Abstract
Purpose
This study sought to determine the effects of microneedle coating formulation, drying time and storage time on antigen stability and in vivo immunogenicity of influenza microneedle vaccines.
Methods
The stability of inactivated influenza virus vaccine was monitored by hemagglutination (HA) activity and virus particle aggregation as a function of storage time and temperature with or without trehalose. In vivo immunogenicity of inactivated influenza vaccines coated onto microneedles was determined in mice by virus-specific antibody titers and survival rates.
Results
In the absence of trehalose, HA activity decreased below 10% and to almost zero after 1 h and 1 month of drying, respectively. Addition of trehalose maintained HA activity above 60% after drying and above 20% after 1 month storage at 25°C. Loss of HA activity generally correlated with increased virus particle aggregation. Administration of microneedles coated with trehalose-stabilized influenza vaccine yielded high serum IgG antibody titers even after 1 month storage, and all animals survived with minimal weight loss after lethal challenge infection.
Conclusions
Inactivated influenza virus vaccine coated on microneedles with trehalose significantly improved the HA activity as well as in vivo immunogenicity of the vaccine after an extended time of storage.
Keywords: inactivated virus vaccine, influenza virus, long-term stability, microneedle, trehalose
INTRODUCTION
Influenza is a major cause of morbidity and mortality worldwide (1). Annual administration of influenza vaccine is the most effective way to control influenza (2). As a result, influenza vaccination is the highest vaccination burden in the public health program due to the recommendation of annual immunization to accommodate the antigenic changes of the virus (3,4). In the United States, trivalent inactivated influenza vaccine and live attenuated influenza vaccine are licensed (5). Inactivated influenza virus vaccines have been widely used in humans and are prepared by adaptation and growth of viruses in embryonated chicken eggs (6). The vaccines consist of purified virus that has been chemically inactivated with formalin; the virus is then detergent-treated to produce soluble forms of the viral surface antigens (7).
Influenza vaccination is routinely performed by intramuscular injection (8). However, this route of administration has limitations. The use of hypodermic needles is limited by needle-phobia (9) and blood-borne pathogen transmission by reuse of needles (10), as well as the requirement of trained medical personnel to give injections. In response, a variety of needle-free vaccination methods have been studied in order to eliminate hypodermic needles (11,12). The intramuscular route may also be sub-optimal. For example, intradermal vaccination with inactivated virus vaccine has been shown to generate stronger immune responses in the elderly (13) and may be dose sparing (14), and nasal vaccination with live attenuated virus has been shown to induce stronger mucosal immune responses (15,16). Our recent work with cutaneous administration of inactivated influenza vaccine has similarly shown enhanced recall immune responses in mice (17).
Microneedles are micon-scale, solid needle structures that can be coated with vaccine in a dried state (18–23). After painless application to the skin (24), the vaccine dissolves off within minutes and can thereby achieve simple vaccination that avoids hypodermic needles and targets immunization to the skin. Preparation of microneedle vaccines involves a drying process, which can cause damage to the influenza vaccines (25). Vaccine immunogenicity can be at least partially retained by adding stabilizers that protect the virus from the stresses of drying. Sugar carbohydrates are well-known stabilizers for proteins (26), liposomes (27), and viruses (28). Among them, the disaccharide trehalose was shown to stabilize influenza vaccines during the preparation processes of freeze-drying (29,30), powder formulation (29,31) and, most recently, coating microneedles (17,25,32,33).
While previous studies have achieved adequate vaccine stabilization to enable vaccine efficacy studies, the stability of microneedle vaccines has not been studied in detail during the drying process or during subsequent long-term storage. Vaccine stability is clearly important to any future use of microneedle-based vaccine in medicine. Moreover, development of thermostable vaccines less dependent on cold-chain storage will make significant impact on vaccine distribution logistics, especially in developing countries that lack adequate refrigeration infrastructure (34,35). A related study coated peptide parathyroid hormone PTH(1–34) onto microneedles and achieved stability for a two-year shelf life by adding sucrose as a stabilizer and controlling moisture, oxygen and outgassed formaldehyde from plastic components of the device (36).
In this study, we coated inactivated influenza vaccine onto microneedles and then investigated the kinetics of vaccine stability over the timescale of minutes, during which microneedle coatings dry, and over the timescale of days and weeks to provide information about long-term storage. We further evaluated the effects of trehalose on stabilizing the vaccine during storage using an in vitro assay of receptor binding functional activity of hemagglutinin (25), which was supplemented with in vivo study of host protective immune responses using a mouse model. We believe that this is the first study to examine the stability of vaccine-coated microneedles during drying and storage.
MATERIAL AND METHODS
Preparation of Inactivated Influenza Virus and Coating Solution
The coating solution contained 1% (w/v) carboxymethyl-cellulose (CMC) sodium salt (USP grade, Carbo-Mer, San Diego, CA) and 0.5% (w/v) Lutrol F-68 NF (BASF, Mt. Olive, NJ), as described previously (20). In some cases, 15% (w/v) trehalose (Sigma Aldrich, St. Louis, MO) was added to serve as a stabilizer. A/PR/8/34 influenza virus was grown in fertilized hen eggs, harvested, and purified using sucrose-gradient ultracentrifugation, as described previously (37). Inactivation of virus was performed with formalin and confirmed by the absence of plaque formation (17). Inactivated virus was mixed with the coating solution prior to coating onto microneedles.
Fabrication and Coating of Microneedles with Inactivated Influenza Virus Vaccine
Rows of solid metal microneedles were fabricated by cutting needle structures from stainless steel sheets (SS304, 75 μm thickness, McMaster-Carr, Atlanta, GA) using an infrared laser, as described previously (25). The microneedles used in this study measured 700 μm in length and 160 μm in width at the base and were aligned in a row of five needles per device (Fig. 1).
Fig. 1.
Image of a microneedle array shown with a United States penny (scale bar = 2 mm).
Each individual row of microneedles was dip-coated by horizontally dipping the microneedles into a dip-coating device containing 10–15 μl inactivated virus vaccine at a concentration of 2 mg/ml. Immersion and withdrawal of the microneedle into a dip-coating device was performed manually by moving the microneedle while viewing under a stereo microscope (SZX12, Olympus America, Center Valley, PA). After vaccine coating, microneedles were left in air for drying at room temperature overnight.
In Vitro Testing of Inactivated Influenza Virus Stability During Drying and Storage
As a measure of antigen stability, we tested hemagglutination (HA) activity of inactivated virus during the drying process of coating. In this test, 1 μL of coating solution with or without trehalose was mixed with 1 μL of 5 mg/ml inactivated virus on a small metal chip (3 mm by 3 mm) made from the same stainless steel sheets used to prepare microneedles. Coating these small metal chips was used as a surrogate for coating microneedles to enable much faster throughput. The mixture was dried in air at 4, 25, and 37°C for specified times of storage up to 1 month without humidity control. The metal chip was then dissolved in 50 μL of phosphate-buffered saline (PBS) for 12 h at 4°C.
Potential changes in vaccine stability were monitored by measuring hemagglutinin receptor binding activity, that is, HA activity of red blood cells in vitro, which has been shown to correlate with in vivo immunogenicity (25). To determine HA activity titers, inactivated influenza virus in solution form or dissolved from metal chips was serially diluted in 100 μL of PBS deficient in Mg2+ and Ca2+, mixed with an equal volume of a fresh 0.5% suspension of chicken red blood cells (Lampire Biological Laboratories, Pipersville, PA), and incubated for 1 h at 25°C. The titers were determined as the endpoint dilutions inhibiting the precipitation of red blood cells (38).
Particle size was measured by similarly dissolving virus coatings from metal chips at a concentration of 0.1 mg/ml in PBS and analyzing by dynamic light scattering (DynaPro Protein Solutions plate reader, Wyatt, Santa Barbara, CA).
Antibody Response and Challenge Study After Immunization Using Microneedles
BALB/c mice (n = 6 animals per group, 8–10 weeks old, female, Charles River Laboratories, Wilmington, MA) were anesthetized intramuscularly with ketamine HCl (Abbott Laboratories, Chicago, IL) and xylazine (Phoenix Scientific, St. Joseph, MO). Although mouse skin differs from human skin in many ways, we believe the mouse model is appropriate for this study assessing vaccine stability. To prepare the site for vaccination, hair was removed from the dorsal surface using depilatory cream (Nair, Princeton, NJ) with a moisturized cotton stick. After washing with a cotton ball soaked with 70% ethanol and drying with a hair dryer, a row of microneedles coated with 0.7±0.05 μg of inactivated virus vaccine was inserted into the skin. To determine the amount of inactivated virus vaccine coated on microneedles, vaccine-coated microneedles were incubated in PBS solution for 12 h at 4°C, and the amount of released protein was measured by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). In our previous study, depilatory cream and 70% ethanol did not affect skin permeability to inactivated influenza virus (39). Microneedles were left in the skin for 10 min to ensure sufficient release of the vaccine antigen coated onto the microneedles, because preliminary studies showed that at least 70% of the vaccine dissolved off within this timeframe (data not shown). All animal studies were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Blood was drawn on weeks 1, 2, and 4 after vaccination. Influenza virus-specific antibodies of different subtypes (IgG, IgG1, IgG2a, and IgG2b) were determined in sera by enzyme-linked immunosorbent assay (ELISA) as described previously (37). Briefly, 96-well microtiter plates (Nunc-immuno plate maxisorp: Nunc Life Technologies, Basel, Switzerland) were coated with 100 μl of inactivated PR8 virus at a concentration of 4 μg/ml in coating buffer (0.1 M sodium carbonated, pH 9.5) at 4°C overnight. The plates were then incubated with horseradish peroxidase-labeled goat anti-mouse IgG, IgG1, IgG2a, and IgG2b (Southern Biotechnology, Birmingham, AL) at 37°C for 1.5 h, and the substrate ophenylenediamine (Zymed, San Fransisco, CA) in citrate-phosphate buffer (pH 5.0) containing 0.03% H2O2 (Sigma) was used to develop color. The optical density at 450 nm was read using an ELISA reader (model 680; Bio-Rad, Hercules, CA)
For challenge infections, isoflurane-anesthetized mice were infected intranasally with 1,000 pfu live influenza virus (A/PR8/34, 20×LD50)in50 μl of PBS per mouse on week 5 after immunization. Mice were observed daily to monitor changes in body weight and to record death. Mice were euthanized to minimize suffering if their body weight loss exceeded 25%.
Statistical Analysis
Every assay was measured using at least four samples, from which the arithmetic mean and standard error of the mean were calculated. A two-tailed Student's t-test (α=0.05) was performed when comparing two different conditions. A value p<0.05 was considered statistically significant.
RESULTS
Kinetics of Influenza Vaccine Stability Loss During Drying
We expect that influenza vaccine can be destabilized over two timescales: on the order of minutes during the drying process and on the order of days and longer during storage. To understand the kinetics and extent of inactivated influenza virus vaccine stability loss during the drying process, vaccine antigenicity, as measured by HA activity, was measured after coating with and without trehalose, which was added as a vaccine stabilizer.
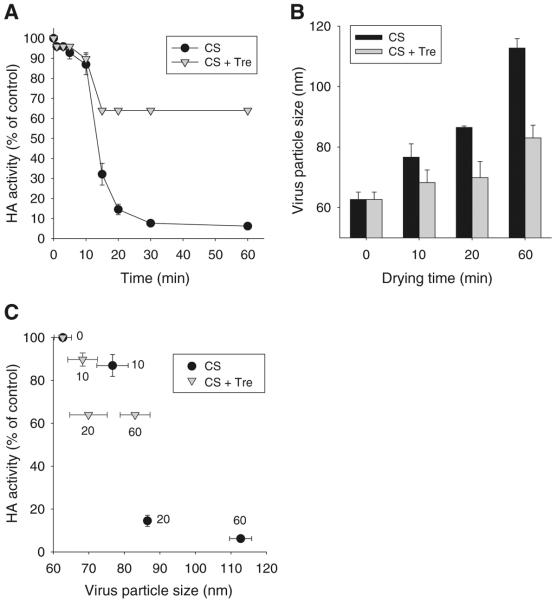
As shown in Fig. 2A, there was a rapid loss of HA activity within 30 min of applying the coating. In the absence of trehalose, there was an initial loss of activity to less than 90% during the first 10 min, which was followed by an additional loss to less than 10% activity within 20–30 min. When treaholse was added to stabilize the formulation, HA activity similarly fell below 90% during the first 10 min and further fell to 64% activity after 15 min. However, after that, HA activity remained constant. Overall, these data show that inactivated influenza vaccine is destabilized to less than 10% HA activity on a timescale of minutes during the drying process and that the addition of trehalose inhibits this destabilization and retains most HA activity. It is interesting to note that the initial, smaller activity loss was not affected by trehalose, whereas the subsequent, bigger loss was largely prevented.
Fig. 2.
Kinetics of influenza vaccine stability loss during drying as measured by HA activity loss. (A) HA activity of inactivated virus and (B) virus particle size as a function of drying time. (C) Correlation between HA activity and virus particle size. Coating solution contained 5 mg/ml inactivated virus and was prepared with or without 15% trehalose. In (C), the numbers next to each data point indicate drying time in minutes. HA activity is normalized relative to a control solution of inactivated virus in PBS at the beginning of the experiment. CS = coating solution, Tre = trehalose. (n=4 replicates expressed as average ± SEM).
To investigate the mechanism of HA activity loss of influenza vaccines over time during drying, we measured virus particle size, which is a measure of virus aggregation (25). As shown in Fig. 2B, the average size of virus particles in the absence of trehalose increased over time during drying. After 1 h, average particle size was almost 1.8 times larger than before drying, which represents an almost 6-fold increase in particle volume, assuming spherical geometry. In contrast, virus particles dried in the presence of trehalose showed much smaller increases in size. After 1 h, average particle size was just 1.3 times larger than before drying, which represents an approximately 2-fold increase in particle volume. Fig. 2C shows a general correlation between HA activity loss and virus particle size, which suggests a role of aggregation in diminishing the HA activities of vaccines during drying and storage. However, aggregation might not be the only factor causing influenza vaccine destabilization.
Stability Kinetics of Influenza Vaccine During Storage in Liquid Formulation
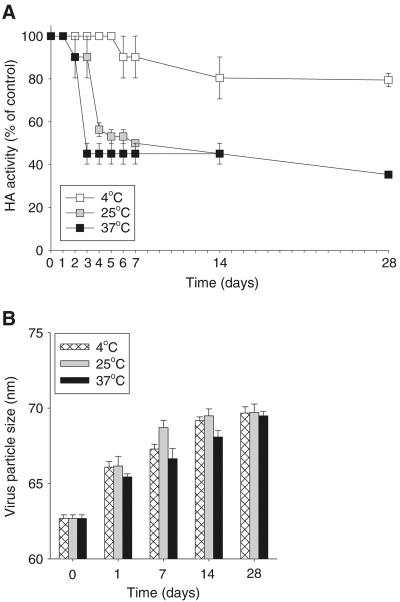
We next investigated influenza vaccine stability, as measured by HA activity, on the timescale of days and weeks during storage. As a reference point, we first tested the stability of inactivated virus formulated as a liquid in PBS at different temperatures over a 4-week period. As shown in Fig. 3A, inactivated virus retained 100% of its HA activity for 5 days at 4°C and then fell to 80% activity after 2 weeks. At 25°C and 37°C, HA activity fell below 50% within 1 week and below 40% after 4 weeks. Interestingly, virus particle size exhibited only small increases, even after 4 weeks storage and considerable HA activity loss (Fig. 3B). This indicates that extensive aggregation of the influenza virus particles did not occur in liquid formulation, even at 37°C. Overall, these results suggest that HA activity loss of inactivated influenza vaccine occurred during storage in a temperature-dependent manner and that virus particle aggregation does not appear to be a significant mechanism of activity loss.
Fig. 3.
Stability kinetics of influenza vaccine during storage in liquid formulation as measured by HA activity loss. (A) HA activity and (B) virus particle size of inactivated virus stored in PBS at different temperatures for up to 28 days. Virus concentration was 0.1 mg/ml. (n=4 replicates expressed as average ± SEM).
Stability Kinetics of Influenza Vaccine During Storage as a Dried Microneedle Coating
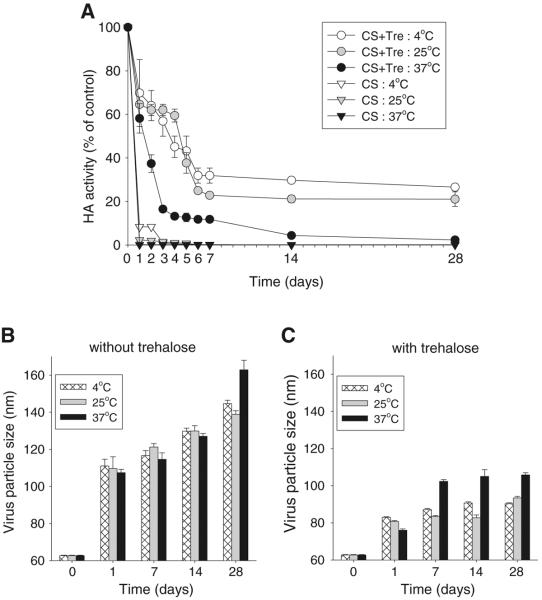
We carried out similar experiments to determine the stability kinetics of vaccine coated onto microneedle surfaces as a function of storage temperature. In the absence of trehalose in the coating solution, HA activity of dried inactivated virus fell below 10% within one day and lost almost all activity within 3 days, regardless of storage temperature (Fig. 4A).
Fig. 4.
Stability kinetics of influenza vaccine during storage as a dried microneedle coating as measured by HA activity loss. (A) HA activity and virus particle size of coatings formulated (B) without and (C) with trehalose stored at different temperatures for up to 28 days. Coating solution contained 5 mg/ml inactivated virus and was prepared with or without 15% trehalose. CS coating solution, Tre trehalose. (n=4 replicates expressed as average ± SEM).
Addition of trehalose to the coating formulation retained HA activity at approximately 60–70% after one day's storage in a temperature-independent manner. Further storage at 4°C reduced activity to approximately 30%, and at 25°C reduced activity to approximately 20% after 1 and 4 weeks. At 37°C, HA activity dropped faster and fell to approximately 10% activity after 1 week and almost no activity after 4 weeks.
Fig. 4B and C show that significant virus particle aggregation occurred during storage of vaccine coatings. In the absence of trehalose, virus particle size increased by up to 2.6-fold to a maximum size of 160 nm after 4 weeks (Fig. 4B), which represents an almost 18-fold increase in particle volume. The addition of trehalose dramatically dampened virus particle aggregation, such that particle size only showed a small, yet significant, increase at 37°C from day 1 to day 28 (Fig. 4C).
In summary, the HA activity of vaccine was almost completely lost during storage of dried inactivated influenza virus either in the absence of trehalose at any temperature or in the presence of trehalose at 37°C. Storage in trehalose at 25°C or 4°C partially maintained HA activity, even after storage for 4 weeks. Evidence of increased virus particle size suggests a role for aggregation in lowering the HA activity in coated vaccine. This contrasts with liquid vaccine, in which only minimal particle aggregation was evident.
Immunogenicity of Influenza Vaccine Coated onto Microneedles After Drying and Storage
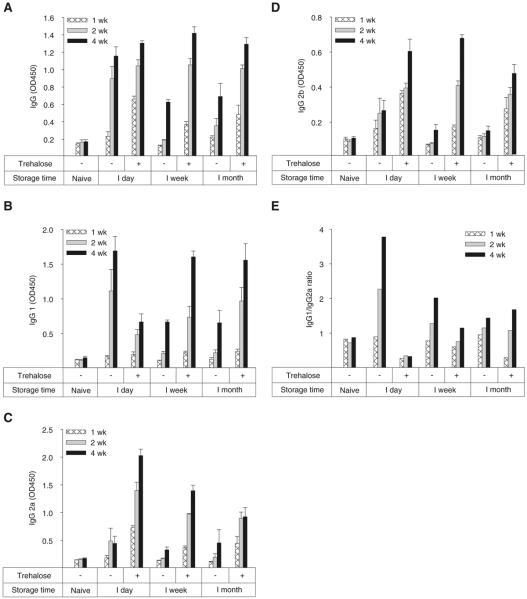
Although in vitro measurements of changes in HA activity and virus particle aggregation are relevant to assessing vaccine antigenicity, we measured antibody responses and protection against lethal virus challenge in mice to provide a direct measure of in vivo immunogenicity and vaccine efficacy. In this study, groups of mice were immunized once with seasonal H1N1 inactivated influenza virus vaccine using microneedles applied to the skin after storage periods of 1 day, 1 week, and 1 month at 25°C using coating formulations with or without trehalose. The levels of influenza virus-specific IgG and isotype IgG antibodies were measured by ELISA at 1, 2, and 4 weeks after vaccination, as shown in Fig. 5.
Fig. 5.
Immunogenicity of influenza vaccine coated onto microneedles after drying and storage. Influenza virus-specific antibody response at 1, 2, and 4 weeks after immunization with coated microneedles formulated with (+) or without (−) 15% trehalose after storage for up to 1 month at 25°C. (A) Total influenza virus-specific serum IgG, (B) subtype IgG1, (C) subtype IgG2a, (D) subtype IgG2b and (E) IgG1/IgG2a ratio. (n=6 replicates expressed as average ± SEM).
After storage for just 1 day, microneedle vaccines stabilized with trehalose elicited high levels of influenza virus-specific IgG antibodies, which was significantly higher than naïve (p<0.01, Fig. 5A). Notably, after storage for 1 week or 1 month, influenza virus-specific IgG levels were similar to microneedles used just 1 day after production. This shows that although in vitro measurements suggested vaccine HA activity loss during storage, the microneedles coated with trehalose-stabilized influenza vaccine remained fully immunogenic in vivo. It is also worth noting that vaccination was carried out using only 0.7 μg of total protein. This small dose suggests that we were not operating with excess vaccine that could accommodate loss of vaccine HA activity without affecting the antibody response.
In contrast, microneedles prepared without trehalose elicited a strong antibody response after just 1 day of storage, but showed lower levels of influenza virus-specific IgG after 1 week or 1 month storage time (Fig. 5A). This shows that influenza vaccine loses immunogenicity during storage and that the presence of trehalose can prevent that loss.
The immune response can be further characterized in terms of influenza virus-specific IgG isotype responses as shown in Fig. 5B–E. After vaccination using trehalose-stabilized microneedles, vaccine stored for just 1 day elicited an antibody response dominated by IgG2a, which was accompanied by lower levels of IgG1 and IgG2b (i.e. IgG1/IgG2a ratio < 1). After longer storage times, the isotype response shifted to stronger IgG1, weaker IgG2a and relatively unchanged IgG2b, which resulted in an IgG1/IgG2a ratio close to one. This corresponds to a balanced Th1/Th2 response by T helper cells (40). This shows that immunogenicity was changed during microneedle storage in the presence of trehalose, but only manifested itself in the form of isotype switching and not in terms of influenza virus-specific IgG.
After vaccination using microneedles lacking trehalose, vaccine stored for just 1 day elicited an antibody response dominated by IgG1 accompanied by much lower levels of IgG2a and IgG2b (i.e. IgG1/IgG2a ratio > 1). After longer storage times, antibody levels for all three isotypes dropped, especially for IgG1, which resulted in a balanced IgG1/IgG2a ratio. In this case, storage of microneedle vaccines without trehalose resulted in two immunogenic effects: reduction of influenza virus-specific IgG and shifting of the isotype profile.
Comparing microneedles with and without trehalose stabilization shows that freshly prepared microneedles (i.e., 1 day storage) generated similar influenza virus-specific IgG, but IgG2a dominated the response for trehalose-positive microneedles, and IgG1 dominated the response for trehalose-negative microneedles. After extended storage (i.e., 1 week and 1 month), influenza virus-specific IgG was much higher for microneedles stabilized with trehalose, but the IgG1/IgG2a ratio was close to one for both types of microneedles. Overall, these results indicate that the in vitro HA activity data of vaccines correctly indicated changes in the in vivo immune response, but these changes manifested themselves in complex ways. Both storage time and the inclusion of trehalose during microneedle coating affected immunogenicity of the vaccine as shown through the induced levels and ratios of antibody isotypes.
Protective Efficacy of Influenza Vaccine Coated onto Microneedles After Drying and Storage
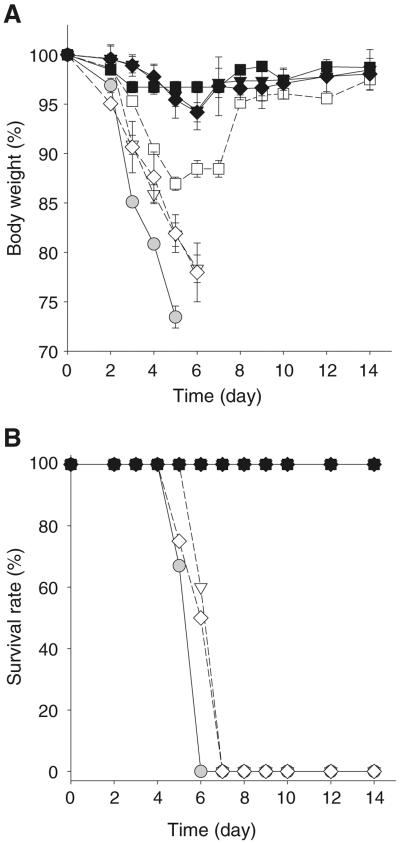
To determine protective efficacy of vaccine-coated microneedles, immunized mice were intranasally challenged with a lethal dose of influenza virus at week 5 post vaccination (Fig. 6). Naïve mice rapidly lost body weight and either died or had to be euthanized within 6 days of challenge. After vaccination with microneedles lacking trehalose and stored for 1 week or 1 month, the mice rapidly lost body weight and either died or were euthanized within 7 days post challenge, similar to naïve mice. Mice immunized with microneedles lacking trehalose and stored for just 1 day showed signs of moderate disease and body weight loss of 10–15% but then recovered to normal body weight, and all mice survived. This is consistent with total IgG data (Fig. 5) indicating that storage of vaccine-coated microneedles without trehalose significantly damages the capacity to confer protective immunity.
Fig. 6.
Protective efficacy of influenza vaccine coated onto microneedles after drying and storage. (A) Body weight and (B) survival rate of immunized mice after lethal challenge infection with 20×LD50 A/PR8/34 live virus. Experimental groups are ( ) naïve; 1 day storage (■) with and (□) without trehalose; 1 week storage (▼) with and (▽) without trehalose; and 1 month storage (◊) with and (♦) without trehalose. Microneedle storage was at 25°C. (n=6 replicates expressed as average ± SEM).
) naïve; 1 day storage (■) with and (□) without trehalose; 1 week storage (▼) with and (▽) without trehalose; and 1 month storage (◊) with and (♦) without trehalose. Microneedle storage was at 25°C. (n=6 replicates expressed as average ± SEM).
In contrast, mice immunized with microneedle vaccine coated with trehalose and stored for 1 day, 1 week or 1 month generally lost less than 5% body weight, and all survived lethal challenge infection without obvious clinical signs of disease. Our results suggest that the addition of trehalose stabilizer when coating microneedles with influenza vaccine is critically important for maintaining protective efficacy, especially after extended storage periods.
DISCUSSION
Microneedle vaccination in the skin can provide a promising vaccine delivery method that avoids the dangers of hypodermic needles and enables improved protection, as demonstrated in recent studies (17,21,22,25,32,33). However, the stability of influenza vaccine after microneedle coating, which involves a drying process, has not been investigated in detail. We previously showed that microneedle coating with inactivated influenza vaccine reduced vaccine activity and that the addition of trehalose to the coating formulation helped retain immunogenicity (25). In this study, we first quantified the kinetics of vaccine activity loss, both with and without trehalose in the formulation, as determined by HA activity and particle aggregation assays. During the first 10 min of drying, there was a small loss of HA activity, which was independent of trehalose. Between 10 and 20 min after coating, there was a dramatic drop in HA activity, which was largely prevented by the presence of trehalose. From 20–60 min, there was a small additional drop in HA activity only in coatings lacking trehalose. Overall, coatings lacking trehalose dropped to less than 10% HA activity, whereas those including trehalose maintained HA activity above 60%.
Over longer time periods of storage ranging from 1 day to 1 month, HA activity was almost zero in coatings lacking trehalose. Vaccine in coatings with trehalose experienced a steady decline in HA activity over the next day up to 1 week, and then experienced much slower activity loss up to 1 month of storage. Activities of 20–30% remained after 1 month for microneedles stored at 4°C or 25°C.
These stability measurements relied on HA activity. Hemagglutinin is the major immunogenic component of influenza vaccines and of live virus, and plays an essential role in receptor binding and entry of virus into target cells (41). HA activity is a measure of HA functionality that assesses the capacity to hemagglutinate red blood cells mediated by binding to sialic acid-containing receptors on the host cell surface (38). The use of HA activity to monitor the integrity of influenza vaccine has been demonstrated in this and previous studies (17,25,32).
It is well known that the stability of influenza vaccines in liquid formulation is dependent on temperature. In general, refrigerated storage at 4°C is required to maintain vaccine stability (42). Our data similarly show that vaccine storage in the liquid state at 4°C was much more stable than when stored at higher temperatures (Fig. 3). In contrast, influenza vaccine stored as a solid coating (with trehalose) was equally stable at 4°C and 25°C during storage up to 1 month. This thermostability suggests the microneedle vaccines could be developed to enable room-temperature storage, which would be critically important to vaccine distribution during a pandemic and in developing countries, where refrigeration is more difficult. It should be noted, however, that coated vaccine was less stable at 37°C and that despite the protective effects of trehalose, coated vaccine was less stable than the liquid formulation. Additional studies are needed to better understand the mechanisms of instability during drying and storage and to further optimize vaccine formulation and processing.
While it is important to characterize in vitro HA activity of influenza vaccines during drying and storage, in vivo immunogenicity and protective efficacy are of still greater interest. After storage for 1 day, levels of influenza virus-specific IgG antibody responses were similar for microneedles prepared with and without trehalose. However, mice immunized with microneedles lacking trehalose stabilization suffered severe loss in body weight after lethal challenge, whereas mice immunized with microneedles coated with trehalose-stabilized influenza vaccine did not. This difference may be explained by the different isotype profiles generated with and without trehalose.
After 1 and 4 weeks of storage, mice immunized with unstabilized microneedles were not protected against lethal challenge and all died. In contrast, mice immunized with microneedles coated with trehalose-stabilized influenza vaccine showed 100% protection. At these time points, the isotype profiles were similar, but influenza virus-specific IgG was much higher in mice immunized with trehalose-stabilized microneedles. Overall, these results indicate that stabilization of influenza vaccines during microneedle coating and storage is critically important to provide protection against lethal infection as well as to modulate host-protective immune responses.
Previous and recent clinical studies on intradermal vaccination indicate that skin is a promising route of vaccine delivery which may provide dose-sparing effects and better protective immunity (13,14). However, conventional intradermal vaccination relies on the difficult and unreliable process of injecting vaccines in liquid formulation into the skin using hypodermic needles (43). Microneedles coated with a solid vaccine formulation can be prepared as a skin patch that is simple and intuitive to apply and, due to the size of the microneedles, inherently targets delivery to the skin. Development of effective microneedle vaccines could provide many logistic advantages, including simple administration, possibly by patients themselves; no dangers associated with hypodermic needles; possibly reduced dependence on cold-chain storage and transportation; and possibly superior protective immunity. Realization of these goals will require, among other things, a vaccine coating that is stable during extended storage to achieve a useful shelf life. This study provides the first assessment of the stability of a microneedle vaccine during storage and identifies parameters influencing antigen stability and strategies to increase it. Additional work can lead to further stabilization of the vaccine to achieve long-term stability.
ACKNOWLEDGMENTS
This work was carried out at the Emory University School of Medicine and the Georgia Tech Center for Drug Design, Development and Delivery and Institute for Bioengineering and Biosciences. It was supported in part by NIH grants R01-EB006369 (M.R.P.), U01-AI0680003 (R.W.C.), SERCEB (R.W.C) and the Georgia Research Alliance Program grant (S.M.K). We thank Dr. Vladimir Zarnitsyn for microneedle fabrication, Dr. Andrew Lyon for dynamic light scattering assay, and Dr. Mark Allen for laser microfabrication facilities. M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. This possible conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
Footnotes
Yeu-Chun Kim and Fu-Shi Quan contributed equally to this work.
REFERENCES
- 1.Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–7. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 2.Monto AS, Ohmit SE. Seasonal influenza vaccines: evolutions and future trends. Expert Rev Vaccines. 2009;8:383–9. doi: 10.1586/erv.09.9. [DOI] [PubMed] [Google Scholar]
- 3.Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–82. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 4.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7:449–55. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 5.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194:S111–8. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 6.Bridges CB, Katz JM, Levandowski RA, Cox NJ. Inactivated influenza vaccines. In: Plotkin SA, Orenstein WA, Offit P, editors. Vaccines. Saunders Elsevier; Philadelphia: 2008. pp. 259–90. [Google Scholar]
- 7.Raviv Y, Blumenthal R, Tompkins SM, Humberd J, Hogan RJ, Viard M. Hydrophobic inactivation of influenza viruses confers preservation of viral structure with enhanced immunogenicity. J Virol. 2008;82:4612–9. doi: 10.1128/JVI.02233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19:95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton JG. Needle phobia—a neglected diagnosis. J Fam Pract. 1995;41:169–75. [PubMed] [Google Scholar]
- 10.Drucker E, Alcabes PG, Marx PA. The injection century: massive unsterile injections and the emergence of human pathogens. Lancet. 2001;358:1989–92. doi: 10.1016/S0140-6736(01)06967-7. [DOI] [PubMed] [Google Scholar]
- 11.Weniger BG, Papania MJ. Alternative vaccine delivery methods. In: Plotkin SA, Orenstein WA, Offit P, editors. Vaccines. Saunders Elsevier; Philadelphia: 2008. pp. 1357–92. [Google Scholar]
- 12.Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005;5:905–16. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 13.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 14.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 15.Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 2004;103:177–85. doi: 10.1016/j.virusres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZY, Santos C, Aspelund A, Gillim-Ross L, Jin H, Kemble G, et al. Evaluation of live attenuated influenza A virus H6 vaccines in mice and ferrets. J Virol. 2009;83:65–72. doi: 10.1128/JVI.01775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to H1N1 influenza vaccination in the skin using vaccine coated-microneedles. J Infect Dis. 2010;201:190–8. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–93. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–64. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–37. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koutsonanos DG, Martin MDP, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu QY, Zarnitsyn VG, Ye L, Wen ZY, Gao YL, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci USA. 2009;106:7968–73. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, et al. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci USA. 2009;106:18936–41. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–94. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–95. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinrichs WLJ, Prinsen MG, Frijlink HW. Inulin glasses for the stabilization of therapeutic proteins. Int J Pharm. 2001;215:163–74. doi: 10.1016/s0378-5173(00)00677-3. [DOI] [PubMed] [Google Scholar]
- 27.Sun WQ, Leopold AC, Crowe LM, Crowe JH. Stability of dry liposomes in sugar glasses. Biophys J. 1996;70:1769–76. doi: 10.1016/S0006-3495(96)79740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bieganski RM, Fowler A, Morgan JR, Toner M. Stabilization of active recombinant retroviruses in an amorphous dry state with trehalose. Biotechnol Prog. 1998;14:615–20. doi: 10.1021/bp980057d. [DOI] [PubMed] [Google Scholar]
- 29.Amorij JP, Meulenaar J, Hinrichs WLJ, Stegmann T, Huckriede A, Coenen F, et al. Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine. 2007;25:6447–57. doi: 10.1016/j.vaccine.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 30.Molina MD, Armstrong TK, Zhang Y, Patel MM, Lentz YK, Anchordoquy TJ. The stability of lyophilized lipid/DNA complexes during prolonged storage. J Pharm Sci. 2004;93:2259–73. doi: 10.1002/jps.20138. [DOI] [PubMed] [Google Scholar]
- 31.Amorij JP, Huckriede A, Wischut J, Frifflink HW, Hinrichs WLJ. Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharm Res. 2008;25:1256–73. doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated-microneedles. Vaccine. 2009;27:6932–8. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS ONE. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berhane Y, Demissie M. Cold chain status at immunisation centres in Ethiopia. East Afr Med J. 2000;77:476–9. doi: 10.4314/eamj.v77i9.46692. [DOI] [PubMed] [Google Scholar]
- 35.Bloom BR. Vaccines for the third-world. Nature. 1989;342:115–20. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- 36.Ameri M, Daddona PE, Maa YF. Demonstrated solid-state stability of parathyroid hormone PTH(1–34) coated on a novel transdermal microprojection delivery system. Pharm Res. 2009;26:2454–63. doi: 10.1007/s11095-009-9960-9. [DOI] [PubMed] [Google Scholar]
- 37.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82:1350–9. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hierholz JC, Suggs MT. Standardized viral hemagglutination and hemagglutination-inhibition test. I. Standardization of erythrocyte suspensions. Appl Microbiol. 1969;18:816–23. doi: 10.1128/am.18.5.816-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24:6110–9. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Spellberg B, Edwards JE. Type 1 type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 41.Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 42.Coenen F, Tolboom JTBM, Frijlink HW. Stability of influenza sub-unit vaccine—does a couple of days outside the refrigerator matter? Vaccine. 2006;24:525–31. doi: 10.1016/j.vaccine.2005.07.081. [DOI] [PubMed] [Google Scholar]
- 43.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]