Abstract
Regional infusions of β2-adrenoceptor (ADRB2) agonist have generally shown that individuals homozygous for Gly16 produces greater vasodilatation than those homozygous for Arg16. Systemic infusions have shown an opposite effect on systemic vascular resistance (SVR), possibly confounded by baroreflexes or interactions between single nucleotide polymorphism (SNP) positions 16 and 27. We tested the hypothesis that ADRB2 gene variation would influence the SVR response to ADRB2 agonist terbutaline (Terb) during ganglionic blockade. Forty healthy young adults were recruited according to the double homozygous haplotypes: Arg16 + Gln27 (n = 13), the rare Gly16 + Gln27 (n = 6), and Gly16 + Glu27 (n = 21). Arterial pressure was measured by brachial arterial catheter, and cardiac output by acetylene breathing. Lymphocytes were sampled for ex vivo analysis of ADRB2 density and binding conformation. Following baroreflex ablation with trimethaphan (3–7 mg min−1), continuous phenylephrine was titrated to restore blood pressure to baseline. Terb was infused i.v. at 33 and 67 ng kg−1 min−1 for 15 min/dose. There was partial evidence to suggest a main effect of haplotype on the change in SVR (P = 0.06). For SNP position 16, the highest dose of Terb produced lower SVR in Gly16 (mean ± s.e.m.: 7.5 ± 0.4) vs. Arg16 (8.9 ± 0.7 units; P = 0.03). Lymphocyte ADRB2 binding conformation was similar but receptor density was greater in Gly16 vs. Arg16 (P = 0.05). We conclude that during ganglionic blockade, the SVR response to systemic ADRB2 agonist is suggestive of augmented ADRB2 function in Gly16 + Glu27 homozygotes, with greater influence from Gly16, providing further evidence that ADRB2 gene variation influences vasodilatation.
Introduction
The β2-adrenoceptor (ADRB2) is ubiquitous in distribution and plays an integral role in cardiovascular regulation. Therefore, variation in the ADRB2 gene has major implications for the physiology and pharmacology of health and disease. Single nucleotide polymorphisms (SNPs) acting alone and in combination are associated with altered cell signalling in vitro and phenotypic effects in humans. Substitution of glycine (Gly) for arginine (Arg) at amino acid position 16 and the substitution of glutamic acid (Glu) for glutamine (Gln) at position 27 influence agonist-mediated desensitization (Green et al. 1994; Dishy et al. 2001; Bruck et al. 2005).
Clinical studies investigating the influence of ADRB2 gene variation on vascular responses to β-agonist infusion have demonstrated conflicting results. Regional infusion studies administering ADRB2 agonists in the brachial artery have demonstrated greater vasodilator responses in Gly16 vs. Arg16 homozygotes (Cockcroft et al. 2000; Garovic et al. 2003). Our laboratory has shown that following a normal sodium diet (150 mmol day−1) the greater forearm vasodilatation in Gly16 homozygotes appears mainly due to ADRB2-mediated release of nitric oxide from the vascular endothelium (Garovic et al. 2003). Additionally, in the hand vein, the maximal venodilator response to isoproterenol was greater in Gly16 than Arg16 homozygotes, but the difference was attributed to effects of the Glu27 variant rather than the Gly16, because greater responses were present only in Gly16 + Glu27 homozygotes, not in Gly16 + Gln27 homozygotes, when compared to Arg16 + Gln27 homozygotes (Dishy et al. 2001).
In contrast to these findings from regional infusion studies, systemic infusions of ADRB2 agonists in humans have shown that Arg16 homozygotes display greater systemic vasodilatation than Gly16 homozygotes (Gratze et al. 1999; Hoit et al. 2000). However, our laboratory previously found no genotype-dependent systemic vasodilator response to incremental infusions of terbutaline (Terb) (Eisenach et al. 2006). An important limitation in the methods used to assess cardiac output and peripheral vasodilatation in these studies is the potential confounding effect of cardiovascular baroreflexes on the haemodynamic responses to vasodilator infusions (Shannon et al. 1998; Jordan et al. 2002). To address this, blockade of autonomic ganglion transmission with the NN-cholinergic antagonist trimethaphan (TMP) prior to the systemic administration of the vasodilator would effectively inhibit both cardiac parasympathetic withdrawal and sympathoadrenal activation during peripheral vasodilatation, thereby ‘isolating’ the peripheral vasodilator response.
Therefore, the purpose of this investigation was to test whether common ADRB2 gene variation influences the cardiovascular and regional vasodilator responses to systemic infusion of ADRB2 agonist during temporary pharmacological autonomic blockade. We recruited only those individuals with the three common homozygous haplotypes that incorporate both SNP positions 16 and 27: Arg16 + Gln27, the rarer Gly16 + Gln27, and Gly16 + Glu27. This would allow analysis of haplotypes and the interaction between individual SNPs. Our hypotheses were: (a) that after temporary autonomic blockade, ADRB2 gene variation would influence systemic vascular resistance during systemic infusion of an ADRB2 agonist; (b) that ADRB2 gene variation would influence the percentage of ADRB2s in the high- and low-affinity binding conformation on lymphocytes sampled from the participants; and (c) that ADRB2 density would be genotype dependent and correlated with haemodynamic variables among the participants.
Methods
Subjects
After providing written informed consent, 40 genotyped males and females between 18 and 40 years of age participated. The study was approved by the Mayo IRB and conformed to the standards set by the latest revision of the Declaration of Helsinki. Only two subjects had participated in the prior investigations from which we formed our hypotheses (Garovic et al. 2003; Eisenach et al. 2006). Subjects were healthy, non-obese non-smokers, did not have a history of any disorder associated with alterations in cardiovascular structure and function, and were not taking any medications (except oral contraceptives). Female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives. Participants were recruited from a pool of subjects (n > 800) that had previously been genotyped for ADRB2 rs1042713 and rs1042714 (SNPs 16 and 27) (Bray et al. 2000). Participants were homozygous for Arg16 + Gln27 (n = 13, ∼14% of our genotyped pool); Gly16 + Gln27 (n = 6, ∼4%); and Gly16 + Glu27 (n = 21, ∼17%). The homozygous combination Arg16 + Glu27 did not occur in our genotyped pool. Thirty-nine subjects reported their ethnicity and race as Non-Hispanic/White, and one Arg16 + Gln27 female reported Latino/White, respectively. Participants were placed on a standardized diet containing 150 mmol of sodium daily for 3 days prior to the study day as previously described (Eisenach et al. 2006). On the morning of the study day, subjects arrived in the clinical research unit (CRU) after at least 8 h fasting and without exercise or caffeine for at least 24 h.
Measurements
A brachial arterial catheter was placed in the non-dominant arm for continuous monitoring of blood pressure and to obtain arterial blood samples for isolation of lymphocytes. In the first four subjects, blood was sampled for lymphocyte analysis from a venous draw and blood pressure was assessed in duplicate with an automated upper arm cuff (CardioCap/5, Datex-Ohmeda, Louisville, CO, USA) and finger plethysmography (Finapres Medical Systems, Amsterdam, the Netherlands). For all subjects, a peripheral intravenous (i.v.) catheter was placed into the dominant arm for administration of study drugs. Heart rate was monitored via electrocardiogram (ECG). Cardiac output (CO) was assessed non-invasively with an open-circuit 8- to 10-breath acetylene re-breathing technique as described previously (Johnson et al. 2000). Forearm blood flow (FBF) was measured using venous occlusion plethysmography.
Experimental protocol
Trimethaphan (TMP) was acquired from Cambridge Laboratories (Wallsend, UK). After 15 min baseline measurement, a continuous i.v. infusion of TMP was adjusted until adequate autonomic blockade was achieved (mean TMP dose 5.0 mg min−1; range 3–7 mg min−1). Blockade was confirmed by minimal HR decrement (<5 bpm) to systolic BP increment ≥25 mmHg via phenylephrine bolus (25 μg) in a manner similar to that described previously (Shannon et al. 1998). When steady-state was reached, haemodynamic measurements were assessed. Then, a continuous i.v. infusion of phenylephrine was titrated to restore blood pressure values to within 10% of the pre-TMP baseline (mean phenylephrine dose 0.2 μg kg−1 min−1; range 0.1–0.5 μg kg−1 min−1). Haemodynamic measurements were again assessed. Finally, three doses of Terb (Novaplus; 33, 67, 100 ng kg−1 min−1) were infused for 15 min at each dose. Haemodynamic measurements were assessed during the final 5 min of each Terb dose. Due to the exquisitely sensitive vasodilator response to Terb during ganglionic blockade, the third dose of Terb was discontinued early in 19 subjects and therefore this dose was excluded from analysis. After discontinuation and de-instrumentation, subjects remained at the CRU for observation for at least 2 h.
Lymphocyte ADRB2 density and binding affinity
From blood sampled (40 ml) at baseline, lymphocytes were isolated by centrifugation as recently described (Snyder et al. 2006b). The cell pellet was re-suspended in Hanks’ solution + 10% dimethyl sulphoxide (DMSO) and stored in liquid nitrogen. Membranes were prepared by homogenization in a Brinkman Polytron and centrifugation at 40,000 g. Final pellets were suspended at ∼1 mg ml−1 in binding buffer (12 mm Tris-HCl, pH 7.6; 60 mm NaCl, 9 mm MgCl2, 1.8 mm EDTA, 3.6 mm sucrose, 4 μg ml−1 bovine serum albumin, and 0.5 mm ascorbic acid) and used immediately. Triplicate assays were performed using 100 μg membrane per 500 μl reaction containing 20 pm125I-labelled cyanopindolol (CYP) and 14 concentrations of isoproterenol from 0.1 nm to 100 μm (Naslund et al. 1990; Snyder et al. 2006b). Specific binding data were fitted to a two-site competition binding equation using Prism4 (GraphPad Software, San Diego, CA, USA). Following incubation for 90 min at 37°C, reactions were terminated and harvested by vacuum filtration over Whatman GF/C filters with a Brandel cell harvester and washed 4 times with 5 ml of 50 mm Tris (pH 7.6) plus 10 mm MgCl2. 125I on the filters was quantified on an automatic gamma counter (Wallac Wizard 1470, Waltham, MA, USA) at 90% counting efficiency. Receptor density was estimated as 2× specific binding at 20 pm [125I]-CYP (approximate Kd under assay conditions) with the assumption that ADRB2 polymorphic variants do not differ in affinity for antagonists (Green et al. 1994).
Data analysis and statistics
Data were sampled at 200 Hz using data acquisition software (Windaq, Dataq Instruments, Akron, OH, USA) and stored for offline analysis. Cardiac output was determined by averaging the duplicate measurements obtained at baseline and during drug infusions. Forearm blood flow (FBF) was determined from venous occlusion plethysmography. Stroke volume was calculated by dividing CO by heart rate, and systemic vascular resistance (SVR) was calculated by dividing mean arterial pressure by CO. In addition to absolute haemodynamic responses, the relative change with each Terb dose was calculated and included in the analysis. Forearm vascular conductance was calculated as FBF/mean arterial pressure (FBF/MAP) × 100 and expressed as arbitrary units, and forearm vascular resistance (FVR) was calculated as (MAP/FBF). Baseline characteristics were compared between haplotype groups using ANOVA for continuous variables and the exact test for categorical variables. Repeated measures ANOVA models were used to assess differences between genotype groups for the within-subject responses to increasing doses of Terb. Each dependent variable was measured repeatedly during the Terb trials and analysed using two-way ANOVA with genotype group as the independent cross classification variable and Terb dose as a repeated factor. Analyses were performed using the raw data and also with data expressed as change from baseline. To supplement the repeated measures ANOVA, one-way ANOVA was used to assess differences across groups at each Terb dose. Repeated-measures modelling was performed using PROC MIXED (SAS version 8.0, SAS Institute, Cary, NC, USA). From previous findings, the magnitude of the observed difference in response between genotypes (expressed in s.d. units) ranged from 0.75 to 1.25 s.d. units for the variables of interest in the current investigation (Garovic et al. 2003). Given our final sample size, the statistical power (two-sided, α = 0.05) to detect differences between Arg16 + Gln27 and Gly16 + Glu27 was 53% for a difference of 0.75 s.d., 77% for a difference of 1.0 s.d., and 92% for 1.25 s.d. All data are presented means ± s.e.m.P values of < 0.05 were considered statistically significant.
Results
There were no group differences in age, sex proportion, or anthropometric values (Table 1). Twenty-four hour urine collection on the morning of study confirmed dietary compliance with sodium intake and yielded no group differences in volume, electrolytes, or creatinine.
Table 1.
Subject characteristics
| Haplotype group |
||||
|---|---|---|---|---|
| Characteristic | Arg16 + Gln27 (n = 13) | Gly16 + Gln27 (n = 6) | Gly16 + Glu 27 (n = 21) | PANOVA |
| Age (years) | 28 ± 2 | 27 ± 3 | 25 ± 1 | 0.6 |
| Sex (f/m) | 8/5 | 3/3 | 13/8 | 0.9 |
| Height (cm) | 168 ± 3 | 173 ± 3 | 173 ± 2 | 0.4 |
| Weight (kg) | 68 ± 3 | 70 ± 5 | 71 ± 2 | 0.7 |
| BMI (kg m−2) | 23.8 ± 0.4 | 23.4 ± 0.9 | 23.2 ± 0.4 | 0.6 |
| BSA (m2) | 1.77 ± 0.05 | 1.84 ± 0.07 | 1.83 ± 0.04 | 0.6 |
| 24 h urine vol. | 2330 ± 197 | 2072 ± 171 | 2395 ± 298 | 0.8 |
| 24 h urine Na+ (mmol) | 121 ± 6 | 128 ± 9 | 130 ± 9 | 0.8 |
| 24 h urine K+ (mmol) | 84 ± 7 | 77 ± 7 | 87 ± 6 | 0.7 |
| 24 h urine Cr (mg) | 1395 ± 123 | 1652 ± 159 | 1646 ± 130 | 0.4 |
Table entries are proportions for sex, or means ± s.e.m. for other characteristics. All values were recorded prior to beginning the study. The urine indices were generated from a 24 h collection on the final day of the normal-sodium diet, prior to the study day. The groups were compared by using Wilcoxon's rank-sum for all variables except sex, which was compared by Fisher's exact test. BMI, body mass index; BSA body surface area.
The systemic haemodynamic values before and during autonomic blockade are listed in Table 2. There was a tendency toward a difference in resting HR based on haplotype (P = 0.09). Trimethaphan increased HR and decreased MAP, SVR and SV (P < 0.01 for all). There were no group differences in the dose of TMP required to achieve autonomic blockade, nor were there differences in the haemodynamic variables once autonomic blockade was reached.
Table 2.
Haemodynamics at baseline and during complete autonomic blockade
| Haplotype group |
|||||
|---|---|---|---|---|---|
| Measure | Condition | Arg16 + Gln27 (n = 13) | Gly16 + Gln27 (n = 6) | Gly16 + Glu 27 (n = 21) | PANOVA |
| HR (bpm) | Baseline | 66 ± 2 | 58 ± 3 | 61 ± 2 | 0.09 |
| Trimeth.* | 87 ± 3 | 80 ± 4 | 82 ± 2 | ns | |
| CO (l min−1) | Baseline | 5.6 ± 0.5 | 5.6 ± 0.4 | 6.6 ± 0.5 | ns |
| Trimeth. | 5.6 ± 0.4 | 6.4 ± 0.6 | 6.0 ± 0.5 | ns | |
| MAP (mmHg) | Baseline | 89 ± 2 | 87 ± 1 | 87 ± 2 | ns |
| Trimeth.* | 74 ± 3 | 72 ± 3 | 71 ± 1 | ns | |
| SVR (units) | Baseline | 19.0 ± 3.0 | 15.8 ± 1.3 | 14.5 ± 1.0 | ns |
| Trimeth.* | 13.6 ± 0.8 | 11.7 ± 1.2 | 12.7 ± 0.9 | ns | |
| SV (ml) | Baseline | 88 ± 10 | 98 ± 3 | 110 ± 9 | ns |
| Trimeth.* | 66 ± 6 | 80 ± 6 | 76 ± 6 | ns | |
Values are means ± s.e.m. HR, heart rate; CO, cardiac output; MAP, mean arterial pressure; SVR, systemic vascular resistance with units = MAP/CO; SV, stroke volume. Trimeth.
indicates a significant effect of trimethaphan on all values for all groups (P < 0.001). PANOVA indicates comparisons across groups in the time-specific condition.
As shown in Table 3, there was a haplotype-dependent difference in HR in the pre-Terb baseline (P = 0.02), and this discrepancy was apparent at both doses of Terb (P = 0.03, P = 0.05, respectively). From pre-Terb baseline to Terb 33 and 67, there was a significant main effect of haplotype on HR (P = 0.02), a significant effect of dose (P < 0.01) but there was no haplotype-by-dose interaction. When comparing the groups for the change (Δ) from pre-Terb baseline, there was no evidence to suggest a main effect of haplotype, indicating that haplotype influenced HR at each condition, but the dose–response relationship to Terb was similar among groups.
Table 3.
Haemodynamics immediately before and during terbutaline infusions
| Haplotype group |
|||||
|---|---|---|---|---|---|
| Condition | Arg16 + Gln27 (n = 13) | Gly16 + Gln27 (n = 6) | Gly16 + Glu 27 (n = 21) | PANOVA | |
| Heart rate (bpm) | BLTrimeth + PE | 86 ± 2 | 79 ± 3 | 78 ± 2 | 0.02 |
| Terb 33 | 101 ± 2 | 90 ± 4 | 91 ± 3 | 0.03 | |
| Terb 67 | 117 ± 3 | 104 ± 4 | 106 ± 3 | 0.05 | |
| Phaplotype/Pdose/Pinteraction | 0.02, < 0.01, 0.46 | ||||
| ΔPhaplotype/Pdose/Pinteraction | 0.23, < 0.01, 0.32 | ||||
| Cardiac Output (l min−1) | BLTrimeth + PE | 6.7 ± 0.6 | 6.7 ± 0.8 | 6.9 ± 0.4 | ns |
| Terb 33 | 7.4 ± 0.6 | 8.8 ± 1.1 | 7.8 ± 0.6 | ns | |
| Terb 67 | 8.0 ± 0.6 | 9.8 ± 1.2 | 9.3 ± 0.7 | ns | |
| Phaplotype/Pdose/Pinteraction | 0.65, < 0.01, 0.10 | ||||
| ΔPhaplotype/Pdose/Pinteraction | 0.09, < 0.01, 0.06 | ||||
| MAP (mmHg) | BLTrimeth + PE | 95 ± 3 | 97 ± 2 | 94 ± 2 | ns |
| Terb 33 | 82 ± 4 | 81 ± 5 | 76 ± 2 | ns | |
| Terb 67 | 68 ± 3 | 67 ± 3 | 66 ± 2 | ns | |
| Phaplotype/Pdose/Pinteraction | 0.47, < 0.01, 0.64 | ||||
| ΔPhaplotype/Pdose/Pinteraction | 0.67, < 0.01, 0.66 | ||||
| SVR (units) | BLTrimeth + PE | 15.1 ± 0.9 | 15.4 ± 1.8 | 14.6 ± 0.8 | ns |
| Terb 33 | 11.8 ± 0.8 | 9.6 ± 0.9 | 10.9 ± 0.9 | ns | |
| Terb 67 | 8.9 ± 0.7 | 7.1 ± 0.5 | 7.6 ± 0.4 | ns | |
| Phaplotype/Pdose/Pinteraction | 0.61, < 0.01, 0.45 | ||||
| ΔPhaplotype/Pdose/Pinteraction | 0.06, < 0.01, 0.29 | ||||
| SV (units) | BLTrimeth + PE | 78 ± 7 | 85 ± 8 | 89 ± 6 | ns |
| Terb 33 | 75 ± 7 | 98 ± 10 | 88 ± 8 | ns | |
| Terb 67 | 70 ± 7 | 96 ± 12 | 91 ± 9 | ns | |
| Phaplotype/Pdose/Pinteraction | 0.30, 0.52, 0.14 | ||||
| ΔPhaplotype/Pdose/Pinteraction | 0.07, 0.67, 0.07 | ||||
Values are means ± s.e.m. HR, heart rate; CO, cardiac output; MAP, mean arterial pressure; SVR, systemic vascular resistance with units = MAP/CO; SV, stroke volume. Analyses were performed using raw values with measures obtained at baseline and each terbutaline dose and also using change from baseline at each terbutaline dose (Δ). Phaplotype indicates main effect of haplotype, Pdose indicates main effect of terbutaline dose, Pinteraction indicates haplotype-by-dose interaction. PANOVA indicates comparisons across groups at the given dose of terbutaline.
Aside from HR, there were no other haplotype-dependent differences in systemic haemodynamic variables during the pre-Terb baseline. Terb increased CO with a tendency toward an effect of haplotype (ΔPhaplotype = 0.09, ΔPinteraction = 0.06), as Arg16 + Gln27 demonstrated evidence of a blunted CO response when compared to the other two haplotypes. Terb decreased MAP but there was no evidence to suggest an influence of haplotype. Terb decreased SVR in all groups, and there was evidence to suggest that the change from pre-Terb baseline was dependent on haplotype (ΔPhaplotype = 0.06). Terb did not significantly affect SV, but there was evidence to suggest that the change from pre-Terb baseline was dependent on haplotype (ΔPhaplotype = 0.07, ΔPinteraction = 0.07).
With reference to previous findings emphasizing the importance of position 16, it can be appreciated from the data in Tables 2 and 3 that Gly16 + Gln27 homozygotes display cardiovascular indices that are in line with the Gly16 + Glu27 group. Therefore, we combined Gly16 homozygotes (n = 27), compared them to the Arg16 group (n = 13), and reported the statistical analyses in the online supplemental Tables 1 and 2. Gly16 had a slower resting HR prior to TMP (P = 0.04) and a tendency toward a lower HR during autonomic blockade (supplemental Table 1). The other systemic variables were consistent with this, because with MAP and CO being similar, resting SVR tended to be lower and SV tended to be greater in Gly16 vs. Arg16.
Online supplemental Table 2 displays the systemic measures based on position 16 in the pre-Terb baseline and the responses to Terb. As shown in Fig. 1, with MAP similar between position 16 groups throughout the protocol, the SVR was decreased in the Gly16 vs. Arg16 subjects at the highest dose of Terb (Pone-tailed = 0.03). Accordingly, as shown in Fig. 2, the CO response at the highest dose of Terb was greater in Gly16 vs. Arg16 homozygotes (Pone-tailed = 0.03). The different CO response to Terb was mainly driven by stroke volume, which tended to be greater in response to Terb in Gly16 vs. Arg 16 homozygotes (Pone-tailed = 0.07).
Figure 1. The absolute systemic vascular resistance (SVR) values in Gly16 and Arg16 homozygotes during baseline, during confirmed autonomic blockade with trimethaphan (Trimeth), during restoration of blood pressure with a steady-state phenylephrine infusion (Trimeth + PE), and in response to terbutaline (Terb) infusions (upper).
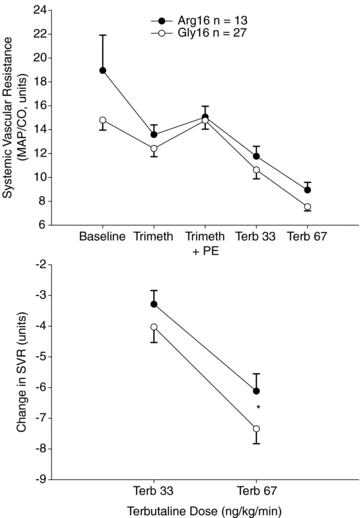
The lower panel indicates the change in SVR between groups. At the highest dose of Terb the decrease in SVR was greater in Gly16 vs. Arg16 homozygotes (*P = 0.03) suggestive of augmented systemic vasodilatation.
Figure 2. The absolute cardiac output values in Gly16 and Arg16 homozygotes during baseline, during confirmed autonomic blockade with trimethaphan (Trimeth), during restoration of blood pressure with a steady-state phenylephrine infusion (Trimeth + PE), and in response to terbutaline (Terb) infusions (upper).
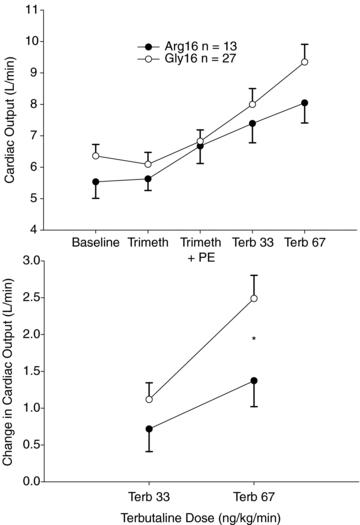
The lower panel indicates the change in cardiac output between groups. At the highest dose of Terb the CO response was greater in Gly16 vs. Arg16 homozygotes (*P = 0.03).
For the forearm blood flow analysis, there were no differences in the forearm measures at rest, during autonomic blockade, or in response to Terb (online supplemental Table 3). Terb significantly increased flow and conductance and decreased resistance, but there was no evidence to suggest an influence of haplotype or position 16 on the forearm measures.
The [125I]-CYP/isoproterenol competition binding curves by group are presented in Fig. 3 and demonstrate that in the resting, pre-study baseline, the fraction of receptors in the high affinity conformation and the affinity constants for the high and low affinity conformations obtained from the fits were similar for all three groups. Lymphocyte ADRB2 receptor concentration per cell membrane mass was Arg16 + Gln27: 10.40 ± 1.26 fmol mg−1; Gly16 + Gln27: 11.88 ± 1.74 fmol mg−1; and Gly16 + Glu27: 12.85 ± 0.82 fmol mg−1 (PANOVA ns). Receptor concentration was significant when comparing Arg16 + Gln27 with Gly16 + Glu27 with evidence of higher values for Gly16 + Glu27. Furthermore, when grouping the subjects according to position 16, the ADRB2 receptor concentration was 10.40 ± 1.26 for Arg16 and 12.67 ± 0.73 fmol mg−1 for Gly16 (Pone-tailed = 0.05).
Figure 3. Blood was sampled for lymphocyte density and analysis of high and low binding conformation.
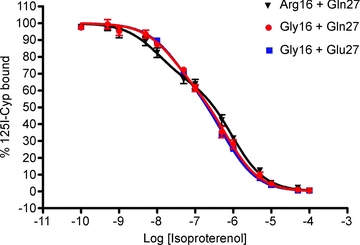
The [125I]-cyanopindolol competition binding curves by group demonstrate that saturation binding in the high and low binding conformation was similar between haplotype groups.
To determine the strength of relationship between lymphocyte density of ADRB2 (drawn at rest) and haemodynamic variables, Fig. 4 (upper) demonstrates a negative correlation between ADRB2 density and resting HR, such that individuals with higher density displayed a lower resting HR (r = −0.38, 95% confidence interval [c.i. −0.65, −0.04]: P = 0.03). Interestingly, when baroreflex ablation was achieved, there was a trend between ADRB2 density and the increase in HR (r = 0.36 [c.i. −0.04, 0.65]; P = 0.07, middle), and a significant association between ADRB2 and the increase in blood pressure (r = 0.43, [c.i. 0.06, 0.70]; P = 0.03; lower). No correlation was present between lymphocyte density and cardiac output.
Figure 4. Lymphocyte density of β2-adrenoceptor (ADRB2) calculated during the assay used forFig. 3.
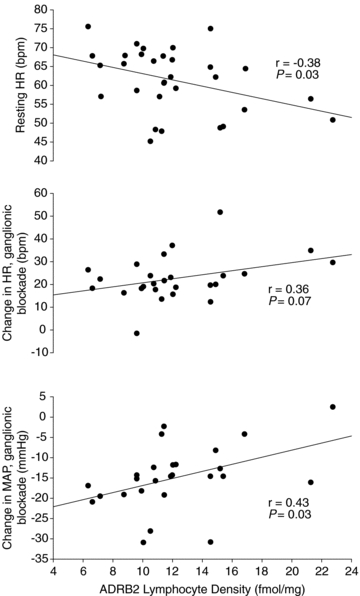
In linear regression analysis, ADRB2 density was negatively correlated with heart rate (HR, upper panel). When autonomic blockade was achieved with trimethaphan, the increase in HR was weakly associated with resting ADRB2 density (middle panel), which may suggest that a higher density of ADRB2 is associated with lower resting HR which responds to autonomic blockade with a greater increase in HR and may be protective against a greater reduction in mean arterial pressure (MAP, lower panel).
Discussion
To our knowledge this is the first study to examine the systemic vascular response to systemic administration of an incremental vasodilator infusion, and the first to determine the influence of ADRB2 gene variation on systemic vasodilatation, both during autonomic blockade. The major finding is that during baroreflex inhibition, both the CO and SVR response to Terb were influenced by ADRB2 gene variation. These measures were trends in the haplotype analysis, but significant when analysing position 16 alone. There was also evidence to suggest that ADRB2 density on lymphocytes was greater in Gly16 vs. Arg16 homozygotes, which may provide partial mechanistic explanation for haemodynamic variables. Finally, in contrast to our hypothesis, neither ADRB2 haplotype nor SNP position 16 influenced the high and low affinity binding conformation on lymphocytes, which may have been due to the controlled ‘normal’ dietary sodium intake because the percentage of receptor in the high affinity conformation has been shown to be altered by dietary sodium restriction and loading (Naslund et al. 1990).
In a regional infusion model, we previously demonstrated that brachial artery administration of a β-agonist evoked greater forearm vasodilatation in Gly16 vs. Arg16 homozygotes and the response was dependent on the endothelial production of nitric oxide (Garovic et al. 2003). We also measured the cardiovascular responses to systemic administration of Terb and found no differences based on genotype before or after dietary sodium restriction; however, there was an effect of sodium restriction on resting myocardial function, as CO decreased, SVR increased, and stroke volume tended to decrease in Gly16, but these values were unaffected in Arg16 (Eisenach et al. 2006). All of the findings from our laboratory were dependent on SNP position 16 and independent of position 27. Finally, we and others have shown enhanced left ventricular function in healthy normotensive Gly16 homozygotes compared to Arg16 homozygotes (Tang et al. 2003; Eisenach et al. 2004; 2005; Snyder et al. 2006a,b;). Together with the present findings during autonomic blockade, these ideas are consistent with the overall concept that compared to Arg16 homozygotes, individuals homozygous for Gly16: (a) have augmented regional ADRB2-mediated vasodilatation; (b) have augmented systemic ADRB2-mediated vasodilatation and augmented ADRB2-mediated cardiac output response during baroreflex inhibition; and (c) may have a greater density of ADRBs on lymphocytes, which has been shown to correlate with the density of ADRBs in cardiac tissue (Qing et al. 1997).
Systemic infusions of selective ADRB2 agonists evoke genotype-dependent differences in systemic vasodilatation following an uncontrolled or unrestricted dietary sodium intake of 8–12 g of salt (137–205 mmol sodium) per day but the genotype effects are in contrast to this investigation. For example, Gratze et al. (1999) showed in Caucasians that during systemic infusion of salbutamol, the total peripheral resistance index in Arg16 homozygotes (n = 12) decreased to a greater extent than in Gly16 (n = 15), and Arg16 group also had a greater increase in HR, cardiac index and stroke index. Hoit et al. (2000) reported that during the highest dose of systemic Terb, calf blood flow was lower and SVR was greater in Gly16 (n = 10) vs. Arg16 (n = 10), while HR and blood pressure responses were similar at baseline and during Terb between groups (race/ethnicity not reported). Finally, Lee et al. (2004) reported that administration of inhaled salbutamol in Caucasian asthmatics evoked a larger decrease in DBP in Arg16 + Gln27 homozygous haplotype (n = 8) when compared to Gly16 + Glu27 homozygous haplotype (n = 8). Collectively, interpretation of these studies may be inconclusive due to variable dietary sodium intake and intact counter-regulatory baroreflexes during systemic drug infusions because blood pressure variables in response to systemic ADRB2 agonist were widely variable or even increased. By inhibiting counter-regulatory baroreflexes, the present findings may reconcile regional vs. systemic discrepancies based on genotype and are consistent with isolated limb models that have generally shown that Gly16 and/or Glu27 are associated with greater vasodilatation than Arg16 and/or Gln27 (Cockcroft et al. 2000; Dishy et al. 2003; Trombetta et al. 2005).
We recruited individuals with homozygous haplotypes to control for the interaction at SNP positions 16 and 27 and improve the physiological predictive power of haplotype when compared to individual SNPs. For instance, Drysdale et al. (2000) reported the bronchodilator response to albuterol was greater in individuals homozygous for the haplotype that includes Gly16 + Glu27 when compared with the homozygous haplotype that includes Arg16 + Gln27. These findings were consistent with the transfection of the corresponding haplotypes into HEK293 cells, resulting in approximately 50% greater mRNA protein expression and ADRB2 density in Gly16 + Glu27 than Arg16 + Gln27 (Drysdale et al. 2000). The rarer homozygous haplotype Gly16 + Gln27 was not found in the Drysdale cohort (designated haplotype 6) and the authors speculated that this group would have had enhanced responsiveness. In the present study, the lymphocyte ADRB2 density was also greater in the Gly16 + Glu27 vs. Arg16 + Gln27 groups. We were able to recruit six individuals with the rare Gly16 + Gln27 haplotype, but we likely did not reach statistical power to detect receptor density differences across all three groups. Importantly, greater ADRB2 density was seen in all Gly16 versus Arg16 homozygotes, suggestive of a dominant influence of position 16 for both receptor density and a potential mechanistic explanation for the haemodynamic differences.
The clinical implications of this investigation may be extended to population-based studies that suggest the Gly16 and/or Glu27 alleles may actually be favourable in cardiovascular health. The Cardiovascular Health Study (CHS) showed that Glu27 carriers had a lower risk of coronary events than Gln27 homozygotes, and there was a suggestion of decreased risk among Gly16 carriers compared with Arg16 homozygotes (Heckbert et al. 2003). A subsequent report from the CHS demonstrated a higher risk of sudden cardiac death in Gln27 homozygotes; similar findings were noted in the Cardiac Arrest Blood Study (CABS) in the same publication (Sotoodehnia et al. 2006). An analysis of multiple gene polymorphisms in heart failure patients demonstrated that the Arg16 + Gln27 diplotype was the only genetic marker of increased risk of death or heart transplantation (Shin et al. 2007).
Genetic variation in ADRB2 may also predict the efficacy of therapeutic regimens (Kaye et al. 2003; Lanfear et al. 2005; Iaccarino et al. 2006). In heart failure patients treated with carvedilol, individuals homozygous for Gln27 represented a significantly lower proportion of ‘good’ responders (improvement in left ventricular function) than individuals who were homozygous or heterozygous for the Glu27 polymorphism (Kaye et al. 2003). Another prospective cohort study in patients with acute coronary syndrome showed that among patients treated with β-blockers, both ADRB2 polymorphisms – independently as well as combined – were predictive of survival in that patients homozygous for Arg16 + Gln27 had the poorest survival, whereas patients homozygous for Gly16 + Glu27 had the favourable survival (Lanfear et al. 2005). Importantly, replication of these findings has been challenged by a recent report showing no influence of SNP positions 16 and 27 on survival of metoprolol or carvedilol-treated heart failure patients (Sehnert et al. 2008).
Limitations
It is unclear why there were no detectable genotype differences in regional (forearm) blood flow in response to Terb. We speculate that the systemic infusion of Terb in this study was a lower effective dose in the forearm, or that the time frame needed to reach steady-state during systemic infusion in this protocol (8–15 min per dose) is not comparable to the time needed to complete a forearm dose–response trial (2 minutes per dose). Additionally, although SNP positions 16 and 27 have garnered the majority of attention in clinical and translational studies, we cannot rule out variability in other genes that govern cardiovascular control. Finally, although our sample sizes exceeded those from previous ADRB2 systemic infusion studies, it was our initial plan to enrol more Arg16 + Gln27 and Gly16 + Gln27 individuals. The need for comprehensive haplotype analysis to improve the predictive power of characterizing physiological and pharmacological loci is limited by the challenge of recruiting individuals with rare homozygous haplotype. This study suggests that the rare Gly16 + Gln27 homozygous haplotype may bear functional semblance to Gly16 + Glu27, but definitive characterization of the physiological relevance of this rare haplotype is inconclusive due to statistical power. Because of larger than expected decreases in blood pressure during administration of TMP (an antiquated ganglionic blocking drug), we analysed the first 40 subjects who completed the protocol and concluded this sample size provided enough power to address the issues of interest.
Perspectives
This investigation adds to the growing body of evidence that polymorphic variation in ADRB2 influences intermediate cardiovascular phenotype in healthy individuals with relevance to distant, more complex phenotypes. When autonomic control of the circulation was temporarily inhibited, the homozygous haplotype associated with Gly16 + Glu27 tended to demonstrate augmented ADRB2 mediated function, an effect that was significant when considering Gly16 alone. Because TMP is no longer in production, isolating the systemic vasodilator response to inhaled or i.v. agonists will require formulation of new baroreflex inhibitors. The present findings may reconcile discrepancies between systemic and regional infusions and, at a minimum, refute augmented vasodilator responses in Arg16 and/or Gln27. In the context of cardiovascular health, it appears that the presence of Gly16 and Glu27 is associated with augmented ADRB2 function and may be protective in hypertension or heart disease.
Acknowledgments
The authors thank all of the study subjects for their enthusiastic participation. We thank Pamela A. Engrav and Cara A. Fernandez for their diligent subject recruitment, Pamela I. Hammond and Jodie L. Van De Rostyne for genotyping, Rachel L. Elvebak, Susan L. Kost, Christopher P. Johnson, Branton G. Walker, and Minelle L. Hulsebus for their protocol assistance, Jean N. Knutson, Karen P. Krucker, and Shelly K. Roberts for study nursing, and the Mayo Clinic CTSA Clinical Research Unit, Dietary Kitchen, and Research Pharmacy. This study was funded by NIH R-01 HL-089331, Deutsche Forschungsgemeinschaft grant He 4605/1 (Dr Hesse, Germany), NIH Clinical and Translational Science Award RR-024150, and the Caywood Professorship via the Mayo Foundation. The authors have nothing to disclose.
Glossary
Abbreviations
- ADRB2
β2-adrenoceptor
- CO
cardiac output
- FBF
forearm blood flow
- FVC
forearm vascular conductance
- HR
heart rate
- MAP
mean arterial pressure
- SNP
single nucleotide polymorphism
- SV
stroke volume
- SVR
systemic vascular resistance
- Terb
terbutaline
- TMP
trimethaphan
Author contributions
Conception and design of the experiments: C.H., W.T.N., A.R.P., S.T.T., M.J.J., J.H.E. Collection, analysis and interpretation of data: C.H., D.R.S., W.T.N., E.C.H., T.B.C., A.R.P., S.T.T., M.J.J., J.H.E. Drafting the article or revising it critically for important intellectual content: C.H., D.R.S., E.C.H., T.B.C., A.R.P., S.T.T., M.J.J., J.H.E. All authors approved the final version for publication.
Supplemental material
Online Supplemental Table 1
Online Supplemental Table 2
Online Supplemental Table 3
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Bray MS, Krushkal J, Li L, Ferrell R, Kardia S, Sing CF, Turner ST, Boerwinkle E. Positional genomic analysis identifies the β2-adrenergic receptor gene as a susceptibility locus for human hypertension. Circulation. 2000;101:2877–2882. doi: 10.1161/01.cir.101.25.2877. [DOI] [PubMed] [Google Scholar]
- Bruck H, Leineweber K, Park J, Weber M, Heusch G, Philipp T, Brodde OE. Human β2-adrenergic receptor gene haplotypes and venodilation in vivo. Clin Pharmacol Ther. 2005;78:232–238. doi: 10.1016/j.clpt.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Cockcroft JR, Gazis AG, Cross DJ, Wheatley A, Dewar J, Hall IP, Noon JP. β2-Adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000;36:371–375. doi: 10.1161/01.hyp.36.3.371. [DOI] [PubMed] [Google Scholar]
- Dishy V, Sofowora GG, Imamura H, Nishimi Y, Xie HG, Wood AJ, Stein CM. Nitric oxide production decreases after salt loading but is not related to blood pressure changes or nitric oxide-mediated vascular responses. J Hypertens. 2003;21:153–157. doi: 10.1097/00004872-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM, Wood AJ. The effect of common polymorphisms of the β2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, Arnold K, Ruano G, Liggett SB. Complex promoter and coding region β2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000;97:10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JH, Barnes SA, Pike TL, Sokolnicki LA, Masuki S, Dietz NM, Rehfeldt KH, Turner ST, Joyner MJ. The Arg16/Gly β2-adrenergic receptor polymorphism alters the cardiac output response to isometric exercise. J Appl Physiol. 2005;99:1776–1781. doi: 10.1152/japplphysiol.00469.2005. [DOI] [PubMed] [Google Scholar]
- Eisenach JH, McGuire AM, Schwingler RM, Turner ST, Joyner MJ. The Arg16/Gly β2-adrenergic receptor polymorphism is associated with altered cardiovascular responses to isometric exercise. Physiol Genomics. 2004;16:323–328. doi: 10.1152/physiolgenomics.00152.2003. [DOI] [PubMed] [Google Scholar]
- Eisenach JH, Schroeder DR, Pike TL, Johnson CP, Schrage WG, Snyder EM, Johnson BD, Garovic VD, Turner ST, Joyner MJ. Dietary sodium restriction and β2-adrenergic receptor polymorphism modulate cardiovascular function in humans. J Physiol. 2006;574:955–965. doi: 10.1113/jphysiol.2006.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garovic VD, Joyner MJ, Dietz NM, Boerwinkle E, Turner ST. β2-Adrenergic receptor polymorphism and nitric oxide-dependent forearm blood flow responses to isoproterenol in humans. J Physiol. 2003;546:583–589. doi: 10.1113/jphysiol.2002.031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratze G, Fortin J, Labugger R, Binder A, Kotanko P, Timmermann B, Luft FC, Hoehe MR, Skrabal F. β2 adrenergic receptor variants affect resting blood pressure and agonist-induced vasodilation in young adult Caucasians. Hypertension. 1999;33:1425–1430. doi: 10.1161/01.hyp.33.6.1425. [DOI] [PubMed] [Google Scholar]
- Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human β2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- Heckbert SR, Hindorff LA, Edwards KL, Psaty BM, Lumley T, Siscovick DS, Tang Z, Durda JP, Kronmal RA, Tracy RP. β2-Adrenergic receptor polymorphisms and risk of incident cardiovascular events in the elderly. Circulation. 2003;107:2021–2024. doi: 10.1161/01.CIR.0000065231.07729.92. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Suresh DP, Craft L, Walsh RA, Liggett SB. β2-Adrenergic receptor polymorphisms at amino acid 16 differentially influence agonist-stimulated blood pressure and peripheral blood flow in normal individuals. Am Heart J. 2000;139:537–542. doi: 10.1016/s0002-8703(00)90099-1. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Izzo R, Trimarco V, Cipolletta E, Lanni F, Sorriento D, Iovino GL, Rozza F, De Luca N, Priante O, Di Renzo G, Trimarco B. β2-Adrenergic receptor polymorphisms and treatment-induced regression of left ventricular hypertrophy in hypertension. Clin Pharmacol Ther. 2006;80:633–645. doi: 10.1016/j.clpt.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- Jordan J, Tank J, Shannon JR, Diedrich A, Lipp A, Schroder C, Arnold G, Sharma AM, Biaggioni I, Robertson D, Luft FC. Baroreflex buffering and susceptibility to vasoactive drugs. Circulation. 2002;105:1459–1464. doi: 10.1161/01.cir.0000012126.56352.fd. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Smirk B, Williams C, Jennings G, Esler M, Holst D. β-Adrenoceptor genotype influences the response to carvedilol in patients with congestive heart failure. Pharmacogenetics. 2003;13:379–382. doi: 10.1097/00008571-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Lanfear DE, Jones PG, Marsh S, Cresci S, McLeod HL, Spertus JA. β2-Adrenergic receptor genotype and survival among patients receiving β-blocker therapy after an acute coronary syndrome. JAMA. 2005;294:1526–1533. doi: 10.1001/jama.294.12.1526. [DOI] [PubMed] [Google Scholar]
- Lee DK, Bates CE, Lipworth BJ. Acute systemic effects of inhaled salbutamol in asthmatic subjects expressing common homozygous β2-adrenoceptor haplotypes at positions 16 and 27. Br J Clin Pharmacol. 2004;57:100–104. doi: 10.1046/j.1365-2125.2003.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund T, Silberstein DJ, Merrell WJ, Nadeau JH, Wood AJ. Low sodium intake corrects abnormality in β-receptor-mediated arterial vasodilation in patients with hypertension: correlation with β-receptor function in vitro. Clin Pharmacol Ther. 1990;48:87–95. doi: 10.1038/clpt.1990.121. [DOI] [PubMed] [Google Scholar]
- Qing F, Rahman SU, Hayes MJ, Rhodes CG, Ind PW, Jones T, Hughes JM. Effect of long-term β2-agonist dosing on human cardiac β-adrenoceptor expression in vivo: comparison with changes in lung and mononuclear leukocyte β-receptors. J Nucl Cardiol. 1997;4:532–538. doi: 10.1016/s1071-3581(97)90012-x. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Daniels SE, Elashoff M, Wingrove JA, Burrow CR, Horne B, Muhlestein JB, Donahue M, Liggett SB, Anderson JL, Kraus WE. Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. J Am Coll Cardiol. 2008;52:644–651. doi: 10.1016/j.jacc.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Shannon JR, Jordan J, Black BK, Costa F, Robertson D. Uncoupling of the baroreflex by NN-cholinergic blockade in dissecting the components of cardiovascular regulation. Hypertension. 1998;32:101–107. doi: 10.1161/01.hyp.32.1.101. [DOI] [PubMed] [Google Scholar]
- Shin J, Lobmeyer MT, Gong Y, Zineh I, Langaee TY, Yarandi H, Schofield RS, Aranda JM, Jr, Hill JA, Pauly DF, Johnson JA. Relation of β2-adrenoceptor haplotype to risk of death and heart transplantation in patients with heart failure. Am J Cardiol. 2007;99:250–255. doi: 10.1016/j.amjcard.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Dietz NM, Eisenach JH, Joyner MJ, Turner ST, Johnson BD. Arg16Gly polymorphism of the β2-adrenergic receptor is associated with differences in cardiovascular function at rest and during exercise in humans. J Physiol. 2006a;571:121–130. doi: 10.1113/jphysiol.2005.098558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Hulsebus ML, Turner ST, Joyner MJ, Johnson BD. Genotype related differences in β2 adrenergic receptor density and cardiac function. Med Sci Sports Exerc. 2006b;38:882–886. doi: 10.1249/01.mss.0000218144.02831.f6. [DOI] [PubMed] [Google Scholar]
- Sotoodehnia N, Siscovick DS, Vatta M, Psaty BM, Tracy RP, Towbin JA, Lemaitre RN, Rea TD, Durda JP, Chang JM, Lumley TS, Kuller LH, Burke GL, Heckbert SR. β2-Adrenergic receptor genetic variants and risk of sudden cardiac death. Circulation. 2006;113:1842–1848. doi: 10.1161/CIRCULATIONAHA.105.582833. [DOI] [PubMed] [Google Scholar]
- Tang W, Devereux RB, Kitzman DW, Province MA, Leppert M, Oberman A, Hopkins PN, Arnett DK. The Arg16Gly polymorphism of the β2-adrenergic receptor and left ventricular systolic function. Am J Hypertens. 2003;16:945–951. doi: 10.1016/s0895-7061(03)01001-x. [DOI] [PubMed] [Google Scholar]
- Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Frazzatto E, Alves MJ, Santos AC, Brum PC, Barretto AC, Halpern A, Villares SM, Negrao CE. Gly16 + Glu27 β2-adrenoceptor polymorphisms cause increased forearm blood flow responses to mental stress and handgrip in humans. J Appl Physiol. 2005;98:787–794. doi: 10.1152/japplphysiol.00503.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


