Summary
During mammary gland development an epithelial bud undergoes branching morphogenesis to expand into a continuous tree-like network of branched ducts [1]. The process involves multiple cell types that are coordinated by hormones and growth factors coupled with signaling events including Wnt and Hedgehog [2-5]. Primary cilia play key roles in the development of many organs by coordinating extracellular signaling (Wnt, Hedgehog) with cellular physiology [6-8]. During mammary development, we find cilia on luminal epithelial, myoepithelial and stromal cells during early branching morphogenesis when epithelial ducts extend into the fat pad and undergo branching morphogenesis. When branching is complete, cilia disappear from luminal epithelial cells but remain on myoepithelial and stromal cells. Ciliary dysfunction caused by intraflagellar transport (IFT) defects results in branching defects. These include decreased ductal extension and decreased secondary and tertiary branching along with reduced lobular-alveolar development during pregnancy and lactation. We find increased canonical Wnt and decreased Hedgehog signaling in the mutant glands, which is consistent with the role of cilia in regulating these pathways [6-11]. In mammary gland and other organs, increased canonical Wnt [12-14] and decreased Hedgehog [15, 16] signaling decreases branching morphogenesis suggesting that Wnt and Hedgehog signaling connect ciliary dysfunction to branching defects.
Keywords: Cilia, intraflagellar transport, branching morphogenesis, mammary, pregnancy, lactation
Results and Discussion
Primary Cilia are Abundant on Multiple Cell Types in the Mammary Gland
In the mammary gland, the ductal epithelium consists of a luminal layer of secretory cells and an outer layer of myoepithelial cells embedded in an extracellular matrix (ECM) and surrounded by a stroma of adipocytes, fibroblasts, vasculature and immune cells. Prior electron microscopy studies documented primary cilia on basally-located myoepithelial cells [17-20] but cilia were rarely observed on the luminal epithelial cells. It is unusual for luminal epithelial cells in vertebrates not to be ciliated and so we re-examined the distribution of cilia. Cilia were present on myoepithelial and stromal cells at similar percentages throughout development (Fig. 1A,B, Supplemental Fig. 1A). Cilia on myoepithelial cells extend from the centrosome toward the stroma and ECM or toward the interstitial space between the myoepithelial cells and luminal epithelial cells (Supplemental Fig. 1B). In contrast to previous reports, we also found cilia on the luminal epithelial cells in terminal end buds (TEB) and mature ductal structures. Luminal epithelial cilia were more abundant early in development (4 weeks: 17% +/- 1.9) and decreased significantly in developed tissues (7 weeks: 2% +/- 1.1, p=6×10-4; adult: 4% +/- 1.9%, p=7×10-4; pregnancy: 4% +/- 1.5%, p=1×10-4) (Fig. 1B, Supplemental Fig. 1C). The loss of cilia with maturity is reminiscent of the cilium on hair cells in the rodent ear. In these cells, a cilium forms initially and appears to be required for proper development of the microvilli bundles but regresses once the microvilli have formed [22]. Thus in these epithelial cell types, it appears that cilia play developmental roles but are not critical once the organ has developed.
Figure 1. Localization of primary cilia within the mammary gland.
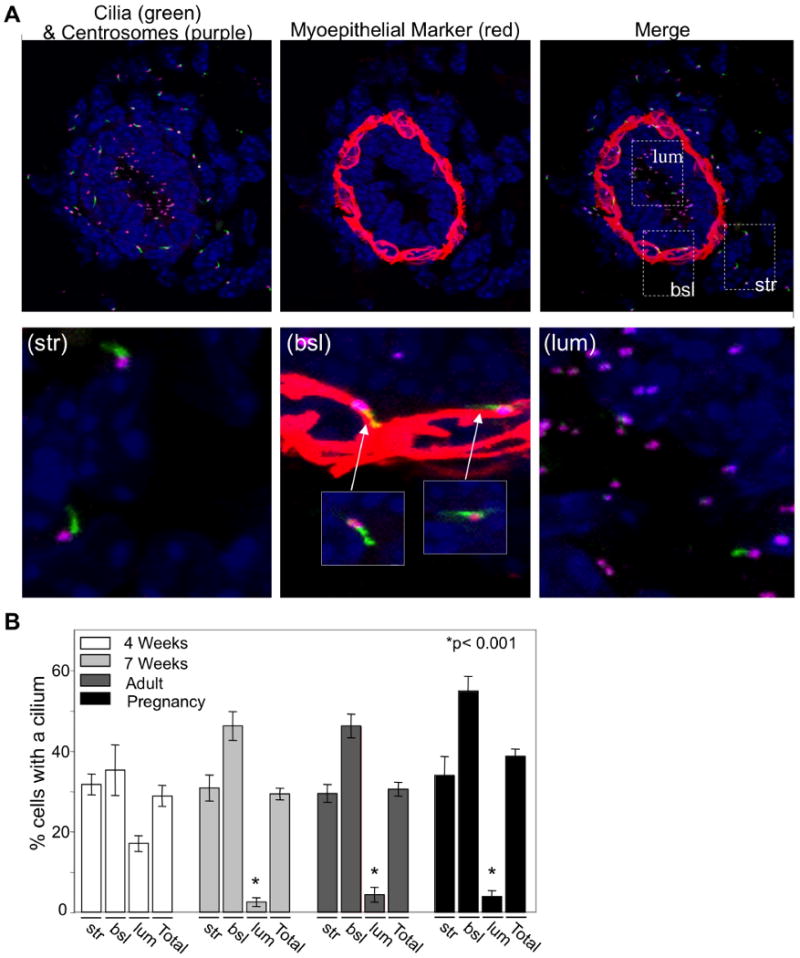
(A) Immunofluorescent confocal projections were acquired for the localization of primary cilia in the murine mammary glands (7weeks). Cilia were detected by an antibody to acetylated α-tubulin (green), centrosomes were detected by an antibody to γ-tubulin (purple) and myoepithelial cells (a basally-located epithelial cell type) were identified with an antibody recognizing smooth muscle actin (red). Insets highlight the localization of cilia on stromal (str), basal epithelial (bsl) and luminal epithelial (lum) cells. (B) Bar graph represents quantitation of the number of cells with a primary cilium in stromal (str), basal epithelial (bsl) and luminal epithelial (lum) cells in murine mammary glands of early development (4 weeks; n value = 4 mice, >500cells), mature (7 weeks; n value = 5 mice, >500 cells and adult; n value = 4 mice, >500 cells) and mid-pregnant (12 days; n value = 3 mice, >500 cells) mammary glands. Student's t-Test (2-sided) was performed to determine statistical significance (*). Statistical significance noted is compared to 4-week luminal epithelial values. See also Figure S1.
Primary Cilia Regulate Branching Morphogenesis
The conservation of cilia in mammary glands across diverse species suggests that cilia have biological significance in this organ but this has not been studied. To address this question, we analyzed branching morphogenesis in the mammary glands of the Tg737orpk mouse, which has a ciliary assembly defect due to a mutation in the IFT88 subunit of the IFT particle. [23-27]. IFT88 is found in control mammary cilia but is not detectable in mutant cilia (Fig 2A). Mutant animals have significantly reduced (4 weeks: p=0.006; 7 weeks: p=7×10-5; pregnant: p=2×10-5) numbers of cilia as compared to controls (Fig. 2B,C). The cilia that remained in the mutants were shorter than those in controls, consistent with ciliary dysfunction (Fig. 2A).
Figure 2. Ciliary dysfunction in mammary glands of the Tg737orpk mutant mouse.
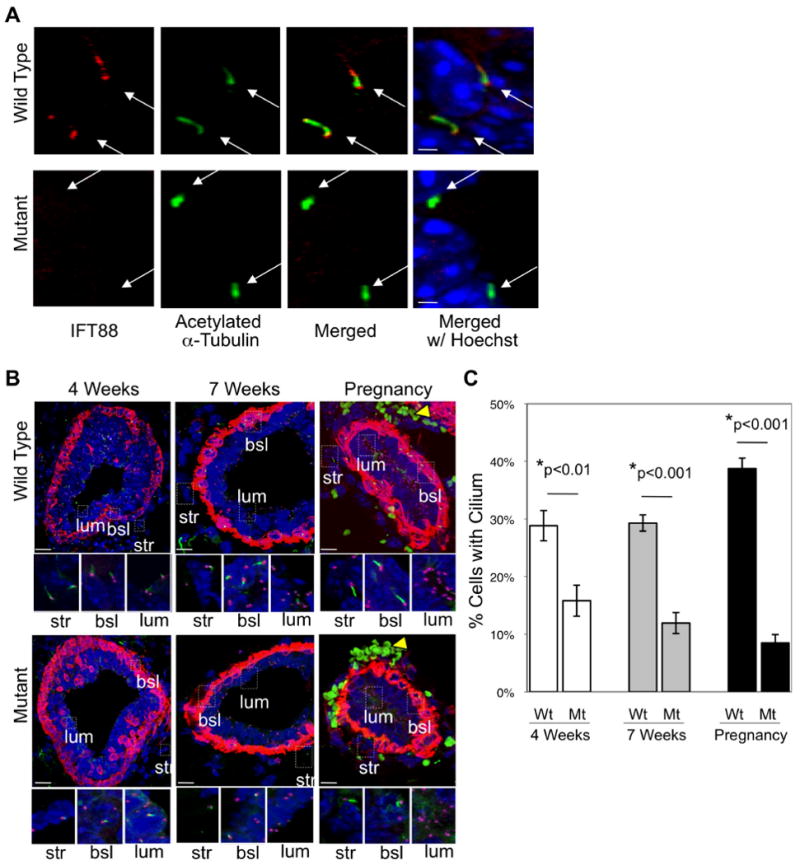
(A) Immunocytochemistry with antibodies that recognize IFT88 (red) and acetylated α-tubulin (green) was performed to determine whether IFT88 is expressed in the cilium (white arrows) in mammary glands of wild type and mutant mice. (B) Mammary glands were harvested at early development (4 weeks), late development (7 weeks) and mid-pregnancy (12 days) from wild type and mutant mammary glands. Immunofluorescent confocal projections were acquired for the localization of primary cilia. Cilia were detected by an antibody to acetylated α–tubulin (green), centrosomes were detected by an antibody to γ-tubulin (purple) and myoepithelial cells (a basally-located epithelial cell type) were identified with an antibody recognizing smooth muscle actin (red). Insets highlight the localization of cilia on stromal (str), basal epithelial (bsl) and luminal epithelial (lum) cells. Note that the green cells (yellow arrowhead) visible during pregnancy are autofluorescent red blood cells. (C) Immunocytochemistry with antibodies to acetylated α-tubulin and γ-tubulin was performed to determine the number of cilia in mammary glands of the wild type (Wt) and mutant (Mt) mice at early development (4 weeks), late development (7 weeks) and mid-pregnancy (12 days). Bar graphs represent quantification of the number of cells containing a primary cilium. These results validate the usefulness of the Tg737orpk model for studying ciliary function in mammary gland biology. Student's t-Test (2-sided) was performed to determine statistical significance (*). (n values: for Wt, 4 weeks = 5 mice, >500 cells; for Mt, 4 weeks = 5 mice, >500 cells; for Wt, 7 weeks = 5 mice, >500 cells; Mt, 7 weeks = 5 mice, >500 cells; for Wt, pregnant = 4 mice, >500 cells; for Mt, pregnant = 2 mice, >500 cells).
Branching morphogenesis begins in the mammary gland with the onset of puberty at 3-4 weeks and continues until the growth of primary ducts accompanied by secondary and tertiary branching fills the fat pad at 8-10 weeks of age [1]. To test whether cilia play a role in ductal extension and branching morphogenesis, mammary glands from mutant and control animals were examined by whole mounts at early (4 weeks) and late (7 weeks) development. At 4 weeks, the glands of the mutant mice had significantly less (p=2×10-5) ductal extension as compared to controls (Fig. 3A, Supplemental Fig. 2A). By 7 weeks, ductal extension and branching morphogenesis in the control animals was nearly complete and the fat pad was almost completely filled (Fig. 3B, Supplemental Fig. 2B). In contrast, ductal extension in the mutant animals was significantly reduced (p=3×10-8) and a large portion of the fat pad was unfilled (Fig. 3B, Supplemental Fig. 2B). At 7 weeks, while the number of tertiary branched points was the same, the number of secondary branch points was significantly less (p=5×10-5) and there was less organization and directionality of the branches in the mutant glands as compared to controls (Fig. 3C, Supplemental Fig. 2C). These results demonstrate that ductal extension and branching morphogenesis of the mammary ductal tree are decreased by ciliary dysfunction due to reduced IFT88.
Figure 3. Primary cilia regulate branching morphogenesis.
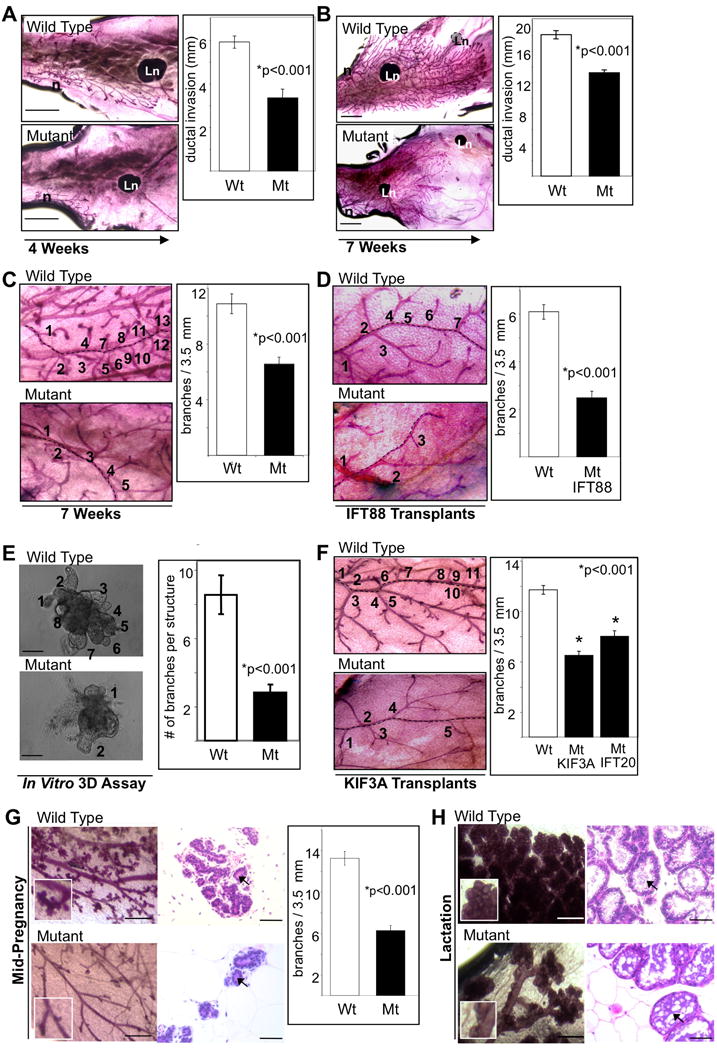
Whole mount analysis was performed on wild type (Wt) and mutant (Mt) mammary glands harvested at (A) early development (4 weeks) and (B and C) late development (7 weeks) to determine the extent of ductal elongation and the number of branch points. Whole mounts were also prepared from mammary glands of mice that had their fat pads cleared of endogenous ductal tissue and transplanted with mammary tissue from (D) wild type or mutant Tg737orpk mice (harvested 8-12 weeks post transplantation). Branching morphogenesis was also assayed (E) in a three-dimensional (3D) in vitro basement membrane culture using wild type or mutant mammary tissue isolated from Tg737orpk mice. (F) Whole mounts of mammary gland transplants from wild type and Kif3A mutant mice. Quantification of branching morphogenesis in transplants from wild type, Kif3A and Ift20 mice is shown in the bar graph. Mammary glands were harvested at (G) mid-pregnancy (12 days) and (H) lactation (5 days of nursing) from wild type and mutant mammary glands. Glands were analyzed by whole mount (left panels) and H&E stain (right panels) at each time point. Abbreviations: Ln, lymph node; n, nipple. Bar graphs represent the quantification of (A and B) the extent of ductal elongation (arrow designates direction of growth) into the fat pad, (C, D, F and G) the number of branch points per 3.5 mm ductal length (numbers and dashed line represent number of branch points from a duct) and (E) the number of branch points per tissue piece. Student's t-Test (2-sided) was performed to determine statistical significance (*). (n values for ductal extension at (A) 4 weeks and (B) 7 weeks for Wt and Mt = 5 mice, 15 ducts each; n values for branch points at (C) 7 weeks for Wt and Mt = 5 mice, 14 and 13 ducts respectively, (D) transplanted tissue for Wt = 4 mice, 12 ducts and Mt = 7 mice, 27 ducts), (E) 3D assay was performed in triplicate counting Wt = 109 tissue pieces and Mt = 92 tissue pieces, (F) transplanted tissue for Wt = 10 mice, 69 ducts and Mt Kif3A = 7 mice, 43 ducts and Mt IFT20 = 4 mice, 34 ducts and (G) mid-pregnancy for Wt = 4 mice, 19 ducts and Mt = 2 mice, 18 ducts). See also Figures S2, S3 and S4.
Decreased ductal extension and branching morphogenesis in the mammary gland of the Tg737orpk mouse could be due to loss of localized ciliary function in the mammary gland or could be a result of secondary effects of ciliary dysfunction at distant organ sites. For example, the loss of cilia could delay the age of puberty, which in turn would affect mammary development. The age of vaginal opening, which correlates with the onset of puberty [28], was similar in control and mutant animals (Supplemental Fig. 3A) suggesting that onset of puberty is not a factor in the phenotype. In addition, we found no significant difference in the estradiol levels (Supplemental Fig. 3B) indicating that the branching morphogenesis defect is not an indirect result of altered estrogen signaling. Weight also is a factor in onset of puberty [28] but we found no statistically significant difference between the weights of the Tg737orpk control and mutant mice (Supplemental Fig. 3C) at puberty.
While the onset of puberty and estrogen signaling did not appear to be contributing factors to the phenotype, the pleiotrophic nature of the Tg737orpk mutation raises concerns about other unanticipated secondary effects. To overcome these concerns and more directly assess how the loss of primary cilia affects branching morphogenesis, we transplanted Tg737orpk control and mutant mammary epithelial tissue into fat pads of wild type recipient mice that had their mammary epithelial buds removed prior to puberty. By transplanting mutant cells on one side of the animal and control cells on the other, we are able to directly compare the ability of control and mutant cells to populate the fat pad and branch without any concerns about secondary effects of the Tg737orpk mutation. Transplanted control cells grow into the fat pad and branch similar to what is observed in normal development but the transplanted mutant cells showed a significant decrease in branching morphogenesis (p=1×10-9) (Fig. 3D). This indicates that the defect in branching morphogenesis is caused by the lack of IFT88 in the mammary epithelial cells and not a secondary defect caused by pathology elsewhere in the mutant animals.
As a further test of the cell intrinsic nature of the defect, we examined branching morphogenesis in an in vitro organotypic culture model. To do this, fragments of mammary tissue (organoids) from Tg737orpk control and mutant mice were cultured in a 3D matrix. Under these conditions, the epithelial cells grow and undergo branching morphogenesis in a process that resembles in vivo development including the formation of a bi-layered epithelium with ciliated myoepithelial cells (Supplemental Fig. 4A). After 7 days of culture, the number of branch points per organoid was significantly (p=2×10-4) decreased in mutant organoids (Mt = 2.8 +/- 0.5) as compared to controls (Wt = 8.5 +/- 1.1) (Fig. 3E). These results further demonstrate that Tg737orpk mutant mammary epithelial tissue has decreased branching morphogenesis under identical conditions to that of control tissue.
To test whether the decreased branching morphogenesis is due to loss of cilia or is due to a non-ciliary function of IFT88, we assessed branching morphogenesis in the Kif3a and Ift20 genetic models of ciliary dysfunction [29, 30]. Mammary ductal tissue harvested from Kif3aflox/flox or Ift20flox/flox mice was infected with adenovirus carrying GFP-Cre to delete Kif3a or Ift20. Control cells were generated by infecting cells with adenovirus carrying GFP without Cre. Flow sorted GFP-positive cells were then transplanted into cleared fat pads of recipient mice. The results were similar to what was observed in the Tg737orpk transplants in that the deletion of Kif3a or Ift20 significantly reduced the number of branch points in the transplanted glands (Mt Kif3a, p=1×10-17; Mt IFT20, p=1×10-8) (Fig. 3F, Supplemental Fig. 4B). Together, this work provides the first genetic evidence that primary cilia play a role in the normal expansion of the mammary ductal tree during development.
During pregnancy, additional secondary and tertiary branches are formed on existing branches. This is followed by differentiation of the branch ends into lobular-alveolar structures that produce milk [1]. At mid-pregnancy, the number of secondary and tertiary branch points was significantly less (secondary: p=6×10-10; tertiary: p=3×10-12) in the mutant glands compared to controls (Fig. 3G, Supplemental Fig. 2C). We were unable to accurately quantitate branching at lactation due to dense ductal tissue in controls. However, the extent of secondary and tertiary branching in the mutant mammary glands during lactation was visibly reduced as compared to controls (Fig. 3H). The pregnant and lactating mutant mammary glands also appear to have delayed or altered alveolar development as compared to the littermate controls (Fig. 3G, 3H). However, histological analysis suggests that both wild type and mutant mammary glands produce milk (Fig. 3G, 3H) indicating that the cells are able to at least partially differentiate.
Loss of Primary Cilia Increases Canonical Wnt Signaling and Decreases Hedgehog Signaling
Wnt signaling is crucial for the development of most mammalian organs. This signaling works through canonical and non-canonical pathways [31] and recent studies have implicated cilia in regulating both branches [8, 9, 32-34]. The role of cilia in regulating Wnt in early mouse development is controversial [9, 35] but is more established in organ development [30, 36]. Given the connections between Wnt signaling and cilia, and since Wnt signaling is linked to branching morphogenesis during mammary gland development [3], we investigated whether cilia regulate Wnt signaling during mammary gland development. β-catenin is a key component of canonical Wnt signaling and increased cytoplasmic levels of the unphosphorylated form correlate with increased signaling. We found increased cytoplasmic levels of unphosphorylated β-catenin (and reduced membrane localization) in Tg737orpk mutant terminal end buds as compared to controls (Fig. 4A). To understand how this affects Wnt signaling, the down-stream transcriptional targets of canonical Wnt signaling, Axin2, Tcf1, Tcf3 and Nkd1, were quantitated by real-time PCR (qPCR) of RNA isolated from Tg737orpk control and mutant mammary tissue and from organoids grown in 3D culture (Fig. 4B). In mammary tissue, Tcf1 and Nkd1 were significantly increased (2.5-fold, p=0.002 and 2.0-fold, p=0.0002 respectively) in the mutants (Fig. 4B). In 3D organ culture, Axin2, Tcf1, Tcf3 and Nkd1 were all significantly increased (1.9-fold, p=0.01; 2.4-fold, p=1×10-7; 2.2-fold, p=0.003 and 1.4-fold, p=0.03 respectively) in the mutant organoids (Fig. 4B). The difference between the two systems is likely due to the large amount of non-epithelial tissue in mammary glands, which may have obscured the signal from the epithelial cells.
Figure 4. Loss of primary cilia increases canonical Wnt signaling and decreases Hedgehog signaling during mammary gland development.
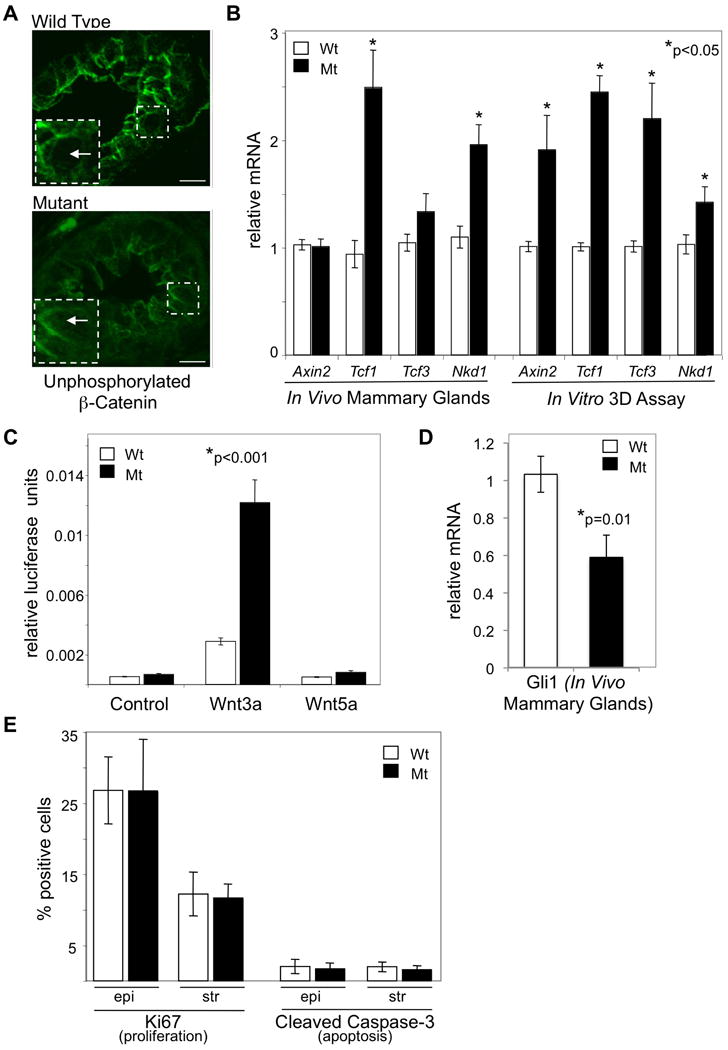
(A) Mammary tissue from wild type (Wt) and mutant (Mt) Tg737orpk mammary glands were stained with an antibody that recognizes the unphosphorylated form of β-Catenin to determine localization of active β-Catenin. Unphosphorylated β-Catenin cytoplasmic localization (white box inset with arrow) is increased in the epithelium of mutant mammary epithelium as compared to wild type. Images are maximum projections of equal confocal z images taken 0.38 μm apart. Images were taken under identical conditions and post-acquisition manipulations were also identical. (B and D) Real-time quantitative PCR was performed on RNA isolated from whole mammary glands or from organoids cultured in a 3D branching assay of wild type (Wt) and mutant (Mt) Tg737orpk mice. Bar graph represents Wt and Mt mRNA levels of the Axin2, Tcf1, Tcf3, Nkd1 and Gli1 genes (normalized to the house keeping gene Gusb). (C) Wnt signaling measured in cells isolated from mammary glands of wild type (Wt) and mutant (Mt) Tg737orpk mice. Cells were infected with a TCF/LEF luciferase reporter and untreated (control) or treated with the Wnt ligands (Wnt3a or Wnt5a). Bar graph represents relative luciferase activity (Wnt signaling). (E) Immunocytochemistry with antibodies that recognize Ki67 (marker of proliferation) and cleaved Caspase-3 (marker of apoptosis) was performed on tissue sections from mammary glands harvested at early development (terminal end buds analyzed at 4 weeks) from wild type and mutant Tg737orpk mammary glands. Bar graphs represent quantitation of percent Ki67 and cleaved caspase-3 positive cells. Student's t-Test (2-sided) was performed to determine statistical significance (*). (n values for qPCR from RNA isolated from tissue: Wt = 5 mice; Mt = 5 mice; n values for qPCR from RNA isolated from cultured organoids: Wt = 3 mice; Mt = 3 mice; n values for luciferase assay: Wt and Mt = cells isolated from 2 mice each; n values for Ki67 and Caspase-3 analysis: Wt = 4 mice, >1000 cells; for Mt = 3 mice, >1000 cells).
We also examined the ability of Tg737orpk mutant mammary ductal tissue to respond to Wnt ligands. Mammary cells isolated from ductal tissue of Tg737orpk wild type and mutant animals were infected with a lentivirus containing a TCF/LEF luciferase reporter for canonical Wnt signaling. After infection with the virus, the cells were treated with Wnt3a, a ligand expected to activate canonical Wnt signaling and Wnt5a, a closely related molecule that activates non-canonical Wnt signaling but is not expected to activate the canonical pathway interrogated by our TCF\LEF reporter construct [37]. As expected, untreated cells from wild type and mutant mammary glands have low luciferase activity and treatment with the control ligand Wnt5a did not significantly increase activity (Fig. 4C). Treatment with Wnt3a increased luciferase activity 5.5-fold in control cells and 18.4-fold in mutant cells (Fig. 4C). This indicates that the Tg737orpk mutant cells were significantly (p=2×10-10) more responsive to Wnt3a stimulation than are wild type cells.
The role of Wnt signaling in mammary gland branching morphogenesis is controversial with some studies finding that increased canonical Wnt signaling leads to decreased branching morphogenesis while other studies came to the opposite conclusion. For example, deletion of APC, which leads to increased levels of β-catenin and increased canonical Wnt signaling, results in reduced ductal extension during mammary gland development [12]. Similarly, during early lung development, expression of stabilized β-catenin in the presumptive epithelium partially inhibited branching morphogenesis [13, 14]. In contrast, over expression of Wnt ligands in mammary tissue or expression of stabilized Xenopus β-catenin in luminal epithelial cells caused increased branching [38-41]. The exact reasons for the discrepancies are not known but may reflect differences in how the non-canonical pathway is affected. For example, overexpression of Wnt ligands may also affect the non-canonical Wnt pathway in ways that deletion of APC does not [3]. In addition, the spatial and temporal control of Wnt signaling may be important during branching morphogenesis. Our in vitro expression studies (Fig. 4C) suggest that basal canonical signaling is not altered in the cilia-defective cells but these cells are hyper responsive to activating ligand. It may be expected that hyper responsiveness will give a different phenotype than overexpression of stabilized β-catenin throughout development. Thus, cilia may function to provide spatial and temporal regulation to Wnt signaling to allow for proper branching morphogenesis.
Defects in Hedgehog signaling lead to decreased branching morphogenesis in the lung and salivary gland [15, 16]. Given that ciliary defects decrease signaling through the Hedgehog pathway [42], we sought to determine if Hedgehog signaling was altered in mammary glands of mice with ciliary dysfunction. Consistent with published studies [6, 7, 10, 11], we found that expression of the Hedgehog target gene, Gli1, was significantly decreased (1.8-fold, p=0.01) in mutant mammary tissue as compared to controls (Fig. 4D). Canonical Wnt and Hedgehog signaling often play a role in proliferation [31], but we do not see alterations in proliferation or apoptosis in the mutant cells (Fig. 4E). It is possible that only small changes are needed in these parameters to cause the observed phenotypes but our assays are not sensitive enough to detect these changes.
Our results demonstrate alterations in Wnt and Hedgehog signaling in the mutant mammary gland but it is likely that primary cilia also regulate additional signaling events. One possibility is communication with the extracellular matrix via integrins. Integrin signaling is important role in mammary gland development [5] and recently it was demonstrated that integrins localize to primary cilia [43, 44]. Our working model is that mammary epithelial cells utilize primary cilia to receive spatial and temporal signals (Wnt, Hedgehog, etc.) to communicate with other cells of the branching ductal epithelium to control orientation of cell division and cell migration in order to develop into the complex three-dimensional structure of the mature organ.
Supplementary Material
Acknowledgments
The authors would like to thank Robert Bloodgood, Curtis Pickering, Geoff Benton and Colleen Fordyce for editorial comments on the manuscript. This work was supported by grants from the National Institutes of Health (CA58207, CA097214 to T.D. Tlsty; GM060992 to G.J. Pazour; K99 HD056965 to K.M. McDermott) and from postdoctoral fellowships from the California Breast Cancer Research Program (9FB-0158 to K.M. McDermott; 12FB-0114 to B.Y. Liu). Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used.
Abbreviations
- orpk
Oak Ridge polycystic kidney
- IFT
intraflagellar transport
- str
stromal cells
- bsl
basal epithelial cells
- lum
luminal epithelial cells
- Ln
lymph node
- n
nipple
- wt
wild type
- mt
mutant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 2.Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- 4.Lewis MT, Veltmaat JM. Next stop, the twilight zone: hedgehog network regulation of mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9:165–181. doi: 10.1023/B:JOMG.0000037160.24731.35. [DOI] [PubMed] [Google Scholar]
- 5.Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol. 2008;130:1105–1118. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 8.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 10.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher RC, Hay T, Meniel V, Naughton C, Anderson TJ, Shibata H, Ito M, Clevers H, Noda T, Sansom OJ, et al. Inactivation of Apc perturbs mammary development, but only directly results in acanthoma in the context of Tcf-1 deficiency. Oncogene. 2002;21:6446–6457. doi: 10.1038/sj.onc.1205892. [DOI] [PubMed] [Google Scholar]
- 13.Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286:270–286. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 16.Jaskoll T, Leo T, Witcher D, Ormestad M, Astorga J, Bringas P, Jr, Carlsson P, Melnick M. Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis. Dev Dyn. 2004;229:722–732. doi: 10.1002/dvdy.10472. [DOI] [PubMed] [Google Scholar]
- 17.Stirling JW, Chandler JA. Ultrastructural studies of the female breast: I. 9 + 0 cilia in myoepithelial cells. Anat Rec. 1976;186:413–416. doi: 10.1002/ar.1091860306. [DOI] [PubMed] [Google Scholar]
- 18.Fiddler TJ, Birkinshaw M, Falconer IR. Effects of intraductal prolactin on some aspects of the ultrastructure and biochemistry of mammary tissue in the pseudopregnant rabbit. J Endocrinol. 1971;49:459–469. doi: 10.1677/joe.0.0490459. [DOI] [PubMed] [Google Scholar]
- 19.Brooker BE. An ultrastructural study of the sinus epithelium in the mammary gland of the lactating ewe. J Anat. 1984;138(Pt 2):287–296. [PMC free article] [PubMed] [Google Scholar]
- 20.Nickerson SC. Cilia on bovine mammary epithelium: ultrastructural observations. Cell Tissue Res. 1989;255:675–677. doi: 10.1007/BF00218809. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen SK, Mollgard K, Clement CA, Veland IR, Awan A, Yoder BK, Novak I, Christensen ST. Characterization of primary cilia and Hedgehog signaling during development of the human pancreas and in human pancreatic duct cancer cell lines. Dev Dyn. 2008;237:2039–2052. doi: 10.1002/dvdy.21610. [DOI] [PubMed] [Google Scholar]
- 22.Sobkowicz HM, Slapnick SM, August BK. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol. 1995;24:633–653. doi: 10.1007/BF01179815. [DOI] [PubMed] [Google Scholar]
- 23.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 25.Pazour GJ. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol. 2004;15:2528–2536. doi: 10.1097/01.ASN.0000141055.57643.E0. [DOI] [PubMed] [Google Scholar]
- 26.Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264:1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- 27.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safranski TJ, Lamberson WR, Keisler DH. Correlations among three measures of puberty in mice and relationships with estradiol concentration and ovulation. Biol Reprod. 1993;48:669–673. doi: 10.1095/biolreprod48.3.669. [DOI] [PubMed] [Google Scholar]
- 29.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol. 2008;183:377–384. doi: 10.1083/jcb.200808137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111–116. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- 32.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 33.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 34.Kishimoto N, Cao Y, Park A, Sun Z. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell. 2008;14:954–961. doi: 10.1016/j.devcel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS ONE. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- 37.Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- 38.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 39.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 40.Roelink H, Wagenaar E, Lopes da Silva S, Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci U S A. 1990;87:4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001;153:555–568. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Praetorius HA, Praetorius J, Nielsen S, Frokiaer J, Spring KR. Beta1-integrins in the primary cilium of MDCK cells potentiate fibronectin-induced Ca2+ signaling. Am J Physiol Renal Physiol. 2004;287:F969–978. doi: 10.1152/ajprenal.00096.2004. [DOI] [PubMed] [Google Scholar]
- 44.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


