Abstract
Activation of spinal microglia and consequent release of pro-inflammatory mediators facilitate pain. Under certain conditions, responses of activated microglia can become enhanced. Enhanced microglial production of pro-inflammatory products may result from priming (sensitization), similar to macrophage priming. We hypothesized that if spinal microglia were primed by an initial inflammatory challenge, subsequent challenges may create enhanced pain. Here, we used a "two-hit" paradigm using two successive challenges, which affect overlapping populations of spinal microglia, presented two weeks apart. Mechanical allodynia and/or activation of spinal glia were assessed. Initially, laparotomy preceded systemic lipopolysaccharide (LPS). Prior laparotomy caused prolonged microglial (not astrocyte) activation plus enhanced LPS-induced allodynia. In this “two-hit” paradigm, minocycline, a microglial activation inhibitor, significantly reduced later exaggerated pain induced by prior surgery when minocycline was administered intrathecally for 5 days starting either at the time of surgery or 5 days before LPS administration. To test generality of the priming effect, subcutaneous formalin preceded intrathecal HIV-1 gp120, which activates spinal microglia and causes robust allodynia. Prior formalin enhanced intrathecal gp120-induced allodynia, suggesting that microglial priming is not limited to laparotomy and again supporting a spinal site of action. Therefore, spinal microglial priming may increase vulnerability to pain enhancement.
Keywords: laparotomy, lipopolysaccharide, microglia, spinal cord, mechanical allodynia, subcutaneous formalin, gp120
Introduction
Within the past two decades, there has been increasing recognition of the importance of glial activation in the creation and maintenance of diverse enhanced pain states46. One important aspect of glial functioning that has not yet been explored in the context of pain is the effect of sensitized, or "primed", microglia. Evidence is accruing from research outside of the field of pain that previous microglial activation can dramatically alter their response to new challenges. Microglia can reach a primed state via a variety of challenges, including trauma/inflammation/infection of peripheral or central nervous system (CNS) tissues.
While in a primed state, microglia can now over-respond to new challenges, stronger and longer than before40. For example, in response to ongoing CNS infection (scrapie), microglia undergo persistent changes consistent with activation26. Despite the maintained appearance of microglial activation with persistent infection, levels of proinflammatory cytokines (interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-alpha (TNF-α)) in the brain and in circulation of animals with persistent infection do not differ from controls9,11,12,45. However, in response to a new challenge such as peripheral or intracerebral injection of lipopolysaccharide (LPS; cell walls of gram negative bacteria), persistently infected animals show enhanced brain IL-1β compared to controls9,12.
Potentiated microglial neuroinflammatory responses are not specific to persistent CNS infection, having also been noted in aged animals13,17 and in response to prior tail-shock stress or stress hormones13,14. Such observations raise the possibility that were microglia to become primed in pain modulatory regions, that these primed microglia may be capable of creating exaggerated pain responses to a subsequent challenge as a consequence of an exacerbated neuroinflammatory response.
There are numerous reports in the experimental literature demonstrating increased sensitivity to painful stimuli when preceded by a conditioning stimulus. Examples of such conditioning stimuli include psychological stress 28,32, subcutaneous inflammation 5,27, nerve injury 24,30 and induction of wind-up within the spinal cord by repeated electrical stimulation of nociceptive afferents20. One area of study that has garnered much interest over the last decade is the effect of immune challenges experienced in the neonatal period on later pain processing and the development of chronic pain disorders. These reports provide compelling evidence that permanent changes in nociceptive processing may occur as a result of neonatal insults including bladder inflammation41, intraplantar injection of complete Freund’s adjuvant 21 and systemic LPS injection 3. From these studies it is unknown whether microglial responses to the second stimuli were augmented. That being said, adult rats treated with LPS as neonates displayed elevated expression of spinal cyclooxygenase-2 (COX-2), an enzyme responsible for prostanoid production that can be induced in microglia3.
The present studies provide an initial exploration of whether prior peripheral inflammatory events may set the stage for exaggerated pain responses to subsequent challenges. Additionally, if such enhanced pain responses were observed, whether spinal microglia may contribute to the effect. To explore this, a "two-hit" paradigm was established with two successive challenges delivered 2 weeks apart. In the first series of studies, the first “hit” modeled routine exploratory abdominal surgery (laparotomy), based on prior evidence that surgery can increase the risk of developing persistent pain4,10,39. Intraperitoneal LPS was chosen as the second “hit” as this activates spinal glia as well as affecting overlapping spinal cord circuitries33,47 as that of the laparotomy18,37. Generality was explored using subcutaneous formalin as the first “hit’ followed 2 wk later by intrathecal HIV-1 gp120. Together, the results of this series of studies provide support that prior spinal microglial activation may contribute to future enhanced pain responses.
Materials and methods
Animals
Pathogen-free adult male Sprague-Dawley rats (325–400 g; Harlan Labs, Madison, WI) were used in all experiments. Before experimental manipulations, rats were allowed one week to acclimate to the University of Colorado animal care facility. Rats were housed two per cage in temperature (23+/−3°C) and light (12:12 light: dark; lights on at 0700 hours) controlled rooms with standard rodent chow and water available ad libitum. All procedures were done during lights on. The Institutional Animal Care and Use Committee of the University of Colorado at Boulder approved all procedures. The care and use of the animals conformed to guidelines of the Office of Laboratory Animal Welfare and International Association for the Study of Pain.
Intraperitoneal (i.p.) LPS
Lyophilized lipopolysaccharide (LPS, 25 mg, Escherichia Coli, serotype 0111: B4, Sigma Lot No. 072k4096) was reconstituted with 0.9% endotoxin-free saline (25 ml) to derive a 1 mg/ml stock solution that was then aliquoted and stored at −20°C. Frozen aliquots were thawed at the time of experimentation and diluted with 0.9% endotoxin-free saline (Abbott Laboratories, North Chicago, IL) yielding a final concentration of 20 µg/ml. Animals were dosed with 20 µg/kg LPS (i.p.) or equal volume 0.9% endotoxin-free saline.
Indwelling intrathecal (i.t.) catheters and drug administration
Lumbosacral i.t. catheters were constructed and implanted by lumbar approach as described previously in detail25,34. Briefly, 3 cm of a 28 cm length of polyethylene tubing was inserted into the spinal subarachnoid space between the fifth and sixth lumbar vertebrae using isoflurane anesthesia (Halocarbon Laboratories, Rivers Edge, NJ). Catheters were anchored to surrounding tissue using 3.0 sterile silk sutures. Catheters were then flushed with sterile pyrogen-free saline, checked for possible occlusion, and sealed. The exterior end of the catheters was threaded beneath the skin on the dorsal surface and exited via a small incision on the nape of the neck. All microinjections were performed as described previously25,34. The sealed tips of the catheters were cut and a sterile, 30-gauge needle, hub removed, was inserted. The needle was attached to a 35 cm length of PE-10 tubing attached at the opposite end to a 50 µl glass Hamilton micro-syringe. A new microinjector was used for each animal and the syringes were thoroughly flushed with sterile water before each use.
Laparotomy
Laparotomy and sham surgeries were performed using aseptic procedures under isoflurane anesthesia (Halocarbon Laboratories, River Edge, NJ). The abdominal region was shaved and thoroughly cleaned with 70% EtOH and Exidine-2 surgical scrub. One of the co-authors (Dr. Thomas J. Martin of Wake Forest University School of Medicine) developed the laparotomy protocol used33. Approximately 0.5 cm below the left, caudal-most rib, a 3 cm diagonal incision was made, penetrating the peritoneal cavity. Wearing sterile latex gloves, the surgeon inserted their index finger up to the second knuckle into the opening and vigorously manipulated the viscera and musculature. Approximately 10 cm of the intestine were then exteriorized and vigorously rubbed between the surgeon’s thumb and index finger for 30 seconds. The intestines were then placed back into the peritoneal cavity. Sterile chromic gut sutures (cuticular 4-0, chromic gut, 27”, cutting FS-2; Ethicon, Comerville, NJ) were used to suture the peritoneal lining and abdominal muscle in two layers. The skin was closed with surgical staples. To prevent infection, the wound was dressed with Polysporin (Pfizer, Morris Plains, NJ) and 0.25 ml TwinPen (150,000 U/ml penicillin G procaine and penicillin G benzathine; AgrilLabs Ltd., St. Joseph, MO) was administered intramuscularly. Sham-operated animals were anesthetized, shaved and injected with TwinPen and remained on isoflurane for the same amount of time as operated rats.
von Frey test for mechanical allodynia
All behavioral training and testing were performed blinded with respect to group assignment. Lowering of response thresholds to light tactile stimuli (mechanical allodynia) was assessed within the sciatic and saphenous innervation areas of the hindpaws using the von Frey test8 as previously described in detail6,34,36. Rats were habituated to the testing apparatus for four consecutive days before testing. A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL) was applied randomly to the left and right hind paws to determine the stimulus intensity threshold stiffness required to elicit a paw withdrawal response. Log stiffness of the hairs is determined by log10 (milligrams×10). The range of monofilaments used in these experiments (0.407–15.136 gm) produces a logarithmically graded slope when interpolating a 50% response threshold of stimulus intensity (expressed as log10 (milligrams×10)7). The behavioral responses were used to calculate the 50% paw withdrawal threshold (absolute threshold), by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method19,44, as described in detail previously35,36. This fitting method allows parametric statistical analyses19,44. No differences between left and right hindpaw withdrawal thresholds were noted in any experiment. Therefore, data obtained from the left and right hindpaws were averaged.
Immunohistochemistry of spinal cord sections
Immunoreactivity for OX-42 (which targets CD11b/c, a component of complement receptor type 3, a marker of microglial activation) and glial fibrillary acidic protein (GFAP, a marker of astrocyte activation) was conducted using the avidin-biotin-horseradish peroxidase (ABC) reaction. On day one, sections were treated with 0.3% hydrogen peroxide/0.1% sodium azide for 30 min at room temperature to suppress endogenous peroxidase activity. The sections were incubated at 4°C overnight in either mouse anti-rat OX-42 (1:100; BD PharMingen, San Jose, CA) or mouse anti-pig GFAP (1:100; MP Biomedicals, Aurora, OH; cross-reactive to rat). On day two, sections were incubated at room temperature for two hours with biotinylated goat anti-mouse IgG antibody (1:200; Jackson ImmunoResearch, West Grove, PA). Finally, sections were reacted using the avidin-biotin complex procedure (ABC, Vector Elite kit; 1:100 in PBS-Triton; 2 hours at room temp; Vector Laboratories, Burlingame, CA) and 3', 3-diaminobenzidine (DAB; Sigma-Aldrich). Glucose oxidase (Sigma-Aldrich; type V-s; 0.02%) and β-D-glucose (0.1%) were used to generate hydrogen peroxide. Nickelous ammonium sulfate was added to the DAB solution (0.025% w/v) to intensify the reaction product. Slides were coverslipped with Permount (Fisher Scientific, Fairlawn, NJ). To ensure comparable analysis, all slides within an experiment were reacted together.
Image analysis
Slides were viewed with an Olympus BX-61 microscope (Olympus America, Melville, NY) using the bright-field setting at 10X magnification. Semiquantitative analyses were performed on images collected using an Olympus MagnaFire SP digital camera running Olympus Microsuite software. Images were opened in NIH Image (version 1.60), converted into grey scale and rescaled from inches to pixels. Signal pixels were defined as having grey values falling three standard deviations above the mean grey value for a cell-poor region near the region of interest (ROI)2. The integrated densitometric value was calculated by multiplying the average grey value for signal pixels within the ROI by the total number of pixels within the ROI. An average of four measurements were made for each ROI, alternating between the right and left spinal hemispheres. Measurements were then averaged to obtain a single integrated density value per rat, per region. All samples were measured blinded as to group assignment.
Data Analysis
All data are presented as mean +/− SEM. All statistical comparisons were computed using Statview 5.0.1 for the Macintosh and consisted of repeated measures for analysis of behavioral time-course studies, and one-way and two-way ANOVA for analysis of integrated densitometry. In the latter, each spinal cord region was analyzed separately. All analyses were followed by Fisher’s protected least significant difference post-hoc comparisons. Paired t-tests were used to compare pre-surgery to post-surgery response thresholds to calibrated mechanical stimuli. Threshold for statistical significance was set at α = 0.05.
Experimental designs
Experiment 1A: Effect of prior laparotomy on LPS-induced mechanical allodynia
In order to assess whether pain arising from a second inflammatory stimulus would be exacerbated by a prior challenge, we used laparotomy of the upper abdomen with gut manipulation as the first “hit” and intraperitoneal injection of LPS as the second “hit”. After assessing pre-surgical threshold responses on the von Frey test, animals underwent either laparotomy or sham surgery as described above. Two wk after surgery, von Frey behavioral measurements were conducted followed 20 min later with either LPS or saline (20 µg/kg, i.p., n = 8/group). Behavioral assessments for mechanical allodynia were conducted 0.5, 1, 1.5, 2, 4, 6, 8, 24 and 168 hours (7 days) after i.p. LPS.
Experiment 1B: Effect of prior laparotomy on spinal glial activation
Based on the results from previous studies demonstrating that laparotomy with gut manipulation led to increased c-fos expression localized to the lumbar and sacral spinal cord29, glial activation in these regions and in a cervical control region were assessed in the present study. The cervical cord was analyzed to ensure no global glial activation had occurred, while the lumbar and sacral cord segments were analyzed to assess the area that innervates the viscera affected by the laparotomy with gut manipulation. In addition, the lumbar region receives input from the area of the hindpaws tested in Experiment 1A.
Two wk after laparotomy or sham surgery (n = 4−5/group), rats were anesthetized by i.p. injection of sodium pentobarbital and transcardially perfused with ice cold heparinized 0.9% saline followed by 4% paraformaldehyde for tissue fixation. The lumbosacral and cervical spinal cord were dissected from the vertebral column and post-fixed for 24 hours in 4% paraformaldehyde. The tissue was then transferred into 30% sucrose containing 0.1% sodium azide and stored at 4°C until sectioned. Spinal cord was sectioned at 30 µm in a −20°C cryostat and mounted onto gelatin subbed glass slides (Fisherbrand Superfrost Slides; Fisher Scientific, Fairlawn, NJ). Five mm sections of cervical (C), lumbar (L3, L4-L5) and lumbosacral (L6-S2) spinal cord were collected. Tissue was processed for OX-42 and GFAP in separate sections and between group comparisons were made separately at each spinal segment. For quantification of both OX-42 and GFAP, regions of interest included the whole dorsal horn and more specifically lamina I and II in the lumbar and sacral regions.
Experiment 1C: Effect of prior laparotomy on expression of OX-42 and GFAP in lumbosacral spinal cord in response to systemic LPS challenge
In order to determine if the glial activation to the second “hit” is influenced by prior laparotomy, LPS or equivolume endotoxin free saline was injected two wk after laparotomy or sham surgery (n = 6/group). Six hours after injection animals were sacrificed and 5 mm segments of lumbar (L3, L4-L5) and lumbosacral (L6-S2) spinal cord were collected. Tissue was processed for immunohistochemistry for OX-42 and GFAP as described above. For quantification, regions of interest included the dorsal horn in L3 through S2.
Experiment 2: Effect of minocycline, a microglial activation inhibitor, on LPS induced mechanical allodynia, when administered for 5 days after laparotomy or 5 days before LPS administration
The prior experiments revealed that the allodynic response to systemic LPS was sensitized by prior laparotomy and that upregulation of microglial activation markers appeared to be associated with this sensitized state. Thus the question arises whether blocking microglial activation around the time of the initial surgery (laparotomy) might prevent the pain sensitization documented above. To test this, repeated intrathecal dosing with the microglial activation inhibitor, minocycline, was used to block microglial activation for five days following laparotomy to define whether this procedure may prove effective in preventing pain sensitization 2 wk later. In addition, in separate groups of rats, minocycline was administered for five days before the LPS administration to assess whether microglial activation plays a role in the potentiated “two-hit” response. Rats underwent combined laparotomy surgery and insertion of indwelling intrathecal catheters. The animals then received either minocycline (90 µg in 6 µl sterile water) or equivolume sterile water, followed by an 8 µl saline flush (n=8–9/group). Over the next four days, the rats received once or twice-daily injections of minocycline or water (6 µl minocycline/ water and 30 µl saline flush). The high dose regimen is in accordance with prior studies31. Each injection was delivered over a four min period to awake and lightly restrained rats. Minocycline (Sigma, St Louis, MO) was made fresh every 24 hours to a concentration 15 mg/ml in sterile water. In a separate group of rats, 90 µg minocycline or water was intrathecally administered (6 µl minocycline/water and 30 µl saline flush) once daily for four days before and one hour before LPS administration. Behavioral assessments for mechanical allodynia were conducted 0.5, 1, 1.5, 2, 4, 6, 8 and 24 hours after i.p. LPS.
Experiment 3: Effect of prior subcutaneous formalin on gp120-induced mechanical allodynia
This last study tested whether the effects observed in Experiment 1would generalize to a different “two-hit” paradigm, versus being specific to laparotomy followed by systemic LPS. Here, a different peripheral first “hit” was followed by a spinally-directed second “hit”. Previous studies have shown that microglia within the dorsal spinal cord are activated by subcutaneous injection of formalin into the plantar or dorsal aspect of the hindpaw for several weeks16. Based on these findings, subcutaneous formalin was chosen as the first “hit”. The second “hit” was intrathecal gp120, a HIV-1 envelope glycoprotein previously documented to activate spinal cord microglia and induce the production and release of proinflammatory cytokines in spinal cord, prevented by minocycline22,31.
Ten-percent formalin (Fisher Scientific, Fairlawn, NJ) was diluted with 0.9% endotoxin-free saline, yielding a final formalin concentration of 5%. Rats were injected with 50 µl of 5% formalin or saline into the dorsal surface of the right hindpaw as described previously16. A pilot study was conducted to compare behavioral responses to the von Frey test prior to and two weeks after formalin injection (n=5). Testing was performed on the plantar surface of the hindpaws to avoid potential confounds of antinociception due to damage to terminal endings in the tissue of the injected dorsal hindpaw that may occur as a result of formalin injection. A paired t-test showed that there was not a significant difference between response thresholds recorded pre-formalin (7.66 +/−0.53 gm) and post-formalin (9.02 +/− 0.98 gm; t(5)=, −0.666, P > 0.5). Thus, baseline behavioral assessments were performed two weeks after injection of formalin or equivolume endotoxin free saline into the right dorsal hindpaw. Twenty min after baseline tests, intrathecal gp120 or equivolume RSA was delivered (n = 5–8/group). Frozen solutions of recombinant gp120 (product 1021; lot number 9AT31/2; ImmunoDiagnostics, Bedford, MA) were thawed, aliquoted at 1 µg/µl, and stored at −75°C. Vehicle, composed of 0.1% rat serum albumin (RSA; Accurate Chemical & Scientific Corp., Westbury, NY) in Dulbecco’s phosphate buffered saline (PBS; 0.1 mm pore-filtered, pH 7.2; Gibco, Grand Island, NY) was aliquoted and stored at −20°C. Frozen aliquots of gp120 and vehicle were thawed immediately before administration, and gp120 was diluted to 0.25 µg/µl with the final concentration containing 0.1% RSA. Aliquots were kept on ice during use and discarded with 30 min. Animals were injected with either 0 or 1.5 µg of gp120 in 6 µl 0.1% RSA followed by an 18 µl saline flush to ensure complete drug delivery. Behavioral assessments for mechanical allodynia were conducted 20 and 60 min after i.t. gp120.
Results
Experiment 1A: Prior laparotomy potentiates later i.p. LPS-induced mechanical allodynia
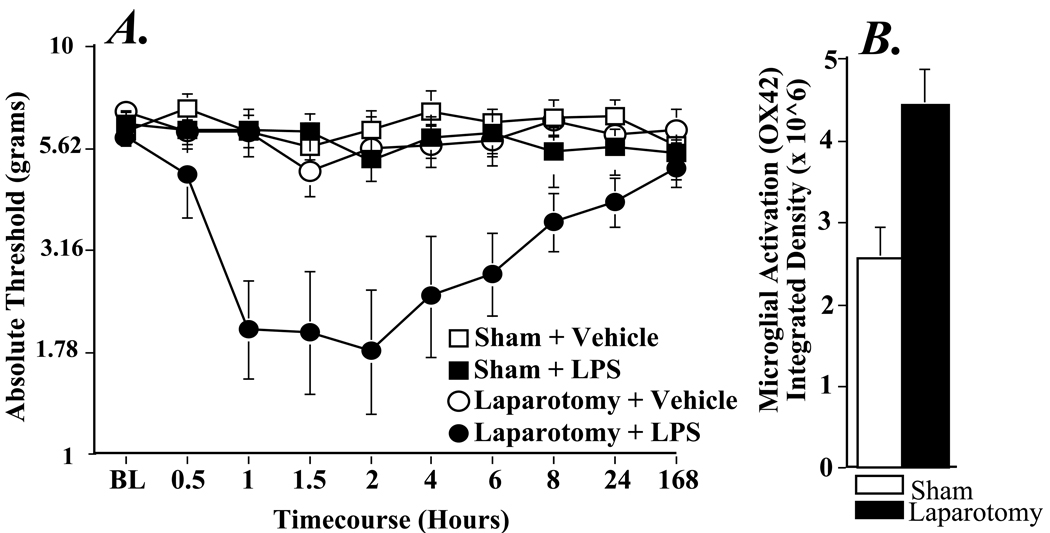
There was no reliable difference in pre-surgery baseline response thresholds in any of the four groups (F1,28=0.8194; P > 0.05). There was also no significant difference in behavioral response to mechanical stimuli to the hindpaws for any of the four groups when baseline assessments were re-tested 2 weeks later, prior to LPS or vehicle (Figure 1A, F1,28 = 0.429, P > 0.05). Further, comparison of the pre-surgery and post-surgery baseline assessments by repeated measures ANOVA revealed no significant effect of surgery (F1,28 = 0.109, P > 0.05). While 20 µg/kg LPS (i.p.) did not alter response thresholds in sham-operated rats, this same LPS dose induced a prolonged mechanical allodynia in rats with prior laparotomy that was evident from 1 hour to 6 hours post injection (P < 0.01 vs. all other groups). Repeated measures ANOVA revealed significant main effects of surgery (F1,28 = 9.166, P < 0.01) and LPS injection (F1,28 = 12.785, P < 0.01) and an interaction between surgery and injection (F1,28 = 5.514, P < 0.05).
Figure 1.
Effect of laparotomy on the behavioral responses to mechanical stimuli following LPS (20 µg/kg, i.p.) administered two wk later (Panel A). LPS causes decreased withdrawal thresholds to mechanical stimuli only when preceded by laparotomy. Animals underwent laparotomy or sham surgery two wk before testing. Low-threshold mechanical sensitivity was assessed by the von Frey test before LPS (20 µg/kg) or vehicle injection (BL) and 30-480 min, 1 and 7 d after injection. Although laparotomy alone (laparotomy + vehicle; black circles) does not affect withdrawal thresholds, when paired with LPS, mechanical allodynia results (laparotomy + LPS; black squares), this is in contrast to the sham group, where LPS had no effect on behavior (sham + LPS; white squares). Values represent average thresholds for the left and right paws (means ± SEM). Panel B shows a significant difference in microglial activation in L4-5 dorsal horn two wk following laparotomy (black bar) compared to sham-operated controls (white bar).
Experiment 1B: Prior laparotomy is associated selectively with enhanced expression of microglial activation markers in L4/L5 spinal cord dorsal horn 2 wk later
Given the induction of LPS-induced mechanical allodynia only in rats receiving a laparotomy 2 wk prior, we assessed whether expression of astrocyte (GFAP) and microglial (OX42) activation markers 2 wk after laparotomy or sham surgery at the region of the lumbar (L3, L4-L5) and lumbosacral (L6-S2) spinal cord and in the cervical spinal cord, an area above the level of injury which served as a control comparison. Separate statistical analyses were performed within each spinal region. There were no reliable differences in GFAP expression between sham and laparotomy groups in any of the regions evaluated. A one-way ANOVA analysis of OX-42 immunoreactivity revealed a significant main effect of laparotomy in the dorsal horn of L4-L5 spinal cord (F1,7 = 13.117, P < 0.001, Figure 1B). The same trend in OX42 expression was observed in lamina I-II of L4-L5 spinal cord, although the effect was not statistically significant (F1,7 = 2.481, P = 0.159). In the L3 and L6-S2 segments, laparotomy had no effect on OX-42 immunoreactivity. In addition, there was no significant change in OX42 expression between sham-operated and laparotomy rats in the cervical dorsal horn (F1,7 = 1.348, P = 0.284).
Experiment 1C: Prior laparotomy does not affect expression of OX-42 in lumbosacral spinal cord dorsal horn in response to i.p. LPS challenge 2 wk later
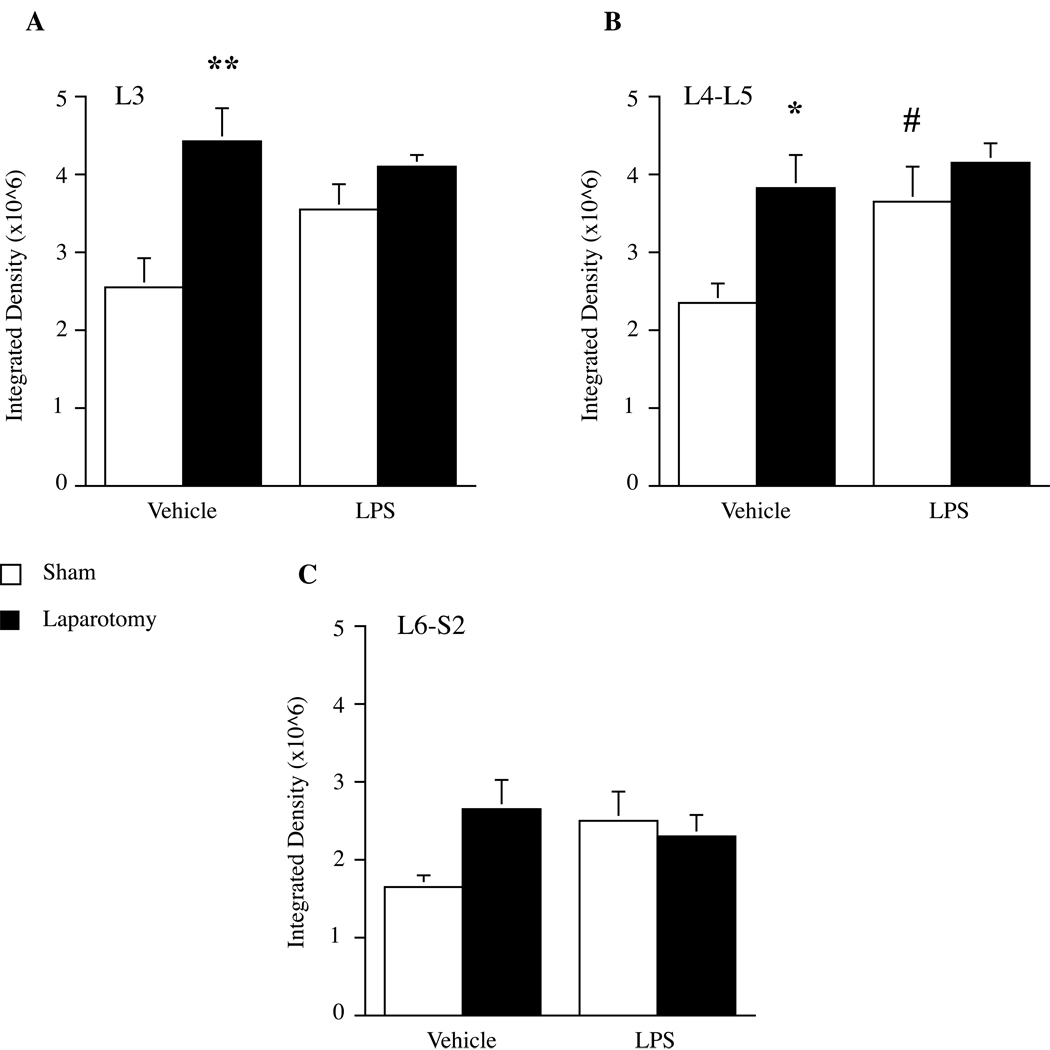
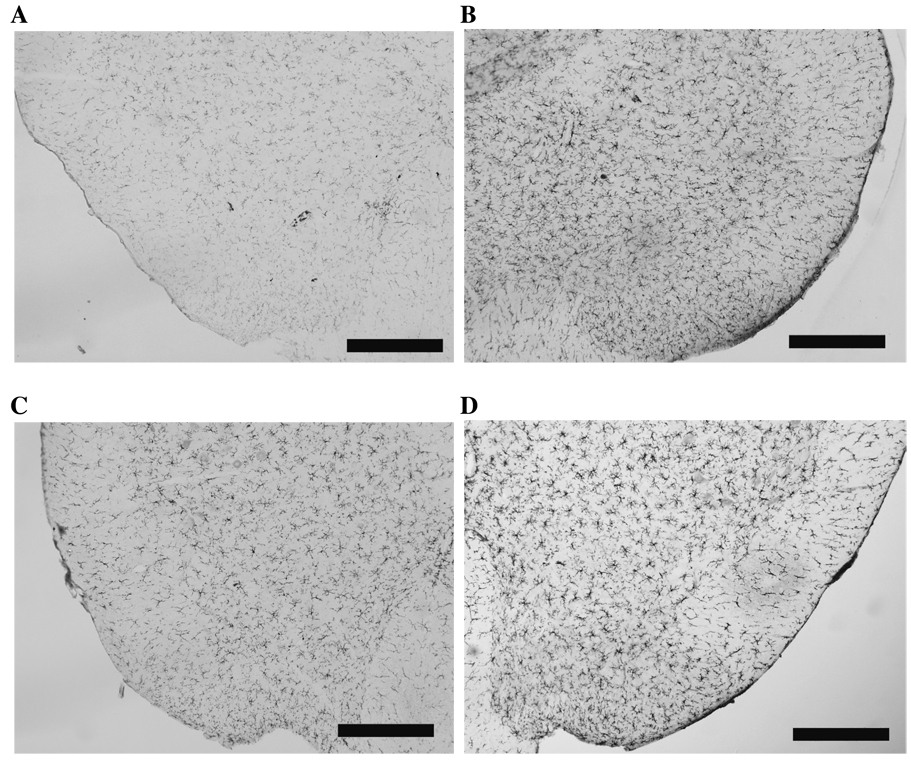
OX-42 and GFAP immunoreactivity were assessed in lumbar (L3, L4-L5) and lumbosacral (L6-S2) spinal cord 6 hours after either LPS or saline vehicle administration to rats that had received either laparotomy or sham surgery 2 wk prior (Figure 2). As in Experiment 1B, no differences in GFAP expression were observed between sham and laparotomy groups in any of the regions examined. In contrast, analysis of OX-42 labeling by two-way ANOVA revealed a significant main effect of laparotomy in L3 (F1,19 = 12.358, P < 0.005) and L4-L5 (F1,20 = 7.625, P < 0.015), but not in L6-S2. Post hoc analyses showed that laparotomy alone increased OX-42 labeling in L3 (P < 0.002 vs. sham + vehicle group) and L4-L5 (P < 0.01 vs. sham + vehicle group). In L4-L5, there was a significant main effect of LPS injection (F1,20 = 4.931, P < 0.04), whereby LPS increased OX-42 labeling in sham animals (P < 0.025 vs. sham + vehicle group). Interestingly, there were no differences in OX-42 immunoreactivity between the laparotomy + vehicle and the laparotomy + LPS groups in any region examined. Representative images showing OX-42 immunoreactivity in L4-L5 dorsal horn in each condition are shown in Figure 3.
Figure 2.
Microglial activation in lumbosacral spinal cord after LPS administered two wk following laparotomy. Laparotomy (black bars) significantly increased OX-42 labeling in L3 a) and L4-L5 b). Systemic LPS induced an increase in OX-42 labeling in L4- L5 that was specific to sham-operated animals. In all areas examined, OX-42 activation was greater in laparotomy + vehicle animals compared to sham + vehicle controls. There was no interaction between laparotomy and LPS. Data represent mean integrated densities of OX-42 immunoreactivity ± SEM. *P < 0.05, **P < 0.05 vs. sham + vehicle; #P < 0.05 vs. sham + vehicle.
Figure 3.
Representative images depicting OX-42 immunoreactivity in L4-L5 dorsal horn following LPS (20µg/kg i.p.) administered two wk after laparotomy. a) sham + vehicle, b) laparotomy + vehicle, c) sham + LPS and d) laparotomy + LPS. Scale bars represent 200 µm.
Experiment 2: Minocycline, a microglial activation inhibitor, suppresses later i.p. LPS induced mechanical allodynia, when minocycline is administered for 5 days after laparotomy or 5 days before LPS administration
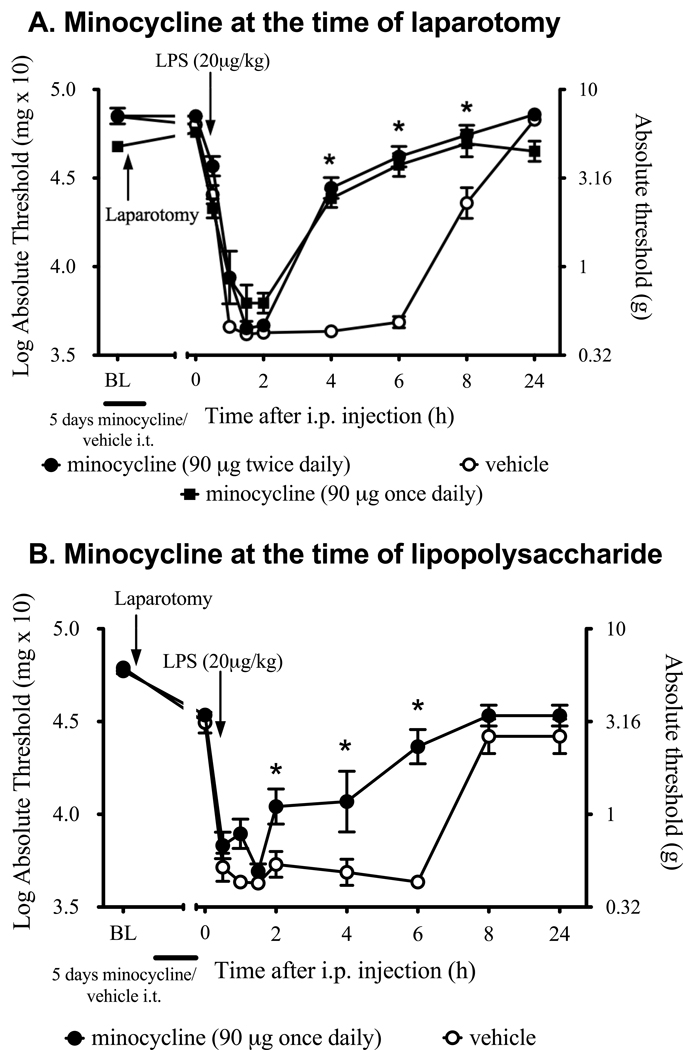
Mechanical allodynia was significantly attenuated in rats that received twice daily minocycline for 5 days after surgery compare to vehicle controls (drug effect: F1, 15 = 64.5, P < 0.001, main effect of time: F9, 135 = 205.1, P < 0.001, interaction: F9,135 = 24.57, P < 0.001). While minocycline-treated rats still became allodynic following LPS administration, the duration of the pain response lasted less than 4 hours. In contrast, LPS-induced allodynia persisted in vehicle treated rats for at least 8 hours (Figure 4A). There was no significant difference between the behavioral responses in the left and right hind paws at any of the time points. While agitation and left hindpaw paresis were briefly observed in response to minocycline when administered twice daily from day 2 after initiation of minocycline treatment onward, only mild left hindlimb reduced motion was detectable 2 wk later which did not impact on the behavioral responses to mechanical stimuli as the left and right hind paw responses were not significantly different. In addition, minocycline given once daily at the time of laparotomy produced the same effect on allodynia induced by the LPS with no motor side effects. This same once daily minocycline regimen administered for 4 days before LPS and given 1 hour before LPS also resulted in significant attenuation in the duration of the LPS induced allodynia following laparotomy (drug effect: F1, 10 = 18.0, P = 0.0017, main effect of time: F9, 90 = 73.9, P < 0.0001, interaction: F9,90 = 5.69, P < 0.0001, Figure 4B) with post-hoc comparisons showing significant differences at 2, 4 and 6 hours after LPS administration (P < 0.05).
Figure 4.
Effect of five-day minocycline administration (twice daily, or once daily, 90 µg, i.t.) or vehicle on a two-challenge paradigm of laparotomy and low dose, systemic LPS. (A) Minocycline administration began at the time of surgery (laparotomy + minocycline +LPS; black circles). (B) Minocycline administration beginning five days before LPS administration, with the last dose 1 h before LPS administration (laparotomy + minocycline +LPS; black circles). Two wk after laparotomy, LPS was administered intraperitoneally. Minocycline significantly reduced the duration of LPS-induced allodynia but not the intensity compared to rats treated with intrathecal vehicle (laparotomy + vehicle +LPS; open circles, P<0.001), irrespective of the dosing regimen. Data are presented as mean ± SEM. Values represent average thresholds for the left and right paws. *P < 0.01 at each respective time point for minocycline against vehicle.
Experiment 3: Generalization of the effect of prior challenge: Prior subcutaneous formalin enhances later intrathecal gp120-induced mechanical allodynia
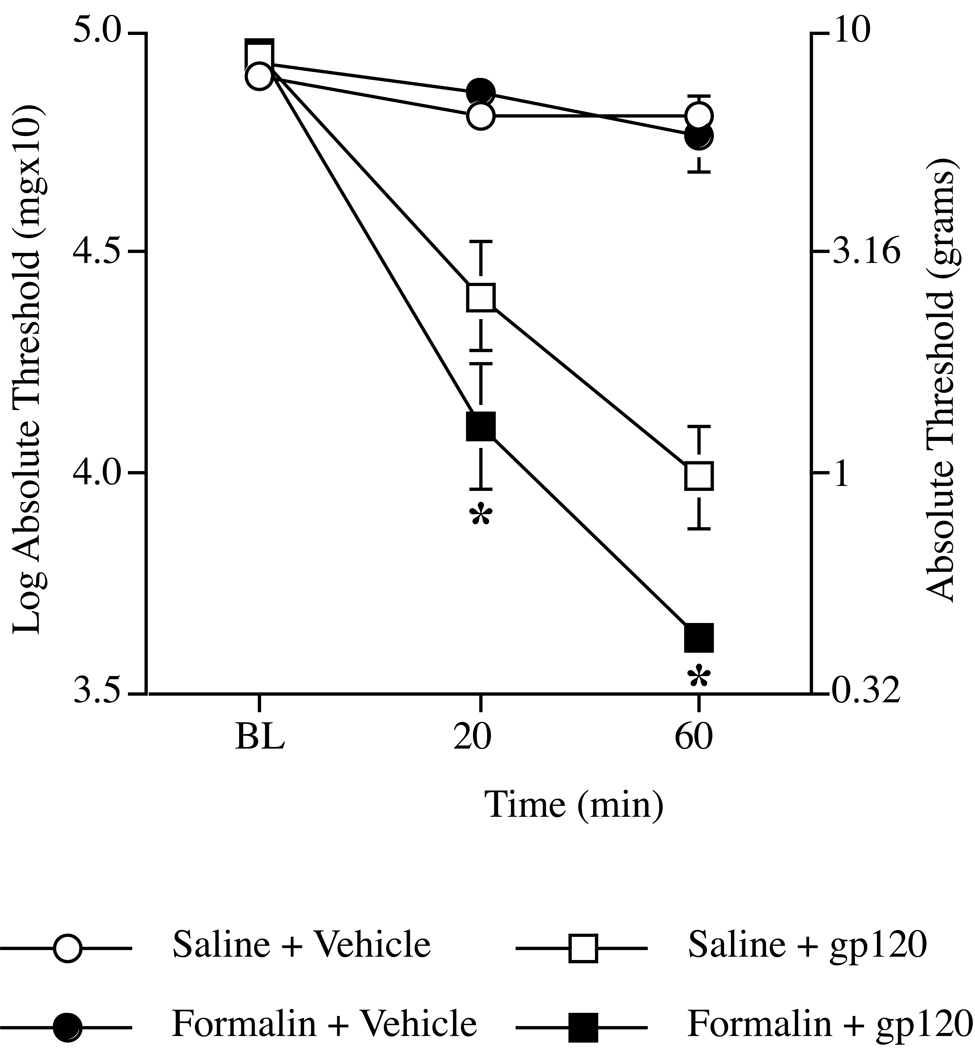
We examined the effect of a different “two-hit” paradigm, with rats tested for intrathecal gp120-induced mechanical allodynia two wk after injection with subcutaneous formalin into the dorsal aspect of the right hindpaw. All groups exhibited comparable baseline behavioral responses immediately prior to i.t. treatment (Figure 5), indicating that formalin injection did not cause mechanical allodynia two wk after injection (F1,22 = 0.326, P > 0.05). In our previous studies, i.t. gp120 (3 µg) delivered to the lumbosacral enlargement produced robust bilateral mechanical allodynia as soon as 20 min after injection and lasting for at least 4 hours22,35,36,43. The dose of gp120 used in the current study is half that used in previous studies, yet it is still potent enough to cause robust allodynia at both time points as determined by repeated measures ANOVA (effect of gp120: F1,22 = 115.664, P < 0.001). The resulting allodynia was exacerbated when gp120 was preceded two wk earlier by subcutaneous formalin (effect of formalin: F1,22 = 4.957, P < 0.04), reflected by a significant interaction between formalin and gp120 (F1,22 = 5.399, P < 0.03). Post hoc means comparison showed that the combination of pretreatment with formalin followed by gp120 resulted in significantly lower mechanical thresholds compared to saline + gp120 group at both 20 (P < 0.04) and 60 (P < 0.008) minutes.
Figure 5.
Effect of intraplantar formalin on i.t. gp120-induced mechanical allodynia tested two wk later. Injection of formalin enhances gp120-induced mechanical allodynia tested two wk later. Formalin (5%) or vehicle was injected into the dorsal aspect of the right hindpaw two wk prior to testing on the plantar aspect of the hindpaw. The von Frey test was used to assess low-threshold mechanical allodynia before (BL), 20 and 60 min after i.t. administration of gp120 (1.5 µg) or vehicle. I.t. gp120 causes allodynia within 20 min of injection. Allodynia is greater in animals injected with formalin (black squares) two wk earlier compared to the vehicle + gp120 group (white squares). Values represent average thresholds of left and right hindpaws (means ± SEM). *P < 0.05 compared to all other groups.
Discussion
The present series of studies demonstrate that acute inflammatory challenges can sensitize pain responses to a second challenge even after resolution of pain sensitivity incurred in response to the first challenge. Using a “two-hit” paradigm, first “hits” invoked by either laparotomy with gut manipulation or subcutaneous formalin potentiated mechanical allodynia induced 2 wk later by low dose of systemic LPS or i.t. gp120, respectively. Importantly, before the second challenge there were no significant differences in behavioral responses to the graded mechanical stimuli between the control groups and the groups subjected to the first hit, either laparotomy or formalin. In addition to enhanced pain following the second challenge, sustained immunohistochemical evidence of upregulated activation markers site-specifically in L4/L5 spinal microglia (OX-42 upregulation), but not for astrocytes, was observed two weeks following laparotomy. As noted above, this was a point in time when behavioral responses to mechanical stimuli had returned to baseline values after laparotomy. The sustained expression of microglial activation markers following laparotomy supports and extends previous observations of multi-week microglial expression of activation markers by a single injection of subcutaneous formalin16 delivered at the same dose and site as employed in the present study. While prior studies have noted persistent expression of microglial activation markers over wk to months, the present studies are the first to challenge microglia in this state and observe effects on behavior. In addition, administration of the glial activation inhibitor, minocycline, significantly decreased the duration of allodynia following laparotomy and LPS compared to the vehicle controls.
Although the number of clinical examples describing pain enhancement to repetitive insults is small, experimental data shows that something as mild as a low-grade infection given within the neonatal period can lead to profound long-lasting changes in pain sensitivity3. Enhanced and/or sensitized pain behavior in response to an immune challenge has been reported previously subsequent to peripheral nerve injury30, subcutaneous inflammation5,38 and repeated sound stress28. These effects have been shown to last at least three1, but not four wk5 after the first challenge. However, if the insult occurred neonatally, exaggerated pain responses to inflammation lasted considerably longer and may represent permanent changes in transduction of nociceptive stimuli42. On the other hand, pain primed by acute peripheral insult in adult animals most likely represents a transient facilitation of pain signaling. In support of this, the sensitization of responses reported here does not occur when the second challenge is presented 4 wk after the first (Hains, Unpub. Observ.).
One explanation that has been forwarded in prior studies to account for how prior trauma may prime pain to later challenge is peripheral sensitization, possibly due to sensitization of the primary afferents via altered signaling to the epsilon isoform of protein kinase C1,38. However, peripheral sensitization alone does not seem to be sufficient to account for priming of pain responses, as a unilateral priming challenge can induce exaggerated pain to a second challenge applied contralaterally within the same dermatome5. However, primed responses were not seen if the first challenge was applied to the tail and the second to the lip, suggesting that afferent signaling for both challenges may need to converge at the same spinal segments so to create local changes in spinal excitability5. These findings, combined with the results of the present study, indicate that segmentally organized central sensitization resulting from the first “hit” may be required for later exaggerated pain responses arising from these same central sites.
Microglial activation restricted to areas of spinal cord receiving nociceptive input from injured or inflamed tissue may be an indication of segmental spinal sensitization. This segmentally organized sensitization has been noted after subcutaneous injection of formalin16, surgical incision15,48,49, and, as reported here, after laparotomy with gut manipulation. It is likely that segmental microglial activation occurs in other animal models of trauma and inflammation, but these are the only examples found where the authors included data describing the lack of activation in spinal levels that are not afferent targets. In addition, the duration of microglial activation varies depending on the nature of the trauma. For example, thoracic surgical incision by itself produced microglial activation lasting only three days15. However, when surgical incision is accompanied by gut manipulation, as in the current experiment, microglial activation persisted for at least 2 weeks. When compared to incision alone, the addition of gut manipulation has been shown to have a greater influence on behaviors related to post-surgical pain 33 and spinal activation. 29 Based on these findings, investigation of glial activation in the present studies was focused on the lumbosacral spinal segments, which receive visceral input from the lower small intestines, as opposed to the lower thoracic segments that innervate the incised skin and muscle.33
In the studies noted above, evidence for microglial activation was determined by increases in immunoreactivity for OX-42 (which targets the CD11b/c component of complement receptor 3)15,16 or the enzyme cyclooxygenase 1 (COX-1)48,49 compared to controls. Describing microglia as being activated or resting has been historically based on the relative degree of expression of activation markers detectable by immunohistochemistry. Other than the fact that microglia appear “activated”, as determined by morphology and expression of classical activation markers, little is known about the functional state of microglia over time following peripheral injury or inflammation. It has often been assumed that microglia that appear activated are producing and releasing pain-enhancing mediators such as nitric oxide and pro-inflammatory cytokines. The current finding, that normal basal pain responses were observed two wk after laparotomy or subcutaneous formalin despite increased OX-42 labeling suggests that this assumption may not always be valid. Indeed, we have observed that IL-1 protein in dorsal spinal cord does not differ between rats receiving laparotomy with gut manipulation 2 wk prior versus sham operated rats, despite upregulated microglial activation marker expression in the operated rats (Hains et al., Unpub. Obs.). Differences in IL-1 protein expression are only revealed upon a subsequent challenge. In response to LPS, a more rapid increase in IL-1 protein in dorsal spinal cord is observed in rats with prior laparotomy with gut manipulation (Hains et al., Unpub. Obs.). Whether such elevations in glial proinflammatory products mediate enhanced pain in response to a second “hit” is currently under investigation. Another example can be found in the aged brain, where microglia show increases in activation markers such as MHC II while production of inflammatory cytokines are at control levels23. Thus there can be a discrepancy between cell surface marker-based microglial activation and functional activation where pro-inflammatory mediators are released.
Recently, our lab has shown that microglia isolated from brains of rats exposed to stress are more reactive and express significantly greater levels of IL-1 mRNA in response to LPS compared to non-stressed controls13. These findings support the hypothesis that responses of primed microglia contribute to exacerbation of LPS-induced cytokine production and sickness behaviors described in prion disease and subsequent to stress. Further, microglial priming may be a key feature of the atypical state of neuroinflammation where pro-inflammatory cytokine levels are low, microglial activation markers are elevated, and response to inflammatory challenge is exaggerated.
In summary, in the first set of experiments, we observed laparotomy-induced expression of microglial activation markers in the absence of mechanical allodynia, two weeks later. Systemic injection of a low dose of LPS caused mechanical allodynia in previously laparotomized rats, but not in sham controls. The enhanced behavioral response to LPS after laparotomy was attenuated by five days administration of minocycline either from the time of surgery or preceding LPS administration. In our final experiment, we found that intraplantar formalin primed pain responses to i.t. gp120, an immune activator that is known to directly activate microglia35. As noted previously, spinal microglia are persistently activated after intraplantar injection of formalin16. Although speculative, results from the current study suggest that prior trauma or inflammation may induce segmental microglial priming in the spinal cord and that, while the microglia remain in this state, such primed microglia have the potential for contributing to amplification of pain to subsequent challenges.
Perspective
Spinal microglia may become “primed” (sensitized) following their activation by disparate forms of peripheral trauma/inflammation. As a result, such primed microglia may over-respond to subsequent challenges, thereby enhancing pain intensity and duration.
Acknowledgments
This work was supported by NIH grants DA015642, DA024044, and DA017670.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. � 2010 The American Pain Society. Published by Elsevier Inc. All rights reserved.
References
- 1.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 3.Boisse L, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ. Neonatal immune challenge alters nociception in the adult rat. Pain. 2005;119:133–141. doi: 10.1016/j.pain.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Bruce J, Drury N, Poobalan AS, Jeffrey RR, Smith WC, Chambers WA. The prevalence of chronic chest and leg pain following cardiac surgery: a historical cohort study. Pain. 2003;104:265–273. doi: 10.1016/s0304-3959(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 5.Cadet R, Aigouy L, Woda A. Enhanced nociceptive behaviour following conditioning injection of formalin in the perioral area of the rat. Brain Res. 1995;676:189–195. doi: 10.1016/0006-8993(95)00055-u. [DOI] [PubMed] [Google Scholar]
- 6.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 7.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 8.Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994;269:1117–1123. [PubMed] [Google Scholar]
- 9.Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 10.Crombie IK, Davies HT, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain. 1998;76:167–171. [PubMed] [Google Scholar]
- 11.Cunningham C, Boche D, Perry VH. Transforming growth factor beta1, the dominant cytokine in murine prion disease: influence on inflammatory cytokine synthesis and alteration of vascular extracellular matrix. Neuropathol Appl Neurobiol. 2002;28:107–119. doi: 10.1046/j.1365-2990.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham C, Wilcockson D, Campion S, Lunnon K, Perry V. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS proinflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Fu D, Guo Q, Ai Y, Cai H, Yan J, Dai R. Glial activation and segmental upregulation of interleukin-1beta (IL-1beta) in the rat spinal cord after surgical incision. Neurochem Res. 2006;31:333–340. doi: 10.1007/s11064-005-9032-4. [DOI] [PubMed] [Google Scholar]
- 16.Fu KY, Light AR. Matsushima GK Maixner W: Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825:59–67. doi: 10.1016/s0006-8993(99)01186-5. [DOI] [PubMed] [Google Scholar]
- 17.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 18.Guo L, Schluesener H. Acute but not chronic stimulation of glial cells in rat spinal cord by systemic injection of lipopolysaccharide is associated with hyperalgesia. Acta Neuropathol. 2006;112:703–713. doi: 10.1007/s00401-006-0135-z. [DOI] [PubMed] [Google Scholar]
- 19.Harvey LOJ. Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- 20.Herrero JF, Laird JMA, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Progress in Neurobiology. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 21.Hohmann AG, Neely MH, Pina J, Nackley AG. Neonatal chronic hind paw inflammation alters sensitization to intradermal capsaicin in adult rats: a behavioral and immunocytochemical study. J Pain. 2005;6:798–808. doi: 10.1016/j.jpain.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Holguin A, O'Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Bravmann C, Martin D, Fleshner M, Maier SF, Watkins LR. HIV-1 gp120 stimulates proinflammatory cytokinemediated pain facilitation via activation of nitric oxide synthase-I (nNOS) Pain. 2004;110:517–530. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt JL, Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Repeated injury to the lumbar nerve roots produces enhanced mechanical allodynia and persistent spinal neuroinflammation. Spine (Phila Pa 1976) 2001;26:2073–2079. doi: 10.1097/00007632-200110010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:9353–9365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato H, Kogure K, Araki T, Itoyama Y. Graded expression of immunomolecules on activated microglia in the hippocampus following ischemia in a rat model of ischemic tolerance. Brain Res. 1995;694:85–93. doi: 10.1016/0006-8993(95)00769-m. [DOI] [PubMed] [Google Scholar]
- 27.Kayser V, Idanpaan-Heikkila JJ, Guilbaud G. Sensitization of the nervous system, induced by two successive hindpaw inflammations, is suppressed by a local anesthetic. Brain Res. 1998;794:19–27. doi: 10.1016/s0006-8993(98)00189-9. [DOI] [PubMed] [Google Scholar]
- 28.Khasar S, Green P, Levine J. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Kreiss C, Birder LA, Kiss S, VanBibber MM, Bauer AJ. COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus. Gut. 2003;52:527–534. doi: 10.1136/gut.52.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaBuda CJ. Donahue R Fuchs PN: Enhanced formalin nociceptive responses following L5 nerve ligation in the rat reveals neuropathy-induced inflammatory hyperalgesia. Pain. 2001;94:59–63. doi: 10.1016/S0304-3959(01)00341-4. [DOI] [PubMed] [Google Scholar]
- 31.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Liebregts T, Adam B, Bertel A, Lackner C, Neumann J, Talley NJ, Gerken G, Holtmann G. Psychological stress and the severity of post-inflammatory visceral hyperalgesia. Eur J Pain. 2007;11:216–222. doi: 10.1016/j.ejpain.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Martin TJ, Kahn WR, Eisenach JC. Abdominal surgery decreases food-reinforced operant responding in rats: relevance of incisional pain. Anesthesiology. 2005;103:629–637. doi: 10.1097/00000542-200509000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90:81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 35.Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 36.Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to post-operative mechanical hypersensitivity in the rat. Anesthesiology. 2006;7:816–822. doi: 10.1016/j.jpain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–190. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 40.Perry V, Cunningham C, Boche D. Atypical inflammation in the central nervous system in prion disease. Curr Opin Neurol. 2002;15:349–354. doi: 10.1097/00019052-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Randich A, Mebane H, Ness TJ. Ice water testing reveals hypersensitivity in adult rats that experienced neonatal bladder inflammation: implications for painful bladder syndrome/interstitial cystitis. J Urol. 2009;182:337–342. doi: 10.1016/j.juro.2009.02.107. [DOI] [PubMed] [Google Scholar]
- 42.Ren K, Anseloni V, Zou S, Wade E, Novikova S, Ennis M, Traub R, Gold M, Dubner R, Lidow M. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Schoeniger-Skinner DK, Ledeboer A, Frank MG, Milligan ED, Poole S, Martin D, Maier SF, Watkins LR. Interleukin-6 mediates low-threshold mechanical allodynia induced by intrathecal HIV-1 envelope glycoprotein gp120. Brain Behav Immun. 2007;21:660–667. doi: 10.1016/j.bbi.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61:87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- 45.Walsh DT, Betmouni S, Perry VH. Absence of detectable IL-1beta production in murine prion disease: a model of chronic neurodegeneration. J Neuropathol Exp Neurol. 2001;60:173–182. doi: 10.1093/jnen/60.2.173. [DOI] [PubMed] [Google Scholar]
- 46.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 47.Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, Conklin D, Eisenach JC. Cyclooxygenase-1 in the spinal cord plays an important role in postoperative pain. Pain. 2003;104:15–23. doi: 10.1016/s0304-3959(02)00465-7. [DOI] [PubMed] [Google Scholar]
- 49.Zhu X, Vincler MA, Parker R, Eisenach JC. Spinal cord dynorphin expression increases, but does not drive microglial prostaglandin production or mechanical hypersensitivity after incisional surgery in rats. Pain. 2006;125:43–52. doi: 10.1016/j.pain.2006.04.027. [DOI] [PubMed] [Google Scholar]