Abstract
In type 1 diabetes (T1D), the breach of central and peripheral tolerance results in autoreactive T cells destroying insulin-producing, pancreatic beta cells. Herein, we identify a critical sub-population of dendritic cells responsible for mediating both the cross-presentation of islet antigen to CD8+ T cells and the direct presentation of beta cell antigen to CD4+ T cells. These cells, termed merocytic dendritic cells (mcDC), are more numerous in nonobese diabetic (NOD) mouse, and when antigen-loaded rescue CD8+ T cells from peripheral anergy and deletion, while stimulating islet-reactive CD4+ T cells. When purified from the pancreatic lymph nodes of overtly diabetic NOD mice, mcDC break peripheral T cell tolerance to beta cells in vivo and induce rapid onset T1D in young NOD mouse. Thus, the mcDC subset appears to represent the long-sought APC responsible for breaking peripheral tolerance to beta cell antigen in vivo.
Keywords: Diabetes, Dendritic Cells, Tolerance
Introduction
The autoimmune attack by T cells on insulin-producing pancreatic beta cells results in type 1 diabetes (T1D) in both humans and NOD mice, reviewed in (1). T cells participate in all phases of the disease from the initial insulitis, to the selective destruction of beta cells by both direct and indirect cytolysis (2, 3). How these rogue T cells escape both central and peripheral tolerance is not fully understood, but both CD4+ and CD8+ T cells are required (4–6).
The naïve islet-reactive T cells are stimulated by dendritic cells that drain from the pancreas and accumulate in the pancreatic lymph nodes (PLN) after acquiring self-antigen from degranulating and apoptotic beta cells (7, 8). While CD4+ T cells are activated by islet antigens presented on class II MHC, CD8+ T cells require cross-presentation on class I MHC class I by a specialized DC subset (9).
The clearance of apoptotic self cells by DC is generally non-inflammatory, even tolerogenic (10). Yet, under certain situations antigen acquired from apoptotic self cells is pro-inflammatory, leading to the priming of self-reactive T cells (11); this appears to be the case in T1D-prone animals. However, which DC subset is responsible and how this process unfolds mechanistically are not well understood.
Recently, we found that the ablation of conventional DC (cDC) in vivo resulted in the loss of CD4+ T cells activation, insulitis and diabetes (12). This cDC subset expressed CD11c but not CD8α or CD317, and had varied levels of CD11b; we referred to these cells as myeloid DC but have since found that they contain not only CD11b+DC but also a novel subset of CD11c+CD11b−/loCD8a− DC that acquired small particulate fragments of antigen from apoptotic cells, and unlike other DC subsets, were capable of processing and presenting antigen in a non-tolerogenic manner to both CD4+ and CD8+ T cells (11). These DC, termed merocytic DC (mcDC), stored antigen from apoptotic cells in discrete, punctate vesicles in their cytoplasm, meros (μερoσ, particle in Greek). Given that mcDC resided within the major stimulatory fraction of cDC, and were particularly effective at breaking tolerance to self antigens, we hypothesized that mcDC may represent the critical cDC subset necessary for the activation of both beta cell-specific CD4+ and CD8+ T cells in vivo.
Materials and Methods
Mice
All mice were purchased from the Jackson Laboratory, except BDC2.5/NOD.Rag1−/− (13) and OT-I (CD45.1) TCR transgenic, B6.PL (CD90.1), RIP-mOVA transgenic B6 (14) and mOVA/B6.Kb−/− (15) mice which were bred in our existing SPF barrier colony. All experiments were performed under approved IACUC guidelines.
Antibodies
All antibodies were purchased from BioLengends (San Diego, CA) except anti-mPDCA-1 which was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany).
DC purification and transfer
DC were expanded in vivo using the B16-Flt3L model described in (16). cDC were purified from spleen and LN, by MACS® cell, followed by cell sorting (to 93 to 97% purity). Cell transfers were performed as described (12). For isolation of DC from recent onset diabetic mice, the PLN were harvested from ~40 NOD mice with blood glucose ≥ 300 mg/dl, cells, the cell were stained with anti-CD11c, anti-CD11b, anti-CD8a and a cocktail of FITC-conjugated mAb to B220, CD19, TCR-β, CD3, NKR-P1C and CD49b to exclude pDC, NKDC, IKDC, B and T cells, NK and NKT cells. CD11c+ cells were sorted into either CD11b− (mcDC) or a pooled of CD11b+DC and CD8α+DC, and transferred (i.v., 4–5 × 105) into 28 day old NOD as above.
Islet cell antigen
B6 Islets were isolated using the technique of Lacy and colleagues for rat islets (17), dispersed using trypsin, irradiated (30 Gy) to induce apoptosis, and labeled with CFSE then mixed at a ratio of 20 cDC to one islet cell overnight. Dead cells were removed using a dead cell removal kit (Miltenyi Biotec).
T cell Proliferation
Antigen-loaded DC were used to stimulate sort-purified naïve T cells (CD62L+ T cells from BDC2.5/Rag1−/− mice). T cell proliferation assays were performed in vitro using 3H-TdR incorporation (ICN Biomedicals, Irvine, CA) as described (12), while in vivo proliferation assays were performed using CFSE decay as described (18).
Type 1 IFN assay
Detection of IFN from DC incubated with irradiated splenocytes were performed using a bioassay described (19).
Imaging Flow Cytometry Analysis
The uptake of CFSE-labeled irradiated islet cells was assessed using an ImageStream® analyzer (Amnis Corp., Seattle, WA). Internalization of CFSE particles from irradiated islet cells was determined by masking DC cytoplasm, followed by a subtracting the nucleus (DRAQ5+, Biostatus, Shepshed, UK) and then applying a spot mask on CFSE particles.
Insulitis and Diabetes
Insulitis and diabetes was assessed as described (20).
Statistics
Statistical analysis was performed using the GNU/Linux application, Qtiplot (ver. 0.9.7)
Results and Discussion
Elevated levels of CD11b−/lo dendritic cells in NOD mice
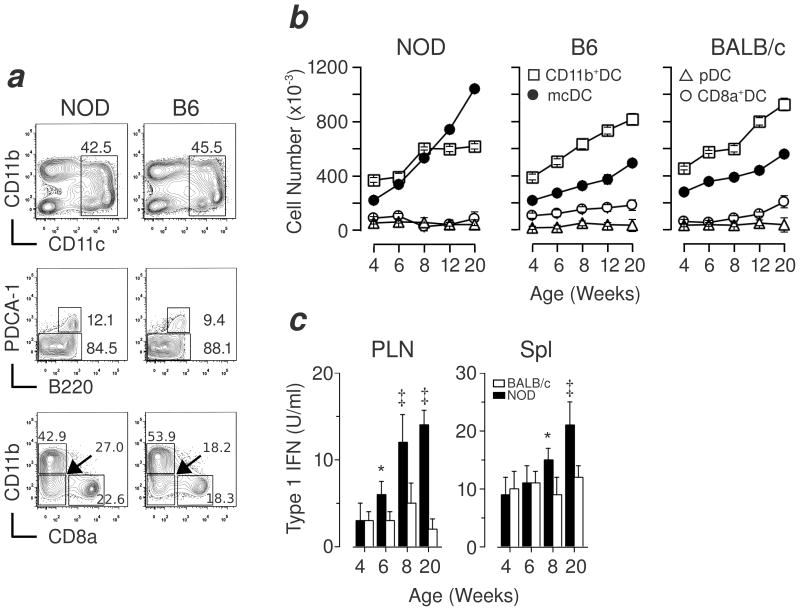
Merocytic DC reside within the CD11b−/lo subset of cDC. Thus, to determine whether NOD mice had altered numbers of mcDC, we compared the frequency of mcDC in the spleen and pancreatic lymph nodes (PLN) of age-matched NOD, C57BL/6 (B6) and BALB/c mice. As shown in Figure 1a and b, the frequency and absolute numbers of mcDC was initially similar between NOD, B6 and BALB/c mice but increased in NOD mice as they aged, this is consistent with the overall rise in DC seen by others in the spleen and blood of aging NOD mice (21). The expansion of mcDC coincided with the appearance of insulitis, reviewed in (2, 22), and it is important to note that the mcDC differed both phenotypically and functionally from the “abnormal” or “altered” DC subsets described by others (21, 23, 24) in the NOD mice, and were found in the “non-activated” DC fraction in all mice strains tested (including NOR, data not shown).
Figure 1. Dynamic rise in frequency of mcDC in NOD mice.
(a) 6-wk old NOD and B6 mice were compared using flow cytometry. Total CD11c+ DC (top panels) were gated for pDC (PDCA-1+B220+) and cDC population (middle), and then the cDC (PDCA-1−B220−) further subdivided into CD11b+DC, CD8α+DC and mcDC (bottom). (b) NOD mcDC increase with age in spleen compared to controls, shown as mean ± SD of 3–5 mice per time-point. Similar results were seen in PLN (not shown). (c) Increased IFN produced by activated mcDC from NOD mice with age as measured from PLN and spleen DC after overnight stimulation with irradiated cells, 3 mice per time-point. * denotes p <0.01, ‡ denotes p <0.001.
Enhanced type 1 interferon production by NOD mcDC
One hallmark of mcDC is their production of type 1 interferons (IFN) upon an encounter with irradiated/dying cells, that is vital for the priming of both CD4+ and CD8+ T cells (11). We assessed IFN production by mcDC from both the PLN and spleen of NOD mice, and found that compared to BALB/c mice, NOD mcDC produced elevated levels of IFN upon co-culture with irradiated splenocytes, Figure 1c. These data suggested not only that NOD mice show elevated levels of mcDC with age but also show elevated production of pro-inflammatory IFN as well. These data agree with recent findings suggesting that type 1 IFN are vital initiators of T1D in NOD mice (25).
Expansion of mcDC in vivo by Flt3L
While the existence of mcDC in NOD mice was considerable, their overall numbers per mouse like that of other DC subsets remain low. Thus to undertake meaningful mechanistic studies, we took advantage of the ability of Flt3L to expand these cells in vivo. This was done using the B16-Flt3L model described in (16). Briefly, the Flt3L-expressing tumor, B16-Flt3L, was injected, s.c. (4–5 × 107), on the back of NOD mice, and produced biologically-active Flt3L that drove the in vivo expansion of several DC subsets, including mcDC by 30 – 35 fold within 7 – 10 days. To assess whether Flt3L altered the phenotype of mcDC, Flt3L-driven and wild type (WT) NOD DC subsets were compared. Flt3L did not alter the expression of co-stimulatory ligands or MHC class I and II on mcDC or any other DC subset (Supplemental Figure 1). Moreover, in vivo-expanded mcDC acquired and presented antigen similarly to WT NOD mcDC (see below). Thus, unless otherwise indicated, subsequent experiments were performed using in vivo Flt3L-expanded mcDC.
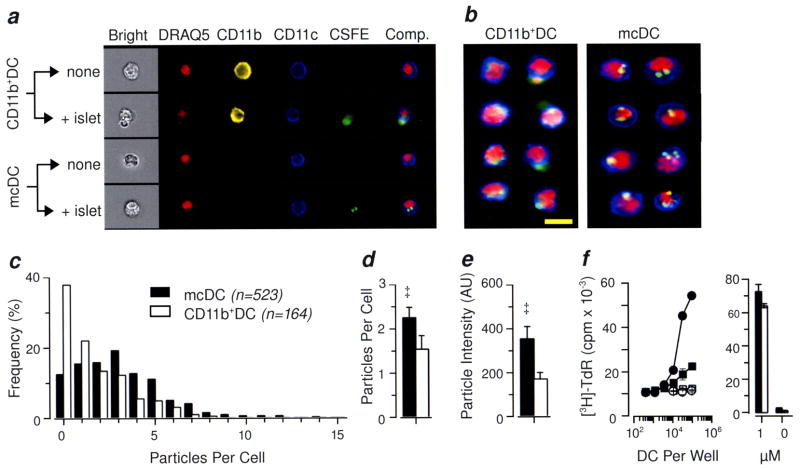
Acquisition of cell fragments from irradiated islet cells by NOD mcDC
Merocytic DC are functionally unique. Unlike their other cDC counterparts, mcDC acquire antigen by merocytosis, or “nibbling” small fragments of antigen from dead or dying cells, rather than by macropinocytosis, hence their original description as nDC for “nibbling” DC (11). This includes cellular antigen from cells that die by either gamma or UV irradiations and CD95-crosslinking (11). Moreover, the antigenic fragments acquired by merocytosis are retained by mcDC as visually-distinct punctate cytoplasmic vesicles. Thus, to establish whether the elevated numbers of CD11b−/loCD8a−PDCA-1− DC observed in NOD mice were in fact functional mcDC, we performed antigen acquisition studies with NOD DC using CFSE-labeled irradiated islet cells as targets. Here, CD11b+DC and mcDC were isolated by magnetic beads from NOD splenocytes, and then mixed with CFSE-labeled irradiated islet cells overnight. After culture, the DC were purified away from dead cells, labeled with mAb to CD11b, CD11c and the nuclear dye, DRAQ-5, and analyzed using an imaging flow cytometry. (Overall DC survival after culture can be found in Supplemental Figure 2). Representative single and composite images for each subset incubated with or without irradiated beta cells are shown in Figure 2a. Note that the CD11b+DC incubated with irradiated, CFSE-labeled islet cells exhibited diffuse CFSE staining within the cytoplasm, typical of phagocytosis and catabolism of ingested cells, while the corresponding mcDC contained cells with distinct punctate vesicles containing CFSE. This is more easily seen in the composite image galleries shown in Figure 2b. Here, several of the CD11b+DC have not only widely-distributed CFSE staining but also cytoplasm-localized apoptotic bodies (DRAQ-5+), consistent with macropinocytosis. In contrast, the mcDC exhibit multiple merocytic vesicles of moderate to high intensity for CFSE.
Figure 2. mcDC have small, high-intensity particle of irradiated islet cells and present islet antigen efficiently to CD4+ T cells.
(a) Representative cell morphology of CD11b+DC and mcDC fed irradiated, CFSE-labeled islet cells in vitro imaged using an imaging flow cytometer. The composite image on the right is an overlay of the CD11c, CFSE and DRAQ-5 (nuclear) images. (b) Representative composite images from CD11b+DC and mcDC fed irradiated CFSE-labeled islet cells. Yellow bar equals 10 microns. (c) Quantitative analysis of particles per cell for mcDC and CD11b+DC from NOD mice. The mcDC subset had a higher mean particle score (d), and higher fluorescent intensity (e). Representative result from 7 independent experiments. * denotes p <0.01, ‡ denotes p <0.001. (f) Efficient presentation of islet cell antigen to naïve CD4+ T cells by mcDC as compared to CD11b+ DC in a 72h proliferation assay (left panel); circles denote mcDC and squares CD11b+DC; filled symbols denote exposure to apopttic islets overnight prior to use in assay. Both subsets were equally effective at presenting peptide antigen to BDC2.5 T cells (right panel). Representative of 3 independent experiments.
To quantify the frequency and intensity of the antigen particles, we applied a spot mask filter described in Supplemental Figure 3. The mcDC fraction had more cells with multiple CFSE-containing cytoplasmic vesicles compared to CD11b+DC (Figure 2c), and the mean particles per cell for mcDC was statistical elevated compared to CD11b+DC (Figure 2d). The CFSE particle intensity was likewise greater in mcDC than in CD11b+DC, reflecting the tight punctate staining seen in the mcDC subset (Figure 2e). Thus, the imaging flow cytometry revealed that the CD11b−/loDC defined in Figure 1 were one in the same with the functionally-defined mcDC subset (Figure 2).
Efficient presentation of islet cell antigen to naïve CD4+ T cells by merocytic DC
To establish if NOD mcDC were more efficient at presenting self-antigen than CD11b+DC on a per cell basis, we undertook antigen presentation studies using mcDC and CD11b+DC as APC and naïve CD4+ BDC2.5 T cells as responders. The mcDC and CD11b+DC were prepared as above and mixed with irradiated islet cells. After 16 hr, the mcDC and CD11b+DC subsets were sort-purified and used as APC in a 3-day T cell proliferation assay. The antigen-exposed mcDC were substantively better APC than their CD11b+DC counterparts (9.2-fold ± 1.2, Figure 2f). The CD11b+DC were, however, equally effective at presenting exogenous synthetic peptide antigen (Figure 2f). These data suggest that mcDC were more effective at presenting self-antigen from irradiated cells. In addition, the mcDC enhanced priming capacity may be due to their ability to sustained antigen presentation, as we have recently found that B6 mcDC stored merocytosed material in non-acidic vesicles which functioned as a longterm depot for antigen, thus allowed prolonged antigen presentation by mcDC (EMJ, manuscript submitted).
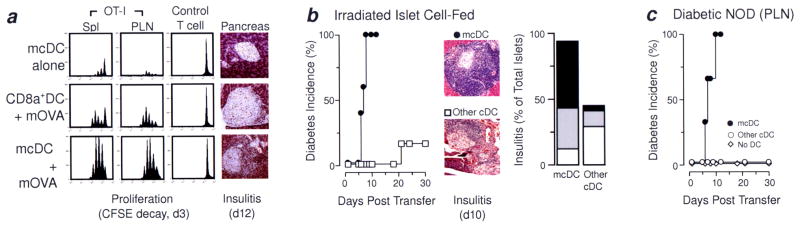
Merocytic DC break CD8+ T cell tolerance by cross-presentation of islet antigen in vivo
To assess the ability of mcDC to break CD8+ T cell tolerance to islet antigen, we used the well-established OT-I model (14, 26). In this model, naïve CD8+ OT-I T cells (OVA-specific) – but not control CD8+ T cells – undergo tolerance by deletion when transferred into mice expressing membrane-bound OVA on the pancreatic beta cells (RIP-mOVA). When OT-I T cells encountered their antigen in these mice, they first showed signs of proliferation (CFSE decay) followed by deletion (total cell loss, (27)) This is likewise true when freshly purified mcDC were transferred one day after the OT-I T cells (Figure 3a, upper panels). However, when the transferred mcDC were first exposed to irradiated cells from Kb−/− mice expressing membrane bound OVA (mOVA/Kb−/−), the OT-I T cell did not undergo deletion in the RIP-mOVA host, but rather showed significant proliferation in both the spleen and pancreatic lymph nodes, resulting in OT-I T cell accumulation and persistence as demonstrated by the increase in the ratio of OT-I/control CD90.1 T cells (Figure 3a. lower panels). Moreover, the activated OT-I T cells induced substantial insulitis by d12 post-transfer (Figure 3a). Importantly, CD8α+DC failed to rescue OT-I T cells from deletion, even when the CD8α+DC were pre-incubated with irradiated mOVA/Kb−/− cells (Figure 3a, middle panels). The rescue of OT-I T cells by mcDC was the result of cross-priming as the antigen-donating mOVA cells came from mice lacking MHC Class I (Kb−/−) and thus could not supply “pre-loaded” Kb complexes for re-presentation. Thus, these results, with those from Figure 2f, suggest that mcDC can present antigen from apoptotic cells to both naïve CD4+ and CD8+ T cells, and can drive immunopathology.
Figure 3. mcDC break T cell tolerance, induce insulitis and transfer diabetes in vivo.
(a) RIP-mOVA (CD45.2) mice received 106 CFSE-labeled CD8+ OT-I T cells (CD45.1) together with CD8+ irrelevant control T cells (CD90.1), d0, and then either mcDC or CD8α+DC exposed to irradiated cells from mOVA/Kb-deficient mice on d1. On d3, proliferation and accumulation of OT-I and control T cells was determined. Only mcDC exposed to mOVA broke T cell tolerance by cross-priming OT-I and induced insulitis (d12). (b) A pool of CD11b+DC, CD8α+DC and mcDC from NOD were exposed to irradiated islet cells in vitro, purified and transferred (5 × 105) to 4-wk old NOD recipients. Recipients that received mcDC but not the other cDC rapidly developed T1D. Mice receiving mcDC had more advanced insulitis, 6 to 9 mice per group. (c) Rapid onset of T1D and insulitis in NOD mice given sort-purified mcDC isolated from the PLN of overtly diabetic female NOD mice. Peri-insulitis, white bars; moderate insulitis, gray bars; severe insulitis, black bars. The mixed CD8α+/CD11b+ cDC subset did not induce T1D, 3 to 5 mice per group.
Transfer of mcDC induces diabetes in NOD mice
To assess whether mcDC were able to break tolerance and induce diabetes in NOD mice, we performed transfer studies in which DC from NOD mice were incubated with irradiated islet cells overnight then sort-purified and transferred into 28-day old NOD recipients. Eight to nine days after transfer, all NOD mice that received antigen-loaded mcDC were diabetic (Figure 3b). In contrast, the pooled antigen-exposed CD11b+DC and CD8α+DC (“other cDC” in Figure 3b) were significantly poorer in transferring disease, exhibiting a statistically slower time to disease with fewer diabetic mice. The overall severity of insulitis was, likewise, markedly enhanced in mice that received antigen-loaded mcDC (Figure 3b).
Until now, the mcDC from Flt3L-treated NOD were exposed to irradiated islet cells ex vivo. Therefore it became critical to determine if mcDC could naturally acquired antigen in vivo and likewise transfer diabetes to young NOD mice. To this end, cDC subsets, either mcDC or a pool of CD8α+DC and CD11b+DC, were sort-purified from PLN of recent-onset diabetic WT NOD females (≥ 18 wks) and immediately transferred without additional manipulation into 28-day old female NOD recipients. Importantly, the recipients had normal levels of mcDC at time of transfer. All NOD mice that received mcDC from the PLN of diabetic mice developed rapid onset diabetes (Figure 3c). The tempo and incidence of disease were similar to those seen when in vitro-loaded mcDC were used (Figure 3b). None of the NOD mice that received pooled (“other cDC”) or were left untreated developed diabetes within the assay period (Figure 3c).
Importantly, these data suggested that the mcDC subset (i) acquire islet antigen naturally in vivo, (ii) were competent antigen presenting cells when transferred to young disease-free recipients, (iii) were capable of breaking of self-tolerance to islet cell antigen, and (iv) were capable of hastening diabetes development in vivo. Moreover, mcDC were not only present but also elevated in NOD mice compared to other strains and accumulated in NOD mice with time. Together, these results lead us to conclude that mcDC are the likely CD11c+ DC subset responsible for the priming of both diabetogenic CD4+ and CD8+ T cells and the functional break in immune tolerance to islet antigens observed in NOD mice.
Supplementary Material
Non-Standard Abbreviations
- cDC
conventional DC
- mcDC
merocytic DC
- PLN
pancreatic LN
- T1D
Type 1 diabetes
Footnotes
This work is supported by JDRF grant (5-2008-944) to JDK and EMJ, NIH grant R01 DK08179 to JDK and NIH grant R21 AI079545 to EMJ.
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.André I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci U S A. 1996;93:2260–2263. doi: 10.1073/pnas.93.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 4.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 5.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 6.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 7.Höglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roos A, Xu W, Castellano G, Nauta AJ, Garred P, Daha MR, van Kooten C. Mini-review: A pivotal role for innate immunity in the clearance of apoptotic cells. Eur J Immunol. 2004;34:921–929. doi: 10.1002/eji.200424904. [DOI] [PubMed] [Google Scholar]
- 11.Janssen E, Tabeta K, Barnes MJ, Rutschmann S, McBride S, Bahjat KS, Schoenberger SP, Theofilopoulos AN, Beutler B, Hoebe K. Efficient T cell activation via a Toll-Interleukin 1 Receptor-independent pathway. Immunity. 2006;24:787–799. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 13.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 14.Kurts C, Heath WR, Kosaka H, Miller JF, Carbone FR. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1) J Exp Med. 1998;188:415–420. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 16.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 20.Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steptoe RJ, Ritchie JM, Harrison LC. Increased generation of dendritic cells from myeloid progenitors in autoimmune-prone nonobese diabetic mice. J Immunol. 2002;168:5032–5041. doi: 10.4049/jimmunol.168.10.5032. [DOI] [PubMed] [Google Scholar]
- 22.Suri A, Katz JD. Dissecting the role of CD4+ T cells in autoimmune diabetes through the use of TCR transgenic mice. Immunol Rev. 1999;169:55–65. doi: 10.1111/j.1600-065x.1999.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 23.Morel PA, Vasquez AC, Feili-Hariri M. Immunobiology of DC in NOD mice. J Leukoc Biol. 1999;66:276–280. doi: 10.1002/jlb.66.2.276. [DOI] [PubMed] [Google Scholar]
- 24.Prasad SJ, Goodnow CC. Cell-intrinsic effects of non-MHC NOD genes on dendritic cell generation in vivo. Int Immunol. 2002;14:677–684. doi: 10.1093/intimm/dxf034. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Orozco N, Chung Y, Chang SH, Wang Y, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belz GT, Behrens GMN, Smith CM, Miller JFAP, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.