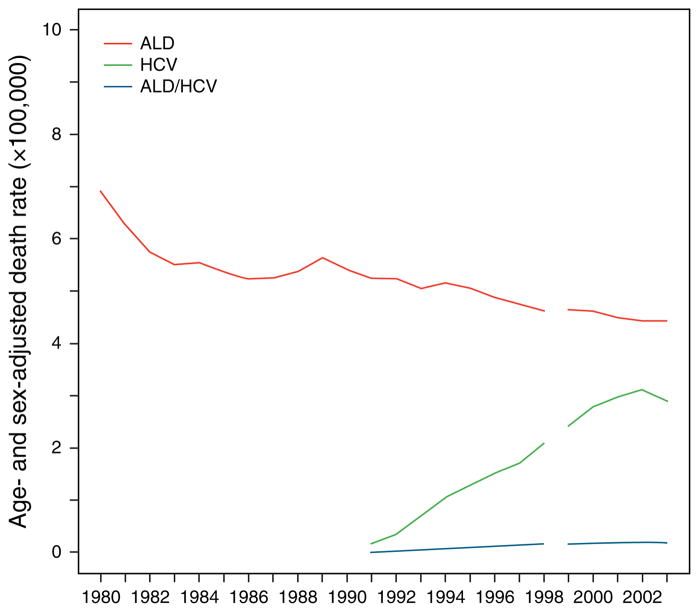
Of all reported ALD-related deaths, deaths attributed to alcoholic hepatitis changed from 8.5 % between 1980 and 1984 to 9.5% between 1990 and 1994 and to 8.7 % between 1999 and 2003 (Table 1). Figure 2 shows the temporal changes in death rate attributed to ALD and HCV. Age- and sex-adjusted ALD-related mortality (per 100,000 persons) decreased from 6.9 in 1980 to 4.6 in 1998 and 4.4 in 2003. HCV diagnostic testing was introduced in 1991. Overall hepatitis C-related age- and sex-adjusted mortality rose to 2.1 in 1998 and 2.9 in 2003. Death rates in the combined ALD/HCV group remained fairly constant from 1999 to 2003 (0.2/100,000 persons for both years).
Figure 2.
Age- and sex-adjusted mortality for patients with alcoholic liver disease (ALD), hepatitis C (HCV), and both ALD/HCV. Age- and sex-adjusted ALD mortality (per 100,000 persons) decreased from 6.9 (1980) to 4.4 (2003). HCV-related age- and sex-adjusted mortality (per 100,000 persons) rose from 2.1 (1998) to 2.9 (2003). Death rates in the combined ALD/HCV group remained fairly constant from 1999 to 2003 (0.2/100,000).
Age-specific changes in ALD-related mortality
Figure 3 examines the change in sex-adjusted mortality related to ALD by age groups, starting at age 15. ALD-related mortality was highest in the ages 45–54 years, followed by 55–64 years and remained relatively unchanged over the 24-year period. Age-specific mortality (per 100,000 persons) was 1.5 and 1.1 for ages 45–54 and 55–64 years in 2003, respectively.
Figure 3.

Age-specific mortality for patients with alcoholic liver disease (ALD).
Race-specific differences in ALD-related mortality
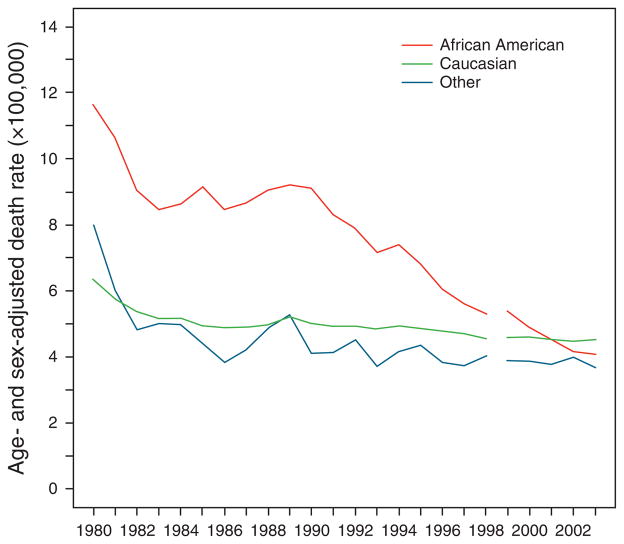
We examined the change in age- and sex-adjusted mortality related to ALD stratified by race (Figure 4). Overall, ALD-related mortality decreased for all races. The age- and sex-adjusted ALD-related mortality (per 100,000 persons) among Caucasians was 6.3 in 1980, which decreased to 4.5 in 1998 and to 4.5 in 2003. With regard to the African-American group, the ALD-related mortality was the highest among the three groups at nearly all time points until 2000. Nonetheless, a decline in mortality was still evident: it decreased from 11.6 in 1980 to 4.0 in 1998 and to 4.1 in 2003. A similar trend was observed among “other” non-African American, non-Caucasian race group, with an age- and sex-adjusted mortality of 8.0 in 1980, which decreased to 4.0 in 1998 and to 3.7 in 2003.
Figure 4.
Age- and sex-adjusted mortality for patients with alcoholic liver disease (ALD) by race. The age- and sex-adjusted ALD-related mortality (per 100,000 persons) decreased from 6.3 (1980) to 4.5 (2003) among Caucasians, 11.6 (1980) to 4.1 (2003) among African Americans, and 8.0 (1980) to 3.7 (2003) among the “other” non-Caucasian, non-African-American race group.
DISCUSSION
Given the divergent data on worldwide ALD-related deaths, we analyzed mortality data from the National Vital Statistics System from 1980 to 2003 (3,9). Several important observations are evident from our study. ALD remains one of the most important causes of liver-related death in the United States. In 2003, mortality rates were 4.4/100,000 for ALD when compared with 2.9/100,000 for HCV. As of 2003, ALD-related mortality affected the three race categories similarly. Finally, patients with concomitant ALD/HCV may be at risk for death at an earlier age.
ALD-related mortality continues to be a significant contributor of liver-related mortality, despite a decline in observed death rate from 6.9/100,000 persons in 1980 to 4.4/100,000 persons in 2003. These trends parallel the reported decline in death rate from liver cirrhosis (any cause) as reported by the NIAAA (15.1/100,000 persons in 1980 to 9.2/100,000 persons in 2003) (6). The decline in ALD-related mortality may be because of several possibilities: decrease in alcohol consumption, improved medical interventions that affect the natural history of disease, or misclassification of cases. Per capita alcohol consumption declined from 1980 to 1998 and the plateau in ALD mortality observed in recent years may be reflective of that change (10). In addition, participation in Alcoholics Anonymous and liver transplantation for ALD have both been associated with improved survival (11–13). Alternatively, it is also possible that liver-related deaths were incorrectly attributed to alcohol rather than chronic hepatitis C infection. When hepatitis C serology testing was introduced in the early 1990s, a rapid increase in HCV-related deaths was observed (14). Therefore, it is possible that alcohol-related mortality may have remained at a constant rate throughout the 1980s and 1990s, but HCV-related deaths may have been mistakenly classified as alcohol related, leading to the apparent decline.
A plateau in the death rate, however, should not provide false assurance. First, although efforts at preventing alcoholic cirrhosis may have improved, the decrease in the median age at death observed in our study is concerning. Second, although the rates of deaths related to alcoholic hepatitis did not increase over the 24-year period (consistent with a recent independent analysis (15)), this may provide an incomplete picture. Alcoholic hepatitis is often misdiagnosed by physicians and by coding specialists and the true burden of alcoholic hepatitis-related deaths may be underestimated (4). Third, quantity, frequency, and pattern of alcohol consumption are all significant determinants of ALD (5). There has been a recent increase in alcohol consumption (1999–2006), and the prevalence of binge drinking in youth aged 12–20 years increased from 12.1 % in 1993 to 19.2% in 2004 to 18.4 % in 2007 (10,16). Among adults, between 1995 and 2001, binge-drinking episodes per person per year increased by 35%, and 69 % of binge-drinking episodes occurred among those aged ≥26 years (17). Fourth, given the burgeoning problem of obesity in the developed world, it is likely that alcohol-related injury will increase (18). In alco holics, predictors for developing liver fibrosis and cirrhosis include obesity and hyperglycemia (18). Obesity potentiates the severity of ALD in all its stages, including fatty liver, alcoholic steatohepatitis, and cirrhosis (19). Although one would hope that a further decline in ALD-related mortality would be observed in the coming years, the contribution of high-risk drinking patterns, the recent increase in alcohol consumption, and the epidemic of obesity needs to be examined (20).
With regard to the racial breakdown of ALD mortality, our data showed that African-Americans’ rate was nearly twice that of Caucasians and “other” race counterparts until the early 1990s, when it started a decrease to a level comparable to others. At least a part of the explanation for this observation is reclassification of hepatitis C cases, as the burden of HCV among African Americans is much higher than others. Whether other factors, such as trends in alcohol consumption, contributed to this trend remains unknown. With regard to the “other” group, we found that rates of ALD-related mortality were lower compared with previous reports. Previous studies have shown that certain minority race groups within the “other” group have a higher mortality. For example, Vong and Bell (14) showed that in 1998, mortality due to chronic liver disease among American Indian and Alaska Natives was more than twice those of African Americans and Caucasians.
Our study had several strengths. First, we analyzed a comprehensive data source over 24 years with a large number of subjects. Previous analyses of similar data sets have been limited by the time period under study or underestimation of the true disease burden (2,6,9,14). We decreased ascertainment bias by examining alcohol and HCV as the immediate and underlying cause of death as well as incorporating a more comprehensive definition of ALD-related mortality. We expanded on a previous estimation of chronic liver disease by including more recent data (1999–2003) that used ICD-10 diagnostic coding system (14).
However, our study may have limitations as well. We may have underestimated the true mortality from ALD or HCV. Alcohol or HCV-related diagnoses may have been underreported on death certificates (21–23). However, the inclusion of death certificates listing manifestations of end-stage liver disease potentially attributed to alcohol minimizes the effect of underreporting. Although the revision from ICD-9 to ICD-10 in 1998 was contemplated as a factor for altering the diagnosis for causes of death, the comparability ratio (the ratio of the number of deaths coded to a cause in ICD-10 to the number coded to the equivalent cause in ICD-9) for chronic liver diseases and cirrhosis changed by only 4% since implementation of ICD-10 (24). Hence, the sudden change in ICD coding would not substantially influence the results.
In summary, our study shows that ALD remains an important cause of mortality. Despite the recent attention given to HCV as a significant cause of death and despite encouraging trends in ALD-related mortality, the pervasive effect of alcohol as a significant cause of liver-related deaths will require continued attention.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Alcohol-related liver disease (ALD) is a significant burden on health.
Data on temporal changes in ALD-related mortality are lacking.
WHAT IS NEW HERE
Over 24 years, ALD-related mortality decreased, whereas hepatitis C virus (HCV)-related mortality increased; however, ALD continues to be a significant cause of mortality.
In 2003, the age- and sex-adjusted mortality rate was 4.4/100,000 for ALD and 2.9/100,000 for HCV.
Acknowledgments
Financial support: This study was supported by grants from the NIH: T32 DK07198 (S.A.), R01DK-34238 (W.K.), and R01 DK059615 (V.S.).
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Vijay Shah, MD.
Specific author contributions: Study concept and design: Helga Paula, Sumeet K. Asrani, Nicholas C. Boetticher, Vijay H. Shah, and W. Ray Kim; acquisition of data: Helga Paula, Sumeet K. Asrani, Nicholas C. Boetticher, and Rachel Pedersen; analysis and interpretation of data: Helga Paula, Sumeet K. Asrani, Nicholas C. Boetticher, Rachel Pedersen, Vijay H. Shah, and W. Ray Kim; drafting of the manuscript: Helga Paula and Sumeet K. Asrani; critical revision of the manuscript for important intellectual content: Sumeet K. Asrani, Rachel Pedersen, Vijay H. Shah, and W. Ray Kim; statistical analysis: Rachel Pedersen; obtained funding: Sumeet K Asrani, Vijay H. Shah, and W. Ray Kim; administrative, technical, or material support: none; study supervision: Vijay H. Shah and W. Ray Kim; and approval of final submitted draft: Helga Paula, Sumeet K. Asrani, Nicholas C. Boetticher, Rachel Pedersen, Vijay H. Shah, and W. Ray Kim.
Potential competing interests: None.
References
- 1.Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Brown RS, Jr, Terrault NA, et al. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–42. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 3.Bosetti C, Levi F, Lucchini F, et al. Worldwide mortality from cirrhosis: an update to 2002. J Hepatol. 2007;46:827–39. doi: 10.1016/j.jhep.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Ceccanti M, Attili A, Balducci G, et al. Acute alcoholic hepatitis. J Clin Gastroenterol. 2006;40:833–41. doi: 10.1097/01.mcg.0000225570.04773.5d. [DOI] [PubMed] [Google Scholar]
- 5.Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–9. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Yi H. Liver Cirrhosis Mortality in the United States, 1970–2005. National Institute on Alcohol Abuse and Alcoholism, Division of Epidemiology and Prevention Research, Alcohol Epidemiologic Data System; Bethesda, MD: 2008. [Google Scholar]
- 7.Safdar K, Schiff ER. Alcohol and hepatitis C. Semin Liver Dis. 2004;24:305–15. doi: 10.1055/s-2004-832942. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics Home page. 2009 December; http://www.cdc.gov/nchswww/mission.htm.
- 9.Hurwitz ES, Holman RC, Strine TW, et al. Chronic liver disease mortality in the United States, 1979 through 1989. Am J Public Health. 1995;85:1256–60. doi: 10.2105/ajph.85.9.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakins NELR, Williams GD, Yi H. Apparent Per Capita Alcohol Consumption: National, State, and Regional Trends, 1977–2006. National Institute on Alcohol Abuse and Alcoholism, Division of Epidemiology and Prevention Research, Alcohol Epidemiologic Data System; Bethesda, MD: 2008. [Google Scholar]
- 11.Kumar S, Stauber RE, Gavaler JS, et al. Orthotopic liver transplantation for alcoholic liver disease. Hepatology. 1990;11:159–64. doi: 10.1002/hep.1840110202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann RE, Smart RG, Rush BR, et al. Cirrhosis mortality in Ontario: effects of alcohol consumption and Alcoholics Anonymous participation. Addiction. 2005;100:1669–79. doi: 10.1111/j.1360-0443.2005.01256.x. [DOI] [PubMed] [Google Scholar]
- 13.Starzl TE, Van Thiel D, Tzakis AG, et al. Orthotopic liver transplantation for alcoholic cirrhosis. JAMA. 1988;260:2542–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Vong S, Bell BP. Chronic liver disease mortality in the United States, 1990–1998. Hepatology. 2004;39:476–83. doi: 10.1002/hep.20049. [DOI] [PubMed] [Google Scholar]
- 15.Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–56. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Yi H, Williams GD, et al. Trends in Underage Drinking in the United States, 1991–2007. National Institute on Alcohol Abuse and Alcoholism, Division of Epidemiology and Prevention Research, Alcohol Epidemiologic Data System; Bethesda, MD: 2009. [Google Scholar]
- 17.Naimi TS, Brewer RD, Mokdad A, et al. Binge drinking among US adults. JAMA. 2003;289:70–5. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 18.Raynard B, Balian A, Fallik D, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–8. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 19.Mathurin P, Beuzin F, Louvet A, et al. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment Pharmacol Ther. 2007;25:1047–54. doi: 10.1111/j.1365-2036.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 20.Naveau S, Giraud V, Borotto E, et al. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–11. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 21.Kircher T, Anderson RE. Cause of death. Proper completion of the death certificate. JAMA. 1987;258:349–52. [PubMed] [Google Scholar]
- 22.Kircher T, Nelson J, Burdo H. The autopsy as a measure of accuracy of the death certificate. N Engl J Med. 1985;313:1263–9. doi: 10.1056/NEJM198511143132005. [DOI] [PubMed] [Google Scholar]
- 23.Pritt BS, Hardin NJ, Richmond JA, et al. Death certification errors at an academic institution. Arch Pathol Lab Med. 2005;129:1476–9. doi: 10.5858/2005-129-1476-DCEAAA. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RN, Minino AM, Hoyert DL, et al. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]