Abstract
Dengue virus has become one of the most important arboviral pathogens affecting the world today. The virus is transmitted among humans by the mosquitoes Aedes aegypti and Ae. albopictus. Like other vector-borne pathogens, this virus encounters innate immune defenses within the mosquito vector that limit infection. We have previously demonstrated the involvement of the Toll pathway in the anti-dengue defense at 7 days after infection. In the present study, we have investigated the activity of this immune signaling pathway against different dengue virus serotypes at the early stages of infection in laboratory and field-derived mosquito strains. Our studies corroborate the importance of the Toll pathway in the anti-dengue defense repertoire at 3 days after an infectious blood meal, when new virions are released from the midgut for dissemination and infection of other mosquito tissues. These immune defenses are furthermore conserved among different Ae. aegypti strains and can act against a broad range of dengue virus serotypes.
Keywords: innate immunity, Toll pathway, Arbovirus, dengue
1. Introduction
Dengue virus is one of the most important arboviral pathogens that affect tropical and subtropical regions of the world. Recent outbreaks in South America and Asia has reached alarming proportions and underscores the need to develop novel dengue control strategies through a better understanding of virus-vector interactions. The dengue virus is transmitted by mosquitoes of the genus Aedes, with Aedes aegypti and Ae. albopictus representing the most important vectors.
The complexity of dengue virus transmission and disease dynamics are exacerbated by the existence of four closely related dengue serotypes and numerous genotypes. Varying infection dynamics of the vector mosquito have been observed for different dengue serotypes [1], [2] and even within genotypes of the same dengue serotype [3], [4], [5]. These differences in infection and virus replication have been shown to be critical in defining the Ae. aegypti’s vectorial capacity [6], [4]. Whether the mosquito is utilizing the same immune defense profiles against different dengue serotypes is still unknown.
Infection and uptake of dengue virions appear to occur rather quickly upon a blood meal, with internalization of the virions taking place within 5 to 7 min upon contact with the mosquito epithelial cells [7]. The dengue virus then enters an extrinsic incubation period that ranges from 4 to 14 days and is thought to be dependent on environmental factors, the mosquito strain and genotype, and tissue-specific susceptibility to different virus serotypes and genotypes [8], [3].
The mosquito midgut is the first tissue that the dengue virus encounters in the vector following an infectious blood meal, with multiple foci of infected midgut epithelial cells readily observed as early as 2 days post-infection [3]. This tissue not only supports replication of the virus but it also provides the first significant barriers to infection through the midgut infection barrier andthe midgut escape barrier, in some mosquito strains [9], [10]. Successful passage through these midgut barriers allows the virus to disseminate into the hemocoel and infect other mosquito tissues. Almost all mosquito cells and tissues, including tissues important for immune function (fat body and hemocytes), are susceptible to dengue virus infection [3].
The multi-tropism of dengue virus represents a complex scenario, with active and direct interaction between the replicating dengue virus and the primordial mosquito immune defense system producing an outcome that most likely will influence subsequent viral dissemination. Transmission to the next human host is ensured once the dengue virus has infected the mosquito salivary glands and overcome the salivary glands’ infection and escape barriers [9], [11]. These intrinsic characteristics of vector competence, infection, and escape barriers have been shown to be genetically determined and are properties of each mosquito population that explain in part the variation in flavivirus vector competence observed in multiple Ae. aegypti populations [9].
Insects rely on their innate immune system to control pathogen infections. These immune responses are highly effective and include the production of antimicrobial peptides, phagocytosis, and the encapsulation and melanization of the invading pathogen [12]. They are regulated by three major immune signaling pathways: the Toll, Imd, and JAK-STAT pathways [13]. While advances have been achieved in understanding the mosquito’s defenses against other pathogens such as Plasmodium, little is yet known about the vector’s antiviral immune responses. We have recently demonstrated the involvement of the Toll and JAK-STAT pathways in the anti-dengue defense repertoire [14], [15]; these pathways appear to operate independently from the mosquito’s RNAi machinery, which has also been shown to represent an important mechanism of viral defense [16]. These studies have provided baseline information on immune signaling pathway-mediated anti-dengue defenses at 7 days after ingestion of infected blood in a specific combination of dengue virus serotype and mosquito laboratory strain.
In the present study, we have now shown that the Toll pathway regulates potent anti-dengue defenses at multiple early stages of infection in both laboratory and field-derived strains of Ae. aegypti, and that these defenses are effective against multiple dengue virus serotypes. The Toll pathway drives the transcription of immune genes through the activation of the Rel1 transcription factor. This transcription factor can be activated through RNAi-based silencing of the Toll pathway’s negative regulator Cactus; we have now shown that such treatment leads to a significant decrease in midgut viral titers at 3 days post-infection, while repression of Rel1 activation through silencing of the Toll pathway factor MYD88 leads to a significant increase in dengue virus titers. Our gene expression assays revealed intriguing dengue virus infection-responsive expression patterns of selected immune marker genes that reflected a dynamic interaction between the virus and the mosquito’s immune system, providing evidence for pathogen detection by the mosquito’s immune surveillance system and potential immune subversion by the dengue virus.
2. Materials and Methods
2.1 Mosquito rearing and mosquito strains
The Ae. aegypti Rockefeller strain and two other field-derived strains (Panamanian strains E-1 and E-4) used in this study were maintained on a 10% sugar solution at 27°C and 95% humidity with a 12-hr light/dark cycle according to standard rearing procedures.
2.2 Cell culture maintenance and DENV infections
The Ae. albopictus cell line C6/36 was used in all plaque assays and for growing infectious dengue virus. It was grown in minimal essential medium (MEM) with 10% heat-inactivated FBS, 1% L-glutamine, and 1% non-essential amino acids at 32°C with 5% CO2. The New Guinea C strain of DENV-2 and 814669 strain of DENV-4 were propagated in C6/36 cells according to standard conditions [17], [14]. In brief, 500-µl aliquots of virus stock were used to infect 75-cm2 flasks of C6/36 cells at 80% confluency with a multiplicity of infection (MOI) of 3.5 virus particles/cell. The infected cells were then incubated for 7 days prior to the infection assays. Cells were scraped and lysed by repeated freezing and thawing in dry CO2 and a 37°C water bath. The virus suspension was then mixed 1:1 with commercially obtained human blood. For the control group, a similar flask with uninfected C6/36 cells was maintained under similar conditions and used to create the noninfectious blood meal. The infectious blood meal was maintained at 37°C for 30 min prior to use for feeding 5- to 6-day-old mosquitoes (http://www.jove.com/index/Details.stp?ID=220).
2.3 Mosquito dissections
Mosquitoes were dissected at either 24 hr, 3 days, or 7 days after the blood meal. Three biological replicates were performed for each experimental procedure. Twenty mosquitoes for each replicate were dissected in RNALater to collect the midguts and fat body tissues. Total mRNA was extracted using the RNeasy kit (Qiagen), and RNA concentrations were measured using the NanoDrop 1000 (Thermo Scientific)
For virus titration, mosquitoes were surfaced-sterilized with 70% ethanol for 1 min and rinsed twice with sterile distilled water. Midguts were then dissected in sterile 1× PBS and transferred to microcentrifuge tubes with 150 µl of MEM. Five midguts per treatment were collected and homogenized with a Kontes pellet pestle motor under sterile conditions.
2.4 Real-time PCR assays
Transcript abundance analysis was conducted by real-time RT-PCR as previously described [14]: RNA samples were treated with Turbo DNase (Ambion) at 37° C for 30 min, adjusted in all samples to 800 ng, and reverse-transcribed using M-MLV Reverse Transcriptase (Promega) with Oligo dt20. Real-time quantification was performed using SYBR® Green PCR Master Mix (Applied Biosystems) and the ABI Detection System ABI Prism 7300 (Applied Biosystems). Three independent biological replicates were used, and all qPCR runs were conducted in duplicate. Relative expression values were normalized against the ribosomal gene S7. Primers used in this assay are listed in Table S1.
2.5 RNAi assay
RNAi-based targeted gene silencing was conducted as previously described ([14], http://www.jove.com/index/Details.stp?ID=230). Approximately 69 nl of dsRNAs (3 µg/µl) resuspended in water were injected into the thorax of cold-anesthetized 4- to 5-day-old female mosquitoes using a nano-injector. Gene silencing validation was performed at 3 days post-injection, before feeding the mosquitoes on a DENV-2 infectious blood meal. Dissection of mosquito midguts was done at 24 hr, 3 days, and 7 days after the blood meal. Samples were homogenized and used for virus titration. Three independent biological replicates were performed for each gene. Synthesis of dsRNA was performed using the T7 Megascript kit (Ambion) and the following primers: for Cactus dsRNA synthesis: Cactus_F: TAATACGACTCACTATAGGG CGAGTCAACAGAACCCGAGCAG, Cactus_R: TAATACGACTCACTATAGGG TGGCCCGTCAGCACCGAAAG; for MyD88 dsRNA synthesis: MyD88_F: TAATACGACTCACTATAGGGGGCGATTGGTGGTTGTTATT, MyD88_R: TAATACGACTCACTATAGGGTTGAGCGCATTGCTAACATC.
2.6 Titration of DENV-infected midguts
Titration of infected tissues homogenates was conducted as previously described (http://www.jove.com/index/Details.stp?ID=220). The homogenates were serially diluted and inoculated into the C6/36 Ae. albopictus cell line in 24-well plates and incubated for 5 days at 32°C and 5% CO2. The inoculated plates were then assayed by peroxidase immunostaining, using a mouse hyperimmune ascitic fluid (MHIAF) specific for DENV-2 and DENV-4 as the primary antibody and a goat anti-mouse HRP conjugate as the secondary antibody.
3. Results
3.1 The Toll pathway regulates anti-dengue defenses at the early stages of dengue virus infection
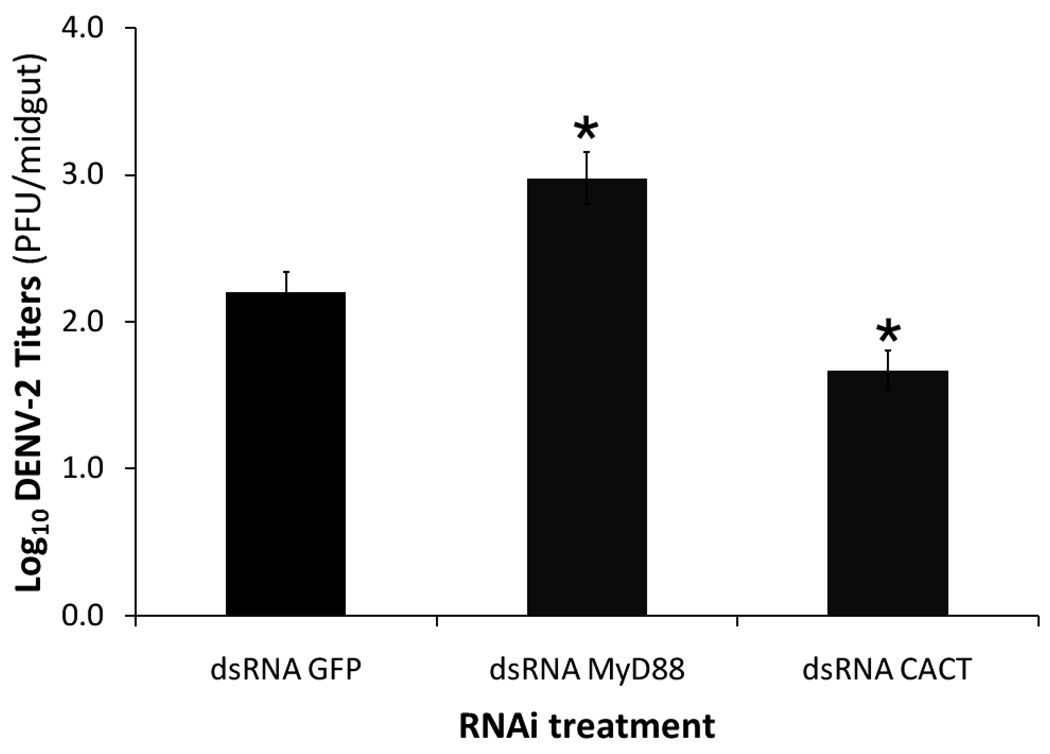
We have previously reported the involvement of the Toll pathway in the anti-dengue defense at 7 days post-blood meal (PBM). Infection of the mosquito midgut is assumed to take place within minutes after ingestion of an infectious blood meal. To determine whether the Toll pathway regulates anti-dengue defense mechanisms at this early stage of infection, we assayed the effects of transient Rel1 activation (through Cactus silencing) and inhibition (through MyD88 silencing) on virus infection at 3 days PBM. As was observed at 7days PBM, silencing of Cactus resulted in a significant decrease in the number of detectable virus particles in the midgut when compared to the GFP dsRNA-treated control mosquitoes, whereas blocking of the Toll pathway via MyD88 silencing resulted in a significantly greater number of virus particles in the midgut (p<0.05 in Mann-Whitney U test) (Fig. 1). Infection assay phenotypes could not be determined with accuracy at 24 hr PBM because of the inherently low number of infectious virions in midgut samples at this stage of infection.
Figure 1.
Dengue virus titers in mosquitoes with transient activation of the Toll pathway through cactus gene silencing (dsRNA CACT) and transientrepression of the Toll pathway (dsRNA MyD88) through MyD88 gene silencing. There is a detrimental effect of Cactus silencing on dengue virus replication in the mosquito midgut at 3 days post-infection. Virus titers were measured by plaque titration in the C6/36 cell line. (*, p<0.05 in Mann-Whitney U test. Error bars represent standard error of the mean).
3.4 The Toll pathway-regulated defenses are active against different dengue virus serotypes
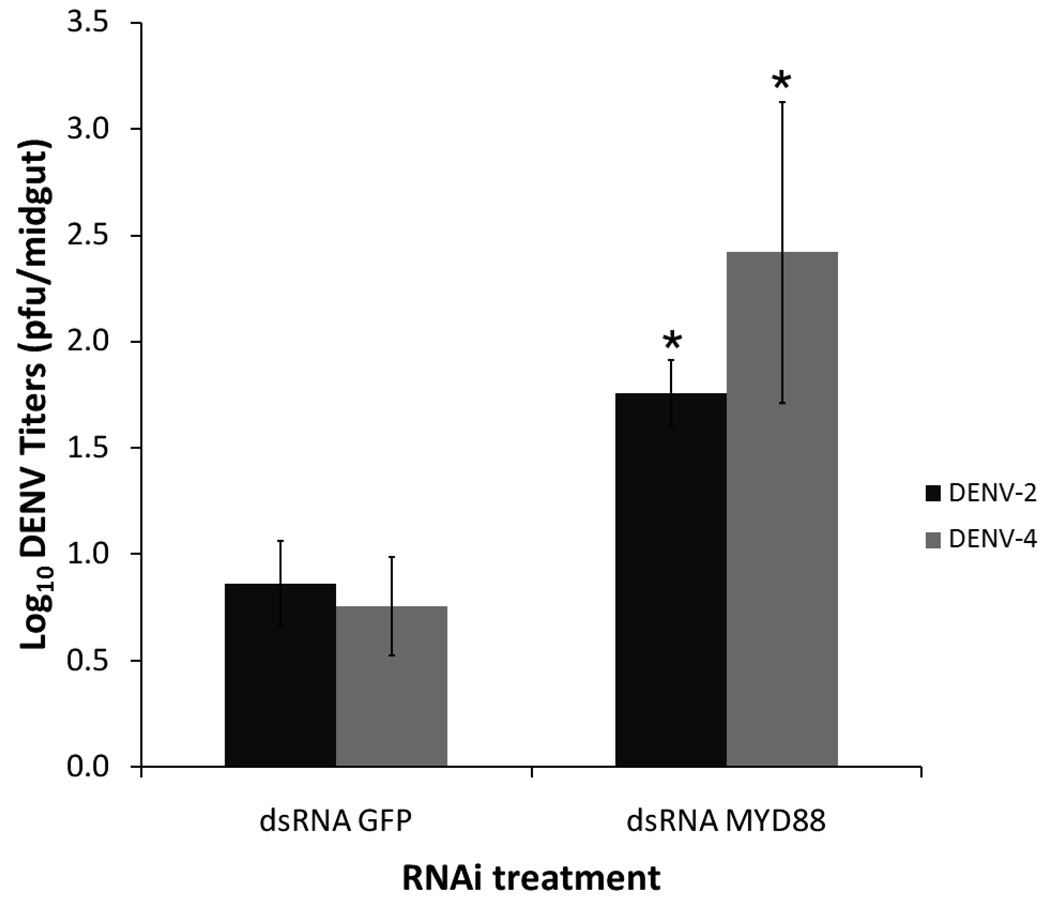
Our knowledge of the insect’s innate immune responses to viruses is still quite limited, and these responses have mainly been studied thus far in the Drosophila system. The fruit fly appears to utilize different immune signaling pathways in its defense against different types of viruses. To determine whether the Toll pathway-mediated anti-viral responses were active against different dengue virus serotypes, we challenged MyD88-depleted mosquitoes with DENV-2 and DENV-4; DENV-4 is the most phylogenetically distinct from the other serotypes [18], [19]. Transient inactivation of the Toll pathway-regulated transcription factor Rel1 resulted in a significant increase in DENV-4 titers at 3 days PBM (p<0.05 in Mann-Whitney U test), as well as in DENV-2 titers (Fig. 2).
Figure 2.
Dengue virus titers in the midgut of MyD88-silenced mosquitoes challenged with DENV-4 (814669 strain) or DENV-2 (NGC strain). Repression of the Toll pathway led to a significant increase in dengue virus titers, demonstrating the importance of this innate immune pathway in the defense against different dengue virus serotypes at the early stages of infection. Virus titers and infection phenotype were assayed by plaque titration in C6/36 cells. (*,p<0.05 in Mann-Whitney U test. Error bars represent standard error of the mean).
3.5 The Toll pathway-mediated anti-dengue defenses are conserved across field-derived Ae. aegypti mosquito strains
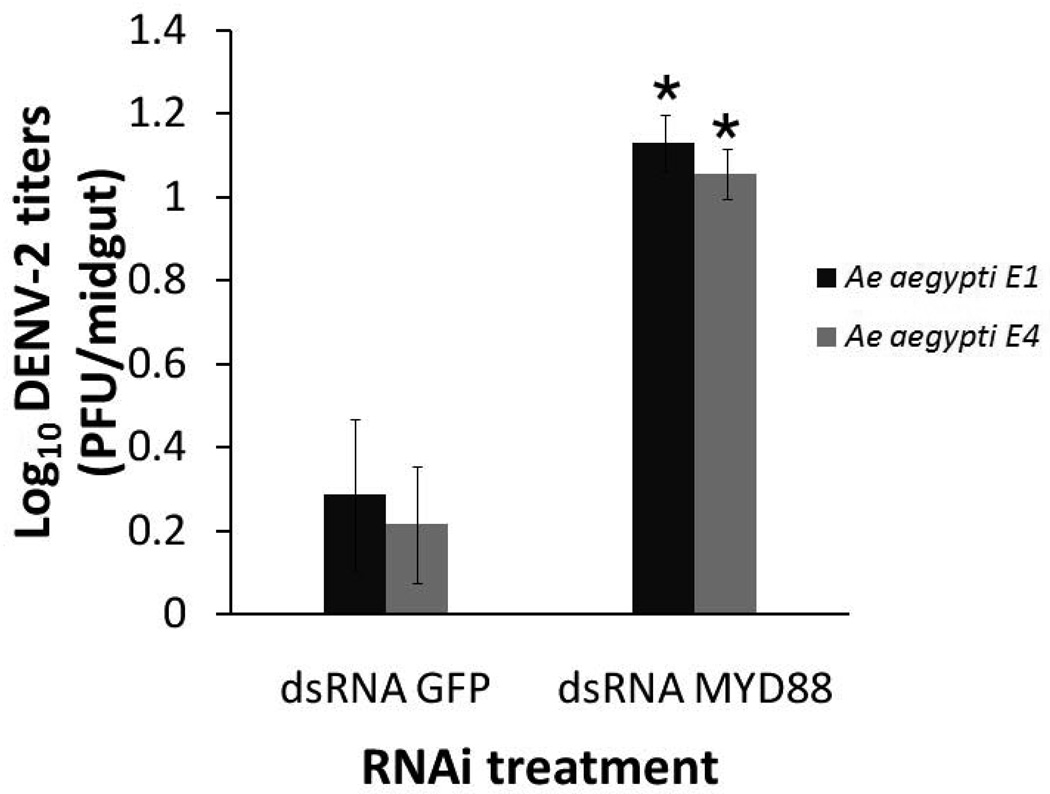
Our standard Ae. aegypti Rockefeller strain has been maintained under artificial laboratory conditions since the 1930s [20] and is assumed to possess a significantly more homogeneous genetic make-up than that of natural field mosquitoes. To test whether field-derived mosquitoes also utilize the Toll pathway-regulated anti-dengue defenses, we challenged two MyD88-depleted Panamanian field-derived mosquito strains, E1 and E2, with DENV2 and compared their susceptibility to infection with that of GFP dsRNA-treated control mosquitoes of the same strains.
As was seen for the Rockefeller Ae. aegypti laboratory strain, the two Panamanian field-derived Ae. aegypti strains showed a significant increase in virus titers when the Toll pathway-regulated Rel1 transcription factor was inhibited (p<0.05 in Mann-Whitney U test) (Fig. 3).
Figure 3.
Dengue virus titers in the midgut of two MyD88-silenced mosquito strains that were derived from field populations. As was seen for the lab strain of Ae. aegypti (Rockefeller strain), the two field-derived strains of Ae. aegypti used in these assays showed a significant increase in viral loads after the Toll pathway was disrupted (via MyD88 silencing), when compared to the GFP control. Virus titers and infection phenotype were assayed by plaque titration in C6/36 cells. (*, p<0.05 in Mann-Whitney U test. Error bars represent standard error of the mean).
3.6 Dengue infection alters mosquito immune gene regulation at the early stages of infection
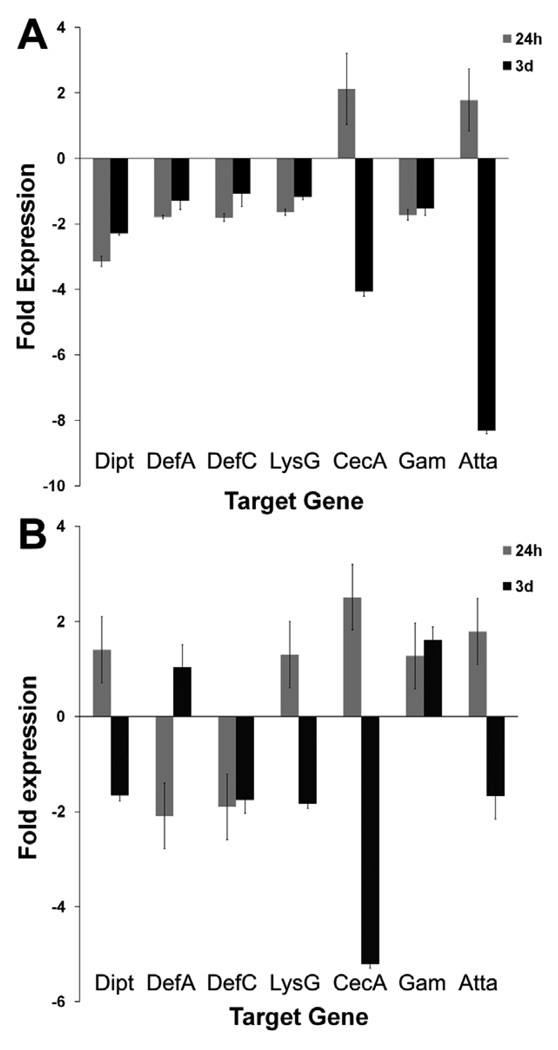
We have previously shown that numerous putative Toll pathway-related genes are up-regulated in dengue virus-infected mosquitoes at 10 days after infection. The involvement of this pathway in the anti-dengue defense during the early stages of infection suggests that a similar transcriptional immune gene induction may occur at 3 days after ingestion of an infected blood meal. To assess whether Toll pathway-related and effector genes respond during the early stage of infection in the midgut and abdominal fat body tissues, we used a quantitative PCR-based approach to compare the transcript abundance of selected immune marker genes between infected and non-infected mosquito tissues at 24 hr and 3 days after ingestion of an infectious blood meal. The fat body displayed the greatest regulation of immune marker genes in the infected mosquitoes (Figure 4). Of particular interest was the induction of the effector genes cecropin-A and attacin at 24 hr and their subsequent repression at 3 days after ingestion of an infectious blood meal in both the midgut and fat body tissues. A similar pattern was observed for diptericin and lysozyme-G in the fat body. Defensin genes were down-regulated at both post-infection time points, with a more profound repression of defensin-C in the fat body. Another interesting observation was the contrasting regulation of gambicin in both tissues, with a slight down-regulation in the midgut and up-regulation in the fat body at both time points.
Figure 4.
Fold expression change in the expression of selected immune genes in the midgut (A) and abdominal fat body (B) tissues of DENV-2-infected mosquitoes, as compared to uninfected mosquitoes at 24 hr and 3 days post-blood meal. Dipt, diptericin; DefA, defensin A; DefC, defensin C; LysG, Lysozyme G; CecA, cecropin A; Gam, gambicin; Atta, attacin. (Error bars represent standard error of the mean)
4. Discussion
Recent studies have shown that the Toll and the JAK-STAT pathways and the RNAi machinery are important for the mosquito’s defense against arboviral infections [14], [16], [15]. Our previous studies have shown that the Toll pathway is involved in limiting dengue virus replication in the midgut at 7 days after an infectious blood meal; the time at which the mosquito midgut has the highest viral load.
In this study, we have assessed the role of the Toll pathway in the antiviral defense at the early stages of infection and have examined its capacity to modulate dengue virus tropism at the early stages of infection. The activation of the Toll pathway-regulated Rel1 transcription factor, through RNAi-targeted depletion of its negative regulator Cactus, resulted in a decreased virus titer in the midgut tissue at 3 days post-ingestion of an infectious blood meal, while Toll pathway repression through MyD88 depletion led to a significant increase in dengue virus replication. These results are reminiscent of what was observed in our previous analysis at 7 days post-infection and show that the Toll pathway regulates potent anti-dengue defenses throughout the virus cycle in the mosquito. This inhibition may be crucial for the duration of the extrinsic incubation period of the dengue virus.
Invertebrate anti-viral immune defenses appear to have a certain degree of specificity. For example, the Drosophila JAK-STAT pathway is involved in limiting infection with the Drosophila C virus, and the Toll pathway is active against the Drosophila X virus [21]. Furthermore, the infection dynamics of dengue viruses are often influenced by their serotype and genotype [4]. Nevertheless, our data show that the Ae. aegypti Toll pathway is controlling universal anti-dengue defenses that are independent of virus serotype specificity. Infection with DENV-4 (a serotype most distantly related to DENV-2, DENV-1, and DENV3) was significantly compromised when the Toll pathway-regulated Rel1 transcription factor was activated and was facilitated when it was inhibited.
Genetically determined variability in the mosquito’s vectorial capacity for human pathogens, including the dengue virus, is common and could indicate that certain anti-viral defense systems are mosquito genotype-specific. Most studies of dengue virus infection in the Ae. aegypti mosquito have utilized highly inbred laboratory strains, such as the Rockefeller strain that has been reared under artificial conditions for more than three decades [20]. It was therefore possible that the anti-dengue activity of the Toll pathway that has been observed was a Rockefeller strain-specific attribute. To investigate this possibility, we have now conducted the same RNAi-based Toll pathway activation and inactivation assays in conjunction with virus infection assays in two Panamanian field-derived Ae. aegypti strains as well as the Ae aegypti Rockefeller strain. The results of these assays indicated that the Toll pathway controls a conserved anti-dengue defense system that is active in Ae. aegypti of diverse origins.
Our transcription analyses of putative Toll pathway regulated effector genes in the midgut and fat body tissues at 24 hr and 3 days after an infectious blood meal revealed intriguing patterns that suggest an immunomodulatory activity by the dengue virus. The transcript enrichment of some effectors at 24 hr and subsequent depletion at 3 days after the infectious blood meal suggest a subversive effect of dengue replication in the midgut and fat body.
Our previous genome-wide transcriptome analyses at 10 days after ingestion of an infectious blood meal showed the up-regulation of numerous effector genes and other Toll pathway-related components [14]. At this stage of infection, the virus titers decline quite significantly in the midgut tissue; this effect may be attributed to the action of the mosquito’s immune system (the Toll pathway and RNAi machinery) [16]. A plausible hypothesis for the down-regulation of putative immune marker genes at 3 days after ingestion of an infectious blood meal is that the dengue virus is capable of a certain degree of immune subversion during these early stages of infection in the midgut tissue, possibly involving a direct interaction with the Toll pathway. Similar results have been observed in the transcriptome immune responses of Aedes aegypti to Sindbis virus infection during the early stages of infection [22].
The apparent incongruence between our gene silencing assays (both Rel1 activation and inactivation infection phenotypes) and the immune gene transcript abundance analyses after ingestion of an infectious blood meal may be a reflection of the complex and dynamic temporal and spatial interaction of the virus with its mosquito host, with local and progressive subversive action occurring as the virus infects other mosquito tissues. Moreover, in our RNAi assays, double-stranded RNA-treated mosquitoes were provided with a dengue virus-laden blood meal 3 days after double-stranded RNA injection. Therefore, the mosquitoes were exposed to dengue virus with either an already activated Toll pathway, which would limit the level of infection, or an already suppressed Toll pathway, which would lower any basal Toll pathway activity and allow higher infection of the midgut tissue, thus temporarily overriding any modulatory effect of the dengue virus replication. For example, the midgut epithelium would already have been enriched with putative Toll pathway-regulated anti-viral factors when the virus was ingested.
In summary, we have shown in the present study that the Toll immune signaling pathway is an important regulator of the anti-dengue defense during the early stages of infection with different dengue serotypes in multiple vector genotypes. This pathway is most likely an important component of the anti-dengue defense repertoire and critical for defining the duration of the extrinsic incubation period. In addition, our studies of the mosquito’s transcriptional responses to dengue virus infection at the early stages of infection and in different tissues further suggests a complex dynamic interaction between the virus and its vector, one that we are just beginning to understand.
Supplementary Material
Table S1.- Primer sequences used for the expression analysis of selected inmune genes by real-time RT-PCR.
Acknowledgements
We are grateful to Janece M. Lovchik, Bhavin Thumar and Anna P. Durbin for providing the techniques support and the materials (DENV-2 and DENV-4 strains and C6/36 cell line), and for sharing the equipment (CO2 incubator and fluorescence microscope) for this study. We are also thankful to the Arbovirus Diseases Branch at the CDC for providing us with the anti-dengue antibodies (Mouse Hyperimmune Ascetic Fluid). We thank the microarray core facility and the insectary personnel at the Johns Hopkins Malaria Research Institute for assistance with the microarray assays and mosquito rearing. We also thank Dr. Deborah McClellan for editorial assistance. We thank Dr. Jorge Motta, Anayansi Valderrama and the Gorgas Institute for providing field derived Ae. aegypti strains. This work has been supported by the National Institutes of Health/National Institute for Allergy and Infectious Disease 1R01AI061576-01A1, RO1 AI059492, RO1AI078997, the Johns Hopkins School of Public Health and the Johns Hopkins Malaria Research Institute. JLR was supported by an individual F31 NRSA training grant from NIH/NIAAD (1F31AI080161-01A1) and by the American Society for Microbiology Robert D. Watkins Graduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gubler DJ, Rosen L. Variation among Geographic Strains of Aedes Albopictus in Suceptibility to Infection with Dengue Viruses. Am J Trop Med Hyg. 1976;25(2):318–325. doi: 10.4269/ajtmh.1976.25.318. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JL. Susceptibility and resistance of vector mosquitoes. The Arboviruses: Epidemiology and Ecology, TP. Monath. 1988;1:87–126. [Google Scholar]
- 3.Salazar MI, Richardson J, Sanchez-Vargas I, Olson K, Beaty B. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiology. 2007;7(1):9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JR, Rico-Hesse R. AEDES AEGYPTI VECTORIAL CAPACITY IS DETERMINED BY THE INFECTING GENOTYPE OF DENGUE VIRUS. Am J Trop Med Hyg. 2006;75(5):886–892. [PMC free article] [PubMed] [Google Scholar]
- 5.Hanley KA, Goddard LB, Gilmore LE, et al. Infectivity of West Nile/Dengue Chimeric Viruses for West Nile and Dengue Mosquito Vectors. Vector-Borne and Zoonotic Diseases. 2005;5(1):1–10. doi: 10.1089/vbz.2005.5.1. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong PM, Rico-Hesse R. Efficiency of dengue serotype 2 virus strains to infect and disseminate in Aedes aegypti. Am J Trop Med Hyg. 2003;68(5):539–544. doi: 10.4269/ajtmh.2003.68.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosso C, Galván-Mendoza IJ, Ludert JE, del Angel RM. Endocytic pathway followed by dengue virus to infect the mosquito cell line C6/36 HT. Virology. 2008;378(1):193. doi: 10.1016/j.virol.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Black WC, Bennett KE, Gorrochótegui-Escalante N, et al. Flavivirus Susceptibility in Aedes aegypti. Archives of Medical Research. 2002;33(4):379. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- 9.Black WC, Bennett KE, Gorrochotegui-Escalante N, et al. Flavivirus Susceptibility in Aedes aegypti. Archives of Medical Research. 2002;33(4):379. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- 10.Richardson J, Molina-Cruz A, Salazar MI, Black WI. Quantitative analysis of dengue-2 virus RNA during the extrinsic incubation period in individual Aedes aegypti. Am J Trop Med Hyg. 2006;74(1):132–141. [PubMed] [Google Scholar]
- 11.Bennett K, Flick D, Fleming KH, Jochim R, Beaty B, Black WC. Quantitative trait loci that control Dengue-2 virus dissemination in the mosquito Aedes aegypti. Genetics. 2005;170:185–194. doi: 10.1534/genetics.104.035634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehane MJ, Aksoy S, Levashina E. Immune responses and parasite transmission in blood-feeding insects. 2004;20(9):433. doi: 10.1016/j.pt.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21(11):2568. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti Toll Pathway Controls Dengue Virus Infection. PLoS Pathog. 2008;4(7):e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez-Vargas I, Scott JC, Poole-Smith BK, et al. Dengue Virus Type 2 Infections of Aedes aegypti Are Modulated by the Mosquito's RNA Interference Pathway. PLoS Pathog. 2009;5(2):e1000299. doi: 10.1371/journal.ppat.1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troyer JM, Hanley KA, Whitehead SS, et al. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg. 2001;65(5):414–419. doi: 10.4269/ajtmh.2001.65.414. [DOI] [PubMed] [Google Scholar]
- 18.Billoir F, de Chesse R, Tolou H, de Micco P, Gould EA, de Lamballerie X. Phylogeny of the genus Flavivirus using complete coding sequences of arthropod-borne viruses and viruses with no known vector. J Gen Virol. 2000;81(3):781–790. doi: 10.1099/0022-1317-81-3-781. [DOI] [PubMed] [Google Scholar]
- 19.Zanotto PM, Gould EA, Gao GF, Harvey PH, Holmes EC. Population dynamics of flaviviruses revealed by molecular phylogenies. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(2):548–553. doi: 10.1073/pnas.93.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RodrÃ-guez MaM, Bisset J, de Fernandez DM, Lauzán L, Soca An. Detection of Insecticide Resistance in Aedes aegypti (Diptera: Culicidae) from Cuba and Venezuela. Journal of Medical Entomology. 2009;38(5):623. doi: 10.1603/0022-2585-38.5.623. [DOI] [PubMed] [Google Scholar]
- 21.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(20):7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders HR, Foy BD, Evans AM, et al. Sindbis virus induces transport processes and alters expression of innate immunity pathway genes in the midgut of the disease vector, Aedes aegypti. Insect Biochemistry and Molecular Biology. 2005;35(11):1293. doi: 10.1016/j.ibmb.2005.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.- Primer sequences used for the expression analysis of selected inmune genes by real-time RT-PCR.