Abstract
CD8 T cells can acquire cytokine-secreting phenotypes paralleling cytokine production from Th cells. IL-17-secreting CD8 T cells, termed Tc17 cells, have been shown to promote inflammation and mediate immunity to influenza. However, most reports have observed a lack of cytotoxic activity by Tc17 cells. In this report, we explored the anti-viral activity of Tc17 cells using a vaccinia virus infection (VV) model. Tc17 cells expanded during VV infection, and TCR transgenic Tc17 cells were capable of clearing recombinant VV infection. In vivo, adoptively transferred Tc17 cells lost the IL-17-secreting phenotype even in the absence of stimulation, but did not acquire IFNγ-secreting potential unless stimulated with a virus-encoded antigen. However, examination of cells following infection demonstrated that these cells acquired cytotoxic potential in vivo even in the absence of IFNγ. Cytotoxic potential correlated with Fasl expression, and the cytotoxic activity of post-infection Tc17 cells was partially blocked by addition of anti-FasL. Thus, Tc17 cells mediate VV clearance through expression of specific molecules associated with cytotoxicity, but independent of an acquired Tc1 phenotype.
Introduction
CD8 T cells can acquire cytokine-secreting phenotypes parallel to those acquired by CD4 T cells and require transcription factors similar to those required for Th cell phenotypes. For example, the development of IFNγ-secreting CTL or Tc1 cells is promoted by IL-12, and the transcription factors T-bet and Eomesodermin (1–4). Recent reports have described the IL-17-secreting Tc17 phenotype. Tc17 cells require Stat3 and RORγt for their development (5, 6). Moreover, factors that promote the development of Tc1 cells inhibit the development of Tc17 cells (7, 8). Apart from the differences in cytokine secretion, Tc17 cells differ from Tc1 cells in that they are not cytotoxic (5, 9–11). In most reports, in vitro generated polyclonal or TCR transgenic Tc17 cells lack cytotoxic activity in 51Cr-release assays. Tc17 cells have low expression of granzyme B (GrB3), perforin and FasL, compared to Tc1 cells (5, 9, 11, 12).
Tc17 cells have been shown to develop in vivo, during the development of EAE and during influenza infection (5, 12). In vivo transfer of in vitro-derived antigen-specific Tc17 cells was shown to be efficacious in clearing lethal doses of influenza, in anti-tumor immunity, and in promoting inflammation, though these cells were not protective against an LCMV infection (7, 8, 10, 12). How Tc17 cells mediate these functions is unclear. While one report demonstrated cytotoxic activity of Tc17 cells that correlated with diabetogenic potential requiring IL-17A and IL-17F (7), most reports have suggested that the ability of Tc17 cells to promote immunity in vivo depends upon the ability of the cells to switch to a Tc1 phenotype (6, 10, 12). The instability of the IL-17-secreting phenotype, and the acquisition of an IFNγ-secreting phenotype, even from highly purified IL-17-secreting CD8 T cells (6), is well documented. In the influenza model, the protective effect of Tc17 cells was partially dependent upon IFNγ (12), though it is likely that other molecules are required for Tc17-mediated immunity.
In this report, we demonstrate that Tc17 cells develop during a VV infection and can promote anti-VV immunity. As with other reports, we observed instability of the IL-17-secreting phenotype of adoptively transferred Tc17 cells. The loss of the IL-17-secreting phenotype occurred in the absence of stimulation in vivo, while the acquisition of IFNγ-secreting potential required both antigen and virus infection. However, the anti-viral activity was present in Ifng−/− Tc17 cells, suggesting that acquiring the Tc1 phenotype is not critical for anti-viral activity. Isolation of cells following adoptive transfer shows an increase in cytotoxic potential, suggesting that the in vivo environment, during infection, re-programs Tc17 cells to a unique effector phenotype.
Materials and Methods
Mice
The generation of Stat4−/− (13), Tbx21−/− (14) mice has been previously described. The derivation of OT-l Ifng−/− (12), OT-l Stat4−/− (2) and Stat4−/−Tbx21−/− (15) mice were previously described. All mice were used on a C57BL/6 background. C57BL/6 and Balb/c mice were purchased from Harlan Bioscience (Indianapolis, IN), OT-I / Rag1−/− mice were purchased from Taconic Farms (Hudson, NY), and Ifng−/− and C3H/HeJ mice were purchased from Jackson Laboratories (Bar Harbor, ME). BoyJ mice were obtained from the IU Simon Cancer Center In Vivo Therapeutics Core. Mice were kept in pathogen-free conditions and all studies were approved by the Indiana University School of Medicine Animal Care and Use Committee.
Viruses
VV (Western Reserve strain) and VV-SIINFEKL (originally provided by J. Yewdell and J. Bennick, LVD, NIAID/NIH) were propagated in the human osteosarcoma TK-143B cell line, followed by sucrose gradient purification and titer determination by the VV Core Facility at the Indiana University School of Medicine as described (16).
Tc cell differentiation
Total CD8+ cells were isolated from spleens and lymph nodes using a MACS isolation system (MACS isolation system; Miltenyi Biotec). For Tc cell differentiation, CD8+ cells were activated with soluble anti-CD3 (4 μg/ml 145–2C11) and anti-CD28 (1 μg/ml; BD Pharmingen) in the presence of CD8+ depleted irradiated splenocytes (1:5). OT-I CD8+ cells were activated with soluble SIINFEKL peptide (1μM; Bio-Synthesis Inc) and anti-CD28 (1 μg/ml). Tc17 primed cells were cultured with hTGFβ1 (2 ng/ml; R&D Systems), IL-6 (100 ng/ml; PeproTech), and anti-IFNγ (10 μg/ml R4/6A2 or XMG) and Tc1 primed cells were differentiated with IL-12 (5 ng/ml; R&D Systems). After 3 days of incubation, cells were expanded in the presence of recombinant hIL-2 (20 units/ml; PeproTech) and analyzed after an additional 2 days of culture. In some experiments, cells underwent 2 rounds of stimulation. For the second five-day culture, cells were re-plated and stimulated with soluble anti-CD3 (1 μg/ml) and anti-CD28 (0.5 μg/ml) in the presence of CD8+ depleted irradiated splenocytes (1:5) with the same cytokine and neutralizing antibody concentrations. OT-I cells were activated with soluble SIINFEKL peptide (0.5 μM) and anti-CD28 (0.5 μg/ml).
Tc cell analysis
CD4+ or CD8+ T cells were stimulated for 4 h with PMA (50 ng/mL; Sigma) and ionomycin (500 ng/ml; Sigma) in the presence of GolgiPlug (BD Biosciences), and OT-I cells were stimulated for 4 h with SIINFEKL peptide (1 μM) in the presence of GolgiPlug before intracellular staining. Cells were fixed for 10 min with 2% formaldehyde, permeabilized in FACS buffer containing 0.1% saponin for cytokine staining, or 100% cold methanol for granzyme B and T-bet, and stained with fluorescently labeled antibodies for IL-17, IFN-γ, granzyme B or T-bet (eBiosciences). For surface staining, cells were stained for CD4, CD8, CD45.1 or CD45.2 (eBiosciences) following treatment with Fc Block (BD Biosciences) for samples containing APCs. All samples were analyzed by flow cytometry using FACScaliber instruments and data were analyzed using WinMDI software. Cytokines were detected using ELISA (reagents from BD Pharmingen), with supernatants of isolated CD8+ T cells stimulated with plate bound anti-CD3 (4 μg/ml) or SIINFEKL peptide (1 μM) for 48 h. Quantitative PCR was performed using TaqMan assays as previously described (15).
Cytolytic activity analysis
Cytotoxic activity of OT-I Tc17 and Tc1 effector cells was measured in a standard 51Cr release assay (17). In brief, 6x106 EL4 and EG.7 (EL4 cells transfected with chicken ovalbumin) target cells were incubated with 200 μCi of Na251CrO4 (Perkin Elmer) in 1 ml for 1 h at 37oC and washed 3 times with supplemented RPMI media. Effector cells were added to a round bottom 96-well microtiter plate in triplicate and serially diluted three fold to generate effector:target cell ratios ranging from 60:1 to 0.1:1. Target cells were then added to the microtiter plate at a concentration of 2x104 cells/well. Reactions were conducted in a total volume of 200 μl per well. Plates were incubated for 6 h at 37oC, spun down, and 100 μl of cell free supernatant was harvested from each well. Radioactivity was counted in a Perkin Elmer gamma counter. The percentage of specific lysis was calculated as (cpmsample – cpmspontaneous)/(cpmmaximum – cpmspontaneous) x 100, where cpm is counts per minute. Spontaneous release represents the radioactivity released by target cells in the presence of media alone, and maximum release represents the radioactivity released by target cells lysed with 5% Triton X-100 (Sigma). In some experiments, as noted in figures, control or anti-FasL (10 μg/ml; BioLegend) was added to cytotoxicity assays.
VV Infections
For standard infections, 2–6 mice per group were infected intraperitoneally (i.p.) with a sublethal dose (1x106 – 5x106 pfu/mouse) of VV-WR. Mice were sacrificed after 5–21 days of infection and ovaries and spleens were collected. For adoptive transfer experiments, 2–7 mice per group were infected either intranasally (i.n.) (2x106 pfu/mouse) or i.p (2x107 pfu/mouse) with VV-SIINFEKL or VV-WR. Infection was performed 1 day before or after cell transfer. Mice were sacrificed 4–8 days after infection and spleens and ovaries were collected. VV was titered using plaque assays as previously described (16).
Adoptive T cell transfer and isolation
BMDCs were cultured as described in Lutz et al. (18) and stimulated with LPS (1 μg/ml; Sigma) in the presence or absence of SIINFEKL peptide (1 μM) for 24 h. Prepared BMDCs (1x106) or 5 day differentiated OT-l Tc17 or Tc1 cells (1x106) were transferred via tail vein injection in 0.5 ml PBS. In some experiments, CD45.2+ cells were isolated from infected mice using anti-CD45.2 antibodies and magnetic cell selection.
Results
Stat4 and T-bet are required for the switch of Tc17 cells to a Tc1 phenotype
Previous reports have shown that the IL-17-secreting T cell phenotype is unstable, particularly in vivo. To explore this phenomenon further, we cultured in vitro-derived Tc17 cells for an additional round of differentiation under various culture conditions. Tc17 cultures maintained for a second round under Tc17 skewing conditions (TGFβ + IL-6) developed a higher percentage of IL-17-secreting cells but still maintained a small population of IFNγ-secreting cells (Fig. 1A). Tc17 cells cultured for a second round in the absence of cytokines (condition labeled as “α-IFNγ”) demonstrated decreased IL-17 production and increased IFNγ production, and culture of the cells in Tc1 conditions (IL-12) enhanced this phenotype (Fig. 1A). When IL-12 was added to cells under Tc17 conditions for the second round, the cells that developed were more heterogeneous, with a significant population positive for both IFNγ and IL-17 (Fig. 1A). The decrease in IL-17 production was largely dependent upon endogenous IFNγ production because culture of Ifng−/− CD8 Tc17 cells resulted in only a modest decrease of IL-17-secreting cells (Fig. 1B). However, IL-12 was able to repress IL-17 production in the absence of endogenous IFNγ (Fig. 1B).
Figure 1.
Tc17 cells are unstable and have increased Granzyme B production in a second round of culture. WT (A) or Ifng−/− (B) CD8+ T cells were primed under Tc17 conditions. After five days, Tc17 cells were restimulated in the presence of α-IFNγ alone, or with the addition of the indicated cytokines, or IL-12 alone for an additional five days. IL-17-, IFN-γ-, and Granzyme B-positive cells were detected using intracellular cytokine staining after the first round or second round of stimulation as indicated.
We also examined GrB expression in these cultures. Only a small percentage of Tc17 cells after one week of culture demonstrated expression of GrB (Fig. 1A). The percentage of GrB+ cells increased after a second round of culture in Tc17 conditions (Fig. 1A). GrB+ cells increased if Tc17 cells were cultured in the absence of cytokines, an effect that was enhanced by culture with IL-12 (Fig. 1A). Moreover, IL-12 induced GrB expression even when cells were maintained in Tc17 culture conditions (Fig. 1A). The induction of GrB was partially dependent on IFNγ, though IL-12 was able to induce GrB+ cells in the absence of endogenous IFNγ (Fig. 1B). Thus, continued exposure to Tc17 skewing cytokines is required to maintain the Tc17 phenotype, while both IFNγ and IL-12 contribute to the conversion of Tc17 cells to a Tc1 phenotype.
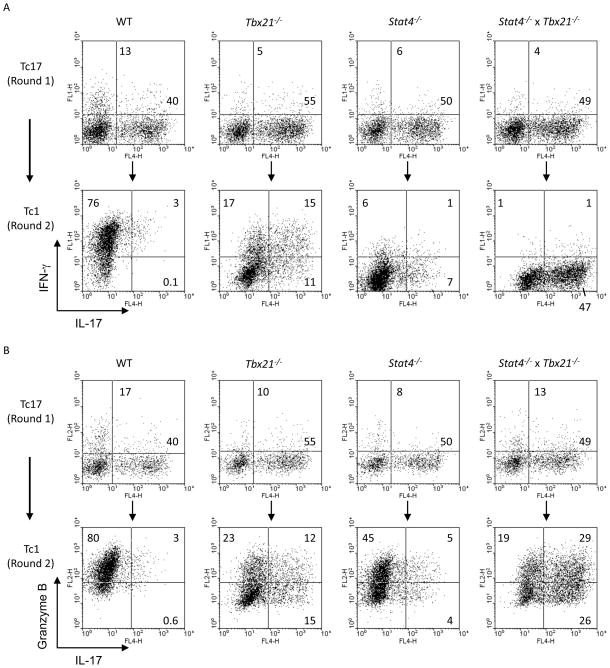
Since IL-12 was able to alter the Tc17 phenotype in the absence of endogenous IFNγ, we further explored the downstream mediators of this response by examining the ability of Tc17 cultures to switch phenotype in response to IL-12 when cells lacked expression of STAT4, T-bet, or both transcription factors. As previously described, differentiation of Stat4−/− and Tbx21−/− cells under Tc17 conditions showed a modest increase in IL-17-secreting cells (7, 8), although this was not further affected by double-deficiency (Fig. 2A). While culture of wild type Tc17 cells for a second round under Tc1 conditions resulted in increased IFNγ production and decreased IL-17, cells deficient in T-bet were unable to completely repress IL-17 or induce IFNγ (Fig. 2A). Stat4−/− cultures showed more repression of IL-17, compared to Tbx21−/− cultures, but less induction of IFNγ. Cells that were doubly-deficient in Stat4 and T-bet could neither repress IL-17 nor induce IFNγ, suggesting that each factor has unique but overlapping functions in switching the phenotype of Tc17 cultures (Fig. 2A). While, IL-12 was also able to induce GrB in wild type cells, an effect that was only modestly attenuated in Stat4−/− cultures, cells that were deficient in T-bet were defective in the induction of GrB (Fig. 2B). Cells that were deficient in both Stat4 and T-bet were capable of inducing GrB expression, and since IL-17 was not repressed in these cultures, there were more IL-17-GrB-double-positive cells (Fig. 2B).
Figure 2.
Tc17 instability is dependent on both Stat4 and T-bet. WT, Tbx21−/−, Stat4−/−, or Tbx21−/− x Stat4−/− CD8+ T cells were cultured under Tc17 conditions. After five days, Tc17 cells were restimulated in the presence of IL-12 (Tc1 conditions) for an additional five days. IL-17, IFN-γ, and Granzyme B were detected using intracellular cytokine staining after the first round of stimulation (A, B-top panel) and also at the end of the second round of stimulation (A, B-bottom panel).
Expression of cytotoxicity genes in Tc17 cells
Since Tc17 cultures switched to Tc1 conditions were able to acquire IFNγ and GrB expression, we then tested further whether other genes in these cultures that are associated with cytotoxicity demonstrated changes in expression. Similar to results of intracellular cytokine staining, Il17a was expressed in Tc17 cells but not in Tc1 cultures, and expression was diminished in Tc17 cells cultured for a second week in Tc1 conditions (Fig. 3). Ifng and Gzmb also showed similar patterns to intracellular staining; higher expression in Tc1 cells and acquired expression in Tc17 cells switched to Tc1 conditions, though still lower than in Tc1 cells. Expression of Tnf was not different between Tc1 and Tc17 cells. Somewhat surprisingly, Tc17 cells switched to culture under Tc1 conditions did not demonstrate increased expression of Prf1 or Tnfsf10 (encoding TRAIL), but rather maintained low expression, compared to Tc1 cells, regardless of culture condition (Fig. 3). In contrast to the pattern of expression of other genes, Fasl, which was expressed at considerably lower levels in cells cultured for one round in Tc17 conditions than in Tc1 cells, demonstrated expression comparable to Tc1 cells in Tc17 cultures that were switched to either Tc1 or Tc17 culture conditions for the second round (Fig. 3).
Figure 3.
Expression of cytotoxicity-associated genes in Tc17 cultures. OT-l CD8+ T cells were cultured under Tc1 or Tc17 conditions. After five days, Tc17 cells were re-stimulated under Tc1 or Tc17 conditions for an additional five days. RNA was isolated from differentiated cells after 4 h of re-stimulation with SIINFEKL peptide. Expression for the indicated genes was measured using quantitative PCR with samples being normalized to the expression of β2-microglobulin mRNA and are relative to levels in Tc1 cells, with the exception of Il17a. A logarithmic scale was used to display the relative expression of all genes with the exception of Il17a and Tnf.
Tc17 cells in vivo
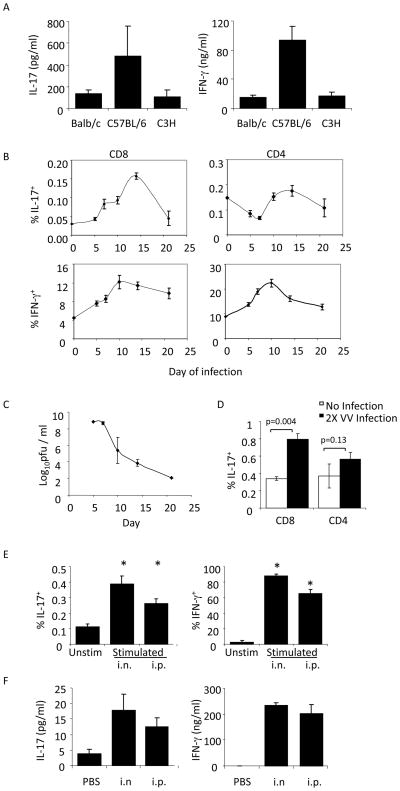
While considerable attention has been paid to the function of in vitro-derived Tc17 cells, little work has been performed on in vivo-derived IL-17-secreting CD8 T cells. To test the ability of CD8 T cells from different strains of mice to produce IL-17, we isolated CD8 T cells from naïve Balb/c, C57BL/6 and C3H mice, stimulated them with anti-CD3 and assessed the production of IL-17 and IFNγ using ELISA. While amounts of IL-17 were significantly lower than IFNγ, IL-17 production was detectable in cultures from all three strains, and highest in C57BL/6 (Fig. 4A). To determine if this population expanded in response to viral infection, we innoculated mice with VV and assessed IL-17 and IFNγ production in CD4 and CD8 cells at time points after infection (Fig. 4B). While both CD4 and CD8 T cells showed transient increases in IFNγ-producing cells, with the decreases following viral clearance (Fig. 4B and C), only CD8 T cells showed an increase in the number of IL-17-secreting cells (Fig. 4B). While the number of IL-17+ cells was much lower than the number of IFNγ-producing cells, the fold-induction of cells, compared to uninfected mice, was similar to that observed for IFNγ-producing cells. We performed a similar experiment in mice that were infected twice with VV to examine the memory response, and while there was no significant increase in IL-17-secreting CD4 T cells, there was a significant increase in IL-17-secreting CD8 T cells, compared to uninfected mice (Fig. 4D).
Figure 4.
Tc17 cells are induced in response to infection with vaccinia virus. (A) CD8+ T cells were isolated from the spleens of Balb/c, C57BL/6, or C3H/HeJ mice and stimulated for 48 h before cell free supernatants were used to measure IL-17 and IFN-γ protein levels using ELISA. Results are presented as the mean ± SEM of CD8+ T cells from 3 mice. (B, C) C57BL/6 mice were infected i.p. with 5x106 pfu VV per mouse. Spleens and ovaries were respectively isolated for cytokine analysis following re-stimulation (B) or viral titers (C) at 5, 7, 10, 14, and 21 days postinfection. The data are presented as an average of values from 4 mice per time point ± SEM. (D) C57BL/6 mice were infected on days 0 and 21 with 1x106 pfu VV, i.p., per mouse at each time point. Five days after the second infection, CD8+ and CD4+ cells were isolated from the spleens and re-stimulated before analyzing IL-17 and IFN-γ production using ICS. The data shown are an average of values from 2–4 mice ± SEM. (E–F) BoyJ homozygous (CD45.1+) or heterozygous (CD45.1+CD45.2+) mice were injected i.v. with 1x106 OT-I / Rag1−/− CD8+ (CD45.2+) T cells. One day later, mice were infected with either 2x106 pfu VV-SIINFEKL i.n., or 2x107 pfu VV-SIINFEKL, i.p. (E) Five days after infection, splenocytes were stimulated with SIINFEKL peptide or left unstimulated. CD45.1-CD45.2+ IL-17+ and IFN-γ+ cells were detected using ICS. The data are the average ± SEM of 5 mice. (F) Splenocytes were stimulated with SIINFEKL peptide for 48 h and IL-17 and IFN-γ protein levels in cell free supernatants were measured using ELISA. Statistics in B and D were performed using a Student’s T test. *, p<0.05 compared to unstimulated condition.
To determine if the increased numbers of IL-17-secreting CD8 T cells elicited in vivo were specific for viral antigen, we adoptively transferred naïve OT-I T cells to recipient mice that were subsequently infected with VV expressing the ovalbumin SIINFEKL peptide. Five days after either intranasal or intraperitoneal infection, splenocytes were stimulated with peptide and analyzed using intracellular cytokine staining and ELISA. A significant increase in epitope-specific IL-17-secreting cells was observed in mice following both routes of infection (Fig. 4E and F).
Anti-viral activity of Tc17 cells in vivo
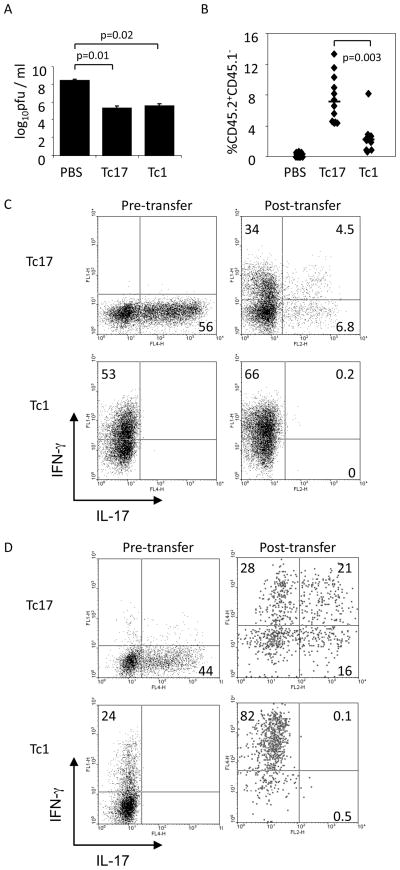
We next tested the ability of Tc17 cells to mediate viral clearance. OT-I cells were differentiated in vitro to Tc1 or Tc17 phenotypes, and cultured cells were adoptively transferred to mice one day after i.p. infection with VV-SIINFEKL. At day 7 post-infection, viral titers in the ovaries were determined. Transferred Tc1 or Tc17 cells were equally capable of enhancing the clearance of virus (Fig. 5A). While transferred Tc17 cells showed greater expansion than Tc1 cells, they lost IL-17-secreting potential and demonstrated increased production of IFNγ; however the levels were still lower than Tc1 cells (Fig. 5B and C). Analysis of post-transfer cells following intranasal VV inoculation showed a switch to more IFNγ and less IL-17 production, though the polarization was less dramatic compared to intraperitoneal infection (Fig. 5C and D).
Figure 5.
Adoptively transferred Tc17 cells promote VV-SIINFEKL clearance and convert to an IFN-γ-secreting phenotype. (A–C) BoyJ (CD45.1+) mice were infected with 2x107 pfu VV-SIINFEKL, i.p., and injected i.v. with differentiated OT-l Tc17 or Tc1 cells (1x106, CD45.2+) or PBS after 24 h. Six days later ovaries were harvested for viral titer determination (A), and splenocytes were surface stained with antibodies to CD45.1 and CD45.2 to identify transferred cells (B). (C) IL-17+ and IFN-γ+ cells were identified in differentiated OT-l Tc17 or Tc1 cells immediately before adoptive transfer (left panels) and in splenocytes six days after adoptive transfer (right panels). Transferred cells in the right panels are gated on CD45.1−CD45.2+ cells. Data are the average of 4–5 mice ± SEM (A) or are representative experiments (B, C). (D) Differentiated OT-I Tc17 or Tc1 cells (1x106) or PBS were injected i.v. into BoyJ mice. One day later, mice were infected with 2x106 pfu VV-SIINFEKL intranasally. IL-17+ and IFN-γ+ cells were determined in Tc17 or Tc1 cells immediately before adoptive transfer (left panels) and five days after adoptive transfer in isolated splenocytes (right panels). Transferred cells in the right panels are gated on the CD45.1−CD45.2+ cells. The data are representative of 6–7 mice. Statistics in A and B were performed using a Student’s T test.
Acquisition of a Tc1 phenotype in vivo requires virus-encoded antigen
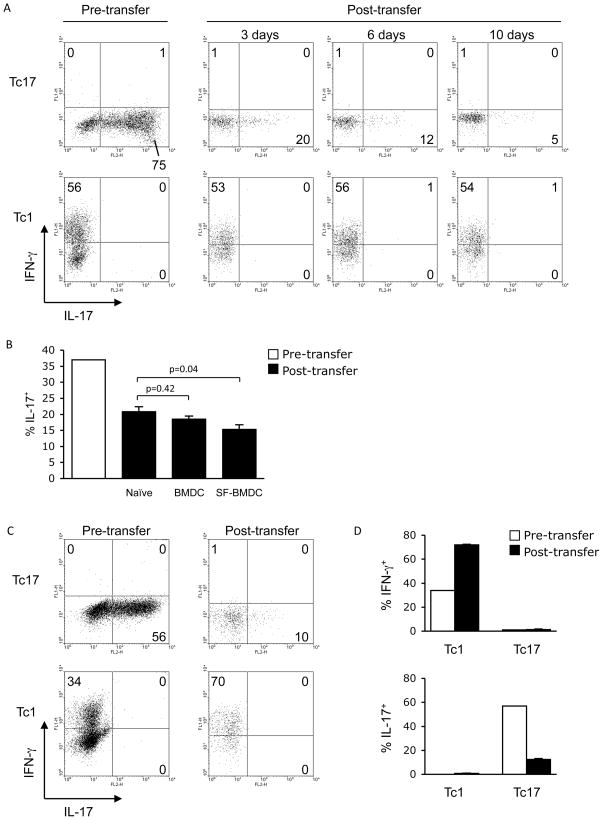
Since we observed that Tc17 cells transferred to VV-infected mice altered their phenotype towards a potential for increased IFNγ production and decreased IL-17 production, we wanted to determine if virus-encoded antigen was required for the altered phenotype. Transfer of Tc17 cells into naïve mice resulted in a time-dependent decrease in IL-17 production with no concomitant increase in IFNγ production, while transferred Tc1 cells maintained the IFNγ-secreting phenotype (Fig. 6A and B). When OT-I Tc17 cells were transferred with BMDC or SIINFEKL-pulsed BMDC, there was a similar loss of IL-17-secreting potential, (significantly more loss of IL-17 in the mice receiving SIINFEKL-pulsed BMDC versus naïve mice) but little induction of IFNγ (Fig. 6B and data not shown), despite increased cell expansion in mice that received SIINFEKL-pulsed BMDCs (1.1 ± 0.06% CD45.2+ cells in spleen versus only 0.31 ± 0.15% for BMDCs alone). We then tested whether VV infection alone, without relevant antigen, would alter the phenotype of the cells. Tc1 cells showed increased IFNγ-secreting potential following infection (Fig. 6C and D). However, transferred Tc17 cells still displayed a marked reduction in the percentage of IL-17-positive cells after infection, with no increase in IFNγ production (Fig. 6C and D). These results suggest that the combination of virus-encoded antigen and infection are required to promote conversion of cells to the Tc1 phenotype in vivo.
Figure 6.
VV-encoded antigen is required for Tc17 cells to convert to an IFN-γsecreting phenotype in vivo. (A) Naïve BoyJ (CD45.1+) mice were injected i.v. with five day differentiated OT-l Tc17 or Tc1 cells (1x106, CD45.2+) or PBS. After an additional 3, 6, or 10 days, spleens were isolated and total splenocytes were stimulated with SIINFEKL (SF) peptide. IL-17+ and IFN-γ+ cells were identified in differentiated Tc17 or Tc1 cells immediately before adoptive transfer (left panels) and 3, 6, and 10 days after adoptive transfer from splenocytes (right panels). Transferred cells in the right panels are gated on the CD45.1−CD45.2+ cells. The data are representative of two experiments. (B) BoyJ mice were injected i.v. with BMDCs untreated or pulsed with SIINFEKL peptide (1x106, CD45.1+) or PBS-treated and injected i.v. with differentiated OT-l Tc17 cells (1x106, CD45.2+) 48 h later. After an additional 5 days, spleens were isolated and total splenocytes were stimulated with SIINFEKL peptide. IL-17+ cells were identified in differentiated Tc17 cells immediately before adoptive transfer and 5 days after adoptive transfer from total splenocytes. Transferred cells are gated on CD45.1−CD45.2+ cells. The data are the average of 3–4 mice ± SEM and are representative of 2 or more experiments. (C, D) BoyJ mice were infected i.p. with 2x107 pfu VV-WR and injected i.v. with differentiated OT-l Tc17 or Tc1 cells (1x106) or PBS one day later. After an additional six days, spleens were isolated and total splenocytes were stimulated with SIINFEKL peptide. (C) IL-17+ and IFN-γ+ cells were analyzed in differentiated OT-l Tc17 or Tc1 cells immediately before adoptive transfer (left panels) and six days after adoptive transfer in spleen (right panels). The transferred cells were gated on CD45.1−CD45.2+ cells. Data are representative of 5 mice (C), or are the average ± SEM of 5 mice (D) and are representative of 2 or more experiments. Statistics in B were performed using a Student’s T test.
Acquisition of cytotoxic function by Tc17 cells in vivo
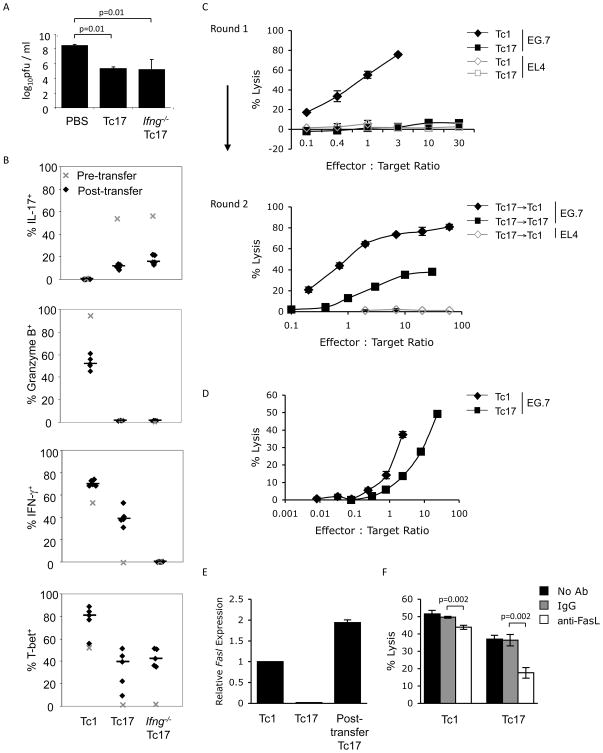
To determine if IFNγ was required for Tc17-mediated viral clearance, we repeated this experiment using both wild type and Ifng−/− OT-I cells. Somewhat surprisingly, WT and Ifng−/− Tc17 cells were equally capable of clearing VV infection (Fig. 7A). While Ifng−/− Tc17 cells demonstrated increased potential for production of IL-17 and modest increases in T-bet expression, neither wild type nor Ifng−/− Tc17 cells showed acquisition of GrB expression (Fig. 7B). Similar results were obtained using Stat4−/− Tc17 OT-I cells, suggesting that Stat4 was also not required for the anti-viral activity of Tc17 cells (data not shown). Thus, conversion to an IFNγ-secreting phenotype is not a crucial component of Tc17-mediated viral clearance.
Figure 7.
Adoptively transferred Tc17 cells acquire cytotoxic potential in vivo. (A, B) BoyJ (CD45.1+) mice were infected with 2x107 pfu VV-SIINFEKL i.p. and injected i.v. with differentiated OT-l Tc17, Ifng−/− Tc17 and Tc1 cells (1x106, CD45.2+) or PBS 24 h later. After six days, ovaries were harvested for viral titer determination (A), and IL-17+, IFN-γ+, Granzyme B+, and T-bet+ CD45.1−CD45.2+ splenic cells were analyzed using ICS (B). Median values are displayed as horizontal lines and symbols represent individual mice. (C) After culture for one or two rounds (as indicated) under Tc17, Tc1 or Tc17 switched to Tc1 conditions, OT-I CD8+ T cells were tested for cytotoxic potential using a 51Cr-release assay. Effector cells were co-cultured with 51Cr-labeled, Ova-expressing EG.7 target cells for 6 h at the indicated effector to target ratios. (D–F) Mice were infected with VV-SIINFEKL and injected with OT-I Tc17 or Tc1 cells as described in (A). Six days later, injected cells were isolated using anti-CD45.2 antibodies and magnetic selection. (D) A 51Cr release assay was performed as in (C). E:T ratios were normalized to the percentages of CD45.1−CD45.2+ cells in the purified populations. Cytotoxicity against EL4 targets was less than 1.5%. (E) Expression of Fasl mRNA was determined in post-transfer Tc17 cells and compared to in vitro derived Tc1 and Tc17 cells using quantitative PCR. (F) A 51Cr release assay was performed as in (D) with the addition of control antibodies or anti-FasL. E:T ratios for post-transfer Tc1 were 2.5:1 and for post-transfer Tc17 were 20:1. Statistics in A and F were performed using a Student’s T test.
The ability of transferred Tc17 cells to mediate viral clearance, regardless of IFNγ production, suggested that Tc17 cells might acquire a cytotoxic phenotype in vivo. As others have shown, and we have reproduced, Tc17 cells, after 1 week of culture, have limited cytotoxic activity in a standard 51Cr-release assay (Fig. 7C). However, after two rounds of culture in Tc17 conditions that maintain the IL-17-secreting phenotype, Tc17 cells acquire cytotoxic activity, though this activity is still comparatively less efficient than killing by cells cultured under Tc1 conditions for two rounds (data not shown), or by cells switched from Tc17 to Tc1 conditions for the second round of culture (Fig. 7C).
We then wanted to determine if the acquisition of cytotoxic activity that potentially contributed to viral clearance also occurred in vivo. Transferred Tc1 and Tc17 OT-I cells were purified after VV-SIINFEKL infection and function in a cytotoxicity assay was tested. Purified Tc17 cells following infection demonstrated in vitro cytotoxic activity following adoptive transfer to virus-infected mice, although they were still less efficient than similarly purified Tc1 cells (Fig. 7D).
Since Fasl expression was increased in long-term Tc17 cultures (Fig. 3), and these cells acquired cytotoxic activity (Fig. 7C), we tested whether there was increased Fasl expression in cytotoxic Tc17 cells isolated post-transfer. We observed that Tc17 cells isolated post-transfer demonstrate Fasl expression comparable to in vitro differentiated Tc1 cells (Fig. 7E). To determine if FasL is responsible for the cytotoxic activity of Tc17 cells following infection we performed a cytotoxicity assay as in Fig. 7D with the addition of control antibodies or anti-FasL. Incubation with anti-FasL, but not the control antibodies, resulted in a modest though significant decrease in Tc1-mediated cytotoxicity (Fig. 7E). Moreover, anti-FasL resulted in a 50% decrease in the cytotoxic activity of post-transfer Tc17 cells. Thus, Tc17 cells can acquire cytotoxic potential in vivo that is at least partially dependent upon FasL.
Discussion
Cytokines are critical effectors of T cell function, and CD8 T cells predominantly produce IFNγ upon stimulation. However, CD8 T cells can acquire various cytokine-secreting potentials, including a phenotype resembling the Th17 phenotype, termed Tc17. Acquisition of this phenotype is directed by similar cytokines and transcription factors as the Th17 subset. However, the function of these cells is still not well defined. In several studies, Tc17 cells have been shown to mediate anti-viral immunity and inflammation. Whether Tc17-mediated immunity is through promoting inflammation or acquiring a cytotoxic phenotype is not clear.
One of the features of in vitro-derived Tc17 cells following in vivo injection is that these cells lose the IL-17-secreting phenotype. As shown in Th17 cells (19, 20), the development of Tc17 cells requires STAT3 and results in cells expressing RORγt ((5, 6, 9, 11) and data not shown). However, signals that generate the Tc1 phenotype appear dominant as retroviral expression of STAT3C or RORγt in developing Tc1 cultures resulted in only modest shifts of phenotype (data not shown). Using in vitro experiments, we determined that IFNγ, STAT4 and T-bet contribute to the ability of Tc17 cells to switch phenotypes (Fig. 1 and 2). IFNγ and T-bet promote the repression of IL-17-secreting potential, while STAT4 is mostly dispensable for this function. This is consistent with the ability of T-bet to cooperate with Eomes in repressing CD8 IL-17 production (8). In contrast, STAT4 is more critical than T-bet in the induction of IFNγ-secreting potential in CD8+ T cells, and this differs with the roles of STAT4 and T-bet in regulating Th17 to Th1 conversion where both factors are equally required (21). Both STAT4 and T-bet contribute to increased GrB after culture of Tc17 in Tc1 conditions, though neither factor is absolutely required (Fig. 2). Yet in vivo, viral clearance and the acquisition of cytotoxic activity were independent of both IFNγ and STAT4, and were not associated with an increase in GrB expression (Fig. 6 and data not shown). Thus the signals that generate cytotoxic Tc17 cells remain undefined.
While Tc17 cells can clearly develop in vitro, their existence in vivo is not well characterized. In this report, we demonstrated IL-17 production from unimmunized CD8 T cells, and an expansion of IL-17-secreting CD8 T cells following infection with VV. This is similar to observations following an influenza virus infection (12). We further show that there is development of IL-17-secreting CD8 T cells specific for a virus-derived peptide following VV infection. However, while this enhancement of IL-17-secreting CD8 T cells is specific, it is unlikely to be the sole mechanism responsible for VV clearance and anti-viral immunity. Accordingly, we observed, respectively, slightly enhanced or normal clearing of VV from Il17−/− and Stat3CD4−/− mice (data not shown). This suggests that IL-17 or Th17/Tc17 cells are not critical for viral clearance. However, IL-17 may regulate anti-viral immunity through additional mechanisms. A recombinant VV encoding IL-17 had increased virulence in a mouse model, suggesting IL-17 impaired anti-VV immunity (22). Moreover, IL-17 has been shown to limit Th1 development (23), and it has been suggested that VV uses induction of IL-17 as a mechanism of immune evasion, particularly in skin with atopic dermatitis (24, 25). In contrast, a recombinant VV expressing IL-23 enhanced anti-viral immunity through a mechanism that was at least partially IL-17-dependent (26). Thus, IL-17 may be involved in regulating the effectiveness of the anti-viral response in a context-dependent manner.
Perhaps the most critical question is how Tc17 cells mediate anti-VV immunity. Our data demonstrate that Fasl is unique among cytotoxicity-associated genes in that expression is lower in Tc17 cells compared to Tc1 cells after 1 week of culture, but expression increases after additional culture in vitro or post-transfer in vivo (Fig. 3 and 7E). We further showed that blocking FasL significantly decreased post-transfer Tc17 cytotoxicity (Fig. 7F), suggesting that Tc17 cytotoxicity is at least partially FasL-dependent. Whether other mediators are involved is still unclear. Hamada et al showed that Tc17-mediated influenza immunity was dependent on IFNγ but not perforin (12). However, we showed that IFNγ was not required for Tc17-mediated immunity to VV (Fig. 7), consistent with a prior report that IFNγ is not required for immunity to VV (27). Similarly, we saw no dependence on STAT4 (data not shown), suggesting that many features of the IL-12-induced genetic program are not required for Tc17-mediated immunity to VV. In agreement with this, we did not see any increase in expression of either perforin or granzyme B when Tc17 cells acquired a cytotoxic phenotype (Fig. 3 and 7). As TNFα expression is not vastly different between Tc1 and Tc17 cells (Fig. 3), it seems unlikely to be a candidate mechanism for Tc17-mediated killing in vitro. However, it could be involved in viral clearance. We observed some increased pulmonary inflammation in mice infected with VV intranasally upon receiving transferred Tc17 cells, compared to mice receiving Tc1 cells, and similar to observations in the influenza model (12). However, this was not uniform among all Tc17 recipients, and there was no correlation between inflammation and viral clearance (data not shown). It is likely that there are multiple overlapping mechanisms of VV clearance and the specific contribution of each mechanism may depend on the pathogen. Importantly, it is clear that Tc17 cells do function after transfer in vivo, and in some experiments function better than matched Tc1 cells (6, 10). In this report, we demonstrate that after extended culture in vitro, or following exposure to antigen-encoded virus in vivo, Tc17 cells can acquire a cytotoxic phenotype that is at least partially dependent upon FasL.
While IL-17-secreting CD4 T cells are established as an important component of inflammatory immunity, the role of Tc17 cells is less clear. Several studies have shown that Tc17 cells expand during infection or inflammation, and that they can function in vivo. Importantly, the current study reveals that Tc17 cells, while initially non-cytotoxic, acquire a cytotoxic phenotype and can mediate clearance of VV in vivo. The requirement for FasL and the mechanisms of cytotoxic phenotype acquisition will be important to determine in the future.
Acknowledgments
The authors wish to thank Jeremy Eltz and Beau Champ in the VV Core for help with experiments.
This work was supported by grants from the NIH (R21 AI077091 and PO1 AI056097 to RRB, JSB and MHK; AI46530 to RWD).
Footnotes
Abbreviations used: BMDC, bone marrow-derived DC; GrB, granzyme B; VV, vaccinia virus
References
- 1.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. J Immunol. 2006;177:7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 3.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 4.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 5.Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hunig T, Mittrucker HW, Brustle A, Kamradt T, Lohoff M. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 6.Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, Liang KL, Bruno TC, Pyle KJ, Chan SL, Anders RA, Trimble CL, Adler AJ, Lin TY, Pardoll DM, Huang CT, Drake CG. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol. 2009;183:7161–7168. doi: 10.4049/jimmunol.0900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182:5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 8.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis MM, Way SS, Wilson CB. IL-23 promotes the production of IL-17 by antigen-specific CD8 T cells in the absence of IL-12 and type-I interferons. J Immunol. 2009;183:381–387. doi: 10.4049/jimmunol.0900939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, Chen HW. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol. 2007;82:354–360. doi: 10.1189/jlb.0207111. [DOI] [PubMed] [Google Scholar]
- 12.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 14.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 15.Thieu VT, Yu Q, Chang HC, Yeh N, Nguyen ET, Sehra S, Kaplan MH. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, Wang N, Zhou D, Yee CS, Chang CH, Brutkiewicz RR, Blum JS. Disruption of MHC class II-restricted antigen presentation by vaccinia virus. J Immunol. 2005;175:6481–6488. doi: 10.4049/jimmunol.175.10.6481. [DOI] [PubMed] [Google Scholar]
- 17.Brutkiewicz RR, Klaus SJ, Welsh RM. Window of vulnerability of vaccinia virus-infected cells to natural killer (NK) cell-mediated cytolysis correlates with enhanced NK cell triggering and is concomitant with a decrease in H-2 class I antigen expression. Nat Immun. 1992;11:203–214. [PubMed] [Google Scholar]
- 18.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 19.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 20.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL- 17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 21.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patera AC, Pesnicak L, Bertin J, Cohen JI. Interleukin 17 modulates the immune response to vaccinia virus infection. Virology. 2002;299:56–63. doi: 10.1006/viro.2002.1400. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, Tagaya Y, Crotty S, Kawakami T. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J Exp Med. 2009;206:1219–1225. doi: 10.1084/jem.20082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyoshi MK, Elkhal A, Kumar L, Scott JE, Koduru S, He R, Leung DY, Howell MD, Oettgen HC, Murphy GF, Geha RS. Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proc Natl Acad Sci U S A. 2009;106:14954–14959. doi: 10.1073/pnas.0904021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohyama S, Ohno S, Isoda A, Moriya O, Belladonna ML, Hayashi H, Iwakura Y, Yoshimoto T, Akatsuka T, Matsui M. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J Immunol. 2007;179:3917–3925. doi: 10.4049/jimmunol.179.6.3917. [DOI] [PubMed] [Google Scholar]
- 27.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]