In the left upper quadrant of the abdomen lies the spleen, functioning in two major capacities—filtering and storing blood cells, and acting as an immune tissue, where antibody synthesis occurs and certain pathogens are eliminated. Yet the spleen lacks the gravitas of neighboring organs because we can survive without it, albeit with some inconveniences. Its surgical removal causes modest increases in circulating white blood cells and platelets, diminished responsiveness to certain vaccines, and increased susceptibility to infection with certain bacteria and protozoa. But on page 612 in this issue, the organ gains some new respect, as Swirski et al. (1) show that in the mouse, the spleen serves as a reservoir for immune cells (monocytes) that function in repairing the heart after myocardial infarction.
Circulating monocytes were postulated by Florence Sabin and Charles Doan, over 80 years ago, to play an important role in defense against infection (2), and recent work has confirmed this (3). Indeed, monocytes are essential for immune defense against potentially lethal microbial pathogens (4). Clearance of microbial infection requires dispatching monocytes from their reservoir, thought to be the bone marrow, in adequate numbers toward the site of infection. Monocytes are guided to their proper destination by chemokines, inflammatory cytokines, and adhesion molecules (3). But how adequate numbers of monocytes are mustered for their mission is less well understood. Swirski et al. demonstrate that after induction of inflammation—in their case, by myocardial infarction in mouse—monocytes rapidly exit the spleen, enter the bloodstream, and infiltrate the inflamed myocardium to remodel damaged tissue.
Circulating monocytes are a heterogeneous population (5), and in humans, can be divided into at least two subsets: one that expresses a high amount of the surface protein CD14 and no CD16, and a more mature subset that expresses a lower amount of CD14 and higher amount of CD16. The latter subset shares similarities with tissue macrophages, which are derived from monocytes. In mice, circulating monocytes also can be divided into subsets on the basis of chemokine receptor expression and the presence of the Ly-6C surface protein (3). One subset of murine monocytes (Ly-6Chigh) expresses high amounts of the CCR2 chemokine receptor and the surface protein Ly-6C, and has been implicated in inflammatory responses. The second murine monocyte subset expresses a high amount of the chemokine receptor CX3CR1 and a low amount of Ly-6C (Ly-6Clow) and is similar to macrophages.
Although Ly-6Chigh monocytes contribute to antimicrobial defense, they have also been implicated in the pathogenesis of atherosclerosis (hardening of the arteries). High blood cholesterol increases the frequency both of circulating monocytes and those that infiltrate lesions (plaques) in arterial walls (6). Furthermore, mice in which the CCR2 chemokine receptor or its major ligand, monocyte chemotactic protein-1 [(MCP-1); also called CCL2] are genetically deleted have markedly reduced atherosclerosis (7, 8). Recruitment of monocytes to plaques depends on CCR2-mediated signaling, perhaps in response to MCP-1 produced by cells within the arterial wall. Ly-6Chigh monocytes lacking CCR2 that are “adoptively transferred” into recipient mice do not traffic as efficiently into plaques of hypercholesterolemic mice as do CCR2-expressing Ly-6Chigh monocytes (6, 9). Although the most obvious explanation for this is that monocytes use CCR2-mediated signals to enter the arterial wall, it is also possible that CCR2-deficient monocytes return to the bone marrow and become trapped there, because CCR2 is required for monocytes to emigrate from the bone marrow (10, 11). Adoptive transfer studies with Ly-6Chigh monocytes have shown that they rapidly return to bone marrow in the absence of active recruitment to sites of inflammation (12).
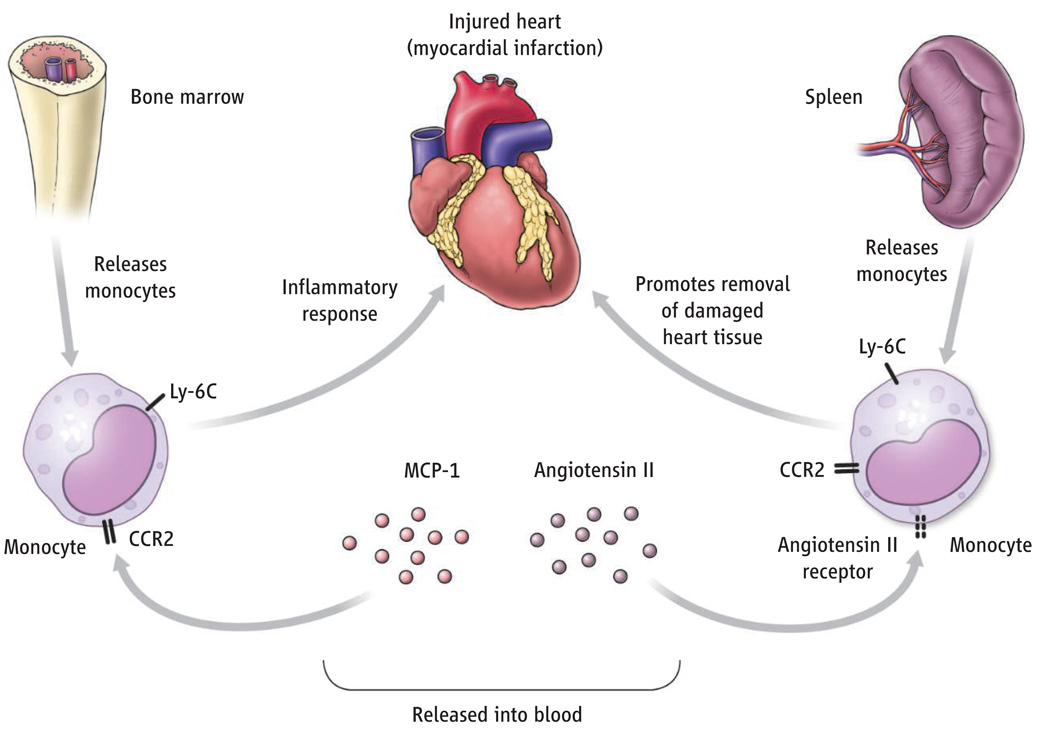
Monocytes have also been implicated in the repair of damaged myocardium after myocardial infarction (13). In this scenario, Ly-6Chigh monocytes are first to infiltrate damaged heart tissue and contribute to the fragmentation and recycling of necrotic and apoptotic tissues, whereas Ly-6Clow monocytes arrive at the scene later to promote revascularization and collagen deposition. Recruitment of Ly-6Chigh monocytes to damaged myocardium is dramatically diminished in CCR2-deficient mice. Swirski et al. used the mouse myocardial infarction model to further characterize Ly-6Chigh monocyte recruitment and identified the subcapsular red pulp of the spleen as a major source of recruited monocytes. Interestingly, angiotensin II, a circulating peptide that regulates vascular tone and blood pressure, promotes CCR2-independent emigration of splenic Ly-6Chigh monocytes into the circulation.
Corticosteroid administration and vigorous physical exertion both result in abrupt increases in the number of circulating white blood cells, including monocytes. It has been assumed in these circumstances that white blood cells are released from endothelial surfaces. The finding by Swirski et al. that an increase in the circulating concentration of angiotensin II after myocardial infarction induces the dimerization of the angiotensin receptor on Ly-6Chigh monocytes reveals a novel mechanism to boost circulating white blood cells in times of stress.
The findings by Swirski et al. raise questions about whether other types of stress or injury draw upon the spleen’s reserve of monocytes as well. In the meantime, although the study does not make the spleen any less dispensable for mammalian survival, it does make this easily dismissed immune system organ seem a bit more purposeful and deserving of recognition.
Calling up the reserves.
In response to heart injury (myocardial infarction), specific subsets of monocytes are recruited from the bone marrow and spleen to remove and repair damaged tissue.
Footnotes
Heart injury triggers the release of monocytes from an unexpected reservoir, the spleen.
References
- 1.Swirski FK, et al. Science. 2009;325:612. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabin FR, Doan CA. J. Exp. Med. 1927;46:627. doi: 10.1084/jem.46.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auffray C, Sieweke MH, Geissmann F. Annu. Rev. Immunol. 2009;27:669. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 4.Serbina NV, Jia T, Hohl TM, Pamer EG. Annu. Rev. Immunol. 2008;26:421. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler-Heitbrock HW, Passlick B, Flieger D. Hybridoma. 1988;7:521. doi: 10.1089/hyb.1988.7.521. [DOI] [PubMed] [Google Scholar]
- 6.Swirski FK, et al. J. Clin. Invest. 2007;117:195. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boring L, Gosling J, Cleary M, Charo IF. Nature. 1998;394:894. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 8.Gu L, et al. Mol. Cell. 1998;2:275. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 9.Tacke F, et al. J. Clin. Invest. 2007;117:185. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serbina NV, Pamer EG. Nat. Immunol. 2006;7:311. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 11.Tsou CL, et al. J. Clin. Invest. 2007;117:902. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varol C, et al. J. Exp. Med. 2007;204:171. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahrendorf M, et al. J. Exp. Med. 2007;204:3037. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]