Abstract
Breast cancer is the most common cancer among women, and despite significant advances in diagnosing and treating it, metastatic spread of cancer cells results in a high mortality rate. Epithelial-to-mesenchymal transition (EMT) is an embryonic program in which epithelial cells lose their characteristics and gain mesenchymal features. Therefore, EMT might play a very important role during malignant tumour progression. In this review we summarise recent advances in breast cancer research with a particular focus on the transcription factors Snail1 and Twist1. Besides discussing the role of EMT in normal mammary gland development, we describe regulatory mechanisms involving newly discovered upstream regulators and microRNAs, the association of EMT with breast cancer stem cells, and the involvement of the tumour microenvironment in breast cancer progression.
Introduction
Cancer metastasis is a multistep process characterised by local invasion, transport by the circulation, and survival and proliferation of metastasising cells in distant tissues. Similarities have been observed between the invasive and metastatic behaviour of cancer cells on the one hand and the long-distance migration of cells during development on the other. Epithelial cells usually form a mono- or multilayer on top of a basement membrane, and by lining the cavities and surfaces of the body, they form a protective barrier. These cells are tightly connected to each other by adhesion proteins (for example, E-cadherin), they express epithelial markers (for example, cytokeratins), and they are apico-basally polarised. Together with the extracellular matrix (ECM), mesenchymal cells fill the interstitial spaces and are a source of growth factors. Mesenchymal cells lack cell-cell contacts, express mesenchymal markers such as vimentin, and exhibit migratory behaviour. During epithelial-to-mesenchymal transition (EMT), epithelial cells lose their epithelial features and acquire a fibroblast-like morphology, with cytoskeletal reorganisation, upregulation of mesenchymal markers, and enhancement of motility, invasiveness and metastatic capabilities [1,2]. Therefore, reactivation of an embryonic EMT program could be the underlying mechanism of tumour invasion. At distant sites, cancer cells can undergo the reverse process, a mesenchymal-to-epithelial transition (MET). This is a transient phenomenon in which cancer cells reacquire epithelial characteristics once the invasion step is finished [1,3]. Understanding these processes is crucial because metastasis is a prominent cause of cancer-related death.
EMT has been studied extensively in cancer cell lines, but pathologists still question the occurrence of EMT in human cancer in vivo. By using different mouse models of mammary cancers in combination with the cre-Rosa26LoxP reporter, Trimboli and colleagues [4] provided strong evidence for an EMT role in breast cancer. Approximately 50% of the tumours from WAP-myc mice showed fibroblast-like cells of mammary epithelial origin adjacent to the tumour site, which proves the existence of early stages of EMT in vivo. The occurrence of EMT in breast cancer in vivo was illustrated by immunohistochemical analysis of human invasive breast carcinomas and carcinosarcomas [5]. Simultaneous upregulation of mesenchymal markers, such as vimentin and proteins involved in motility and ECM remodelling, together with downregulation of epithelial markers such as E-cadherin, were predominant in breast tumours with a basal-like phenotype. Breast carcinosarcomas are supposed to have undergone complete EMT and show a basal-like phenotype, which suggests that EMT occurs in specific tumour subtypes [5].
This review will focus mainly on the specific role of the transcription factors Snail1 (encoded by SNAI1) and Twist1 (encoded by TWIST1) during EMT in breast cancer. Snail1 is a zinc-finger transcription factor belonging to the Snail superfamily and characterised by a strongly conserved carboxy-terminal region containing four to six C2H2-zinc fingers. Snail family members Snail1 and Snail2 (Slug) act as transcriptional repressors when their fingers bind to E-box motifs (5'-CANNTG-3') in target promoters, including the E-cadherin gene (CDH1) promoter. Snail1 plays an essential role during gastrulation and neural crest formation, which explains the death of Snail1 knock-out mice at the gastrula stage [6]. Mammals have two Twist-like proteins with strong structural homology. Twist proteins possess an evolutionarily conserved basic helix-loop-helix domain, which allows protein-protein interaction with other basic helixloop-helix proteins. When Twist molecules dimerise, they bind to E-box sequences in target promoters [7]. Gene deletion experiments showed that Twist1 is important for closure of the neural tube during embryogenesis [8]. On the other hand, the elevated expression of proinflammatory cytokines in Twist2 knock-out mice caused perinatal death [9]. In Drosophila, Snail1 acts as a repressor of ectodermal genes and Twist1 as a positive regulator of mesoderm-specific genes, and together they define the borders between the mesoderm and the surrounding tissue [10]. Stable Snail1 knock-down in breast and skin carcinoma cell lines causes a partial MET with weak invasiveness and tumorigenicity, but these effects were not observed in Snail2 knock-down cells. This suggests that Snail1 and Snail2 have complementary roles in the induction of tumour growth [11]. In human cancers, Twist1 has been linked to metastasis [12] and Snail1 to recurrence [13].
Snail1 and Twist1 during mammary gland development
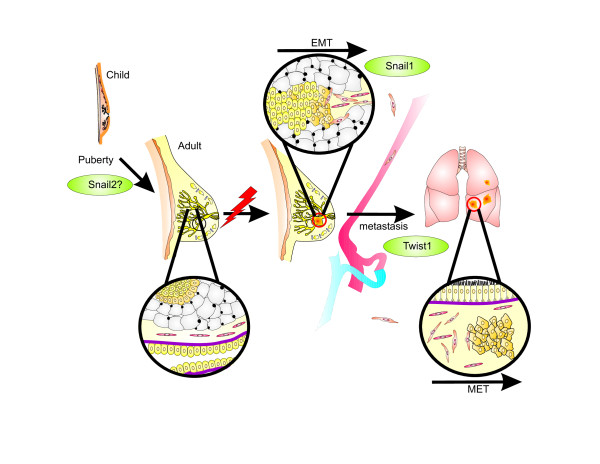
Most vertebrate organs are patterned during embryogenesis and maintain their basic structure throughout adult life, but the structure of breast tissue in reproductive females changes continuously [14]. Before birth, the specified mammary epithelium invades from the nipple into the fat pad to form a small, branched ductal network. Snail1 and Snail2 control expression of aromatase, which converts androgens to oestrogens. The latter are necessary for ductal outgrowth, and so SNAI genes might play a role in ductal network development [15]. The release of ovarian hormones in puberty causes the distal ends of the mammary ducts to swell into bulbous structures composed of multiple layers of cuboidal epithelial cells called terminal end buds (Figure 1) [16]. Experimental evidence indicating a role for Snail family members during acini differentiation is very limited. In this respect, mammary epithelial MCF-10A cells cultivated on matrigel form acinar structures with characteristics found in glandular epithelium in vivo [17]. Gene expression analysis in this cellular model system revealed substantial amounts of Snail2 and E-cadherin mRNA, but very little Snail1 mRNA (Foubert E, Berx G, unpublished observation). Similar results were obtained by Côme and colleagues using human mammary epithelial cells cultivated on matrigel, which suggests that Snail2 has an active role during the lobuloalveolar phase [18]. The mammary gland reaches its final developmental stage during pregnancy and lactation. Reproductive hormones induce the expansion and terminal differentiation of the mammary epithelium into secretory, milk-producing, lobular alveoli, while large fat cells dedifferentiate into tiny adipocytes [19]. During involution, the mammary gland regresses due to apoptosis of alveolar epithelial cells and remodelling of the basement membrane, and this restores the breast to its previous state. Snail2 has an anti-apoptotic function and might be involved in the final stages of this process as a break to stop involution [18].
Figure 1.
Role of Snail1 and Twist1 during mammary gland development and breast cancer progression. In females, release of ovarian hormones induces further development of the mammary gland. Snail1 and Snail2 control aromatase expression, indicating that they have a role in development of the ductal network. Somatic mutations and/or aberrant expression of oncogenes can cause proliferation of mammary epithelial cells. During malignant cancer progression, Snail1 induces an epithelial-to-mesenchymal transition (EMT) of epithelial breast cancer cells, which grants them invasive and migratory capacities. Twist1 plays a role in the development of distant metastasis by prompting cancer cells to enter the bloodstream. At distant organs, these cells undergo a mesenchymal-to-epithelial transition (MET).
Many processes during mammary gland development have some features of tumour progression, such as invasion, reinitiation of cell proliferation, resistance to apoptosis, and angiogenesis. Inhibition of stromal regulators or secreted growth and differentiation factors disturbs the interaction between the epithelium and ECM. This disruption can induce and promote breast cancer (Figure 1).
Snail1 and Twist1 as criteria in breast cancer classification?
Breast cancer can be classified on the basis of different criteria. Classically, two main histological subgroups are defined morphologically, ductal and lobular carcinomas, which together represent 90% of all breast cancers. Ductal carcinoma in situ is the most common type of non-invasive breast cancer and arises inside the milk ducts, whereas lobular carcinoma in situ is characterised by abnormal cell growth in the lobules. When tumour cells invade the surrounding tissue and give rise to metastasis, ductal carcinoma in situ can progress to invasive ductal carcinoma, and lobular carcinoma in situ to lobular carcinoma [20]. Ductal carcinoma in situ and lobular carcinoma in situ can be distinguished by the expression of E-cadherin. Positive but heterogeneous E-cadherin expression is observed in invasive ductal carcinomas. In contrast, there is often no E-cadherin expression in infiltrating lobular carcinomas due to somatic mutations in CDH1, loss of heterozygosity, or CDH1 promoter methylation [21].
At least five molecular subtypes of breast cancer can be distinguished by their gene expression profiles: luminal A, luminal B, normal breast-like, HER-2+/ER-, and basallike [5,22]. Luminal tumours are positive for the oestrogen receptor (ER) and express luminal epithelial markers such as cytokeratin 8 and 18. Based on differences in histological grade and prognosis, luminal tumours are classified as luminal A or luminal B. The clinical significance of normal breast-like tumours is still questionable because these lesions consistently cluster together with samples of fibroadenomas and normal breast samples [22]. The contribution of the ER pathway to EMT is well described as the ER status has an impact on E-cadherin biosynthesis. In response to oestrogen signalling, ER indirectly activates MTA3 (metastasis associated 1 family, member 3), which forms a transcriptional corepressor complex with Mi-2/NuRD. One direct function of this complex is to inhibit Snail1, which leads indirectly to transcriptional activation of E-cadherin [23]. More recently, it was shown that the ER pathway can regulate Snail2 expression. Ligand-activated ERα forms a transcriptional inhibitory complex with histone deacetylase 1 (HDAC1) and the nuclear receptor corepressor (N-CoR), which leads to repression of the Snail2 promoter and results in E-cadherin expression. Alternatively, Snail2 expression can be inhibited by glycogen synthase kinase 3-beta inactivation through phosphoinositide 3-kinase (PI3K)/AKT activation upon ERα activity [24].
ER-negative tumours are composed of HER-2 tumours (which overexpress HER-2 and genes associated with the HER-2 pathway) and the basal-like subgroups. These tumours express genes usually expressed in myoepithelium of the normal mammary gland, such as basal cytokeratins and epidermal growth factor receptor. They are usually characterised by high histological grade, resistance to chemotherapy, and poor prognosis [5,22]. Basal-like tumours are often incorrectly described in the literature as triple-negative cancers (negative for ER, progesterone receptor and HER-2) [25]. Indeed, most triple-negative cancers have a basal-like phenotype, but several do not express basal markers. On the other hand, a small sub group of basal-like cancers expresses either hormone receptors or HER-2 [26].
Immunohistochemical analysis illustrated that aggressive, poorly differentiated tumours usually express basal markers typical of the basal-like phenotype [5]. Statistical analysis of breast adenocarcinoma samples correlated high ZEB1 expression with clinicopathological features such as poorly differentiated tumours, meta stasis, and poor survival. [27]. Microarray analysis revealed Snail2 expression in basal-like breast cancer cell lines [28]. It was demonstrated that tumours expressing high levels of SNAI2 mRNA have a basal-like phenotype [29]. These data indicate that EMT preferentially occurs in more aggressive breast tumours of the basal phenotype.
Interplay of transcription factors in the control of EMT in breast cancer
One key molecular change in EMT is E-cadherin downregulation, which results in reduction of cell-cell adhesion and destabilisation of the epithelial architecture. Several transcription factors, so-called EMT inducers, act as transcriptional repressors of E-cadherin and directly modulate the expression of many genes involved in cancer invasion and metastasis, consequently promoting EMT in vitro [30]. These transcription factors include members of the Snail family (SNAI1/Snail1 and SNAI2/Snail2/Slug) and ZEB (zinc finger E-box binding homeobox) family (ZEB1/δEF1 and ZEB2/SIP1), basic helixloop-helix factors, such as E12/E47 and Twist1, and the recently identified factors CBF-A (CArG box-binding factor-A), FOXC2 (forkhead 1), HOXB7 (homeobox gene B7), Goosecoid, and KLF8 (Krüppel-like factor 8) [2,30,31]. Most of these transcription factors have been shown to bind and repress the CDH1 promoter, but direct interaction of Twist1 with this promoter has not been proven [12,32].
Peinado and colleagues [33] suggested a model in which different E-cadherin repressors participate during EMT/invasion. In this model, Snail1 and ZEB2 play a role in inducing the first EMT steps that lead to the initiation of the invasive process, whereas Snail2, E47 and ZEB1 favour the maintenance of the migratory, invasive phenotype, and Twist1 has a critical role in the development of distant metastases by prompting cancer cells to enter the bloodstream [12]. EMT is a transient, reversible process and most likely occurs only in small groups of cells or isolated cells in the invasive areas of tumours. Tumour cells undergoing partial EMT, and so exhibiting only some features of the developmental program, are therefore difficult to distinguish from tumour-associated fibroblasts sharing similar characteristics. Nevertheless, many potential markers have been described for monitoring EMT in tissue samples and in biological fluids [34].
In addition, other recent findings provide further evidence for the involvement of different EMT mechanisms in breast cancer, including signalling pathways, the micro-environment, and the newly discovered developmental proteins acting upstream of EMT inducers. Several classical signalling cascades (transforming growth factor (TGF)-β, Wnt, and receptor tyrosine kinase signalling) that lead to the expression of the EMT inducers are active during both development and cancer progression [35]. Figure 2 is a schematic overview of upstream regulators of Snail1/Snail2 and Twist1, and their corresponding downstream effects.
Figure 2.
Snail1 and Twist1 contribute to a series of normal processes and cancer related progression in the mammary gland. Snail1 and Twist1 contribute to different developmental and pathological outcomes in the mammary gland. Several epithelial-to-mesenchymal transition (EMT)-inducing signals in breast epithelial cells induce Snail1 and Twist1 transcription factors. Examples of effector or direct target genes that are regulated by Snail1 and Twist1 to produce the indicated outcomes are shown. Note that in many cases numerous targets that mediate a specific outcome have been identified, but only one example target or effector gene is shown here. AKT2, v-akt murine thymoma viral oncogene homolog 2; CAR, coxsackie virus and adenovirus receptor; HIF-1α, hypoxia inducible factor-1 alpha; IL-6, interleukin-6; LBX1, ladybird homeobox 1; MiR- 10b, microRNA-10b; NF-κB, nuclear factor-κB; p21, cyclin dependent kinase inhibitor 1A; p16, cyclin dependent kinase inhibitor 2A; Src-1, steroid receptor co-activator-1; TGF-β, transforming growth factor-beta; TNF-α, tumour necrosis factor-alpha; TrkB, neutrophic tyrosine kinase receptor; Wnt, wingless-type MMTV integration site family; YB-1, Y-box binding protein 1; ZEB1, zinc finger E-box-binding homeobox.
Despite its tumour suppressor function in normal conditions, TGF-β is a potent EMT inducer (Figure 2). It has been reported that NMuMG cells, a mouse mammary gland epithelial cell line, undergo EMT upon TGF-β treatment [36]. The phosphorylated Smad proteins translocate to the nucleus and control the expression of target genes [2]. Smads have low affinity for DNA and interact with DNA-binding cofactors to gain high affinity and selectivity for specific target genes [37]. Co-immunoprecipitation and chromatin immunoprecipitation experiments identified Snail1 as a cofactor for Smad3/4. TGF-β leads to translocation of Snail1 to the nucleus, where it interacts with activated Smad3/4. This complex binds the promoters of CDH1 and the Coxsackie- and adenovirus receptor (CAR), which have an E-box and a Smad-binding element nearby. In vivo, the Snail1-Smad3/4 complex was found in the nucleus of tumour cells at the invasive front [38]. Another protein that interacts with the Smads is high mobility group protein A2 (HMGA2), a non-histone chromatin binding factor containing three A/T hook domains, which enable it to bind to A/T-rich sequences in the minor groove of DNA [39]. In mammary epithelial cells, TGF-β induces HMGA2 via the Smad pathway [40]. In turn, HMGA2 binds the SNAI1 promoter in cooperation with Smads and induces SNAI1 expression, CDH1 repression, and TGF-β-induced EMT. HMGA2 acts as a specific regulator of Snail1 and possibly also of Twist1, Snail2, ZEB1 and ZEB2, probably by general chromatin reorganisation and DNA binding of the A/T hook domains [41].
A novel upstream regulator of Snail1 is Ladybird homeobox 1 (LBX1), a transcription factor implicated in normal myogenesis and neurogenesis. LBX1 over expression in MCF-10A cells elicits EMT, enhances migration, and increases the CD44+/CD24- population. A considerable increase of endogeneous mRNA levels of TGF-β2, SNAI1 and ZEB1/2 was observed, and promoter analysis proved that LBX1 directly activates the SNAI1 and ZEB1 promoters. Based on RNA microarray and protein immunohistochemistry, LBX1 expression was associated with triple-negative basal-like tumours [42].
The role of mammalian Y-box binding protein-1 (YB-1) in breast tumorigenesis is well studied. Elevated YB-1 expression in mammary glands causes chromosomal instability and induces breast carcinomas in lactating transgenic mice [43], whereas YB-1 overexpression in MCF7 adenocarcinoma cells enhances their proliferation and formation of colonies in soft agar [44]. YB-1 is involved in fundamental processes, such as DNA repair, mRNA transcription, splicing, translation and stabilisation [45]. Overexpression of YB-1 in H-Ras-transformed MCF-10A cells induces EMT accompanied by enhanced metastatic potential and decreased proliferation rates, but the cells fail to form tumours in vivo. Microarray gene analysis revealed that YB-1 increases TWIST1 expression on the transcriptional and translational levels and directly activates cap-independent translation of Snail1 mRNA. In vivo, YB-1 expression was associated with potentially metastatic breast cancer cells and poor clinical outcome and was inversely correlated with CDH1 expression levels in breast cancer specimens [46].
From a recent screening of an RNA interference library in cells defective in the early steps of metastasis (migration and invasion), KLF17 was identified as a metastasis suppressor in human breast cancer. Loss of KLF17 leads to metastasis through direct regulation of Id1. Moreover, an inverse correlation was found between KLF17 and Id1 expression in human breast cancer samples. This relation ship can potentially be used to predict the metastatic state of primary breast cancer [47].
Steroid receptor coactivator-1 (Src-1) and hypoxiainducible factor-1α (HIF-1α) are newly discovered upstream regulators of Twist1 (Figure 2) [48,49]. Src-1 promotes transcription by interacting with nuclear receptors and transcription factors. Src-1 is strongly expressed in HER-2-positive breast cancers and correlates with disease recurrence and resistance to endocrine therapy [50]. Together with PEA3 (polyomavirus enhancer activator 3), Src-1 binds to and co-activates the proximal TWIST1 promoter, enhancing breast cancer invasiveness and metastasis [48]. It has been reported that HIF-1α induces Snail1, ZEB1, ZEB2 and E47 [51,52]. HIF-1α and TWIST1-null mice show phenotypic similarities, which points to a possible link between these genes [8,53]. This suggestion is supported by studies showing that HIF-1α can bind and activate the TWIST1 promoter via the hypoxia-response element. This might represent an early step and a critical mechanism causing hypoxia-induced tumour progression and metastasis [49].
Snail1 and Twist1: potent protection against anoikis and senescence
Mounting experimental evidence indicates that the Snail1 and Twist1 transcription factors control cell proliferation and survival, which has major consequences for cancer progression. In fact, upregulation of Snail1 could be a rapidly induced epigenetic variation aimed at genetically inhibiting cell death. Indeed, expression of Snail1 seems to protect cells from caspase-mediated programmed cell death elicited by serum depletion or by signals downstream of therapeutic agents, TNF-α, and DNA damage [54,55]. In this context, it is worth mentioning that the highly homologous Snail2 gene seems to be a target of p53 and acts as an antagonist of PUMA (p53-upregulated modulator of apoptosis) [56]. Like Snail1, Twist1 also seems to be able to regulate resistance of breast cancer cells to chemotherapeutics such as paclitaxel. Twist1 transactivates AKT2, which results in increased survival, migration and invasiveness [57]. Furthermore, chemotherapeutic treatment of breast cancer cells with adriamycin results in upregulation of Twist1 and its interaction with p53-MDM2. Only the cells undergoing EMT display enhanced invasiveness and multidrug resistance [58]. Twist1 and Snail1 seem to play a central role in the metastasis induced by TrkB, a neutro phic tyrosine kinase receptor, mainly by suppressing anoikis [59]. Furthermore, Twist proteins were recently found to be responsible for bypassing ErbB2 or Ras oncogene-induced senescence. This is explained at least in part by Twist proteins repressing both transcription of p21CIP1 (in a p53-independent manner) and p16Ink4a [60]. These results indicate that Snail1 and Twist proteins have a doubly damaging effect with potent prosurvival functions that, in conjunction with EMT, provide an explanation for the strong contribution towards tumour progression. The relationships between Snail1, Twist1 and AKT2, TrkB, p21 and p16 are depicted in Figure 2.
Snail1 and Twist1 under control of the tumour micro-environment
In addition to the role played by the induction of EMT by growth factors and developmental signalling pathways in cancer progression, the tumour micro-environment is involved as well. The inflammatory tumour micro-environment evolves as tumours grow, with infiltration of immune cells and activation of the inflammatory responses. Inflammatory cells, particularly tumour-associated macrophages (TAMs), are usually found at the invasive front of more advanced tumours [61]. TAMs facilitate angiogenesis, ECM breakdown and tissue remodel ling, and thereby they promote tumour cell motility. TAMs also secrete pro-inflammatory cytokines, such as TNF-α. Wu and colleagues [62] demonstrated that Snail1 can be stabilised by TNF-α through the activation of the NF-κB pathway. TNF-α and NF-κB induce the COP9 signalosome 2 (CSN2), the second and most conserved subunit of the COP9 signalosome, which inhibits ubiquitination and degradation of Snail1. These researchers also showed that knockdown of Snail1 suppresses both intrinsic and inflammation-enhanced migration, which provides a plausible mechanism for inflammation-induced metastasis [62]. Another study focused on IL-6, a pleiotropic cytokine that participates in acute inflammation [63]. Elevation of serum IL-6 has already been shown to be correlated with advanced breast tumour stage, metastasis and poor prognosis [64,65]. MCF7 cells that constitutively express IL-6 exhibit an EMT phenotype characterised by upregulation of Snail1 and Twist1. Alternatively, they also observed that MCF7 cells overexpressing Twist1 produce more IL-6 due to aberrant activation of STAT3 (signal transducer and activator of transcription 3), which illustrates the role of IL-6 in breast cancer progression and eventually in metastasis [63].
There is a strong association between inflammation and tumorigenesis. In inflammatory diseases, NF-κB is one of the key pathways generating a loop that maintains the inflammatory signals by inducing a wide range of pro-inflammatory cytokines, chemokines and growth factors. The recruitment of immune cells, TAMs and cancer-associated fibroblasts producing NF-κB and HIF-1α generates a micro-environment capable of driving tumour progression. In cancer development, NF-κB is linked to resistance to apoptosis and increased angiogenesis [66]. Although TGF-β is described as an antiinflammatory cytokine, it contributes to the formation of cancer-associated fibroblasts via the activation of resident fibroblasts. TGF-β is the most potent inducer of Snail1, which seems able to upregulate the expression of pro-inflammatory interleukins [66].
Besides inflammatory cytokines, matrix metalloproteinases (MMPs) are also important participants in tumour progression because they degrade structural components of the ECM, which allows tumour invasion and metastasis. In breast tumours, MMP-3 is frequently upregulated. It can induce Snail1 expression and EMT through increased production of cellular reactive oxygen species. MMP-3-induced EMT causes DNA damage and genomic instability [67].
Snail1 and Twist1 contribute to EMT and breast cancer stemness
Relapse and subsequent metastatic spread to distant sites is the main cause of cancer death. Nevertheless, metastasis formation is considered an inefficient process because thousands of cancer cells are shed into the circulation, but only a few cells can survive, reach secondary organs, and colonise them [68]. There is recent growing interest in one particular cell population of socalled cancer stem cells (CSCs) because they could be responsible for therapy failure and cancer recurrence. Stem cell features include self-renewal, ability to differentiate, and resistance to chemotherapeutic drugs and radiation [69]. CSCs were first identified in the hematopoietic system [70], and more recently they were also described in solid tumours of breast, colon and brain [71,72]. Al-Hajj and colleagues [71] initially described the CD44+/CD24-/low phenotype as a feature of human breast CSCs. This cell population, which was fractionated from a primary invasive breast cancer and metastatic pleural effusions, has classical features of normal stem cells and can form tumours in immunocompromised nonobese diabetes/severe combined immunodeficiency (NOD/SCID) mice. The CD44+/CD24- population is associated with the expression of basal/mesenchymal or myoepithelial markers and is enriched in basal-like and BRCA1 mutant breast cancers [71]. The origin of breast CSCs (BCSCs) is still unclear. One hypothesis is that BCSCs are derived from transformed, resident tissue stem cells, which occasionally produce a copy of themselves but most often generate daughter cells with limited tumorigenicity. Alternatively, CSCs might be derived from transformed, differentiated epithelial cells that acquire stem cell characteristics. Recurrence of cancer after therapy suggests that treated patients still have a small population of tumorigenic CSCs [69]. The cellular transformations needed for resistance show similarities to some changes required for the acquisition of a more aggressive pheno type. In this respect, several studies link EMT with CSCs and therapy failure.
The Wnt signalling pathway is also thought to be necessary for cancer cell self-renewal. The triple-negative SUM1315 cancer cell line is known for its strong Wnt activity and its ability to metastasise to the lung in mice [73]. This cell line exhibits a CD44+/CD24- profile and strong expression of SNAI2 and TWIST1. Inhibition of the Wnt pathway increases the CD44-/CD24- population and blocks tumour formation because Snail2 and Twist1 levels are decreased and expression of epithelial markers is increased [68]. Further studies are needed to determine if therapies targeting the Wnt pathway will affect tumour recurrence and/or metastasis.
A novel subtype of breast cancer was recently described, namely metaplastic breast cancers (MBCs), which are aggressive, chemoresistant tumours associated with poor outcome. MBCs are frequently triple-negative and express basal epithelial markers. Based on an integrated genomic-proteomic approach, MBCs represent an independent subtype that is distinct from basal-like cancers. Their transcriptional profiles are closely related to claudin-low cancers [74]. Claudin-low cancers are a novel subgroup of receptor-negative breast cancers characterised by loss of genes involved in cell-cell adhesion and strong expression of mesenchymal markers such as vimentin [75]. It has been reported that the gene expression patterns of CD44+/CD24- cells showed a significant correlation with the claudin-low subgroup. More-over, residual cancer cells after conventional therapy are the tumour-initiating cells that could be more resistant and have more mesenchymal-like features, which are characteristics of claudin-low tumours [76]. Additionally, claudin-low tumours and MBCs are enriched in stem cell-like markers (high CD44/CD24 and CD29/CD24 ratios) and EMT markers (strong SNAI2 and TWIST1 expression in MBCs and strong SNAI3 in claudin-low tumor cells) [74].
Assuming that metastasis requires dissemination of tumour stem cells or tumour cells undergoing EMT, it seems likely that such cells should be detectable among circulating tumour cells (CTCs) found in breast cancer patients. Patient blood samples positive for CTCs were analysed for EMT markers (Twist1, Akt and PI3Kα) and the BCSC marker aldehyde dehydrogenase 1, a detoxifying enzyme responsible for the oxidation of intracellular aldehydes [77]. Expression of the EMT markers and aldehyde dehydrogenase 1 was correlated with poor response to breast cancer-related therapies. A major proportion of CTCs of MBC patients shows EMT and tumour stem cell features, which is indicative of therapy-resistant cell populations. Detection and characterisation of CTCs exhibiting EMT or stem cell-like metabolism might be a powerful diagnostic tool for patient stratification, early identification of therapy failure, or the potential risk of resistance to a given therapeutic intervention [77].
The relationship between EMT and CSCs has been studied as well. Mani and colleagues [78] proposed that cells that have undergone EMT behave in many respects like stem cells isolated from normal or neoplastic cell populations. When SNAI1 and TWIST1 were expressed in human mammary epithelial cells (HMLEs), Her2/neu-transformed HMLEs, and V12H-Ras-transformed HMLEs, the cells went through EMT and acquired a greater mammosphere-forming ability and a CD44+/CD24- expression pattern. Although these enriched CD44+/CD24- cells were considered as stem cells, tumour formation in vivo was only observed in V12H-Rastransformed HMLEs upon overexpression of SNAI1 or TWIST1 [78]. Comparable findings were reported by Morel and colleagues [79], which demonstrates that CSCs can develop from HMLEs upon aberrant activation of the Ras/mitogen-activated protein kinase pathway.
Epithelial-to-mesenchymal transition and microRNAs
In the past few years, considerable evidence has shown that small RNA species are involved in the control of RNA stability or translation. MicroRNAs (miRNAs) are involved in physiological processes, such as muscle differentiation, and in the onset and/or progression of several pathologies, such as cancer. More than 50% of human miRNAs are located in fragile chromosomal regions that are prone to mutations during tumour development [80]. Functional characterisation revealed that miRNAs can act as oncogenes (miR-21, miR-155, miR-17-92 cluster) or as tumour suppressor genes (miR-34a, let-7) by silencing target genes encoding tumour-suppressors or oncogenic proteins, respectively [80].
The miR-200 family consists of two subgroups located on chromosomes 1 (miR-200b, miR-200a and miR-429) and 12 (miR-200c and miR-141) and predicted to target a large common group of genes [81]. Expression of the miR-200 family is enriched in differentiated epithelial tissues. Several studies have demonstrated an inverse correlation between expression of the miR-200 family and the ZEB transcription factors [82,83]. Suppression of endogeneous miR-200 family members is sufficient to induce EMT, whereas their ectopic expression induces MET in normal and cancer cell lines through direct targeting of ZEB1/2 [83]. miRNA screening in human breast cancer revealed metastatic suppressor miRNAs (miR-335, miR-126) [84] and pro-metastatic miRNAs (miR-10b, miR-373, miR-520c) [85,86]. It was shown that miR-10b was needed for in vitro invasiveness and in vivo metastasis. Twist1 could bind and activate the MIR10B promoter, leading to upregulation of the pro-metastatic gene RHOC and to the translational inhibition of HOXD10, an inhibitor of genes involved in cell migration and ECM remodelling [86]. Growing evidence shows the importance of miRNAs in stem cells and CSCs. The embryonic stem cell factors Oct4, Nanog and Sox2 can occupy the promoters of many transcription factors and the regulatory sequences of 14 miRNAs [87]. Moreover, embryonic stem cells deficient in miRNA processing enzymes had a diminished capacity for differentiation and self-renewal [88]. Isolation of tumour-initiating cells from breasts of patients before and after chemotherapy indicated that after chemotherapy these cells were resistant to drugs and did not express let-7 and miR-200 family members. Experiments proved that these miRNAs were upregulated when differentiation was favoured, indicating that stem-like cancer cells lack expression of both the let-7 and miR-200 families [89]. Some of the predicted targets of the miR-200 family members, such as Sox2, KLF4 and the polycomb repressor Bmi1, are involved in maintaining or inducing the stem cell phenotype [90,91]. It has been shown that miRNAs in pancreatic and colorectal cancer cells can control stemness properties. Strong ZEB1 expression was associated with Bmi1 overexpression in undifferentiated tumour cells while Bmi1 was negatively regulated by miR-200 family members (particularly miR-200c) and by miR-203, and to a lesser extent by miR-183, the so-called stemnessinhibiting miRNAs [92]. It was also recently shown that normal mammary stem cells and breast cancer stem cells with reduced expression of miR-200 family members had elevated Bmi1 expression [93].
Conclusion
Breast cancer, the most common cancer among women, is a heterogeneous disease in terms of tumour histology, clinical presentation and response to therapy. Because metastatic spread of tumour cells is responsible for almost all breast cancer deaths, considerable interest has grown in gaining a full understanding of the molecular processes in order to develop risk assessment schemes and suitable markers for evaluating the efficacy of therapy. EMT is the biological morphogenetic process by which epithelial cells undergo morphological changes by losing their epithelial characteristics and gaining mesenchymal features. The switch in certain differentiation markers is accompanied by functional changes required for cells to migrate and invade the ECM. In pathological conditions, EMT is considered as the reactivation of a developmental process controlled by a network of transcriptional regulators. Accumulating evidence supports the notion of a relationship between key EMT molecules such as Snail1 and Twist1 and bad cancer prognosis, resistance to chemotherapy and the initiation of the early steps of metastasis. Interest has been growing in investigating the importance of inflammation during tumorigenesis. Inflammatory signals produced by NF-κB, HIF-1α and TGF-β recruit immune cells, TAMs and cancer-associated fibroblasts, which generates a microenvironment that drives cancer progression. Moreover, regulation of proliferation and survival in cancer cells might link EMT to stemness. Cancer stem cells are believed to be responsible for therapy failure and cancer recurrence. Knowing the molecular signature of the EMT program would help to understand the complexity of these different features. This might eventually open new avenues to the development of targeted therapies to restore the epithelial state and immunocompetence in order to decrease the rate of death from breast cancer.
Abbreviations
BCSC: breast cancer stem cell; CSC: cancer stem cell; CTC: circulating tumour cell; ECM: extracellular matrix; EMT: epithelial-to-mesenchymal transition; ER: oestrogen receptor; HER: human epidermal growth factor receptor; HMLE: human mammary epithelial cell; HIF-1α: hypoxia inducible factor-1 alpha; HMGA2: high mobility group protein A2; IL: interleukin; KLF: Krüppel-like factor; LBX1: ladybird homeobox 1; MBC: metaplastic breast cancer; MET: mesenchymal-to-epithelial transition; miRNA: microRNA; MMP: matrix metalloproteinase; NF: nuclear factor; PI3K: phosphoinositide 3-kinase; Src-1: steroid receptor co-activator-1; TAM: tumour-associated macrophage; TGF: transforming growth factor; TNF: tumour necrosis factor; YB-1: Y-box binding protein 1; ZEB: zinc finger E-box-binding homeobox.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
This research was funded by grants from the FWO, the Geconcerteerde Onderzoeksacties of Ghent University, the Belgian Federation against Cancer, the Association for International Cancer Research (Scotland), and FP7 (TUMIC) of the European Union. We acknowledge Dr Amin Bredan for critical reading of the manuscript and the members of our research groups for valuable discussions.
Contributor Information
Ellen Foubert, Email: Ellen.Foubert@dmbr.vib-ugent.be.
Bram De Craene, Email: Bram.Decraene@dmbr.vib-ugent.be.
Geert Berx, Email: Geert.Berx@dmbr.vib-ugent.be.
References
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–787. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, Creasap N, Rosol TJ, Robinson ML, Eng C, Ostrowski MC, Leone G. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Firulli AB, Conway SJ. Phosphoregulation of Twist1 provides a mechanism of cell fate control. Curr Med Chem. 2008;15:2641–2647. doi: 10.2174/092986708785908987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/S0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Alberga A, Boulay JL, Kempe E, Dennefeld C, Haenlin M. The snail gene required for mesoderm formation in Drosophila is expressed dynamically in derivatives of all three germ layers. Development. 1991;111:983–992. doi: 10.1242/dev.111.4.983. [DOI] [PubMed] [Google Scholar]
- Olmeda D, Montes A, Moreno-Bueno G, Flores JM, Portillo F, Cano A. Snai1 and Snai2 collaborate on tumor growth and metastasis properties of mouse skin carcinoma cell lines. Oncogene. 2008;27:4690–4701. doi: 10.1038/onc.2008.118. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Truong TK, Yu B, Itoh T, Zhao J, Grube B, Zhou D, Chen S. Down-regulation of promoter 1.3 activity of the human aromatase gene in breast tissue by zinc-finger protein, snail (SnaH) Cancer Res. 2001;61:1338–1346. [PubMed] [Google Scholar]
- Silberstein GB. Postnatal mammary gland morphogenesis. Microsc Res Tech. 2001;52:155–162. doi: 10.1002/1097-0029(20010115)52:2<155::AID-JEMT1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/S1046-2023(03)00032-X. [DOI] [PubMed] [Google Scholar]
- Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1:467–475. doi: 10.1016/S1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- Debnath J, Brugge JS. Modelling glandular epithelial cancers in threedimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14:6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer FC, Marchio C, Reis-Filho JS. The role of molecular analysis in breast cancer. Pathology. 2009;41:77–88. doi: 10.1080/00313020802563536. [DOI] [PubMed] [Google Scholar]
- Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/S0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- Ye Y, Xiao Y, Wang W, Yearsley K, Gao JX, Shetuni B, Barsky SH. ERalpha signaling through slug regulates E-cadherin and EMT. Oncogene. pp. 1451–1462. [DOI] [PubMed]
- Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, Birnbaum D. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adélaïde J, Cervera N, Fekairi S, Xerri L, Jacquemier J, Birnbaum D, Bertucci F. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25:2273–2284. doi: 10.1038/sj.onc.1209254. [DOI] [PubMed] [Google Scholar]
- Storci G, Sansone P, Trere D, Tavolari S, Taffurelli M, Ceccarelli C, Guarnieri T, Paterini P, Pariali M, Montanaro L, Santini D, Chieco P, Bonafé M. The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J Pathol. 2008;214:25–37. doi: 10.1002/path.2254. [DOI] [PubMed] [Google Scholar]
- van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- De Wever O, Pauwels P, De Craene B, Sabbah M, Emami S, Redeuilh G, Gespach C, Bracke M, Berx G. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130:481–494. doi: 10.1007/s00418-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene B, van Roy F, Berx G. Unraveling signalling cascades for the Snail family of transcription factors. Cell Signal. 2005;17:535–547. doi: 10.1016/j.cellsig.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, Crystal RG, de Herreros AG, Moustakas A, Pettersson RF, Fuxe J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco A, Fedele M. Roles of HMGA proteins in cancer. Nat Rev Cancer. 2007;7:899–910. doi: 10.1038/nrc2271. [DOI] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelialmesenchymal transition. J Cell Biol. 2006;174:175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-tomesenchymal transition. J Biol Chem. 2008;283:33437–33446. doi: 10.1074/jbc.M802016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Smolen GA, Zhang J, Wittner B, Schott BJ, Brachtel E, Ramaswamy S, Maheswaran S, Haber DA. A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes Dev. 2009;23:1737–1742. doi: 10.1101/gad.1809309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S, Royer-Pokora B, Fietze E, Jürchott K, Hildebrandt B, Trost D, Leenders F, Claude JC, Theuring F, Bargou R, Dietel M, Royer HD. YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res. 2005;65:4078–4087. doi: 10.1158/0008-5472.CAN-04-4056. [DOI] [PubMed] [Google Scholar]
- Sutherland BW, Kucab J, Wu J, Lee C, Cheang MC, Yorida E, Turbin D, Dedhar S, Nelson C, Pollak M, Leighton Grimes H, Miller K, Badve S, Huntsman D, Blake-Gilks C, Chen M, Pallen CJ, Dunn SE. Akt phosphorylates the Y-box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene. 2005;24:4281–4292. doi: 10.1038/sj.onc.1208590. [DOI] [PubMed] [Google Scholar]
- Evdokimova VM, Ovchinnikov LP. Translational regulation by Y-box transcription factor: involvement of the major mRNA-associated protein, p50. Int J Biochem Cell Biol. 1999;31:139–149. doi: 10.1016/S1357-2725(98)00137-X. [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Tognon C, Ng T, Ruzanov P, Melnyk N, Fink D, Sorokin A, Ovchinnikov LP, Davicioni E, Triche TJ, Sorensen PH. Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer cell. 2009;15:402–415. doi: 10.1016/j.ccr.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Gumireddy K, Li A, Gimotty PA, Klein-Szanto AJ, Showe LC, Katsaros D, Coukos G, Zhang L, Huang Q. KLF17 is a negative regulator of epithelialmesenchymal transition and metastasis in breast cancer. Nat Cell Biol. 2009;11:1297–1304. doi: 10.1038/ncb1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Z, Chen H, Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009;69:3819–3827. doi: 10.1158/0008-5472.CAN-08-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- Fleming FJ, Myers E, Kelly G, Crotty TB, McDermott EW, O'Higgins NJ, Hill AD, Young LS. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol. 2004;57:1069–1074. doi: 10.1136/jcp.2004.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AJ, Russell RC, Roche O, Burry TN, Fish JE, Chow VW, Kim WY, Saravanan A, Maynard MA, Gervais ML, Sufan RI, Roberts AM, Wilson LA, Betten M, Vandewalle C, Berx G, Marsden PA, Irwin MS, Teh BT, Jewett MA, Ohh M. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol Cell Biol. 2007;27:157–169. doi: 10.1128/MCB.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of Ecadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, Chen ZQ, Liu XP, Xu ZD. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15:2657–2665. doi: 10.1158/1078-0432.CCR-08-2372. [DOI] [PubMed] [Google Scholar]
- Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol. 2009;29:3722–3737. doi: 10.1128/MCB.01164-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, Radisky DC, Ferrone S, Knutson KL. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SA, Graham TA, Schier S, Wright NA, Alison MR. Stem cells and solid cancers. Virchows Arch. 2009;455:1–13. doi: 10.1007/s00428-009-0783-1. [DOI] [PubMed] [Google Scholar]
- Kuperwasser C, Dessain S, Bierbaum BE, Garnet D, Sperandio K, Gauvin GP, Naber SP, Weinberg RA, Rosenblatt M. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 2005;65:6130–6138. doi: 10.1158/0008-5472.CAN-04-1408. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, Liu W, Stivers D, Baggerly K, Carey M, Lluch A, Monteagudo C, He X, Weigman V, Fan C, Palazzo J, Hortobagyi GN, Nolden LK, Wang NJ, Valero V, Gray JW, Perou CM, Mills GB. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Nat Acad Sci USA. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Puré E, Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemnessinhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, Dirbas FM, Somlo G, Pera RA, Lao K, Clarke MF. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]