Abstract
OBJECTIVES
Esophageal adenocarcinoma (EAC) is a devastating disease that has risen in incidence over the past several decades. Barrett's esophagus (BE) is an associated premalignant lesion. Current preventative efforts rely on endoscopic screening of individuals with gastroesophageal reflux disease (GERD) symptoms and surveillance endoscopy for those with BE. However, some recent studies have found a high prevalence of BE in patients without GERD, and others have found little or no association with GERD. We hypothesized that studies of higher-quality design show weaker associations of GERD with BE, and that GERD is only weakly associated with short-segment Barrett's esophagus (SSBE).
METHODS
We performed a systematic literature search in multiple online electronic databases regardless of language. Eligible studies required visualization of columnar mucosa and histological confirmation of intestinal metaplasia, and GERD symptoms ascertained by questionnaire or interview. The highest-quality sampling design was defined a priori by both cases and controls identified among unselected research volunteers (“research design”) rather than by patients selected for endoscopy for clinical indications (“clinical design”), which introduces selection and ascertainment bias. A priori, heterogeneity was defined by Cochrane's Q P < 0.20 and the inconsistency index (I2; 25% low, 50% moderate, and 75% high). Heterogeneity of results can reflect significant differences in study design or effect modification by strata of outcomes.
RESULTS
Systematic review identified 13,392 citations. Evaluation identified 108 potentially relevant journal articles, of which 26 met eligibility. Of these, 14 studies identified cases of BE and controls based on clinical indication (“clinical design”), and 6 used the “research design.” The remaining six studies identified cases of BE from patients undergoing endoscopy for clinical indication and controls among patients without known BE (“cases clinical/controls research”). The summary odds ratio (OR) for the association of GERD with BE from all studies was 2.90 (95% confidence interval (CI), 1.86–4.54), but the results were very heterogeneous (P = 0.0001; I2 = 89%). When stratified by BE length and sampling design, the studies with clinical design showed substantial, but heterogeneous, associations with SSBE (OR, 2.38; 95% CI, 1.21–4.70; P = 0.02; I2 = 62%), and stronger and homogeneous association with long-segment BE (LSBE; fixed effects OR, 2.96; 95% CI, 1.69–5.19; P = 0.25; I2 = 25%). In the research study design, stratifying by length of BE resolved the heterogeneity and showed a strong association between GERD and LSBE (fixed effects OR, 4.92; 95% CI, 2.01–12.0; P = 0.30; I2 = 19%) and no association with SSBE (fixed effects OR, 1.15; 95% CI, 0.763–1.73; P = 0.84; I2 = 0%). Funnel plots showed potential evidence for bias against dissemination of small negative studies.
CONCLUSIONS
In the highest-quality studies, GERD symptoms are not associated with SSBE, but increased the odds of LSBE by fivefold. GERD symptoms can serve as a reliable predictor of LSBE, but not SSBE. If SSBE is considered worthy of identification, then current screening practices do not select patients at risk for endoscopy, and alternative methods of selection for screening need to be developed.
INTRODUCTION
Over the past three decades, the incidence of esophageal adenocarcinoma (EAC) has risen rapidly (1,2). Symptoms of gastroesophageal reflux disease (GERD), such as heartburn or regurgitation, have been associated with an increased risk of cancer(3–9). However, approximately 50% of EAC patients report no previous history of symptoms of GERD (3,10,11). Barrett's esophagus (BE) is a metaplastic change in the esophageal mucosal lining from squamous to specialized intestinal mucosa (12). It is believed that BE is an intermediate step in the development of cancer and that prevention or intervention targeting BE might prevent the development of EAC. The ability to reliably screen for and treat BE would have broad implications in esophageal cancer prevention.
The screening practices for BE largely rely upon identification of patients with GERD symptoms (13,14). Although the definition of BE has changed over time, the earliest reports of the condition were in patients with symptoms of GERD (15,16). Perhaps as a result, the association of BE with GERD symptoms had largely been assumed as axiomatic for decades. Many of the first studies examining the risk factors for BE only compared cases of BE with control subjects with symptoms of GERD, rather than sampling groups of cases and controls without GERD (17–20). As a result, earlier studies were limited to examining the duration of GERD symptoms or measures of severity of GERD symptoms as risk factors rather than the mere presence of GERD symptoms. However, a few recent studies have reported surprisingly high prevalences of BE in patients without GERD symptoms. In 2002, Gerson et al. (21) reportedfinding BE in 25% of patients without GERD symptoms who underwent a research upper endoscopy at the time of colon cancer screening. In 2006, Ward et al. (22) reported a 15% prevalence of BE in asymptomatic patients, in a similarly designed study. Although a high prevalence of BE in non-GERD patients has not been consistently reproduced, a number of recent population-based studies have found little, if any, association between GERD symptoms and the presence of BE (23,24).
There are a number of potential explanations for these recent findings. First, GERD might truly be a much weaker risk factor for BE than previously appreciated; the higher-quality design of these recent population-based studies may be better estimates of the strength of the association, by avoiding selection and ascertainment biases. Second, shorter segments of BE may be more weakly associated with GERD symptoms than longer segments, and the recent studies are more likely to include short segments within their definition of BE than older studies. Finally, geographic differences in the origin of study subjects or changes over time might explain these recent findings.
We aimed to synthesize the available data across multiple studies to precisely estimate the association between GERD symptoms and BE. In light of the results of recent population-based studies, we expected to find substantial heterogeneity between study results. As outlined above, we hypothesized that this heterogeneity would be at least partially explained by the selection of cases and controls, and the length of BE.
METHODS
Study protocol
We performed a systematic literature search in MEDLINE (1950 to August 2008), EMBASE (1947 to August 2008), Web of Science (1900 to August 2008), Cochrane Central Register of Controlled Trials (third-quarter of 2008), BIOSIS preview (1926 to August 2008), Data Abstracts of Review of Effect (third-quarter of 2008), and ACP Journal Club (1991 to August 2008) to identify studies evaluating the prevalence of BE in patients with and without GERD symptoms, without regard to language of the publication. Medical subject headings for our literature review included (“gastroesophageal reflux,” or “GERD,” or “esophageal reflux,” or “esophagitis,” or “heartburn,” or “pyrosis,” or “regurgitation”) and (“esophageal neoplasm,” or “adeno-carcinoma,” or “carcinoma,” or “Barrett*,” or “metaplasia,” or “meta-plastic”). British spelling of terms were also searched. This process included electronic searching of supplemental abstracts published in Gastroenterology and Gut. Supplemental abstracts from the American Journal of Gastroenterology between the years 2000 and 2008 were manually searched for relevant studies. Citations from identified articles were cross-referenced for completeness.
Study selection and data abstraction
The studies met inclusion criteria if the following were satisfied: (i) BE determined by endoscopic suspicion and confirmed by histological evidence of intestinal metaplasia, (ii) GERD symptoms ascertained by questionnaire or interview, (ii) the presence of four groups: both with and without GERD and with and without BE, (iv) data presented as odd ratios (ORs) or in a format from which the OR could be derived, and (v) study subjects were unique to that publication. GERD was broadly defined as experiencing heartburn and/or acid regurgitation for at least 1 day a week.
Study references and citations were collected in Endnote software application version 8.0 (Thomson Reuters, New York, NY). One investigator (J.T.) reviewed all titles, and of those that appeared eligible, both investigators independently reviewed the abstracts to assess eligibility. A data collection form was designed in Microsoft Access 2007 (Microsoft, Redmond, WA). Abstracts, and if deemed appropriate, full articles, were translated into English as needed. For abstracts that appeared eligible on first review, both investigators independently abstracted data from the full articles. The database collected information on study design, summary measures for participants with and without BE, definition of GERD symptoms, method of GERD symptom ascertainment, histological confirmation, definition of BE, BE length, and country of origin. Data abstraction was confirmed by consensus. Studies examined outcomes of short-segment BE (SSBE), long segment BE (LSBE), or both. One study examined 36 patients of whom 35 were short segment; for the purposes of this meta-analysis, all 36 patients were classified as short segment (25).
Study quality
Quality of study design was classified by sampling of subjects. Most studies identified both cases of BE and controls without BE among patients undergoing upper endoscopy for clinical indication (Table 1). We labeled these studies as “clinical design.” A few recent studies minimized potential selection bias and ascertainment bias by sampling a general population, and inviting them to undergo a research upper endoscopy irrespective of any symptoms, thereby prospectively classifying subjects as cases of BE or control subjects (Table 1). We labeled these studies as “research design.” Finally, in some other studies, the cases of BE were obtained from patients undergoing clinically indicated upper endoscopy, but the control group was obtained from a relevant general population without undergoing upper endoscopy to exclude BE (Table 1).We labeled this study design as“case-clinical/control-research.”
Table 1.
Study characteristics
| Authors | Year | Study design | No. of BE cases | % Of BE that are short | % With BE in GERD/no GERD | % With GERD in BE/no BE | % Male in BE/no BE | Mean age BE/no BE | GERD threshold | Country | OR (95% CI) for any length of BE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spechler et al. (29) | 1994 | Clinical | 16 | 0 | 14/9 | 75/63 | 63/43 | 53/51 | — | United States | 1.75 (0.530–5.78) |
| Chalasani et al. (31) | 1997 | Clinical | 12 | 58 | 39/0 | 100/25 | — | — | 2×/week | United States | 70.7 (3.99–1,260) |
| Triadafilopoulos and Sharma (40) | 1997 | Clinical | 36 | — | 16/9 | 42/53 | — | — | — | United States | 0.629 (0.316–1.25) |
| Pereira et al. (35) | 1998 | Clinical | 19 | 100 | 37/14 | 74/43 | 56/48 | 59/— | — | United States | 3.73 (1.18–11.79) |
| Hirota et al. (33) | 1999 | Clinical | 101 | 62 | 14/6 | 87/72 | 77/53 | 59/54 | — | United States | 2.58 (1.41–4.72) |
| Csendes et al. (32) | 2000 | Clinical | 43 | 67 | 11/0 | 100/52 | 87/37 | 58/47 | 1×/week | Chile | 79 (4.84–1,290) |
| Voutilainen et al. (39) | 2000 | Clinical | 11 | 0 | 3/1 | 36/11 | 71/38 | 63/56 | 6 Months | Finland | 4.63 (1.34–16) |
| Romero et al. (44) | 2001 | Research | 45 | — | 12/8 | 84/79 | — | — | 1×/week | United States | 1.47 (0.634–3.43) |
| Castro et al. (30) | 2002 | Clinical | 41 | 100 | 28/24 | 49/43 | 49/42 | 55/49 | — | Spain | 1.25 (0.612–2.54) |
| Conio et al. (45) | 2002 | CC/CR | 149 | 73 | 81/9 | 82/9 | 76/50 | 59/61 | 1×/week | Italy | 46.4 (26.1–82.4) |
| Rex et al. (43) | 2003 | Research | 66 | 82 | 9/6 | 21/15 | 72/59 | — | 1×/week | United States | 1.53 (0.826–2.85) |
| Rajendra et al. (36) | 2004 | Clinical | 123 | 26 | 9/4 | 62/41 | 43/52 | 51/60 | 1×/week | Malaysia | 2.37 (1.63–3.45) |
| Toruner et al. (37) | 2004 | Clinical | 29 | 83 | 5/8 | 10/15 | 55/42 | 51/48 | — | Turkey | 0.637 (0.186–2.18) |
| Johansson et al. (34) | 2005 | Clinical | 25 | 80 | 5/3 | 40/32 | 43/— | — | 50×/year | Sweden | 1.40 (0.619–3.17) |
| Kim et al. (25) | 2005 | Clinical | 36 | 100 | 4/4 | 19/16 | 67/52 | 51/46 | — | South Korea | 1.23 (0.529–2.85) |
| Ronkainen et al. (24) | 2005 | Research | 16 | 69 | 2/1 | 56/40 | 56/49 | 57/54 | 3 Months | Sweden | 1.95 (0.720–5.28) |
| Smith et al. (50) | 2005 | CC/CR | 117 | — | 86/21 | 43/3 | 64/66 | 56/63 | — | Australia | 23.6 (10.7–52.2) |
| Corley et al. (46) | 2006 | CC/CR | — | — | — | — | 73/68 | — | 1×/week | United States | 5.80 (3.10–10.9) |
| Veldhuyzen et al. (38) | 2006 | Clinical | 25 | — | 4/1 | 64/37 | 68/— | 53/— | — | Canada | 3.01 (1.32–6.88) |
| Ward et al. (22) | 2006 | Research | 50 | 92 | 20/15 | 42/34 | 55/— | — | 2×/week | United States | 1.41 (0.756–2.61) |
| Corley et al. (47) | 2007 | CC/CR | 320 | — | 73/22 | 80/29 | 75/75 | — | 1×/week | United States | 9.63 (6.69–12.9) |
| Johansson et al. (49) | 2007 | CC/CR | 21 | 0 | 38/7 | 48/10 | 29/34 | 60/62 | 50×/year | Sweden | 10.7 (3.5–33.4) |
| Esquivel et al. (42) | 2008 | Research | 11 | 100 | 5/5 | 45/49 | — | — | 2×/week | United States | 0.858 (0.254–2.90) |
| Jacobson and Fuchs (48) | 2008 | CC/CR | — | — | — | — | 0/0 | — | — | United States | 2.79 (1.96–3.97) |
| Tseng et al. (41) | 2008 | Clinical | 12 | 92 | 0/0 | 8/16 | 75/55 | 62/52 | — | Taiwan | 0.477 (0.0615–3.70) |
| Zagari et al. (23) | 2008 | Research | 11 | 85 | 2/1 | 54/44 | 46/— | — | — | Italy | 1.47 (0.49–441) |
BE, Barrett's esophagus; CC/CR, cases clinical/controls research; CI, confidence interval; GERD, gastroesophageal reflux disease; OR, odds ratio.
In CC/CR, cases of BE were sampled from patients undergoing endoscopy for clinical indications, but controls were sampled among research volunteers without regard to symptoms.
Analysis
Meta-regression of the year of the study for the effect of GERD on the risk of BE was performed in SAS v.9.2 (SAS Institute, Cary, NC), using mixed linear regression of log-transformed ORs. Meta-analyses were conducted to evaluate the summary OR of combined studies using MIX v.1.7 software (Leon Bax, Kanagawa, Japan) (26,27). Using a fixed effects model, heterogeneity of the pooled estimate was tested with the Cochrane's Q statistic, and P<0.20 was considered to indicate heterogeneity. The inconsistency index (I2) was used to measure the degree of heterogeneity; the low, moderate, and high degrees of heterogeneity correlated with I2 values of 25, 50, and 75% , respectively (28). In settings in which there is evidence of minimal heterogeneity, we report the results of the fixed effects models. Unless otherwise stated, all other summary ORs are reported from random effects models. Heterogeneity of results can reflect differences in study design or effect modification across strata of outcomes. Heterogeneity might be resolved by performing series of predetermined stratified analyses, thereby identifying strata with homogenous results with more reliable estimates of the effects of exposure within those strata than the estimates for effects in all of the studies combined. Evidence of potential dissemination bias or other small study effects was examined by plotting the log of the OR (effect size) vs. the standard error of the log OR (inversely proportional to study size); patterns that skew away from a funnel shape indicate possible dissemination bias.
RESULTS
Study characteristics
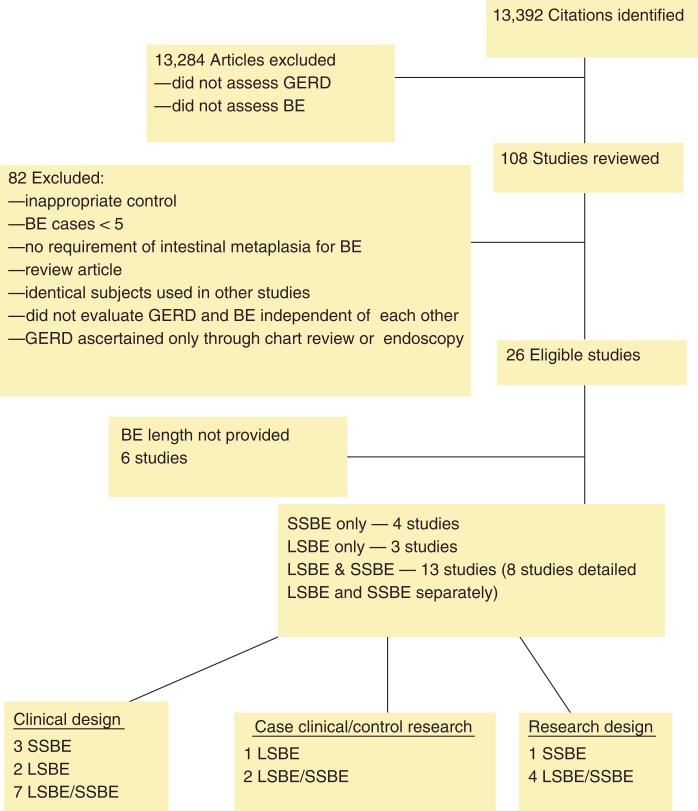
Our systematic review identified 13,392 citations; titles and abstracts were manually evaluated for relevancy (Figure 1). Of these, we reviewed 108 journal articles and abstracts and found 26 studies eligible for inclusion (Table 1). Of these 26 studies, 14 identified cases of BE and controls based on upper endoscopies performed for clinical indication (“clinical design”) (25,29–41) and 6 studies sampled the general population (23,24), colorectal screening patients (22,42,43), or family members of patients with BE (44) to identify cases and controls (“research design”). The remaining six studies identified cases of BE among patients undergoing upper endoscopy for clinical indication, and sampled a population without known BE as the control group (case-clinical/control-research)(45–50).
Figure 1.
Flow chart of study eligibility and classification. BE, Barrett's esophagus; GERD, gastroesophageal reflux disease; LSBE, long-segment Barrett's esophagus; SSBE, short-segment Barrett's esophagus.
Overall results
The summary OR for the association of GERD with BE from all eligible studies was 2.90 (95% confidence interval (CI), 1.86–4.54), but as expected, these results were very heterogeneous (P<0.0001; I2 =89%).Overall, there was a nonsignificant secular trend toward decreasing OR (β-estimate for natural log of OR = −0.019peryear, s.d. = 0.065, P = 0.77).In 12 studies, GERD was defined as present if symptoms occurred at least weekly (22,31–32,34,36,42–47,49). The summary OR for these studies was 4.45 (95% CI, 2.13–9.29) and the results were heterogeneous (P<0.0001; I2 =93%). In 14 studies, the frequency of GERD symptoms was not reported (23–25,29,30,33,35,37–41,48,50).
Stratifying by continent of origin did not resolve the heterogeneity, but showed weaker associations in Asia than in Europe. The summary OR for Asian studies was 1.62 (95% CI, 0.813–3.24; P=0.14; I2=49%)(25,36,41). European-based studies had a summary OR of 3.00 (95% CI, 0.901–9.99; P<0.0001; I2=93%)(23,24,30,34,37,39,45,49). US-based studies had a summary OR of 2.44 (95% CI, 1.42–4.23; P<0.0001; I2=87%) (22,29,31,33,35,40,42–44,46–48).
Length of BE
Six studies did not report the length of BE (Figure 1) (38,40,44,46,48,50). In all, 3 studies only reported the association with LSBE (29,39,49), 4 only reported the association with short segment (25,30,35,42), 5 included patients with both long and short segments but only reported overall results (36–37,41,45,47), and 8 reported the association with short and long segments separately (22–24,31–34,43). Studies stratified BE in short and long segmentsby 2cm (23,24,31,39,49) or 3cm (22,25,29–30,32–37,41,42,43,45,47). Of the 15 studies that provided data specified by length of BE, the summary OR was 2.06 (95% CI, 1.46–2.89; P =0.0092; I2=46%) for any length of BE, still showing heterogeneity (22–25,29–35,39,42,43,49).Stratifying results of these 15 studies by the length of BE resulted in a weaker association between GERD and SSBE (12 studies, OR, 1.59; 95% CI, 1.07–2.38; P =0.04; I2 =46%)(22–25,30–35,42–43) than with LSBE(11studies,OR,4.16; 95% CI, 2.43–7.12; P=0.19; I2=27%)(22–24,29,31–34,39,43,49). Although the results for LSBE were fairly homogeneous, the results for SSBE still showed moderate heterogeneity.
Quality of study design
We further analyzed the studies by stratifying by study design. Studies using the clinical design showed substantial, but heterogeneous, associations with SSBE (OR, 2.38; 95% CI, 1.21–4.70; P=0.02; I2=62%) (25,30–35), and stronger and homogeneous association with LSBE (fixed effects OR, 2.96; 95% CI, 1.69–5.19; P=0.25; I2=25%)(29,31–34,39).There was only one study of case-clinical/control-research design with outcomes specified by BE length (49). In that study, there was a strong association between GERD and LSBE (OR, 10.7; 95% CI, 3.3–33.4). SSBE was not evaluated in that study.
We a priori had defined the “research design” as the highest-quality sampling design because of minimization of selection and misclassification biases. The five studies that stratified by BE length and used the “research design” included the ones performed by Ronkainen et al. (24),Rex et al. (43),Ward et al. (22), Esquivel et al. (42), and Zagari et al. (23). All of the studies used a cross-sectional study design. Rex et al. (43),Ward et al. (22),and Esquivel et al. (42) were US studies that invited patients undergoing colonoscopy for clinical indications (typically colon cancer screening) to undergo a research upper endoscopy. Ronkainen et al. (24) invited residents of two Swedish municipalities by mail to undergo a research of upper endoscopy. Zagari et al. (23) used a similar design in two Italian villages. In total, among these five studies, 3,510 subjects underwent research upper endoscopies, identifying LSBE in 23 subjects and SSBE in 132 subjects. In three of the five studies, GERD symptoms were defined as occurring at least one time per week (22,42,43). In the other two studies, GERD symptoms were defined as occurring with any frequency (23,24).
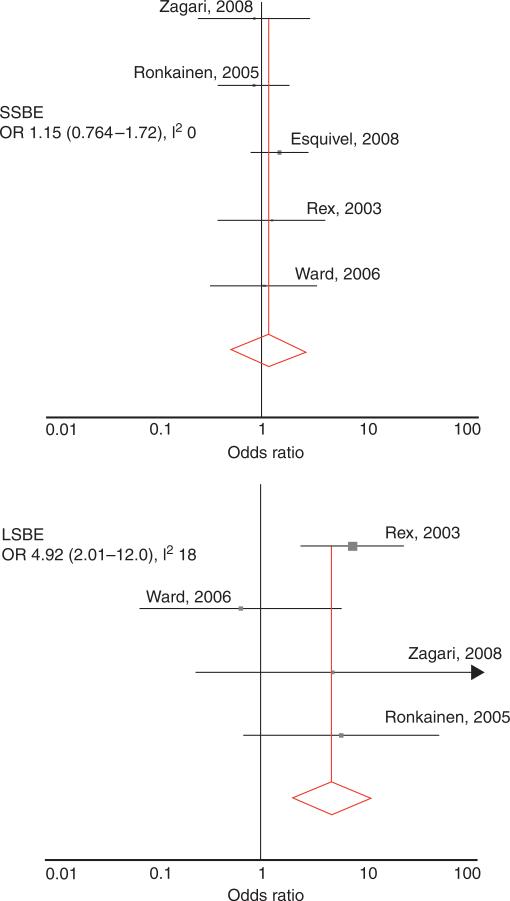
The results from these studies stratified by length of BE were homogenous. For SSBE, the fixed effects summary OR was 1.15 (95%CI, 0.763–1.73; P=0.84; I2=0%),showing no association of SSBE with GERD symptoms (Figure 2). For LSBE, the fixed effects summary OR was 4.92 (95% CI, 2.01–12.0) and the results were also homogenous (P=0.30; I2=19%; Figure 2).
Figure 2.
Forest plots of studies with research design. The top panel represents results from studies for associations of gastroesophageal reflux disease (GERD) symptoms with short-segment Barrett's esophagus (SSBE), showing no association. The bottom panel represents the association with long-segment Barrett's esophagus (LSBE), showing a fivefold increased odds of LSBE with GERD symptoms. Note the scale is logarithmic. I2, inconsistency index; OR, odds ratio.
Funnel plot asymmetry
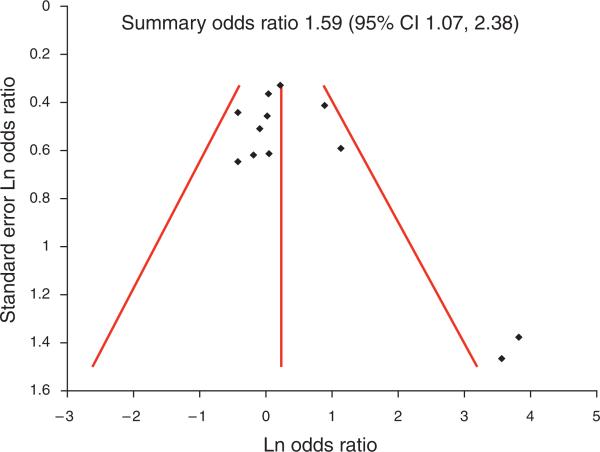
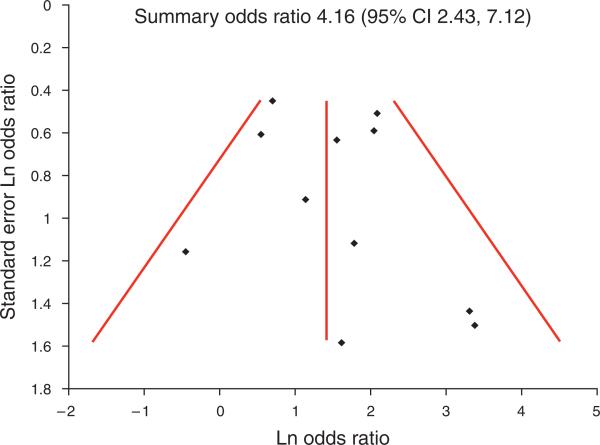
A funnel plot including studies of all quality for the association of GERD with SSBE showed a number of small studies with strong associations that were not balanced by small studies showing no or inverse associations (Figure 3), suggesting possible bias against disseminating or performing small negative studies. A funnel plot for the association with LSBE did not show such evidence (Figure 4).
Figure 3.
Funnel plot for relation with short-segment Barrett's esophagus (SSBE). Two small, but strongly positive studies for the relation with SSBE, were identified (bottom right quadrant of plot), without balancing small studies showing no or inverse associations (none in bottom left quadrant). This suggests that such small studies may have been completed but were either never submitted for publication or rejected in the review process. CI, confidence interval.
Figure 4.
Funnel plot for relation with long-segment Barrett's esophagus (LSBE). Studies were balanced across the summary odds ratio and size of the studies. Therefore, there was no evidence for dissemination bias among studies examining the relation with LSBE. CI, confidence interval.
DISCUSSION
We performed a systematic review of the literature and a series of meta-analyses to estimate the relation between GERD symptoms and BE. As we had expected, we found substantial heterogeneity in the study results overall. We explored multiple sources for this heterogeneity, and as we had hypothesized, we found that it was because of study sampling design and the length of BE. In the highest-quality study design, there was no association between GERD symptoms and SSBE, but an approximately fivefold increased odds of LSBE in individuals with GERD symptoms.
The studies of poorer-quality sampling design found moderate twofold increased odds of SSBE with GERD symptoms, but with substantial heterogeneity in the study results. We believe that the results of the higher-quality “research” sampling design studies are a more reliable estimate of the relation. The “clinical” sampling design included only cases and controls undergoing endoscopy for some clinical indication. Most of the BE cases were undergoing endoscopy for GERD symptoms. Th e controls were undergoing endoscopy for some other clinical indication (for instance, Helicobacter pylori- associated peptic ulcer disease), and that indication may actually be associated with a decreased risk of BE, thereby biasing the study results toward finding that GERD is associated with BE. Furthermore, endoscopists performing a clinical upper endoscopy for an indication of GERD may be more likely to notice or biopsy SSBE than when performing the endoscopy for some other indication.
In the funnel plots, we found potential evidence of bias against dissemination of small studies showing no association between GERD and SSBE. Multiple reasons could explain this pattern, including bias among reviewers or editors against publishing such studies, bias among investigators against submitting such findings for publication, and importantly the logistical and financial barriers against performing even small studies of the “research” design.
Why would GERD symptoms be associated with LSBE but not with SSBE? Pathophysiologically, both LSBE and SSBE might be due to reflux of gastroduodenal contents. As SSBE is in the distal 2 to 3 cm of the esophagus (typically confined at, or below, the level of the lower esophageal sphincter, but above the esophagogastric junction), physiologic amounts of reflux in this region are less likely to be sensed as GERD symptoms than more proximal reflux. Patients with SSBE may not have an abnormal amount of reflux, but their distal esophageal epithelium may still pathologically respond to the physiologic amount of reflux, resulting in metaplasia.
We found evidence to suggest that the relation between GERD and BE may be weaker in Asian populations than in American or European populations. However, this could merely reflect a higher proportion of SSBE vs. LSBE in Asia. Other unknown genetic or behavioral factors might also modify the effect of GERD on the risk of BE. For instance, although African Americans have a similar prevalence of GERD symptoms as European Americans, they are less likely to develop complications of GERD (51).
Current screening efforts for EAC are focused on patients with GERD symptoms. However, our study shows that such efforts largely miss patients with SSBE. A recent meta-analysis found similar risk of progression to cancer in SSBE and LSBE (52). If SSBE does warrant diagnosis, then screening programs would need to be altered to reliably identify these patients.
The limitations of our analysis extend from the various forms of bias within each individual study, primarily because of confounders. Although some of the studies adjusted for potential confounders (such as age, gender, and obesity), most did not. Our summary ORs are primarily based on crude ORs from the individual studies, and hence do not reflect adjustment for potential confounders. In addition, given the available data, we were unable to test for a dose-response relation in terms of frequency, duration, or severity of GERD. However, we were able to show a dose-response relation in terms of length of BE. Finally, although there were a large number of eligible studies, there were small numbers of cases of LSBE from studies of “research” design, resulting in broad confidence intervals for that stratum.
In summary, our results show that GERD symptoms are significantly associated with LSBE, but there is no association between GERD symptoms and SSBE. These findings have broad implications for screening programs for esophageal adenocarcinoma.
ACKNOWLEDGMENTS
We thank Julian Abrams and Rocco Maurizio Zagari for graciously providing data in a format not presented in their published studies. We also thank Sandra Hoogerwerf, John Kao, Tommomi Takeuchi, and Andrea Todisco who assisted with translation of foreign language abstracts and articles and Brenda Vibbart who provided administrative assistance in obtaining abstracts and articles. We thank Jack Colford and Vincent Yau for technical guidance.
Financial support: J.H.R. is the Damon Runyon-Gordon Family Clinical Investigator funded (in part) by the Damon Runyon Cancer Research Foundation (CI-36-07), and by the National Institutes of Health (K23 DK079291).
Footnotes
CONFLICT OF INTEREST Guarantor of the article: Joel H. Rubenstein, MD, MSc, FACG.
Potential competing interests: None.
REFERENCES
- 1.Bytzer P, Christensen PB, Damkier P, et al. Adenocarcinoma of the esophagus and Barrett's esophagus: a population-based study. Am J Gastroenterol. 1999;94:86. doi: 10.1111/j.1572-0241.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Welch HG. The role of over diagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 3.Lagergren J. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 4.Chak A, Faulx A, Eng C, et al. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer. 2006;107:2160. doi: 10.1002/cncr.22245. [DOI] [PubMed] [Google Scholar]
- 5.Cameron AJ, Romero Y. Symptomatic gastro-oesophageal reflux as a risk factor for oesophageal adenocarcinoma. Gut. 2000;46:754. doi: 10.1136/gut.46.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrow DC, Vaughan TL, Sweeny C, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–8. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 7.Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer. 2003;98:940–8. doi: 10.1002/cncr.11568. [DOI] [PubMed] [Google Scholar]
- 8.Whiteman DC, Sadeghi C, Pamdeua N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 9.Anderson LA, Watson RG, Murphy SJ, et al. Risk factors for Barrett's oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–94. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reavis KM, Morris CD, Gopal DB, et al. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Ann Sur. 2004;239:849–56. doi: 10.1097/01.sla.0000128303.05898.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Spechler SJ. Barrett's esophagus. N Engl J Med. 2002;346:836. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 13.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 14.Prateek S, Kenneth M, John D, et al. A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago Workshop1. Gastroenterology. 2004;127:310. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Allison PR, Johnston AS. The oesophagus lined with gastric mucous membrane. Thorax. 1953;8:87–101. doi: 10.1136/thx.8.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett NR. Chronic peptic ulcer of the esophagus and esophagitis. Br J Surg. 1950;38:157. doi: 10.1002/bjs.18003815005. [DOI] [PubMed] [Google Scholar]
- 17.Cannon RA, Cox KL, Sanders KD. Barrett's epithelium resulting from chronic gastroesophageal reflux in children. Clin Res. 1984;32:A97. [Google Scholar]
- 18.Eisen G, Sandler G, Murray S, et al. The relationship between gastroesophageal disease and its complications with Barrett's esophagus. Am J Gastroenterol. 1997;92:27–31. [PubMed] [Google Scholar]
- 19.Gerson LB, Edson R, Lavori PW, et al. Use of a simple symptom questionnaire to predict Barrett's esophagus in patients with symptoms of gastroesphageal reflux. Am J Gastroenterol. 2001;96:2005–12. doi: 10.1111/j.1572-0241.2001.03933.x. [DOI] [PubMed] [Google Scholar]
- 20.Mann N, Tsaj M, Nair P. Barrett's esophagus in patients with symptomatic reflux esophagitis. Am J Gastroenterol. 1989;84:1494–6. [PubMed] [Google Scholar]
- 21.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology. 2002;123:4617. doi: 10.1053/gast.2002.34748. [DOI] [PubMed] [Google Scholar]
- 22.Ward EM, Wolfsen HC, Achem SR, et al. Barrett's esophagus is common in older men women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol. 2006;101:12–7. doi: 10.1111/j.1572-0241.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 23.Zagari RM, Fucci L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–9. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 24.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 25.Kim JY, Kim YS, Jung MK, et al. Prevalence of Barrett's esophagus in Korea. J Gastroenterol Hepatol. 2005;20:633–6. doi: 10.1111/j.1440-1746.2005.03749.x. [DOI] [PubMed] [Google Scholar]
- 26.Bax L, Yu LM, Ikeda N, et al. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bax L, Yu LM, Ikeda N, et al. MIX: comprehensive free software for meta-analysis of causal research data—version 1.7. 2008 doi: 10.1186/1471-2288-6-50. http://www.mix-for-meta-analysis.info. [DOI] [PMC free article] [PubMed]
- 28.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spechler SJ, Zeroogian JM, Antonioli DA, et al. Prevalence of metaplasia at the gastroesophageal junction. Lancet. 1994;344:1533–6. doi: 10.1016/s0140-6736(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 30.Castro ML, Fachal C, Pineda JR, et al. Intestinal metaplasia at the esophagogastric junction. Prevalence and association in patients undergoing endoscopy. J Gastroenterol Hepatol. 2002;25:487–92. [PubMed] [Google Scholar]
- 31.Chalasani N, Wo JM, Hunter JG, et al. Significance of intestinal metaplasia in different areas of esophagus including esophagogastric junction. Dig Dis Sci. 1997;42:603–7. doi: 10.1023/a:1018863529777. [DOI] [PubMed] [Google Scholar]
- 32.Csendes A, Smok G, Burdiles P, et al. Prevalence of Barrett's esophagus by endoscopy and histologic studies: a prospective evaluation of 306 control subjects and 376 patients with symptoms of gastroesophageal reflux. Dis Esophagus. 2000;13:5–11. doi: 10.1046/j.1442-2050.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 33.Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology. 1999;116:277–85. doi: 10.1016/s0016-5085(99)70123-x. [DOI] [PubMed] [Google Scholar]
- 34.Johansson J, Hakannson HO, Melblom L, et al. Prevalence of precancerous and other metaplasia in the distal oesophagus and gastro-oesophageal junction. Scand J Gastroenterol. 2005;40:893–902. doi: 10.1080/00365520510015692. [DOI] [PubMed] [Google Scholar]
- 35.Pereira AD, Suspiro A, Chaves P, et al. Short segments of Barrett's epithelium and intestinal metaplasia in normal appearing oesophagogastric junctions: the same or two different entities? Gut. 1998;42:659–62. doi: 10.1136/gut.42.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajendra S, Kutty K, Karim N. Ethnic differences in the prevalence of endoscopic esophagitis and Barrett's esophagus: the long and short of it all. Dig Dis Sci. 2004;49:237–42. doi: 10.1023/b:ddas.0000017444.30792.94. [DOI] [PubMed] [Google Scholar]
- 37.Toruner M, Soykan I, Ensari A, et al. Barrett's esophagus: prevalence and its relationship with dyspeptic symptoms. J Gastroenterol Hepatol. 2004;19:535–40. doi: 10.1111/j.1440-1746.2003.03342.x. [DOI] [PubMed] [Google Scholar]
- 38.Veldhuyzen Van Zante JO, Thomson ABR, Barkun AN, et al. The prevalence of Barrett's oesophagus in a cohort of 1040 Canadian primary care patients with uninvestigated dyspepsia undergoing prompt endoscopy. Aliment Pharmacol Ther. 2006;23:595–9. doi: 10.1111/j.1365-2036.2006.02813.x. [DOI] [PubMed] [Google Scholar]
- 39.Voutilainen M, Sipponen P, Mecklin JP, et al. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion. 2000;61:6–13. doi: 10.1159/000007730. [DOI] [PubMed] [Google Scholar]
- 40.Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92:2007–11. [PubMed] [Google Scholar]
- 41.Tseng PH, Lee YC, Chiu M, et al. Prevalence and clinical characteristics of Barrett's esophagus in a Chinese general population. J Clin Gastroenterol. 2008;42:1074–9. doi: 10.1097/MCG.0b013e31809e7126. [DOI] [PubMed] [Google Scholar]
- 42.Esquivel RF, Boolchand V, Kumar N, et al. The prevalence of Barrett's esophagus in veteran patients with and without GERD symptoms undergoing outpatient colonoscopy. Gastrointest Endosc. 2008;67:AB172. [Google Scholar]
- 43.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–7. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 44.Romero Y, Cameron A, McDonnell SK, et al. Barrett's esophagus: prevalence in relatives with and without frequent symptoms. Gastroenterology. 2001;120:A410–1. [Google Scholar]
- 45.Conio M, Filiberti R, Blanchi S, et al. Risk factors for Barrett's esophagus: a case-control study. Int J Cancer. 2002;97:225–9. doi: 10.1002/ijc.1583. [DOI] [PubMed] [Google Scholar]
- 46.Corley DA, Levin TR, Habel LA, et al. Barrett's esophagus and medications that relax the lower esophageal sphincter. Am J Gastroenterol. 2006;101:937–44. doi: 10.1111/j.1572-0241.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 47.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson BC, Fuchs CS. A prospective study of body mass index and Barrett's esophagus. Gastroenterology. 2008;134:A437–8. [Google Scholar]
- 49.Johansson J, Hakansson HO, Mellblom L, et al. Risk factors for Barrett's oesophagus: a population-based approach. Scand J Gastroenterol. 2007;42:148–56. doi: 10.1080/00365520600881037. [DOI] [PubMed] [Google Scholar]
- 50.Smith KJ, Obrien SM, Smither BM, et al. Interactions among smoking, obesity, and symptoms of acid reflux in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:2481–6. doi: 10.1158/1055-9965.EPI-05-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Serag H, Petersen N, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126:1692–9. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 52.Yousseff F, Cardwell C, Cantwell M. The incidence of esophageal cancer and high grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237–49. doi: 10.1093/aje/kwn121. [DOI] [PubMed] [Google Scholar]