Abstract
This study tested whether children’s eating behavior and parental feeding prompts during a laboratory test meal differ among children born at high risk (HR) or low risk (LR) for obesity and are associated with excess child weight gain. At 4 years of age, 32 HR children (mean maternal prepregnancy BMI = 30.4 kg/m2) and 29 LR children (maternal BMI = 19.6 kg/m2) consumed a test meal in which their eating behavior was assessed, including rate of caloric consumption, mouthfuls/min, and requests for food. Parental prompts for the child to eat also were measured at year 4, and child body composition was measured at ages 4 and 6 years. T-tests, and logistic and multiple regression analyses tested study aims. Results indicated that HR and LR children did not differ in eating rate or parental feeding prompts. Greater maternal BMI, child mouthfuls of food/min, and total caloric intake/min during the test meal predicted an increased risk of being overweight or obese at age 6, whereas greater active mealtime was associated with a reduced risk of being overweight or obese. Regression analyses indicated that only mouthfuls of food/min predicted changes in BMI from 4 to 6 years, and mouthfuls of food/min and gender predicted 2-year changes in sum of skinfolds and total body fat. Thus, a rapid eating style, characterized by increased mouthfuls of food/min, may be a behavioral marker for the development of childhood obesity.
INTRODUCTION
The prevalence of obesity in children has increased threefold in the past 20 years (1,2), a particularly disturbing trend because childhood obesity persists into adult life (3). An attempt to understand the causes of childhood obesity gave rise to the “Infant Growth Study” (IGS), a cohort of infants of overweight mothers and infants of lean mothers who have been followed prospectively throughout childhood (4–9). The design of the study makes it possible to identify familial, behavioral, and metabolic factors contributing to obesity onset in children who were born with or without a familial predisposition to obesity.
Previous reports on this cohort established significant differences in the growth and obesity status of “high risk” (HR) and “low risk” (LR) children in this cohort through age 6 years (4,5,7). At 2 years of age, the size and body composition of HR and LR children did not differ (5). By age 4, the weight, BMI (kg/ m2), and lean body mass of the HR children were significantly greater than those of the LR children, but the groups did not differ in fat mass. By 6 years of age, the fat mass of the HR group had become much greater than that of the LR group (6.7 ± 5.7 kg vs. 3.8 ± 1.2 kg, P < 0.02) while increasing further its difference from the LR group in weight, BMI, and lean body mass (4).
A critical question is how obesity-promoting genes express themselves behaviorally in developing children (10,11). In the IGS cohort, analysis of food records indicated that HR children consumed more soft drinks (including fruit juice) at ages 3, 4, and 5 years and more soda at age 6 than LR children (12). At age 5 years, HR boys consumed more food in the absence of hunger than LR boys (13). On the other hand, the energy density of the children’s free-living diet did not differ between the two groups (8). Few other behavioral phenotypes for obesity have been identified in young children (14,15). One trait that may be pertinent is rate of eating. Drabman et al. (16,17) reported that a rapid eating style was associated with increased adiposity in children. Obese boys (18) and girls (19) ate twice as rapidly as nonobese control subjects. Recently, child eating rate (kcal/min), as assessed by laboratory multi-item meal, was reported to be highly heritable and associated with increased child BMI (20). Interestingly, nutritive sucking rate in infants also was associated with excess weight gain in the first several years of life in the IGS and another cohort (7,21). Children’s satiety recognition and food enjoyment were found to be heritable, and associated with weight status and FTO genotype, in a series of studies by Wardle and colleagues (22–24).
Familial influences on the development of childhood obesity also may occur through the child-rearing practices of the parents. Specifically, parental feeding attitudes and practices may impact the development of child eating practices (25). A review of 19 studies indicated that parental restriction of child eating was the only parental behavior that was consistently associated with child eating or weight status (26). The authors concluded that there is a pressing need for observational methods to measure eating styles and food intake, for prospective designs, and for the use of samples that are genetically informative regarding obesity risk status. For example, recent studies indicated that the association between maternal restriction of child eating and excess child weight gain only was present among children who were born at HR for obesity (6) or who have obese mothers (27).
The present study had two main aims. The first aim was to test whether child rate of eating and parental prompts during a laboratory meal at age 4 years differ among children born at HR and LR for obesity, in the IGS cohort. The second aim was to test whether these same child eating and parental feeding measures were associated with an increased risk of child overweight status at age 6 years. We hypothesized that children with more rapid eating during a test meal, and whose parents displayed more eating prompts, would more likely be overweight at age 6 and would have greater increases in BMI from year 4 to 6.
METHODS AND PROCEDURES
Participants
Subjects were 61 of the 71 6-year-old children enrolled in the IGS cohort, as described elsewhere (4), consisting of 32 HR (16 boys, 16 girls) and 29 LR (15 boys, 14 girls) participants. Mothers of the HR subjects had a prepregnancy BMI of 30.4 ± 4.2 kg/m2, whereas mothers of the LR subjects had a prepregnancy BMI of 19.6 ± 1.12 kg/m2. All children were whites, born without complications following an uneventful, full-term pregnancy. They did not differ in any measure from the 10 children who did not participate in the present study. Additional details on recruitment and procedures of the IGS cohort are provided elsewhere 4–7,28.
Measurements
Behavioral assessments
Parent and child behavioral measures were assessed in the laboratory via a videotaped test meal at 4 years of age using the mealtime observation scale (29). Specifically, after at least 4 h without eating (confirmed by parent report), the children were served a weighed test meal at about 5 pm. Parents (48 mothers and 12 fathers) were present at the meal, during which they were asked to interact with their children as they usually did, not to mention the camera, and not to eat any of the food. Child and parent behaviors were videotaped by a concealed camera. There were no differences in measures of children accompanied by their mothers from those accompanied by their fathers. The meal continued as long as the subject desired and lasted as long as 40 min. Following the meal, the remaining food was weighed, and the amount and nutritional content of the ingested food was calculated (i.e., by subtracting postmeal weights from premeal weights).
The following foods were served to children in the specified weights (in grams): spaghetti (105), marina sauce (120), hamburger (115), hamburger bun (22), roasted skinless chicken breast (75), macaroni and cheese (138), French fries (65), yellow corn (103), peas (100), carrot and celery sticks (55), applesauce (167), grapes (138), mandarin orange (138), cupcake (49), Teddy Grahams (50), chocolate pudding (255), skim milk (255), 2% milk (255), whole milk (258), apple juice (130), fruit punch (129), butter (10), grape jelly (15), mixed fruit (10), low-calorie ranch dressing (66), regular ranch dressing (63), ketchup (47), mustard (12), and mayonnaise (14). The meals were designed to be familiar foods and typical of the children’s usual meals except for their size, which was far larger than usual in order to present several different types of food. Parents completed an eight-item questionnaire evaluation of the “typicality” of the laboratory meal relative to their child’s general meal experience. Typicality ratings of the laboratory meal included assessments of the amount, type, and presentation of food consumed by the child, the length and duration of the meal, and the child’s mealtime behavior. Parents’ assessments of each of these typicality domains were recorded on a five-point scale, with “5” being a “typical meal item.” The mean score of seven of the eight items on the typicality scale was 3.7 ± 1 out of a possible five, indicating that parents rated the meal as typical. Only one item (presentation of the food) was rated as atypical, with a score of 2.4 ± 1.3. The goals of the videotaped meal were met. All other variables were rated as typical.
The videotapes of the meals were coded by three research assistants using a standard method. Five behavior measures were coded during four viewings of each videotape: parental prompts (for the child to eat), parental discouragements (of eating), child’s requests for and refusals of food, and the child’s number of mouthfuls of food. Operational definitions for each behavior were adapted from those used in the mealtime observation scale (29). Previous studies have expressed behavior both as frequencies of types as well as rates of behavior (30). Accordingly, we computed both the total number of behaviors within each category, and the rate of each category in behavior/min. In addition, calories consumed during the standardized meal were calculated (total kcal) as was the duration of the meal (min) and a rate of consumption (kcal/min). Inter-rater reliability of the coding was calculated during each phase of the study in order to preclude drift over time. The reliability of mouthfuls was very high (98.1%) and that of maternal prompts and discouragements almost as high (95.8%) whereas that of the children’s requests and refusals were somewhat lower (85.4%).
In addition to the laboratory measures of child eating behavior, their average daily energy intake at 4 years of age was calculated from weighed 3-day records of food intake kept by the parents, as described elsewhere (8,28,31).
Body composition assessments
At ages 4 and 6 years, the following anthropometric and body composition measurements were taken at the Nutrition and Growth Laboratory of the Children’s Hospital of Philadelphia: height and weight, from which BMI was calculated. Children at or above the 85th percentile according to the Centers for Disease Control and Prevention growth charts were considered overweight or obese (32); sum (mm) of the biceps, triceps, subscapular, and suprailiac skinfold thicknesses; total and percentage fat mass (kg) and lean mass as measured by dual-energy X-ray absorptiometry (4).
Statistical analyses
Descriptive statistics are presented as means and standard deviations. To test aim 1, t-tests compared the two risk groups in terms of anthropometric measurements, parental feeding behaviors, and child eating behaviors. To test aim 2, a series of analyses were conducted to identify the predictors of child overweight status at age 6 years and weight gain from ages 4 to 6 years. These analyses were conducted on all children and did not stratify by risk group. First, univariable logistic regression analyses were conducted using prepregnancy maternal BMI, year 4 parental feeding behaviors, and year 4 child eating behaviors as predictors of child overweight status at age 6 years. The outcome was overweight status, which was coded as 0 = healthy weight (BMI <85th percentile) and 1 = overweight or obese (BMI ≥85th percentile). Second, Pearson’s correlations and multivariable regression models were conducted for continuous outcomes. Zero-order correlations assessed the relationships among year 4 parent measures, year 4 child behavioral measures, and changes in child BMI, waist circumference, total fat mass, and total lean mass from year 4 to 6. Due to the large number of associations being tested, only those that were moderately to highly correlated (i.e., r > 0.40, P < 0.005) were presented. Next, multivariable regression using the stepwise procedure simultaneously tested all parent and child behavioral measures, child gender, prepregnancy maternal BMI, and paternal BMI as predictors of 2-year changes in child BMI, waist circumference, total body fat measures, and lean body mass. For these regression analyses, an α level of 0.05 was considered significant. All analyses were performed using the statistical software package SAS, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Comparison of LR and HR families
Descriptive statistics for LR and HR children and their parents are presented in Table 1. The BMI of the 32 mothers of HR children (30.4 kg/m2) was significantly greater than that of the 29 mothers of LR children (19.6 kg/m2, P < 0.001). At ages 4 and 6 years, HR children weighed significantly more and had a significantly greater BMI than LR children. At age 4 years, HR children had significantly greater total lean mass (P = 0.01) and waist circumference (P = 0.03) than the LR children. At age 6 years, HR children had a significantly greater total skinfold thickness (P = 0.02), total body fat (P = 0.02), total % body fat (P = 0.03), and waist circumference (P = 0.01) than LR children. From ages 4 to 6, HR children displayed a significantly greater gain in total body fat (2.25 kg vs. 0.56 kg, P = 0.03) and % body fat (2.2% vs. −1.3%, P = 0.02) than LR children. HR and LR children did not significantly differ on any of the child and parent behavioral measures at child aged 4 years.
Table 1.
Child anthropometrics, and child and parent behavioral measures by risk group
| Variable | Low risk (N = 29) | High risk (N = 32) | t-Test P value |
|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | ||
| Maternal BMI at child’s birth | 19.6 (1.12) | 30.4 (4.20) | <0.0001 |
| Paternal BMI at child’s birth | 24.92 (2.49) | 27.25 (4.06) | 0.009 |
| Child anthropometrics | |||
| Height at 4 years (cm) | 101 (3.17) | 103 (3.82) | 0.07 |
| Height at 6 years (cm) | 115 (3.90) | 117 (4.85) | 0.06 |
| Weight at 4 years (kg) | 15.92 (1.36) | 17.51 (2.74) | 0.006 |
| Weight at 6 years (kg) | 20.04 (1.99) | 23.55 (6.44) | 0.006 |
| BMI at 4 years | 15.64 (0.99) | 16.57 (2.00) | 0.03 |
| BMI at 6 years | 15.12 (0.99) | 16.99 (3.56) | 0.008 |
| Change in child BMI from year 4 to 6 | −0.53 (0.76) | 0.41 (2.03) | 0.02 |
| Body fat variables | |||
| Total skinfold thickness 4 years (mm) | 25.0 (7.3) | 29.3 (10.9) | 0.13 |
| Total skinfold thickness 6 years (mm) | 23.7 (7.5) | 36.9 (28.2) | 0.02 |
| Change in skinfold from year 4 to 6 (mm) | −0.71 (3.6) | 5.91 (16.9) | 0.09 |
| Total lean mass 4 years (kg) | 12.17 (0.86) | 13.05 (1.46) | 0.01 |
| Total lean mass 6 years (kg) | 15.59 (1.28) | 16.57 (2.07) | 0.05 |
| Change in total lean mass from year 4 to 6 (kg) | 3.22 (0.624) | 3.40 (0.80) | 0.39 |
| Total body fat 4 years (kg) | 3.23 (0.84) | 3.71 (1.6) | 0.19 |
| Total body fat 6 years (kg) | 3.79 (1.2) | 6.55 (5.6) | 0.02 |
| Change in total body fat from year 4 to 6 (kg) | 0.56 (0.7) | 2.25 (3.5) | 0.03 |
| Total % body fat 4 years | 20.3 (3.4) | 21.0 (6.23) | 0.64 |
| Total % body fat 6 years | 18.8 (4.5) | 24.5 (11.6) | 0.03 |
| Change in % body fat 4–6 | −1.3 (2.6) | 2.24 (6.2) | 0.02 |
| Waist circumference 4 years (cm) | 52.2 (2.6) | 54.6 (5.1) | 0.03 |
| Waist circumference 6 years (cm) | 55.0 (3.0) | 59.8 (9.2) | 0.01 |
| Change waist circumference 4–6 (cm) | 2.6 (2.0) | 5.0 (5.3) | 0.05 |
| Child and parent eating behaviors—4 yearsa | |||
| Total daily energy intake (kcal) | 1,240 (318) | 1,243 (300) | 0.98 |
| Total meal energy intake (kcal/min) | 11.9 (4.0) | 13.9 (6.5) | 0.18 |
| Total meal energy intake (kcal) | 430 (136) | 463 (195) | 0.47 |
| Parental prompts/min | 3.20 (1.2) | 2.9 (1.5) | 0.41 |
| Parental discouragements/min | 0.22 (0.3) | 0.16 (0.13) | 0.25 |
| Child refusals/min | 0.47 (0.41) | 0.50 (0.39) | 0.80 |
| Child requests/min | 0.53 (0.25) | 0.56 (0.28) | 0.79 |
| Child mouthfuls/min | 2.16 (0.96) | 2.60 (1.08) | 0.10 |
| Total active mealtime | 37.8 (11.8) | 36.9 (15.7) | 0.82 |
| Gender | n (%) | n (%) | 0.89 |
| Boy | 15 (48) | 16 (52) | |
| Girl | 14 (47) | 16 (53) |
Variables were measured as part of an ad libitum laboratory feeding protocol, except for total daily energy intake, which was estimated from 3-day food records.
Predictors of child overweight status at age 6
At year 6, 10 of the 32 HR children (31.3%) were overweight or obese vs. only 1 of 29 LR children (3.4%). The mean (s.d.) child BMI at year 4, child and parent behaviors at year 4, and changes in child BMI and body composition measures from 4 to 6 years are presented by child overweight status at year 6 in Table 2. We note that mean BMI of mothers of children who were overweight/obese vs. normal weight at age 6 (i.e., 30.98 kg/m2 vs. 24.03 kg/m2) (see Table 2) differs from the mean BMIs of mothers of HR vs. LR children (see Table 2), as these are different comparisons. Interestingly, 10 out of the 11 overweight/obese youth at age 6 came from the HR group, compared to only 12 out of the 50 normal-weight youth.
Table 2.
Child and parent behavioral measures by overweight status at age 6 years
| N | Overweight (BMI >85th %) |
Normal weight (BMI <85th %) |
Univariable logistic P value |
|---|---|---|---|
| 11 | 50 | ||
| Continuous variablesa | Mean (s.d.) | Mean (s.d.) | |
| Child BMI (kg/m2), year 4 | 18.6 (1.7) | 15.6 (1.1) | 0.001 |
| Absolute change in BMI (kg/m2) from year 4 to 6 | 2.2 (2.6) | −0.52 (0.73) | 0.002 |
| Change in skinfold from year 4 to 6 (mm) | 23.3 (19.4) | −1.6 (4.0) | 0.03 |
| Change in total lean mass from year 4 to 6 (kg) | 3.97 (1.0) | 3.18 (0.58) | 0.03 |
| Change in total fat from year 4 to 6 (kg) | 6.0 (4.3) | 0.540 (0.6) | 0.01 |
| Change in % fat 4–6 | 8.7 (7.1) | −1.1 (2.5) | 0.005 |
| Change in waist circumference from year 4 to 6 (cm) | 9.6 (6.7) | 2.7 (2.2) | 0.002 |
| Maternal BMI at child’s birth | 30.98 (5.50) | 24.03 (5.76) | 0.003 |
| Paternal BMI at child’s birth | 27.92 (4.80) | 25.78 (3.19) | 0.08 |
| Total daily energy intake (kcal), year 4 | 1,261 (376) | 1,237 (296) | 0.84 |
| Child eating and parental behavior during test meal at year 4 | |||
| Total meal energy intake/min (kcal/min) | 16.58 (6.36) | 12.22 (5.05) | 0.04 |
| Total meal energy intake (kcal) | 438 (200) | 449 (163) | 0.85 |
| Parental prompts/min | 3.47 (1.42) | 2.95 (1.35) | 0.28 |
| Parental discouragements/min | 0.20 (0.13) | 0.18 (0.22) | 0.83 |
| Child refusals/min | 0.60 (0.49) | 0.46 (0.38) | 0.34 |
| Child requests/min | 0.68 (0.23) | 0.52 (0.26) | 0.10 |
| Child mouthfuls/min | 3.12 (1.17) | 2.24 (0.95) | 0.02 |
| Total active mealtime (min) | 28.53 (14.4) | 39.19 (13.19) | 0.03 |
| Categorical variables | n (%) | n (%) | |
| Risk group | |||
| Low | 1 (3) | 28 (97) | 0.02 |
| High | 10 (31) | 22 (69) | |
| Gender | |||
| Boy | 3 (10) | 28 (90) | 0.10 |
| Girl | 8 (27) | 22 (73) | |
Unless otherwise noted, measures were summarized per min.
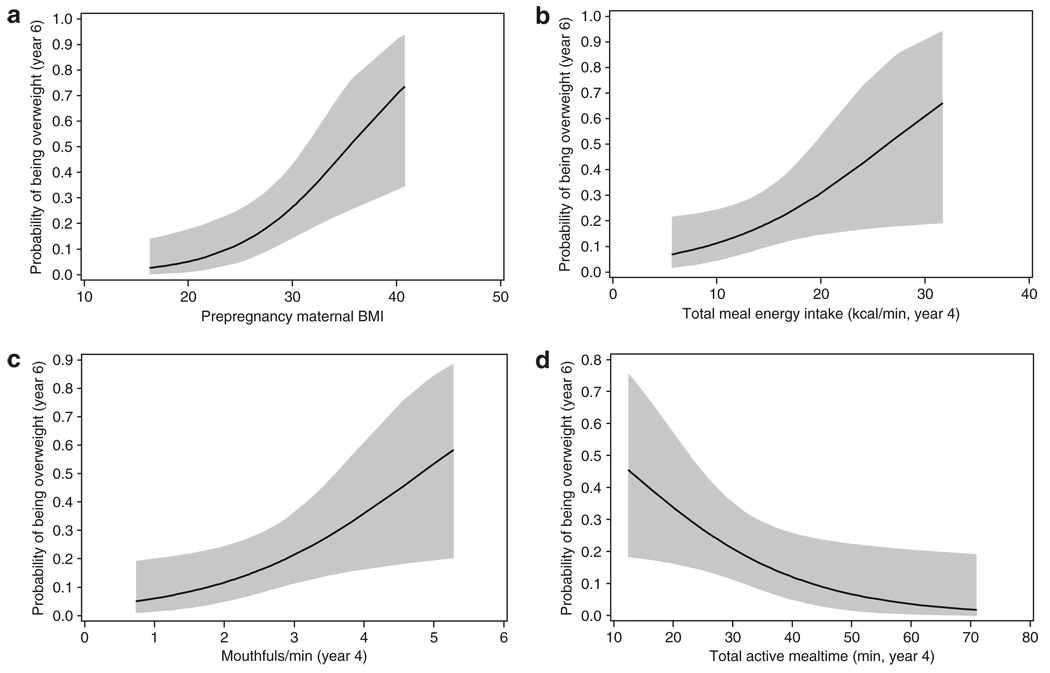
Univariable logistic regression analyses indicated that prepregnancy maternal BMI (P = 0.003), year 4 child energy intake in the laboratory (kcal/min) (P = 0.04), year 4 child mouthfuls (mouthfuls/min) (P = 0.02), and total active mealtime (P = 0.03) were significant predictors of child’s overweight status at age 6 years (Table 2). Specifically, increasing maternal BMI, child kcal/min intake at year 4, and child mouthfuls/min at year 4 were associated with an increased probability of child overweight or obesity at year 6 (see Figure 1a–c); in contrast, greater total active mealtime was associated with a reduced probability of child overweight or obesity at age 6 (Figure 1d). When using child risk status as the predictor variable instead of maternal BMI, comparable results were found. Of the 11 children who were overweight or obese at age 6, 10 were from the HR group and 1 was from the LR group (P = 0.006).
Figure 1.
Probability of child overweight or obesity at age 6 years as a function of (a) maternal BMI, (b) total meal energy intake/min, (c) mouthful of food/min, and (d) total active mealtime. These four were the only significant predictors in univariable logistic regression models where outcome was overweight status (yes/no) at age 6 years.
In an exploratory analysis restricted to the HR group, we compared children who were normal weight vs. overweight/ obese at year 6. There was a nonsignificant trend for two variables: child mouthfuls/min (P = 0.06) and active mealtime (P = 0.08). Thus, HR children who were overweight/obese compared to normal weight at year 6 tended to eat more rapidly (3.21 ± 1.21 mouthfuls/min vs. 2.35 ± 0.94 mouthfuls/min) and have shorter meals (28.86 ± 15.27 min vs. 40.23 ± 15.27 min).
Correlation and regression analyses
Associations among the behavioral variables that were significant and at least moderate effects (i.e., r ≥0.40) are presented in Table 3. Child mouthfuls/min was significantly correlated with change in BMI (r = 0.30, P = 0.02), total skinfold thickness (r = 0.32, P = 0.04), total fat (r = 0.35, P = 0.02), and % fat (r = 0.37, P = 0.01) from ages 4 to 6 years. Total daily energy intake (kcal) from food records was positively correlated with change in waist circumference from 4 to 6 years (r = 0.35, P = 0.02). In addition, maternal BMI at child’s birth was significantly correlated with paternal BMI at child’s birth (r = 0.42, P = 0.0009).
Table 3.
Child and parent eating behaviors at child age 4 that are significantly correlated at Pearson’s r ≥0.40
| Variable 1 with | Variable 2 | Pearson r |
|---|---|---|
| Maternal BMI at child’s birth | Paternal BMI at child’s birth | 0.42 |
| Active mealtime (min) | Total meal energy intake/min (kcal) | −0.51 |
| Total meal energy intake (kcal) | 0.50 | |
| Parental prompts | −0.67 | |
| Child refusals | −0.55 | |
| Child requests | −0.54 | |
| Parental Discouragements | Parental prompts | 0.46 |
| Child requests | 0.46 | |
| Total meal energy intake (kcal) | Child active mealtime | 0.50 |
| Total meal energy intake/min (kcal) | 0.40 | |
| Total meal energy intake/min (kcal/min) | Parental prompts | 0.48 |
| Child requests | 0.50 | |
| Child mouthfuls | 0.62 | |
| Child active mealtime | −0.51 | |
| Total daily energy intake (kcal) | 0.42 | |
| Total meal energy intake (kcal) | 0.40 | |
| Child mouthfuls | Total meal energy intake/min (kcal) | 0.62 |
| Parental prompts | Total meal energy intake/min (kcal) | 0.48 |
| Parental discouragements | 0.46 | |
| Child refusals | 0.73 | |
| Child requests | 0.55 | |
| Child active mealtime | −0.67 | |
| Child refusals | Parental prompts | 0.73 |
| Child active mealtime | −0.55 | |
| Child requests | Total meal energy intake/min (kcal) | 0.50 |
| Parental prompts | 0.55 | |
| Parental discouragements | 0.46 | |
| Active mealtime | −0.54 |
All P values ≤0.005; measurements were summarized per min, unless otherwise noted.
Stepwise multiple regression models were conducted to identify predictors of change in child body composition measures from ages 4 to 6 years. Mouthfuls/min was the only significant predictor of change in BMI (β = 0.50, P = 0.03), with each additional mouthful of food/min ingested associated with an additional ½-unit BMI gain over 2 years. Mouthfuls/min of food ingested, and gender, were the only significant predictors of changes in child total skinfold thickness, total body fat, and total % body fat (P < 0.04 for all) (Table 4). Girls showed greater increases in body fat measures than boys. There were no significant predictors of change in total lean mass or in waist circumference.
Table 4.
Results of stepwise regression analyses testing predictors of change in BMI, change in total skinfold thickness, total body fat, and total percent body fat from ages 4 to 6 years
| Outcome variable |
Intercept | Gender | Mouthfuls/min | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β (s.e.) | P value | β (s.e.) | Partial R2 | P value | β (s.e.) | Partial R2 | P value | ||
| Change in | |||||||||
| BMI | −1.19 (0.53) | 0.03 | Not significant | 0.50 (0.22) | 8.8% | 0.03 | |||
| Total skinfold | −12.6 (1.02) | 0.03 | 8.72 (4.04) | 10.2% | 0.04 | 4.89 (2.04) | 11.4% | 0.02 | |
| thickness | |||||||||
| Total body fat | −1.60 (1.85) | 0.12 | 1.63 (7.70) | 9.1% | 0.04 | 0.96 (3.56) | 13.7% | 0.01 | |
| % Body fat | −5.46 (1.85) | 0.005 | 3.94 (1.39) | 13.9% | 0.008 | 1.75 (0.64) | 13.9% | 0.01 | |
DISCUSSION
The main finding from this study was that children’s rate of eating at 4 years of age, specifically, mouthfuls of food/min at a single laboratory test meal, predicted overweight status at age 6 years and excess weight gain from ages 4 to 6 years. In multiple regression analyses, the effect of child mouthfuls/min was independent of maternal BMI that, as expected, predicted changes in child body fat indexes. These findings indicate the importance of rapid eating rate in the development of childhood obesity, consistent with several prior studies. Drabman et al. (16,17) reported that a rapid eating style was associated with adiposity in children. Obese boys (18) and girls (19) ate twice as rapidly as nonobese control subjects in other reports. Eleven-year-old obese children ate much more rapidly than nonobese children and, unlike nonobese children, their eating rate did not decelerate during the course of the meal (33). This lack of deceleration of eating rate suggests an inadequate satiety signal or inadequate response to that signal.
A rapid eating style in infancy also has been found to be a marker for excess weight gain (7). In the IGS cohort, nutritive sucking rate at 3 months of age was significantly greater among HR than LR children, and increased sucking rate predicted increased weight gain up to 24 months of age (7). In a separate prospective cohort study of infants, rapid sucking rate predicted excess weight gain up to age 6 years (21,34). In a recent UK-based study, child eating rate was found to be highly heritable and positively associated with child overweight status (20). In conjunction with the present findings, these results implicate rapid eating rate as a behavioral precursor to obesity in childhood. Additional prospective studies using larger samples are needed to confirm this causal pathway, as opposed to rapid eating being a correlate or consequence of rapid weight gain.
When comparing children who actually became overweight or obese at age 6 years, 32% of HR children had a BMI ≥85th percentile compared with only 3.4% of LR children. Thus, in this subsample of the IGS families who participated in the video-recorded meals at age 4 years, the effect of risk group on overweight status was strong, which is consistent with our previous report (4). What is noteworthy from the present study was the differences in the preceding eating rate and energy intake at 4 years of age. Overweight and obese children at age 6 had 3.12 (1.17) mouthfuls/min compared with 2.24 (0.95) for those who were of normal weight. Similarly, in measures of total calories consumed/min, overweight and obese children consumed 16.58 (6.36) kcal/min compared with the normal-weight child who consumed 12.22 (5.05) kcal/min. Thus, the child’s eating more vigorously in just one test meal and maternal BMI predicted increased weight gain (increased in BMI) over a 2-year period. Similarly, childhood mouthfuls/min during the test meal predicted body fat at age 6.
Curiously, despite the prospective effect of child eating rate on child anthropometric measures, there was no significant effect of risk group on eating rate measures at year 4. Thus, a primary hypothesis was not supported. Although this may reflect a “true” null finding, inspection of means in Table 2 suggests that the means were in the expected directions and could not attain significance given our sample size. Specifically, there was a trend for mouthfuls of food/min (2.16 vs. 2.60, P = 0.10) and kcal/min (11.9 vs. 13.9, P = 0.18) to be greater among HR than LR children. A larger sample size may have provided the power for these mean differences to achieve significance.
To explore this further, a post hoc power analysis was conducted based on the data in Table 2. This analysis estimated the number of children per risk group that would have been needed in this study, and may be necessary in future investigations, to detect significant risk group differences in eating rate and parental prompts. Assuming a two-tailed significance test and α = 0.05, the number of youth per risk group needed to attain significance with 80% power would be 86 (mouthfuls/min), 116 (kcal/min), 235 (discouragements/ min), and 323 (encouragements/min). Thus, the lack of support for our primary hypotheses may have been due, in part, to low power.
Another main finding from the present study was the significant intercorrelations among child feeding behaviors and parental prompts to eat during the test meal. Parental prompts to eat was associated with increased total energy intake/min at the test meal (r = 0.48), increased child requests for food (r = 0.55), and a shorter child meal duration (r = −0.67). Similar findings were reported by Drucker et al. (30), who found that greater parental encouragements to eat, discouragements to eat, and total prompts were associated with increased total energy intake by young children at a laboratory buffet meal. In a study of 4–8-year-old children whose home meals with parents were directly observed, greater parental prompts to eat were associated with increased food ingestion by children (35). In yet another report, Klesges et al. (36) found that total parental food prompts during meals was associated with increased child eating time. These studies suggest that a more appetitive or vigorous eating style by children is associated with greater feeding prompts by parents during the meal, although cause and effect cannot be determined. Future research should address this question.
It is noteworthy that parental eating prompts, despite being associated with child eating rate, and food requests and refusals, was not a risk factor for child overweight at age 6 years or excess weight gain from 4 to 6 years in the present study. It may be the case that child mouthfuls/min and calories eaten/min are stronger determinants of future weight status than feeding prompts by parents. It may also be the case that parental prompts are secondary to the child eating traits with which they are associated. For example, parental restriction of child eating is elicited by child overeating and parental perceptions that their child is overweight (37). Indeed, maternal restrictive feeding at child age 5 years predicted excess child BMI at age 7 years in the IGS cohort, but only among HR children (6). Future studies need to identify parental feeding behaviors that have a casual influence on the development of childhood obesity.
Girls showed greater accretions in fat mass compared to boys in the present study, consistent with other reports in the literature (38,39). Future analyses of this cohort will need to continue to model sex effects in analyses. Controlling for sex, however, the effect of child eating rate was significant in the regression analyses, suggesting that the effect was not simply due to potential sex differences in eating styles that may have impacted on results.
A noteworthy finding in our study was that children’s total meal energy intake/min was negatively correlated with active mealtime (i.e., a shorter duration) and positively associated with total daily energy intake from the 3-day food records. Thus, children eating faster during the test meal consumed more calories as recorded from the 3-day food records kept by parents. Interestingly, these children also spontaneously requested more food during the test meal.
The present findings should be interpreted in light of study strengths and limitations. Strengths include use of a prospective cohort design, investigation of children born with and without a familial predisposition to obesity, and precise measurement of child eating and parental feeding behaviors that were evaluated under controlled laboratory conditions. Limitations include a relatively smaller sample size and investigation of a single ethnic group. In addition, physical activity was not measured at year 4 and therefore was not included as a predictor variable. Total energy expenditure was not examined in the present study, although, in prior analyses of this cohort, HR and LR children did not differ in total energy expenditure (5,7). Finally, behavioral measures at year 6, such as food intake outside of the home and physical activity, were not obtained.
In summary, the vigorous feeding style among overweight children described in this study includes more rapid eating (i.e., mouthfuls/min) and greater food consumption (i.e., kcal/min). This eating style may be a behavioral phenotype for a genetically based predisposition for the development of obesity. Future studies are needed to understand the potential genetic and physiological factors, such as hormonal or neuro-biological factors, associated with this vigorous feeding style in young children at HR for obesity. Rapid eating could relate to the construct of food reward (40). From a clinical viewpoint, future intervention research should address strategies for targeting a vigorous eating style in obese prone children. Possibilities include the promotion of less energy-dense foods as a target for vigorous eating (41), satiety training procedures (42), or teaching a slowing eating rate to help minimize excessive weight gain. The development of clinically effective protocols to assist parents of children at risk for obesity with their eating behavior is also needed.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grant DK068899, the clinical and Translational Research center (grant RR00240), and the Nutrition Center of the Children’s Hospital of Philadelphia.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz RI, Stallings VA, Maislin G, Stunkard AJ. Growth of children at high risk of obesity during the first 6 y of life: implications for prevention. Am J Clin Nutr. 2005;81:140–146. doi: 10.1093/ajcn/81.1.140. [DOI] [PubMed] [Google Scholar]
- 5.Stunkard AJ, Berkowitz RI, Schoeller D, Maislin G, Stallings VA. Predictors of body size in the first 2 y of life: a high-risk study of human obesity. Int J Obes Relat Metab Disord. 2004;28:503–513. doi: 10.1038/sj.ijo.0802517. [DOI] [PubMed] [Google Scholar]
- 6.Faith MS, Berkowitz RI, Stallings VA, et al. Parental feeding attitudes and styles and child body mass index: prospective analysis of a gene-environment interaction. Pediatrics. 2004;114:e429–e436. doi: 10.1542/peds.2003-1075-L. [DOI] [PubMed] [Google Scholar]
- 7.Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, not energy output, is a determinant of body size in infants. Am J Clin Nutr. 1999;69:524–530. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- 8.Kral TV, Berkowitz RI, Stunkard AJ, et al. Dietary energy density increases during early childhood irrespective of familial predisposition to obesity: results from a prospective cohort study. Int J Obes (Lond) 2007;31:1061–1067. doi: 10.1038/sj.ijo.0803551. [DOI] [PubMed] [Google Scholar]
- 9.Kral TVE, Stunkard AJ, Berkowitz RI, Stallings VA, Faith MS. Eating frequency is inversely related to weight status in a cohort of children born at different risk for obesity. Obesity (Silver Spring) 2006;14 Suppl:460-P. A146. [Google Scholar]
- 10.Faith MS. Development and modification of child food preferences and eating patterns: behavior genetics strategies. Int J Obes (Lond) 2005;29:549–556. doi: 10.1038/sj.ijo.0802981. [DOI] [PubMed] [Google Scholar]
- 11.Faith MS, Keller KL. Genetic architecture of ingestive behavior in humans. Nutrition. 2004;20:127–133. doi: 10.1016/j.nut.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Kral TV, Stunkard AJ, Berkowitz RI, et al. Beverage consumption patterns of children born at different risk of obesity. Obesity (Silver Spring) 2008;16:1802–1808. doi: 10.1038/oby.2008.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faith MS, Berkowitz RI, Stallings VA, et al. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obesity (Silver Spring) 2006;14:131–138. doi: 10.1038/oby.2006.16. [DOI] [PubMed] [Google Scholar]
- 14.Wardle J, Guthrie C, Sanderson S, Birch L, Plomin R. Food and activity preferences in children of lean and obese parents. Int J Obes Relat Metab Disord. 2001;25:971–977. doi: 10.1038/sj.ijo.0801661. [DOI] [PubMed] [Google Scholar]
- 15.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42:963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 16.Drabman RS, Cordua GD, Hammer D, Jarvie GJ, Horton W. Developmental trends in eating rates of normal and overweight preschool children. Child Dev. 1979;50:211–216. [PubMed] [Google Scholar]
- 17.Drabman RS, Hammer D, Jarvie GJ. Eating styles of obese and nonobese black and white children in a naturalistic setting. Addict Behav. 1977;2:83–86. doi: 10.1016/0306-4603(77)90023-5. [DOI] [PubMed] [Google Scholar]
- 18.Waxman M, Stunkard AJ. Caloric intake and expenditure of obese boys. J Pediatr. 1980;96:187–193. doi: 10.1016/s0022-3476(80)80800-6. [DOI] [PubMed] [Google Scholar]
- 19.Waxman M. Fat Families and Thick Description: A Naturalistic Study of Obese Girls and Their Nonobese Sisters. Unpublished Doctoral Dissertation. University of Pennsylvania; 1988. [Google Scholar]
- 20.Llewellyn CH, van Jaarsveld CH, Boniface D, Carnell S, Wardle J. Eating rate is a heritable phenotype related to weight in children. Am J Clin Nutr. 2008;88:1560–1566. doi: 10.3945/ajcn.2008.26175. [DOI] [PubMed] [Google Scholar]
- 21.Agras WS, Kraemer HC, Berkowitz RI, Hammer LD. Influence of early feeding style on adiposity at 6 years of age. J Pediatr. 1990;116:805–809. doi: 10.1016/s0022-3476(05)82677-0. [DOI] [PubMed] [Google Scholar]
- 22.Carnell S, Haworth CM, Plomin R, Wardle J. Genetic influence on appetite in children. Int J Obes (Lond) 2008;32:1468–1473. doi: 10.1038/ijo.2008.127. [DOI] [PubMed] [Google Scholar]
- 23.Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr. 2008;88:22–29. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- 24.Wardle J, Carnell S, Haworth CM, et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3643. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 25.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–549. [PubMed] [Google Scholar]
- 26.Faith MS, Scanlon KS, Birch LL, Francis LA, Sherry B. Parent-child feeding strategies and their relationships to child eating and weight status. Obes Res. 2004;12:1711–1722. doi: 10.1038/oby.2004.212. [DOI] [PubMed] [Google Scholar]
- 27.Francis LA, Birch LL. Maternal weight status modulates the effects of restriction on daughters’ eating and weight. Int J Obes (Lond) 2005;29:942–949. doi: 10.1038/sj.ijo.0802935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kral TV, Stunkard AJ, Berkowitz RI, et al. Daily food intake in relation to dietary energy density in the free-living environment: a prospective analysis of children born at different risk of obesity. Am J Clin Nutr. 2007;86:41–47. doi: 10.1093/ajcn/86.1.41. [DOI] [PubMed] [Google Scholar]
- 29.Sanders MR, Patel RK, Le Grice B, Shepherd RW. Children with persistent feeding difficulties: an observational analysis of the feeding interactions of problem and non-problem eaters. Health Psychol. 1993;12:64–73. doi: 10.1037//0278-6133.12.1.64. [DOI] [PubMed] [Google Scholar]
- 30.Drucker RR, Hammer LD, Agras WS, Bryson S. Can mothers influence their child’s eating behavior? J Dev Behav Pediatr. 1999;20:88–92. doi: 10.1097/00004703-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Kral TV, Berkowitz RI, Stunkard AJ, Stallings VA, Faith MS. Caloric beverage consumption in children: a behavioral phenotype for obesity? Appetite. 2006;46:364. [Google Scholar]
- 32.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 33.Barkeling B, Ekman S, Rössner S. Eating behaviour in obese and normal weight 11-year-old children. Int J Obes Relat Metab Disord. 1992;16:355–360. [PubMed] [Google Scholar]
- 34.Agras WS, Kraemer HC, Berkowitz RI, Korner AF, Hammer LD. Does a vigorous feeding style influence early development of adiposity? J Pediatr. 1987;110:799–804. doi: 10.1016/s0022-3476(87)80029-x. [DOI] [PubMed] [Google Scholar]
- 35.McKenzie TL, Sallis JF, Nader PR, et al. BEACHES: an observational system for assessing children’s eating and physical activity behaviors and associated events. J Appl Behav Anal. 1991;24:141–151. doi: 10.1901/jaba.1991.24-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klesges RC, Coates TJ, Brown G, et al. Parental influences on children’s eating behavior and relative weight. J Appl Behav Anal. 1983;16:371–378. doi: 10.1901/jaba.1983.16-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francis LA, Hofer SM, Birch LL. Predictors of maternal child-feeding style: maternal and child characteristics. Appetite. 2001;37:231–243. doi: 10.1006/appe.2001.0427. [DOI] [PubMed] [Google Scholar]
- 38.Ackerman A, Thornton JC, Wang J, Pierson RN, Jr, Horlick M. Sex difference in the effect of puberty on the relationship between fat mass and bone mass in 926 healthy subjects, 6 to 18 years old. Obesity (Silver Spring) 2006;14:819–825. doi: 10.1038/oby.2006.95. [DOI] [PubMed] [Google Scholar]
- 39.Freedman DS, Wang J, Maynard LM, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 40.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105:S98–103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 42.Johnson SL. Improving Preschoolers’ self-regulation of energy intake. Pediatrics. 2000;106:1429–1435. doi: 10.1542/peds.106.6.1429. [DOI] [PubMed] [Google Scholar]