Abstract
Telomere shortening is a marker of cellular aging and has been associated with risk of Alzheimer’s disease. Few studies have determined if telomere length is associated with cognitive decline in non-demented elders. We prospectively studied 2,734 non-demented elders (mean age: 74 years). We measured cognition with the Modified Mini-Mental State Exam (3MS) and Digit Symbol Substitution Test (DSST) repeatedly over 7 years. Baseline telomere length was measured in blood leukocytes and classified by tertile as “short”, “medium”, or “long”. At baseline, longer telomere length was associated with better DSST score (36.4, 34.9 and 34.4 points for long, medium and short, p <0.01) but not for change in score. However, seven-year 3MS change scores were less among those with longer telomere length (−1.7 points vs. −2.5 and −2.9, p = 0.01). Findings were similar after multivariable adjustment for age, gender, race, education, assay batch, and baseline score. There was a borderline statistically significant interaction for telomere length and APOE e4 on 3MS change score (p=0.06). Thus, telomere length may serve as a biomarker for cognitive aging.
Keywords: Cognitive Decline, Biomarker, Genetics, Telomeres, Epidemiology
1. Introduction
Telomere length is emerging as an important biomarker for aging-related processes. Telomeres are simple stretches of DNA that “cap” the ends of chromosomes. Each time a cell divides, end nucleotides are lost and telomere length decreases. Telomeres shorten not only with each cell division, but also with aging and oxidative stress, both important factors in cognitive function.(Aviv, 2006) The cumulative shortening of telomere length has been linked to lifespan(Cawthon, et al., 2003) and various age-related conditions including cardiovascular disease,(Benetos, et al., 2004,Brouilette, et al., 2007,Fitzpatrick, et al., 2007,van der Harst, et al., 2007) osteoarthritis,(Zhai, et al., 2006) osteoporosis(Valdes, et al., 2007) and obesity.(Valdes, et al., 2005)
A small number of studies have investigated telomere length and risk of developing dementia. Findings have suggested an association between shortened telomere length and risk of vascular dementia(von Zglinicki, et al., 2000) and Alzheimer’s disease (AD).(Panossian, et al., 2003) More recently, Honig and colleagues reported that relative telomere length was shorter among elders with reduced survival and with a diagnosis of AD. Furthermore, they found an interaction with apolipoprotein E (APOE) e4 in that with persons with an e4 allele had more marked reductions in survival and greater risk of AD.(Honig, et al., 2006) Shorter telomere length has also been linked to an increased risk of dementia among stroke survivors.(Martin-Ruiz, et al., 2006) Most of these studies used a case-control design which may be more sensitive to bias. Few studies have prospectively examined the association of telomere length and cognitive function or rate of cognitive decline in non-demented elders.
We determined if leukocyte telomere length was associated with cognitive aging in an ongoing prospective study of non-demented elderly men and women. We also assessed if there was an interaction between APOE e4, telomere length and cognitive aging. Our hypothesis was that shorter telomere length would be associated with worse baseline cognitive function and greater rate of cognitive decline.
2. Methods
2.1 Study population
Participants were part of the Health Aging and Body Composition (Health ABC) Study, a prospective cohort study beginning in 1997 of 3,075 community-dwelling older adults aged 70–79 years and living in Memphis, TN or Pittsburgh, PA. To identify potential participants, a random sample of white and black Medicare-eligible elders within designated zip code areas were contacted. To be eligible for the study, participants had to report no difficulties performing activities of daily living, walking a quarter of a mile, or climbing 10 steps without resting. All participants were free of life-threatening cancers and intentions to move out of the study area for at least 3 years. All participants signed an informed written consent, approved by the institutional review boards at the clinical sites. This study was approved by the institutional review boards of the two field centers (University of Pittsburgh and University of Tennessee, Memphis) and that of the coordinating center, the University of California, San Francisco. After excluding the 328 participants without telomere length measurement and 13 participants without cognitive testing, our analytic cohort consists of 2,741 Health ABC participants.
2.2 Measurements
The Modified Mini-Mental State Examination (3MS), a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory,(Teng and Chui, 1987) was administered to participants during the baseline visit (Year 1) and repeated at the Year 3, 5 and 8 follow-up visits. The maximum or best score is 100. The Digit Symbol Substitution Test (DSST), administered in Years 1, 5 and 8, measures attention, psychomotor speed, and executive function.(Wechsler, 1997) The DSST score was calculated as the total number of test items correctly coded in 90 seconds with a maximum (best) score possible of 90.
Genomic DNA was extracted from blood leukocytes by standard procedures. Average telomere lengths were measured in each sample by quantitative polymerase chain reaction (PCR) in the Department of Human Genetics at the University of Utah. Thermal cycling was conducted using the Corbett Research Rotor-Gene 3000 machine and the Rotor-Gene 5.0.2 software. Relative average telomere lengths were determined by comparing each DNA sample to a reference DNA sample using the standard curve method. The coefficient of variation is 5.8%.(Cawthon, 2002)
Covariates included participant baseline age, race, gender and whether or not they achieved a high-school level of education. Body mass index (BMI) (kg/m2) was calculated from direct height and weight measurements at baseline. Presence of diabetes mellitus and hypertension, and history of stroke or transient ischemic attack (TIA) or myocardial infarction (MI) were determined using prevalent disease algorithms based on a combination of self-report of physician diagnoses, recorded medications and laboratory findings. Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D), with a score ≥16 consistent with possible depression.(Radloff, 1977) Apolipoprotein E (APOE) genotyping was performed at Bioserve.com (Laurel, MD) using standard SNP analyses. For our analyses, we determined the presence of one or more e4 alleles.
2.3 Statistical analyses
We first categorized telomere lengths into tertile as telomere length was not normally distributed. We next determined if there were associations between telomere tertile and baseline characteristics, using chi-square tests for categorical and ANOVA for continuous variables.
We analyzed the association between telomere tertiles and baseline and change in 3MS and DSST scores using repeated measures mixed effects models. Random intercepts and slopes were estimated for each participant, assuming an unstructured covariance matrix. Covariates entered as fixed effects in multivariable adjusted models included age, race, gender and education because they were significantly associated (p<0.05) with both telomere tertile and cognitive test scores. We also included a covariate indicating assay batch to account for interassay variability. In addition, we tested for an interaction between telomere tertile and APOE e4 genotype on cognitive function. We also tested for interactions with gender and race and telomere tertile and cognitive function. Since 3MS scores were negatively skewed, we log transformed them for analysis and then back-transformed them for reporting purposes. Mean 3MS and mean DSST scores were calculated from the mixed model results by telomere tertile for presentation. All analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC) and Stata 10.1 (StataCorp LP, College Station, TX).
3. Results
The mean age of the study population was 73.6 (± 2.9) years at baseline and 80.4 (± 2.8) years at follow-up; 52% were women and 41% were Black. The “short” telomere group was composed of 910 individuals with mean telomere length of 3510 base pairs. The “medium” telomere group was composed of 902 individuals with mean telomere length of 4740 base pairs and the “long” telomere group was composed of 922 individuals with mean telomere length of 6280 base pairs. Those with longer telomeres were younger on average and more likely to be female, Black, and a high school graduate (p<.05 for all) (Table 1).
Table 1.
Baseline characteristics by telomere length tertile
| Telomere Length Tertile | ||||
|---|---|---|---|---|
| Characteristic | Short (n=910) | Medium (n=902) | Long (n=922) | p-value* |
| Age (mean, sd) | 73.9 (2.8) | 73.6 (2.9) | 73.4 (2.9) | 0.001 |
| Black (%) | 37.0 | 40.9 | 44.3 | 0.007 |
| Female (%) | 41.0 | 52.0 | 61.6 | <0.001 |
| Education < high school (%) | 25.7 | 27.1 | 21.7 | 0.02 |
| Depression score ≥16 (%) | 4.4 | 4.6 | 4.7 | 0.96 |
| Body mass index (mean, sd) | 27.6 (4.9) | 27.3 (4.9) | 27.5 (4.8) | 0.55 |
| Hypertension (%) | 62.6 | 61.3 | 65.6 | 0.15 |
| Diabetes (%) | 23.9 | 25.3 | 22.8 | 0.44 |
| Myocardial infarct history (%) | 12.2 | 12.2 | 11.6 | 0.91 |
| Stroke/TIA history (%) | 6.9 | 8.4 | 8.1 | 0.46 |
| APOE e4 Carrier (%) | 29.8 | 30.4 | 26.6 | 0.16 |
P-value by ANOVA for continuous variables and chi-square for categorical variables.
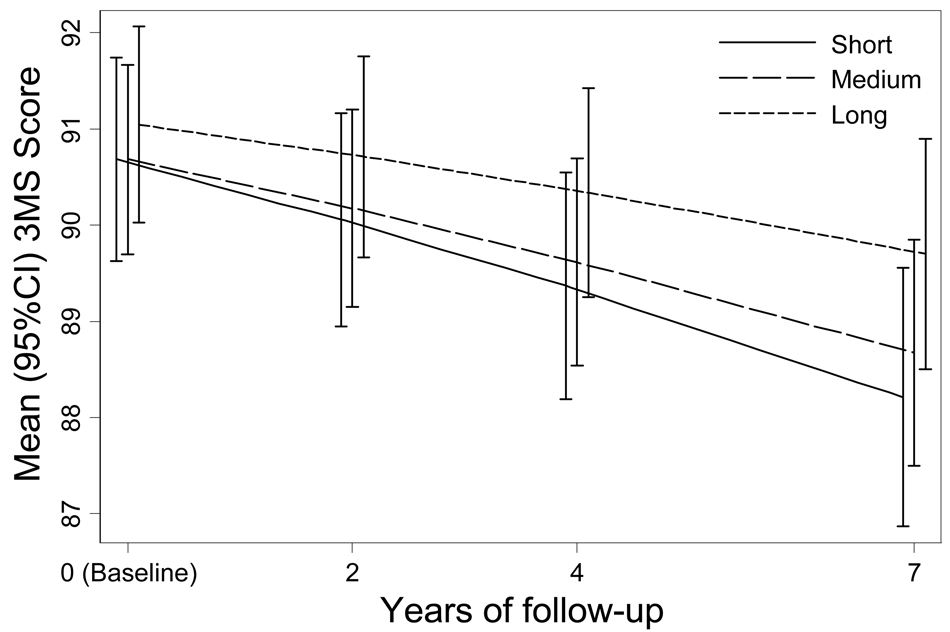
At baseline there was no difference in 3MS score by telomere length. Those with long telomere length had a mean (±standard error) score of 91.2 (±0.2) points vs. 90.9 (±0.1) and 90.8 (±0.2) for those with medium and short length (p=0.28) (Table 2). However, 7-year 3MS score decline was less among elders with long telomere length compared to those with medium or short telomere length (−1.7 ± 0.3 points vs. −2.5 ± 0.2 and −2.9 ± 0.3 respectively, p=0.01). In multivariable models adjusted for age, gender, race, education, and assay variability, results were similar (−1.3 ± 0.3 points vs. -2.0 ± 0.2 and −2.5 ± 0.3 respectively, p = 0.01) (Figure 1).
Table 2.
Unadjusted mean (standard error) baseline and 7-year change 3MS and DSST scores
| Cognitive Measure | Telomere Length Tertile | |||
|---|---|---|---|---|
| p-value | ||||
| Short | Medium | Long | ||
| Baseline 3MS score (SE) | 90.8 (0.2) | 90.9 (0.1) | 91.2 (0.2) | 0.28 |
| 3MS 7-year change score (SE) | −2.9 (0.3) | −2.5 (0.2) | −1.7 (0.3) | 0.01 |
| Baseline DSST score (SE) | 34.4 (0.4) | 34.9 (0.3) | 36.4 (0.4) | <0.01 |
| DSST 7-year change score (SE) | −4.3 (0.4) | −4.2 (0.2) | −4.3 (0.4) | 0.97 |
Figure 1.
The association between multivariable adjusted 3MS scores and telomere length tertile. Models were adjusted for age, gender, race, education, and assay variability.
*P for trend = 0.98 for baseline and 0.01 for change score.
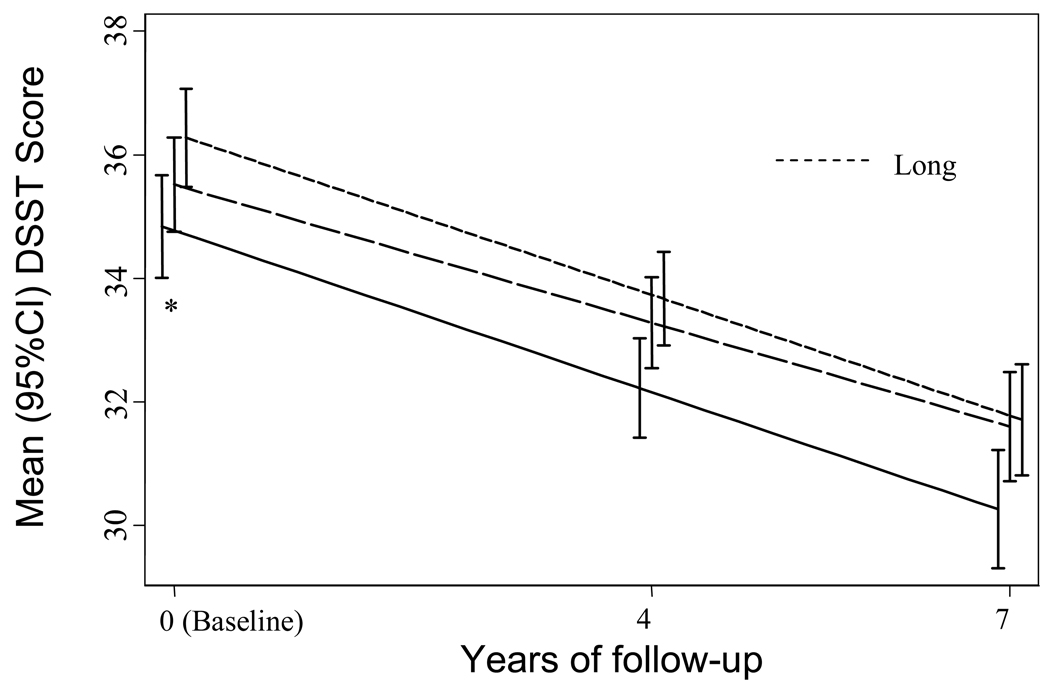
Baseline DSST scores varied by telomere length tertile. Those with longer telomere lengths had a mean score of 36.4 (± 0.4) points vs. 34.9 (± 0.3) and 34.4 (± 0.3) for those with medium and short lengths (p<0.01) (Table 2). Average 7-year change score, however, did not vary across the three groups (−4.3 ± 0.4 points, −4.2 ± 0.2 points, and −4.3 ± 0.4 points, p=0.97). In multivariable models adjusted for age, gender, race, education, and assay variability, results were similar with statistically significant differences in baseline score (36.4 ± 1.0, vs 34.8 ± 1.0 and 34.4 ± 1.0, p=0.02) (Figure 2).
Figure 2.
The association between multivariable adjusted DSST scores and telomere length tertile. Models were adjusted for age, gender, race, education, and assay variability.
*P for trend = 0.02 for baseline and 0.99 for change score.
There were no significant interactions between telomere length and APOE e4 on DSST at baseline (p=0.67) or over time (0.33). There was a borderline statistically significant interaction (p=0.06) for 3MS score over time in which elders with an APOE e4 allele and shorter telomere length had greater decline but not for baseline score (p=0.96). There were no significant interactions with gender or race and telomere length and cognitive scores (p<0.10 for all). Additional analyses using race and gender specific tertile of telomere length resulted in almost identical results. Participants with longer telomere length were less likely to have died by the final visit compared to those with medium and short telomere lengths (18.1% versus 20.5% and 23.0%, p=0.04).
4. Discussion
Community resident elders with longer telomere length exhibited better baseline attention and psychomotor speed, dimensions of cognitive function and less decline in global cognitive functioning over 7 years relative to elders with short or medium telomere length. These results were independent of factors that varied by telomere length including demographic characteristics.
These results are consistent with previous studies reporting an association between telomere length and risk of dementia.(Grodstein, et al., 2008,Honig, et al., 2006,Martin-Ruiz, et al., 2006,von Zglinicki, et al., 2000) In a nested case-control study, Honig and colleagues observed that mean telomere length was shorter among patients with AD compared to controls.(Honig, et al., 2006) Further, in those who developed dementia, telomere length was associated with reduced survival.(Honig, et al., 2006) Interestingly, the interaction between APOE e4 and telomere length on risk of AD and survival reported in that study was not present in the current work although there was a similar trend level finding for 3MS change score suggesting that this needs further exploration.(Honig, et al., 2006) Our findings are also consistent with a prospective study which found longer telomere length associated with less cognitive decline, as measured by the MMSE, and reduced risk for dementia and death.(Martin-Ruiz, et al., 2006) Finally, our results are consistent with a cross-sectional study of older women in which a correlation was observed between worse cognitive function and shorter telomere length.(Valdes, et al., 2008)
However, other studies have not reported an association between telomere length and cognitive outcomes. Harris and colleagues observed no significant association between age-related cognitive decline or mortality.(Harris, et al., 2006) This could, however be due to the fact that the study was small (N=190) and the mean age was 79 years old. The authors postulate that the stability of telomeres lessens with age, and thus there may be a reduction in the strength of the association with telomere length and outcomes as people get much older.(Harris, et al., 2006)
Several mechanisms may explain the association between telomere length and cognition. The association may be due to an indirect mechanism such as increased oxidative stress(Barnham, et al., 2004) or inflammation.(Jones, 2001,Yaffe, et al., 2003) Previous studies have demonstrated that telomere length is associated with indices of oxidative stress,(Demissie, et al., 2006,Epel, et al., 2004) and with markers of inflammation.(Bekaert, et al., 2007,Fitzpatrick, et al., 2007) Both of these are believed to be key mechanisms for the neurodegeneration seen in AD and other dementias.(Barnham, et al., 2004,Jones, 2001,Perry, et al., 2000,Yaffe, et al., 2003) Another possible mechanism is that cognitive aging and telomere length are strongly influenced by similar genetic factors.(Andrew, et al., 2006,Nawrot, et al., 2004) Based on twin and family studies, telomere length has been noted to be heritable, with heritability estimated to be around 36%.(Andrew, et al., 2006) Finally, it may be that telomere length may contribute to a third factor such as hypertension or cardiovascular disease, which in turn has an effect on cognitive function.(Bekaert, et al., 2007,Fitzpatrick, et al., 2007,Jeanclos, et al., 2000)
This study has many strengths including a prospective design and large and diverse sample. Nearly all prior studies have had case-control or cross-sectional design(Honig, et al., 2006,Panossian, et al., 2003,Valdes, et al., 2008,von Zglinicki, et al., 2000) with limited ability to draw causal inferences. We were also able to control for possible confounding by conducting multivariable models adjusting for those characteristics that differed across telomere length and might also affect cognitive function.
There are also several limitations to be considered when interpreting the results, including the large inter-individual variability of telomere length.(Demissie, et al., 2006,Honig, et al., 2006,Jeanclos, et al., 2000) We could not test all cognitive domains and lacked of information on the etiology underlying cognitive decline. Finally, there was differential survival related to telomere length and this could affect our longitudinal results. However, we anticipate that since participants with shorter telomere length had reduced survival, the findings might have even been more pronounced had they remained in the study.
In this study we found that telomere length was associated with cognitive decline and mortality in a diverse cohort of non-demented elders. As identification of biomarkers for dementia and cognitive decline is critical, it will be essential to further study the association between telomere length and cognition. The possible advantage of telomere length as a biomarker is its relative ease of measurement and association with a wide variety of aging-related conditions. However, an issue that needs to be resolved is that there is not a clear “cut-off” telomere length that can be used as a prognostic marker at the individual level. Future studies should prospectively evaluate if changes in an individual’s telomere length correspond to incident disease including the development of cognitive impairment and dementia.
Acknowledgements
This study was conducted on behalf of the Health, Aging and Body Composition (Health ABC) Study. This work was funded by: AG-6-2101, AG-6-2103, AG-6-2106, and AG021918. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Dr. Yaffe was supported in part by AG031155 and an anonymous foundation. The authors have no disclosures.
Footnotes
Disclosure Statement
The authors declare not conflicts of interest.
References
- Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, Kimura M, Kato BS, Valdes AM, Spector TD. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. The American Journal of Human Genetics. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A. Telomeres and human somatic fitness. J Gerontol A Biol Sci Med Sci. 2006;61(8):871–873. doi: 10.1093/gerona/61.8.871. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, Cooman L, Van Damme P, Cassiman P, Van Criekinge W, Verdonck P, DeBacker GG, Gillebert TC, Van Oostveldt P. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43(2):182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. PNAS. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, De Vivo I. Shorter telomeres may mark early risk of dementia: Preliminary analysis of 62 participants from the Nurses' Health study. PLoS ONE. 2008;3(2):1–3. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neuroscience Letters. 2006;406(3):260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. 2006;60(2):181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- Jones RW. Inflammation and Alzheimer's disease. Lancet. 2001;358:436–437. doi: 10.1016/S0140-6736(01)05667-7. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60(2):174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging. 2003;24(1):77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Jones PK, et al. Oxidative imbalance is a major feature of Alzheimer disease. Curr Biochem Res. 2000;3:151–156. [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;(1):385–401. [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Deary IJ, Gardner JP, Kimura M, Lu X, Spector TD, Aviv A, Cherkas LF. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiology of Aging. 2008:1–7. doi: 10.1016/j.neurobiolaging.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, Aviv A, Spector TD. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int. 2007;18(9):1203–1210. doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, van Gilst WH, van Veldhuisen DJ. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49(13):1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Serra V, Lorenz M, Saretzki G, Lenzen-Grossimlighaus R, Gessner R, Risch A, Steinhagen-Thiessen E. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80(11):1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Zhai G, Aviv A, Hunter DJ, Hart DJ, Gardner JP, Kimura M, Lu X, Valdes AM, Spector TD. Reduction of leucocyte telomere length in radiographic hand osteoarthritis: a population-based study. Ann Rheum Dis. 2006;65(11):1444–1448. doi: 10.1136/ard.2006.056903. [DOI] [PMC free article] [PubMed] [Google Scholar]