Abstract
There is growing evidence that activation of high affinity extrasynaptic GABAA receptors in the brain, cerebellum and spinal cord substantia gelatinosa results in a tonic inhibition controlling postsynaptic excitability. The aim of the present study was to determine if GABAA receptors mediating tonic inhibition participate in the modulation of monosynaptic reflex (MSR) in the vertebrate spinal cord. Using an in vitro turtle lumbar spinal cord preparation, we show that conditioning stimulation of a dorsal root depressed the test monosynaptic reflex (MSR) at long condition–test intervals. This long duration inhibition is similar to the one seen in mammalian spinal cord and it is dependent on GABAA as it was completely blocked by 20 μm picrotoxin (PTX) or bicuculline (BIC) or 1 μm gabazine, simultaneously depressing the dorsal root potential (DRP) without MSR facilitation. Interestingly 100 μm picrotoxin or BIC potentiated the MSR, depressed the DRP, and produced a long lasting motoneurone after-discharge. Furosemide, a selective antagonist of extrasynaptic GABAA receptors, affects receptor subtypes with α4/6 subunits, and in a similar way to higher concentrations of PTX or BIC, also potentiated the MSR but did not affect the DRP, suggesting the presence of α4/6 GABAA receptors at motoneurones. Our results suggest that (1) the turtle spinal cord has a GABAA mediated long duration inhibition similar to presynaptic inhibition observed in mammals, (2) GABAA receptors located at the motoneurones and primary afferents might produce tonic inhibition of monosynaptic reflex, and (3) GABAA receptors modulate motoneurone excitability reducing the probability of spurious and inappropriate activation.
Introduction
GABA, the major inhibitory neurotransmitter in the central nervous system, exerts its action by activating the widely distributed GABAA and metabotropic GABAB receptors. Unlike GABAB receptors the ionotropic synaptic GABAA receptors are formed by a pentamer of subunits that form ligand-gated chloride channels, and play an important role in signalling with high temporal and spatial precision as well as producing synchronization and oscillatory behaviour (Farrant & Nusser, 2005). Recently in neurons of hippocampus and cerebellum a new type of GABAA receptor has been described. Residing at extrasynaptic or perisynaptic sites, these receptors are activated persistently by the GABA spillover, producing a form of signalling termed tonic inhibition (Farrant & Nusser, 2005; Walker & Semyanov, 2008). Importantly, all tonic extrasynaptic GABAA receptor-mediated conductances are blocked by high concentrations of bicuculline, picrotoxin or SR-95531 (Semyanov et al. 2004; Farrant & Nusser, 2005); for example, in hippocampal interneurones picrotoxin produces a maximal change in holding current at 100 μm (Semyanov et al. 2004). However, a submicromolar concentration of SR-95531 selectively blocks only phasic currents mediated by the classical α1β2γ2 synaptic GABAA receptor (Semyanov et al. 2004). Few GABAA receptor antagonists show clear subunit selectivity, but the diuretic furosemide has ∼100-fold selectivity for α6 over α1 subunit-containing receptors (Korpi et al. 1995), and has been used to determine the role of synaptic and extrasynaptic α6-containing receptors in cerebellar granule cells.
In the spinal cord of adult mice the presence of extrasynaptic GABAA receptors in substantia gelatinosa neurones has been described recently (Takahashi et al. 2006; Ataka & Gu, 2006). However, until now electrophysiological evidence regarding the presence of these receptors at either motoneurones or primary Ia afferents has not been reported. In mammalian spinal cord, there is evidence to show that the monosynaptic reflex (MSR) is modulated by presynaptic inhibition of primary afferents mediated by synaptic activation of GABAA receptors at axo-axonic synapses with GABAergic interneurons (Schmidt, 1971). Presynaptic inhibition has been associated with primary afferent depolarization (PAD); this signal is propagated to the dorsal root afferents and is recorded as the dorsal root potential (DRP) (Eccles et al. 1962; Schmidt, 1971; Rudomin & Schmidt, 1999). In the bullfrog spinal cord a GABAA agonist, muscimol, depresses the MSR by inducing pre- and postsynaptic inhibition (Peng & Frank, 1989). Interestingly, the muscimol effect was reversely blocked by bicuculline (100 μm), although at that time it was not known that two classes of GABAA receptors existed. Our previous studies also have shown that muscimol decreased by 60% the motoneurone input resistance and the time constant, whereas bicuculline increased the amplitude of EPSPs in motoneurones by 20% (Delgado-Lezama et al. 2004), confirming the presence of GABAA receptors on turtle lumbar cord motoneurones. In the lamprey GABAA receptors play an important role in burst frequency regulation during fictive locomotion, increasingly significantly the frequency in the presence of picrotoxin (100 μm) or gabazine (100 μm) (Schmitt et al. 2004).
Morphological evidence from immunohistochemical and in situ hybridization also supports the presence of distinct GABAA receptors in afferent terminals and motoneurones composed of α2, α3, α5 and γ2 subunits (Persohn et al. 1991, 1992; Wisden et al. 1991; Ma et al. 1993; Alvarez et al. 1996; Bohlhalter et al. 1996; Yang et al. 1997). In supraspinal neurons extrasynaptic GABAA receptors include the α5-subunit (Farrant & Nusser, 2005; Walker & Semyanov, 2008). However, the role of these receptors in MSR modulation in the spinal cord is unknown.
In this study, we were able to answer this question pharmacologically with different concentrations of antagonists like picrotoxin, bicuculline and gabazine as well as furosemide, a selective extrasynaptic GABAA antagonist. Moreover we also observed for the first time that a GABAA mediated long duration inhibition of the MSR exists in the adult turtle, suggesting that there is indeed presynaptic inhibition of primary afferents and that it resembles the one observed in adult mammals (Eccles et al. 1963). We also demonstrated that GABAA receptor antagonists at concentrations known to preferentially block synaptic receptors (i.e. picrotoxin 20 μm, bicuculline 20 μm and gabazine 1 μm) abolished presynaptic inhibition without facilitation of MSR and partially depressed the DRP. However, a higher concentration of bicuculline or picrotoxin (100 μm) facilitated the MSR producing at the same time an additional depression of the DRP. Importantly, the MSR was followed by a prolonged after-discharge. Likewise, furosemide facilitated the MSR without affecting the DRP, suggesting the presence of GABAA receptors with α4/6 subunits in motoneurones.
Overall, these results suggest that (1) the turtle has a mammalian-like presynaptic inhibitory system in the spinal cord, and (2) GABAA receptors, probably of the extrasynaptic type, located on motoneurones and/or primary afferents produce tonic inhibition of the MSR.
Methods
All experimental procedures followed the guidelines set out in The Journal of Physiology (Drummond, 2009) and were carried out with the approval of the Cinvestav Experimental Ethics Committee and in accordance with the current Mexican Norm for Care and Use of Animals for Scientific Purposes. The animals were provided by the National Mexican Turtle Centre located in Mazunte, Oaxaca (Mexico) with the authorization (DGVS-03821/0907) by the Federal Mexican Government Ministry of Environment and Natural Resources (Secretaría de Medio Ambiente y Recursos Naturales, Semarnat).
Preparation
Fifty adult turtles (Kinosternon leucostomun and Pseudemis scripta, 15–20 cm carapace length) were anaesthetized with pentobarbitone (100 mg kg−1, i.p.). The plastron was opened and the blood removed by intraventricular perfusion with Ringer solution (∼10°C) of the following composition (mm): 120 NaCl, 5 KCl, 15 NaHCO3, 3 CaCl2, 2 MgCl2 and 20 glucose, saturated with 2% CO2–98% O2 to give pH 7.6. The laminectomy was made to isolate lumbar spinal enlargement containing segments from D7 to D10 in continuity with the ventral and dorsal roots. For the recordings, the preparation was placed in a recording chamber and perfused with Ringer solution (20–22°C). At the end of the dissection the animals were killed by decapitation.
Recordings
Two dorsal (DRD8 and DRD9) and one ventral root (VRD9) were mounted in suction electrodes (Fig. 1A). The test MSR recorded from VRD9 was evoked by stimulation of the ipsilateral dorsal root (DRD9). Long duration inhibition was induced by applying a conditioning stimulus to DRD8 before the test MSR was evoked; in some experiments the sensory evoked dorsal root potential (DRP) was recorded from DRD9. The recording suction electrodes were connected to a differential AC amplifier (Grass Instruments, Quincy, MA, USA) with a bandwidth of 0.1 Hz to 1 kHz. The dorsal roots were stimulated with a rectangular current pulse (0.5 ms). The threshold was defined as the minimum stimulus intensity that elicits a measurable ventral root potential (VRP). Unless otherwise stated the recordings shown were the average of four to eight stimuli applied every 30 s.
Figure 1. Simultaneous recordings of the monosynaptic reflex (MSR) and the dorsal root potential (DRP) in the turtle spinal cord.
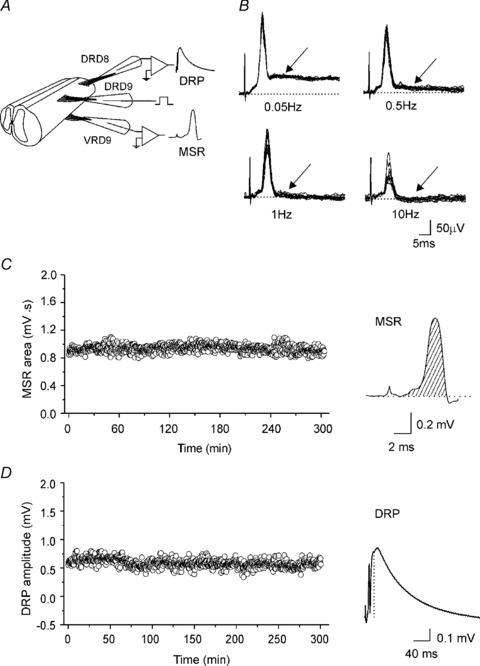
A, scheme of two spinal cord segments in continuity with the dorsal and ventral roots attached to suction electrodes for stimulation and recording of the DRP and VRP. B, VRP evoked at four different frequency stimulations (2T) applied to the ipsilateral dorsal root. The earliest component of VRP is the MSR because it followed one to one frequency stimulation at 10 Hz without jittering and failure. The slower VRP component failed to follow one to one at 1 Hz of dorsal root stimulation. Striped area under the MSR curve (C) and the DRP amplitude (D) recorded simultaneously every 30 s plotted versus time. Traces, average of the MSR and DRP recordings of 10 sweeps.
Drugs and analysis
Bicuculline (20–100 μm), picrotoxin (20–100 μm), gabazine (1 μm) and furosemide (100–200 μm) were applied to the bath solution to block GABAA receptors. All the drugs were purchased from Sigma. The action of GABAA antagonists was quantified by measuring the changes of the area under the MSR curve (Fig. 1C) and DRP amplitude (Fig. 1D). Values are presented as the mean ± s.e.m. The statistical difference between means was determined by Student's t test. Means were considered statistically different when P < 0.05.
Results
MSR characterization
Ventral root potential was evoked by stimulation (2.5 times threshold) of the homonymous dorsal root (D9); the earliest component presented a peak latency of 7.41 ± 0.10 ms (n = 40). These results are in agreement with previous studies showing similar latencies for monosynaptic EPSPs in turtle motoneurones (Yamashita, 1986), and as a result we considered this component to be the monosynaptic reflex (MSR). Likewise, the MSR also followed one to one high frequency stimulation (>10 Hz) without jittering (Fig. 1B). At higher stimulation frequencies ≥1 Hz, slow VRP components were depressed, but attenuated MSRs remained (Fig. 1B).
Viability of the preparation and effect of GABAA antagonists on the MSR and DRP
In order to know if GABAA receptor antagonists at different concentrations produce any reliable action on the MSR and the DRP we first tested the stability of the preparation by recording both signals every 30 s during 4 h in normal Ringer solution. Figure 1C and D shows plots of the MSR area and DRP amplitude, respectively. During this time both variables did not change (P > 0.05). Therefore, we proceeded to evaluate the action of the drugs.
Modulation of MSR and motoneurone excitability by GABAA postsynaptic receptors has received little attention (Curtis et al. 1971; Peng & Frank, 1989; Vinay & Clarac, 1999; Delgado-Lezama et al. 2004). The reported data suggest the presence of GABAA receptors in motoneurones, which probably are tonically activated by GABA and might modulate the MSR. To assess this possibility, the MSR was recorded in the presence of picrotoxin (20 μm) or bicuculline (20 μm), concentrations reported to block mostly synaptic GABAA receptors (Semyanov et al. 2004; Farrant & Nusser, 2005). Figure 2A and B shows that the MSR area was not significantly affected (P > 0.05), while the DRP was depressed to 46.4 ± 1.5% (n = 11) by picrotoxin. Similar actions were observed in the presence of bicuculline (20 μm) (data not shown). However, previous data from our lab reported that motoneurone EPSPs evoked by stimulation of the dorsolateral funiculus increased in amplitude in the presence of bicuculline (40 μm) (Delgado-Lezama et al. 2004). Therefore, we decided to investigate if the MSR could be modulated by the extrasynaptic GABAA receptors reported to be sensitive to high concentrations of picrotoxin or bicuculline (100 μm) (Semyanov et al. 2004; Farrant & Nusser, 2005; Walker & Semyanov, 2008). Unexpectedly, 100 μm picrotoxin facilitated the MSR by about 20 ± 2.4% (n = 11) with respect to the control (Fig. 2A; P < 0.05), while the sensory evoked DRP presented an additional depression to 38 ± 2.7% (Fig. 2B; n = 11; P < 0.05). Similar changes were recorded with bicuculline 100 μm (n = 9; data not shown). Interestingly, gabazine at 1 μm, a competitive antagonist which has affinity for synaptic GABAA receptors, also depressed the DRP by 44 ± 2.5% without facilitating the MSR (P > 0.05; n = 8). These results suggest the presence of synaptic GABAA receptors in the terminals of primary afferents producing PAD due to the effect of low concentrations of picrotoxin (20 μm), bicuculline (20 μm) and gabazine (1 μm) in the DRP. On the other hand the MSR remained unchanged with low concentrations of picrotoxin (20 μm), bicuculline (20 μm) and gabazine (1 μm), suggesting that GABAA receptors located on motoneurones are not sensitive to low concentrations of antagonist, excluding the possibility of these being synaptic.
Figure 2. Action of picrotoxin on the MSR and DRP.
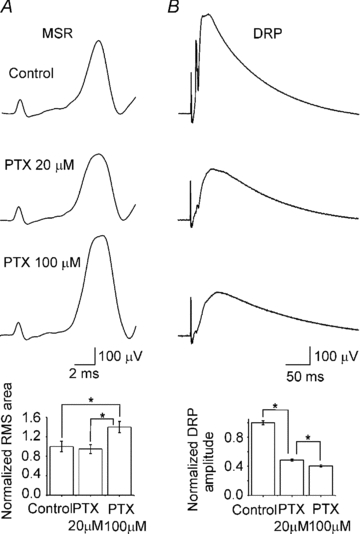
A and B, top to bottom, representative MSR and DRP evoked by dorsal root stimulation (2T) and simultaneously recorded in control Ringer solution and in the presence of picrotoxin (PTX) at 20 and 100 μm. Bar graphs, normalized MSR area and DRP amplitude in control Ringer solution and in the presence of PTX. Vertical lines indicate s.e.m. Asterisks indicate statistical difference with respect to the control for the MSR and with respect to the control and between PTX at 20 and 100 μm for the DRP (n = 11; P < 0.05).
Long duration inhibition in the turtle spinal cord
It is well documented that sensory flow information in mammals is modulated by presynaptic inhibition of primary afferents. Sensory evoked presynaptic inhibition is mediated by activation of GABAA receptors at axo-axonic synapses producing PAD (Schmidt, 1971; Rudomin & Schmidt, 1999). Previous studies in mammals have shown that presynaptic inhibition lasts at least 300 ms (Eccles et al. 1962). Therefore we attempted to study sensory evoked presynaptic inhibition by applying conditioned stimulation of the MSR at different time intervals (30–300 ms). We observed that a long latency inhibition mediated by GABAA receptors regulated transmission from primary afferents. A test MSR evoked by one stimulus to DRD9 was conditioned by applying one stimulus to DRD8 at interstimulus intervals of 30, 60, 100 and 300 ms (scheme, Fig. 3A). The conditioning–test MSR in normal Ringer solution was depressed at all interstimulus intervals tested with a maximal depression of about 92% at 30 ms (Fig. 3B and C). However, in the presence of picrotoxin (20 μm), a conditioning stimulation did not depressed the MSR in the range of interstimulus intervals assessed (Fig. 3B and C). Interestingly, the sensory evoked DRP recorded on DRD9 and evoked by stimulation of DRD8 presented almost the same duration as the long latency inhibition of the MSR and was depressed about 40% (Fig. 3B). A similar result was observed in another three preparations. Blockade of presynaptic inhibition and depression of DRP were also observed in the presence of bicuculline (20 μm; n = 4) and gabazine (1 μm; n = 4). The fact that gabazine at this concentration is considered to block mainly synaptic GABAA receptors (Semyanov et al. 2004; Farrant & Nusser, 2005) strongly suggests that a long latency inhibition in the turtle, like in mammals, might be produced by activation of synaptic GABAA receptors located at primary afferent terminals, which consequently might mediate the fraction of DRP depressed by the blockers. As suggested by Eccles et al. (1963), the DRP sensitive to picrotoxin, and in our results also sensitive to bicuculline (20 μm) or gabazine, could correspond to the PAD associated with presynaptic inhibition of primary afferents. Consequently, we might conclude that sensory evoked presynaptic inhibition of low threshold afferents in the turtle spinal cord might be mediated by GABA release from GABAergic interneurones establishing axo-axonic contacts with primary afferents, as has been shown in mammals and amphibians (Rudomin & Schmidt, 1999; Peng & Frank, 1989; Vesselkin et al. 2001). This inhibition is not tonically active as occurs in the case of presynaptic inhibition of high threshold cutaneous afferents in the cat spinal cord (Rudomin et al. 2007).
Figure 3. Long duration inhibition of the MSR.
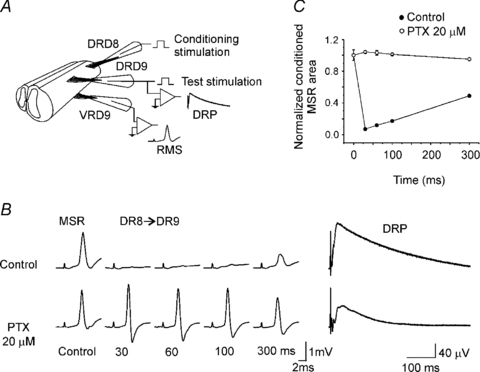
A, scheme showing the protocol to induce long duration inhibition of the MSR. MSR recorded from D9 ventral root (VRD9) was evoked by stimulation of the ipsilateral dorsal root (DRD9). Conditioning stimulation was applied through D8 dorsal root (DRD8). B, MSR control and conditioned (DR8→DR9) recorded in control Ringer solution (top traces) and in presence of picrotoxin (20 μm; lower traces) at interstimulus intervals indicated bellow. To the right, DRP recorded from DRD9 in control Ringer solution and in the presence of picrotoxin. C, plot of normalized MSR area versus interstimulus interval in control Ringer solution (filled circles) and in presence of picrotoxin (open circles). Vertical bars indicate s.e.m.
Effect of high concentration of picrotoxin on the long duration inhibition of the MSR
Having shown that long duration inhibition was abolished by GABAA receptor antagonists at low concentrations, which preferentially blocks synaptic GABAA receptors at supraspinal nuclei (Farrant & Nusser, 2005; Walker & Semyanov, 2008), we decided to assess the action of picrotoxin at 100 μm on the MSR conditioned by stimulation of an adjacent dorsal root. Figure 4 shows the time course of the conditioned MSR area recorded at interstimulus intervals of 30, 60, 100, 150 and 300 ms. As previously shown, picrotoxin (20 μm) abolished the long duration inhibition without any significant facilitation of the MSR (P > 0.05). However higher concentrations of picrotoxin (100 μm) facilitated the conditioned MSR by 20 ± 1% (n = 4). The same result was observed in the presence of bicuculline (100 μm; n = 4; not shown). This result suggests the existence of two types of GABAA receptors on primary afferents and motoneurones. One type, sensitive to low concentration of the antagonists, is probably located on primary afferents, where they are activated synaptically and mediate presynaptic inhibition. It appears that motoneurones do not have this type because the MSR was not facilitated. A second type, sensitive to high concentration of the antagonists, might be located in motoneurones and also in primary afferents.
Figure 4. Faciliation of the conditioned MSR by higher concentrations of picrotoxin.
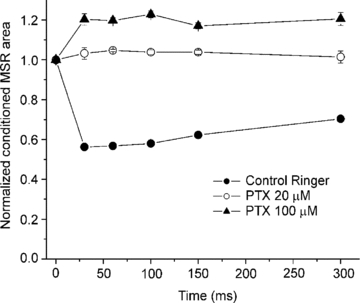
Plot shows the normalized area of MSR evoked by stimulation of the DRD9 conditioned by stimulation of an adjacent dorsal root (DRD8) at interstimulus intervals of 30, 60, 100, 150 and 300 ms in control Ringer solution (filled circles) and in the presence of picrotoxin at 20 (open circles) and 100 μm (filled triangles). Vertical bars indicate the s.e.m.
Facilitation of MSR by furosemide sensitive GABAA receptors
Immunohistochemical and in situ hybridization studies have revealed the presence of diverse GABAA receptors in dorsal horn layers and dorsal root ganglia as well as in motoneurones (Persohn et al. 1991; Wisden et al. 1991; Ma et al. 1993; Alvarez et al. 1996; Bohlhalter et al. 1996). However, the physiological roles and pharmacological implications of this receptor diversity have not yet been determined (Rekling et al. 2000). Therefore we decided to assess the action of furosemide, an antagonist of α4/α6 subunit-containing GABAA receptors at concentration that do not affect the Cl− transporter (Hochman & Schwartzkroin, 2000), on the MSR and the DRP. In order to block synaptic GABAA receptors involved in the long duration inhibition of the MSR, we first applied picrotoxin (20 μm) (n = 4) or gabazine (1 μm) (n = 4). As previously shown, gabazine (1 μm) did not facilitate the MSR (P > 0.05) and depressed the DRP amplitude 44 ± 2.58% (P < 0.05; n = 4, Fig. 5). Then, we added furosemide (100 and 200 μm). Interestingly the MSR was facilitated by 11 ± 2.6 and 20 ± 2% respectively, as observed in the presence of picrotoxin and bicuculline (100 μm); however the DRP was not affected (n = 4; P > 0.5; Fig. 5B and C). Additionally, similar to the action of picrotoxin during the long duration inhibition, furosemide also facilitated the conditioned MSR (not shown). Therefore, these results suggest that α4/6 subunit-containing GABAA receptors might be located at motoneurones and regulate their excitability by shunting the motoneurone membrane.
Figure 5. Furosemide effect on the MSR and DRP.
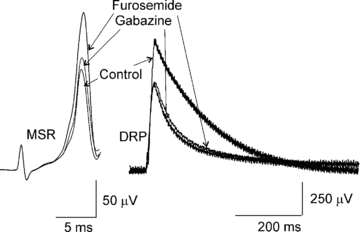
The MSR and DRP recorded in control Ringer solution, in the presence of gabazine 1 μm, and plus furosemide 200 μm.
Long-lasting after-discharge in motoneurones induced by picrotoxin, bicuculline and furosemide
MSR evoked by one stimulus (2T) applied to the ipsilateral dorsal root was followed by some polysynaptic reflexes not lasting more than 10 ms (Fig. 6A). Nonetheless, in the presence of picrotoxin (20 μm) or bicuculline (20 μm; not shown) as well as gabazine (1 μm; not shown) the MSR was not affected, but polysynaptic reflexes were facilitated and a 40 ms latency long-lasting post-discharge of 2 s duration was evident (Fig. 6B). When the antagonist concentration of picrotoxin or bicuculline was increased to 100 and 200 μm, respectively, the post-discharge was facilitated and the latency was shortened (20 ms) (third and fourth traces from the top in Fig. 6A; n = 16).
Figure 6. Motoneurone after-discharge in the presence of picrotoxin.
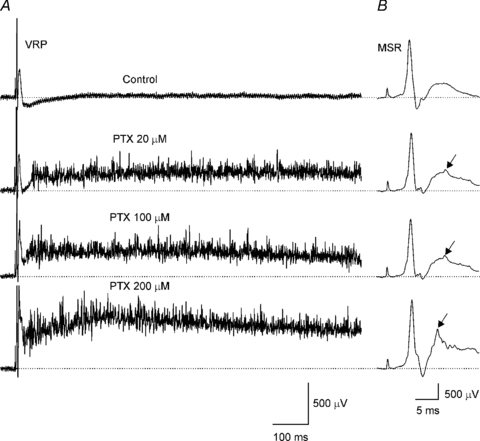
A, VRP evoked by ipsilateral dorsal root stimulation (2T) recorded, from top to bottom, in control Ringer and in the presence of picrotoxin (PTX) at 20, 100 and 200 μm. B, the MSR from A at a different time and voltage scale. Arrows indicate polysynaptic reflex.
Increase of the excitability of motoneurones might be due to two factors, first a large decrease of presynaptic inhibition leading to an increase of excitatory synaptic inputs to the motoneurone, and second, blockade of postsynaptic inhibitory control due to extrasynaptic or synaptic GABAA receptors that would drive the membrane potential close to the firing threshold through an increase in membrane resistance (Delgado-Lezama et al. 2004). This has been observed in hippocampus and cerebellar granule cells where activation of extrasynaptic GABAA receptors has a profound effect on neuronal excitability (Semyanov et al. 2004), as well as a gain modulation control in cells with high variability of synaptic input (Mitchell & Silver, 2003).
Discussion
The modulation of the MSR by GABAA receptors is mediated by two mechanisms: presynaptic inhibition of Ia afferent terminals and postsynaptic inhibition of motoneurones (Kellerth & Szumski, 1966; Schmidt, 1971; Peng & Frank, 1989; Jonas et al. 1998; Rudomin & Schmidt, 1999; Kullmann et al. 2005). Despite the morphological and pharmacological evidence available on GABAA receptors on the motoneurones, the role of these receptors in the postsynaptic modulation of the MSR has been scarcely studied. Although presynaptic inhibition has gained much attention in the past decades, and is one of the most studied mechanisms in the spinal cord, it seems that both presynaptic and postsynaptic sites play a complementary role in maintaining the stability of reflex activity (Solodkin et al. 1984). Our results show that GABAA receptors, located probably at extrasynaptic sites and activated by ambient GABA, along with synaptic GABAA receptors, might have a relevant role in controlling primary afferents and motoneurone excitability.
Monosynaptic reflex in the turtle spinal cord
The early component of the VRP, evoked by dorsal root stimulation, was considered as a MSR because it followed one to one dorsal root stimulation without failure and presented a time to peak similar to the value reported for turtle motoneurone EPSP (Yamashita, 1986). Interestingly, the polysynaptic reflex in control Ringer solution and in the presence of GABAA antagonists failed to follow frequency stimulation higher than 1 Hz while the MSR was able to followed one to one frequencies higher than 10 Hz. Furthermore, stable recordings of the MSR and DRP were observed for even 300 min, which gives confidence in the action of the drugs.
MSR and DRP modulation by synaptic GABAA receptors
Our results show that blockade of GABAA receptors with bicuculline and picrotoxin at 20 μm or gabazine at 1 μm did not facilitate the MSR but depressed the DRP. It is worth mentioning that gabazine at 1 μm, which selectively blocks only synaptic GABAA receptors at supraspinal nuclei neurons (Farrant & Nusser, 2005; Walker & Semyanov, 2008), like picrotoxin and bicuculline at 20 μm, also depressed the DRP, suggesting that a fraction of the DRP probably is mediated by activation of synaptic GABAA receptors located at primary afferent terminals. Therefore, like in mammals (Schmidt, 1971; Rudomin & Schmidt, 1999), an important part of the DRP could be produced by synaptic GABAA receptors located on primary afferents and activated by GABA released at axo-axonic synapses with interneurones. This is supported by previous immunohistochemical studies carried out in mammals and amphibians showing that focal accumulation of β subunit-containing GABAA receptors on primary afferents is opposed by glutamic acid decarboxylase (GAD)-immunoreactive terminals (Alvarez et al. 1996; Vesselkin et al. 2001), although this is a point that needs to be addressed further in the turtle in future experiments.
MSR and DRP modulation by extrasynaptic GABAA receptors
Interestingly, when picrotoxin or bicuculline concentration was increased to 100 μm, the MSR was facilitated while the DRP resistant to picrotoxin, bicuculline (20 μm) or gabazine (1 μm) presented an additional depression. It is well known that in many neurons from supraspinal nuclei these concentrations block preferentially GABAA receptors that mediate inhibitory tonic current and possess an important role controlling excitatory synaptic integration (Semyanov et al. 2004; Chadderton et al. 2004; Farrant & Nusser, 2005; Walker & Semyanov, 2008). Therefore, MSR facilitation and DRP depression might have been produced by blockade of tonic GABAA receptors located at primary afferents and/or motoneurones. This conclusion is partly supported by the action of furosemide, which selectively antagonizes α4/α6 subunit-containing GABAA receptors (Korpi et al. 1995; Hamann et al. 2002), at a concentration that does not block the Na+–K+–2Cl− cotransporter (Hochman & Schwartzkroin, 2000) and facilitates the MSR without affecting the DRP. This result is in agreement with the finding that α4/6 subunit mRNA is not expressed in dorsal root ganglia (Ma et al. 1993; Maddox et al. 2004). In addition, in adult cerebellar granule cells α6 subunit-containing GABAA receptors were found to mediate a tonic current that represents 58% of the total current blocked by bicuculline (40 μm) and picrotoxin (100 μm) (Semyanov et al. 2004; Farrant & Nusser, 2005; Walker & Semyanov, 2008). Consequently, lack of effect of furosemide on the DRP strongly indicates that MSR facilitation might be produced by blockade of α4/6 subunit-containing GABAA receptors located in motoneurones.
Presynaptic inhibition mediated by synaptic GABAA receptors
Like in mammals, long latency inhibition of the conditioned MSR presented a time course of 30–300 ms and was blocked by picrotoxin, bicuculline or gabazine (Eccles et al. 1963; Schmidt, 1971; Deshpande & Warnick, 1988; Rudomin & Schmidt, 1999). Likewise, the sensory evoked DRP recorded from one dorsal root presented a similar time course to the long duration inhibition of the MSR. Moreover, as occurs in cat and neonate rat, the DRP was not completely blocked by 20 μm bicuculline or picrotoxin (Schmidt, 1971; Kremer & Lev-Tov, 1998; Rudomin & Schmidt, 1999). We therefore assume that the long latency inhibition observed in this study corresponds to sensory evoked presynaptic inhibition of the MSR. Interestingly, removal of presynaptic inhibition and depression of DRP were also produced by gabazine at 1 μm concentration, which has been shown to block only synaptic GABAA receptors mediating fast IPSCs in hippocampal and cerebellar neurons (Semyanov et al. 2004; Farrant & Nusser, 2005; Walker & Semyanov, 2008). This result indicates that the DRP sensitive to GABAA receptor antagonists at low concentrations probably was mediated by activation of synaptic GABAA receptors at axo-axonic synapses on primary afferent terminals and might correspond to the PAD associated with presynaptic inhibition as was proposed in mammals initially by Eccles et al. (1963), and others (Schmidt, 1971; Rudomin & Schmidt, 1999). In conclusion, our results demonstrate that presynaptic inhibition of turtle primary afferents presents similar properties to that found in cat (Schmidt, 1971; Rudomin & Schmidt, 1999) and neonate rat (Deshpande & Warnick, 1998), and therefore it might also be produced by GABA release from axo-axonic synapses (Rudomin & Schmidt, 1999).
Motoneurone after-discharge in presence of GABAA receptor antagonist
Control VRP evoked by an afferent volley was composed of an early synchronous MSR sometimes accompanied by a polysynaptic reflex, as has been recorded in mammals (Deshpande & Warnick, 1998; Jiang & Heckman, 2006). However, in the presence of GABAA receptor antagonists, the MSR was followed by a long latency post-discharge lasting up to 20 s; this activity of the motoneurones has been reported to occur in the presence of picrotoxin (100 μm) and strychnine (5 μm) in the in vitro spinal cord preparation from adult mice (Jiang & Heckman, 2006). We speculate that long-lasting activity of motoneurones might be produced by synaptic activation of a plateau potential mediated by L-type Ca2+ channels of last order excitatory interneurones involved in the pathway of primary afferents and motoneurones. Interestingly, similar long-lasting after-discharges have also been evoked in dorsal horn neurons by stimulation of the dorsal root after blockade by bicuculline of GABAA receptors, which facilitated the plateau potential expression mediated by L-type Ca2+ channels (Russo et al. 1988). In motoneurones the plateau potential also mediated by L-type Ca2+ channels is down-regulated by activation of GABAA receptors (Alaburda et al. 2005). However, we cannot rule out the possibility of a GABAA tonic conductance in the motoneurone that simply allows a decrease in the membrane resistance reducing the excitability produced by a constant synaptic inflow of segmental and descending pathways. Regardless of the origin of the motoneurone after-discharge, it is clear that GABAA receptors play an important role in controlling motoneurone excitability, which is crucial to muscle activity.
Functional implications
In this work, we show that GABAA receptors sensitive to a high concentration of bicuculline, picrotoxin and furosemide are preventing over-excitation of motoneurones and probably interneurones involved in motor activity. Synaptic strength in the spinal cord is a mechanism to control information flow on motoneurones and is regulated accurately by different neurotransmitters and modulators in order to allow motoneurones to respond suitably to the ambient requirements. GABAA receptors could play an important role as modulators of synaptic strength, as has been shown in nociception. Decreasing with bicuculline the inhibitory control by GABAergic neurones leads to a hyperactivity of dorsal horn neurones projecting to supraspinal nuclei (Woolf & Doubell, 1994; Russo et al. 1998). At the motoneurone level, tonic activation of GABAA receptors can be a mechanism to control information processing by reducing its membrane time constant, thereby narrowing the time window to integrate excitatory synaptic inputs, working as a filter to maintain accuracy of response of motoneurones. Furthermore, the tonic inhibitory conductance mediated by GABAA receptors might be a low-cost metabolic mechanism to control excitability of motoneurones and interneurones involved in networking spinal activity. Additionally, the proposal of a likely tonic inhibitory state at the motoneurones responsible for modulating the reflex activity during locomotion in cat spinal cord has been proposed before (Gosgnach et al. 2000), and there have been experiments on humans which point towards the motoneurone as a possible site for GABAA reflex activity modulation (Guissard et al. 2001).
Acknowledgments
Master fellowships from Conacyt to B.W. and L.A.J.E. are gratefully acknowledged. We thank R. Leyva and R. Panfilo for technical assistance with animals and the National Mexican Turtle Centre for providing the turtles. We also thank Dr Pablo Rudomín and Dr Ricardo Felix for comments and suggestions to improve the paper and Dr David McCrea for revising the manuscript. This work was supported by grant no. 50864-Q from the National Council of Science and Technology (Conacyt, Mexico).
Glossary
Abbreviations
- BIC
bicuculline
- DRP
dorsal root potential
- MSR
monosynaptic reflex
- PTX
picrotoxin
- VRP
ventral root potential
Author contributions
W.B.: design of the experimental protocol; collection, analysis and interpretation of data; drafting the manuscript; J.E.L.A.: collection, analysis and interpretation of data. J.A.: design of the experimental protocol; collection, analysis and interpretation of data. R.D.L.: conception and design of the experimental protocol; collection, analysis and interpretation of data; drafting the manuscript; final approval of the manuscript. The work was carried out in the Department of Physiology, Biophysics and Neuroscience of the Centre for Research and Advanced Studies of the National Polytechnic Institute (Cinvestav-IPN), Mexico City.
References
- Alvarez FJ, Taylor-Blake B, Fyffe RE, De Blas AL, Light AR. Distribution of immunoreactivity for the β2 and β3 subunits of the GABAA receptor in the mammalian spinal cord. J Comp Neurol. 1996;365:392–412. doi: 10.1002/(SICI)1096-9861(19960212)365:3<392::AID-CNE5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Alaburda A, Russo R, MacAulay N, Hounsgaard J. Periodic high-conductance states in spinal neurons during scratch-like network activity in adult turtles. J Neurosci. 2005;27:6316–6321. doi: 10.1523/JNEUROSCI.0843-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataka T, Gu JG. Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Mol Pain. 2006;27:2–36. doi: 10.1186/1744-8069-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16:283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Duggan AW, Felix D, Johnston GA. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971;32:69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Aguilar J, Cueva-Rolon R. Synaptic strength between motoneurons and terminals of the dorsolateral funiculus is regulated by GABA receptors in the turtle spinal cord. J Neurophysiol. 2004;91:40–47. doi: 10.1152/jn.00569.2003. [DOI] [PubMed] [Google Scholar]
- Deshpande SB, Warnick JE. Temperature-dependence of reflex transmission in the neonatal rat spinal cord, in vitro: influence on strychnine- and bicuculline-sensitive inhibition. Neuropharmacology. 1988;27:1033–1037. doi: 10.1016/0028-3908(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt R, Willis WD. Pharmacological studies on presynaptic inhibition. J Physiol. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Magni F, Willis WD. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962;160:62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABAA receptors. Nat Rev Neurosciences. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hochman DW, Schwartzkroin PA. Chloride-cotransport blockade desynchronizes neuronal discharge in the ‘epileptic’ hippocampal slice. J Neurophysiol. 2000;83:406–417. doi: 10.1152/jn.2000.83.1.406. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Quevedo J, Fedirchuk B, McCrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol. 2000;526:639–652. doi: 10.1111/j.1469-7793.2000.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guissard N, Duchateau J, Hainaut K. Mechanisms of decreased motoneurone excitation during passive muscle stretching. Exp Brain Res. 2001;137:163–169. doi: 10.1007/s002210000648. [DOI] [PubMed] [Google Scholar]
- Jiang MC, Heckman CJ. In vitro sacral cord preparation and motoneuron recording from adult mice. J Neurosci Methods. 2006;156:31–36. doi: 10.1016/j.jneumeth.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kellerth JO, Szumski AJ. Effects of picrotoxin on stretch-activated post-synaptic inhibitions in spinal motoneurones. Acta Physiol Scand. 1966;66:146–156. doi: 10.1111/j.1748-1716.1966.tb03179.x. [DOI] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. GABA-receptor-independent dorsal root afferents depolarization in the neonatal rat spinal cord. J Neurophysiol. 1998;79:2581–2592. doi: 10.1152/jn.1998.79.5.2581. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Lüddens H. Selective antagonist for the cerebellar granule cell-specific γ-aminobutyric acid type A receptor. Mol Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Saunders PA, Somogyi R, Poulter MO, Barker JL. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1993;338:337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- Maddox FN, Valeyev AY, Poth K, Holohean AM, Wood PM, Davidoff RA, Hackman JC, Luetje CW. GABAA receptor subunit mRNA expression in cultured embryonic and adult human dorsal root ganglion neurons. Brain Res Dev Brain Res. 2004;149:143–151. doi: 10.1016/j.devbrainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Peng Y, Frank E. Activation of GABAA receptors causes presynaptic and postsynaptic inhibition at synapses between muscle spindle afferents and motoneurons in the spinal cord of bullfrogs. J Neurosci. 1989;9:1516–1522. doi: 10.1523/JNEUROSCI.09-05-01516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiological Reviews. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Jimenez I, Quevedo J, Solodkin M. Pharmacologic analysis of inhibition produced by last-order intermediate nucleus interneurons mediating nonreciprocal inhibition of motoneurons in cat spinal cord. J Neurophysiol. 1990;63:147–160. doi: 10.1152/jn.1990.63.1.147. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Hernández E, Lomelí J. Tonic and phasic differential GABAergic inhibition of synaptic actions of joint afferents in the cat. Exp Brain Res. 2007;176:98–118. doi: 10.1007/s00221-006-0600-x. [DOI] [PubMed] [Google Scholar]
- Russo RE, Nagy F, Hounsgaard J. Inhibitory control of plateau properties in dorsal horn neurones in the turtle spinal cord in vitro. J Physiol. 1998;506:795–808. doi: 10.1111/j.1469-7793.1998.795bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RF. Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- Schmitt DE, Hill RH, Grillner S. The spinal GABAergic system is a strong modulator of burst frequency in the lamprey locomotor network. J Neurophysiol. 2004;92:2357–2367. doi: 10.1152/jn.00233.2004. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walter MC, Kullman DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Solodkin M, Jiménez I, Rudomin P. Identification of common interneurons mediating pre- and postsynaptic inhibition in the cat spinal cord. Science. 1984;224:1453–1456. doi: 10.1126/science.6328657. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Tokunaga A, Yamanaka H, Mashimo T, Noguchi K, Uchida I. Two types of GABAergic miniature inhibitory postsynaptic currents in mouse substantia gelatinosa neurons. Eur J Pharmacol. 2006;553:120–128. doi: 10.1016/j.ejphar.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Vesselkin NP, Adanina VO, Rio JP. Axo-axonic GABA-immunopositive synapses on the primary afferent fibres in frogs. J Chem Neuroanat. 2001;22:209–217. doi: 10.1016/s0891-0618(01)00132-6. [DOI] [PubMed] [Google Scholar]
- Vinay L, Clarac F. Antidromic discharges of dorsal root afferents and inhibition of the lumbar monosynaptic reflex in the neonatal rat. Neuroscience. 1999;90:165–176. doi: 10.1016/s0306-4522(98)00435-7. [DOI] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABAA receptors. Results Probl Cell Differ. 2008;44:29–48. doi: 10.1007/400_2007_030. [DOI] [PubMed] [Google Scholar]
- Wisden W, Gundlach AL, Barnard EA, Seeburg PH, Hunt SP. Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Brain Res Mol Brain Res. 1991;10:179–183. doi: 10.1016/0169-328x(91)90109-b. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Doubell TP. The pathophysiology of chronic pain: increased sensitivity to low threshold Aβ-fibre inputs. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Yamashita M. Monosynaptic connexions of low threshold muscle afferents with hindlimb motoneurones in the turtle spinal cord. Exp Brain Res. 1986;63:519–529. doi: 10.1007/BF00237475. [DOI] [PubMed] [Google Scholar]
- Yang HW, Appenteng K, Batten TF. Ultrastructural subtypes of glutamate-immunoreactive terminals on rat trigeminal motoneurones and their relationships with GABA-immunoreactive terminals. Exp Brain Res. 1997;114:99–116. doi: 10.1007/pl00005627. [DOI] [PubMed] [Google Scholar]


