Abstract
Background
Increased intake of sugar-sweetened beverages and fruit juice has been associated with overweight in children.
Objective
This study prospectively assessed beverage consumption patterns and their relationship with weight status in a cohort of children born at different risk for obesity.
Methods and Procedures
Participants were children born at low risk (n = 27) or high risk (n = 22) for obesity based on maternal prepregnancy BMI (kg/m2). Daily beverage consumption was generated from 3-day food records from children aged 3–6 years and coded into seven beverage categories (milk, fruit juice, fruit drinks, caloric and noncaloric soda, soft drinks including and excluding fruit juice). Child anthropometric measures were assessed yearly.
Results
High-risk children consumed a greater percentage of daily calories from beverages at age 3, more fruit juice at ages 3 and 4, more soft drinks (including fruit juice) at ages 3–5, and more soda at age 6 compared to low-risk children. Longitudinal analyses showed that a greater 3-year increase in soda intake was associated with an increased change in waist circumference, whereas a greater increase in milk intake was associated with a reduced change in waist circumference. There was no significant association between change in intake from any of the beverage categories and change in BMI z-score across analyses.
Discussion
Children’s familial predisposition to obesity may differentially affect their beverage consumption patterns. Future research should examine the extent to which dietary factors may play a role in pediatric body fat deposition over time.
INTRODUCTION
The prevalence of overweight among children has reached an all time high. Recent data from the National Health and Nutrition Examination Survey (2003–2004) indicate that ~14% of children between 2 and 5 years of age and 19% of children between 6 and 11 years of age are overweight (1). Consumption of sugar-sweetened beverages and fruit juice has increased considerably among children and adolescents over the past few decades (2). Causality cannot be determined from these associations although increased consumption of sugar-sweetened beverages may be contributing to excessive weight gain among children. A recent systematic review of cross-sectional, prospective cohort, and experimental studies showed that in most studies increased intake of sugar-sweetened beverages among children and adults was positively associated with increased weight status and obesity (3). For example, Ludwig et al. (4) enrolled 548 ethnically diverse schoolchildren in a prospective, observational study. They found that for each additional serving of a sugar-sweetened drink consumed per day, both BMI and the incidence of obesity increased after adjusting for anthropometric, demographic, dietary, and lifestyle variables. Specifically, for each additional can or glass of a sugar-sweetened drink consumed per day, 11-year-old children were 1.6 times more likely to become overweight. Associations between sugar-sweetened beverage consumption and the prevalence of overweight among children, however, have been inconsistent as noted in recent reviews (5,6).
Besides sugar-sweetened beverages such as soda and fruit drinks, an increased consumption of 100% fruit juice has also been associated with increased energy intake and weight gain in some studies. For example, Dennison et al. showed that a daily consumption of 12 oz of fruit juice by preschool age children was associated with growth extremes such as overweight and short stature (7,8). In a prospective cohort study, Faith et al. (9) found that among children, aged 1–4 years, who were initially either at risk for overweight or overweight, increased fruit juice consumption was associated with excess adiposity gain. Other studies, however, found no association between fruit juice consumption and anthropometric indices (10), height and weight (11,12), and weight change (13) in children.
Data on milk and dairy consumption in relation to children’s weight status are scarce and conflicting. Although some studies show no association between calcium/dairy intake and excessive weight gain and percent body fat in children (14,15), other studies showed either an inverse (16–18) or a positive relationship (19) between these variables.
Beverage consumption patterns may be influenced by genetic factors. In a study with adult twin pairs, De Castro (20) showed that both the amounts and the types of fluids ingested were influenced by heredity and environmental factors. Wardle et al. (21) showed that children, aged 4–5 years, from obese families had higher scores for “desire for drinks” as assessed with the parent-rated Children’s Eating Behavior Scale (22) than did children from lean families eluding to the possibility that familial obesity risk status may be implicated in the development of beverage preferences.
The aims of this longitudinal study were twofold. Aim one was to compare whether children who were born at either high risk or low risk for obesity differ in their beverage consumption patterns (percent energy consumed from beverages, as well as types and amounts of beverages consumed) at ages 3–6 years. Aim two was to test whether changes in beverage consumption patterns from ages 3 to 5 years were associated with changes in children’s BMI z-score and waist circumference from ages 5 to 6 years. The specific hypotheses were that (i) high-risk children would consume greater amounts, both as a percentage of their daily energy intake as well as an absolute intake, of sugar-sweetened beverages than did low-risk children and (ii) greater increases in the consumption of sugar-sweetened beverages would be positively associated with greater increases in children’s anthropometric measures.
METHODS AND PROCEDURES
Subjects
Families in this study were participants in an ongoing longitudinal investigation designed to examine the growth and development in a cohort of white children born at high or at low risk for obesity. Children’s risk status for obesity was based on maternal prepregnancy BMI (kg/m2) with mothers of the high-risk children averaging a BMI of 31.2 kg/m2 and those of low-risk children averaging a BMI of 19.4 kg/m2 (P < 0.001). Children were enrolled in the study at the age of 3 months and have undergone yearly assessments of growth and development. Details of parental characteristics and study design were reported previously (23–27). These analyses were based on the participants for whom food records were available during ages 3, 4, 5, and 6 years: 45 (23 low risk, 22 high risk), 48 (27 low risk, 21 high risk), 42 (23 low risk, 19 high risk), and 42 (22 low risk, 20 high risk), respectively. Written informed consent was obtained from the parents. The study protocol was approved by the Institutional Review Boards of the University of Pennsylvania and The Children’s Hospital of Philadelphia.
Anthropometry
Each year, children’s height was measured using a wall-mounted stadiometer (Holtain, Crymych, UK), weight using a digital scale (model 6002; Scaletronix, Carol Stream, IL), and waist circumference in the Nutrition and Growth Laboratory at the Children’s Hospital of Philadelphia. All research anthropometrists in the laboratory underwent an extensive, standardized training and retraining by a lead anthropometrist. They followed standardized procedures (28) to measure the waist circumference of children. Specifically, the anthropometrist placed an inelastic tape around the subjects at the level of the natural waist which is the narrowest part of the torso. Waist circumference was measured at the end of a normal expiration. Children’s height, weight, and waist circumference were measured in triplicate. The mean for each parameter was used for statistical analyses. BMI was computed and converted to z-scores for analyses (29).
Dietary methodology
Each year, at ±2 weeks of each child’s birthday, the primary caretaker of each child completed 3-day weighed food records (2 weekdays, 1 weekend day). The primary caretaker in relation to food was identified for each family. For most subjects, the primary caretaker was the mother. Families received extensive training on how to complete weighed food records and were provided with electronic food scales. They were instructed to preweigh all foods and beverages (except water) that were served to the child and postweigh the leftovers. Food records were analyzed by registered dietitians of the General Clinical Research Center of the Children’s Hospital of Philadelphia using the Food Processor Nutrition Analysis software (version 7.0; ESHA Research, Salem, OR). Food records which consisted of at least 2 days of reported intake were included in the analyses (30).
Beverages were stratified into the following seven categories: (i) milk containing all milk and milk-based beverages including chocolate milk, milk with powder/syrup, buttermilk, and milkshakes, (ii) fruit juice including 100% fruit juice, (iii) fruit drinks including fruit punches, sports drinks, lemonade, instant and iced tea, (iv) soda including carbonated caloric beverages, (v) diet soda including carbonated noncaloric beverages, (vi) soft drinks including soda, diet soda, and fruit drinks, and (vii) soft drinks and fruit juice including soda, diet soda, fruit drinks, and fruit juice. The rationale for combining the sugar-sweetened beverages (and fruit juice) is based on the finding that soft drinks and fruit juice have been implicated in some studies in increased energy intake and weight gain among children (3,4,7). We therefore extended our examination to clusters of putatively obesity-promoting beverages instead of only assessing the individual beverages alone.
Water intake was not recorded. All intakes were averaged across the number of days of food records for each child to derive a mean daily intake for each beverage category.
Statistical analysis
Descriptive statistics are presented as means ± s.e.m. To test research aim one, a 2 (risk group) × 4 (age) repeated measures ANOVA using a mixed linear model tested for differences in daily beverage consumption patterns across the beverage categories and percent energy consumed from beverages as a function of risk group and child age. Risk group was a between-subjects variable with two levels (i.e., high risk vs. low risk), and child age was a within-subjects variable with four levels (i.e., 3, 4, 5, and 6 years). The model used restricted maximum likelihood estimates and a compound symmetry error structure due to the repeated measures structure of the data. The compound symmetry structure is a covariance structure used in mixed models to allow for correlated errors (31). Contrast statements were used to test for potential linear effects of time across all 4 years of age.
Post hoc power calculations for aim one were performed based on the between-group mean differences found in the mixed model analyses. The power analysis was performed using a power calculation specified for longitudinal mixed models (31). In order to compare the beverage consumption variables on a common unit, Cohen’s d effect sizes (32) were computed for each of the between-group mean differences found in the analysis of aim one (Table 1). We also calculated the sample size required to replicate all significant findings found in our study.
Table 1.
Amount (least square mean ± s.e.m.) of beverages consumed by risk group and age and between-group effect sizes and power by age
| Age 3 | Age 4 | Age 5 | Age 6 | |
|---|---|---|---|---|
| Percent energy consumed from beverages (%) | ||||
| Low risk | 20.0 ± 1.9 | 22.9 ± 1.8 | 18.9 ± 1.9 | 19.8 ± 2.0 |
| High risk | 26.7 ± 2.0* | 22.7 ± 2.1 | 20.3 ± 2.1 | 17.2 ± 2.1 |
| Effect size | 0.45* | 0.01 | 0.09 | 0.17 |
| Power | 0.66 | 0.05 | 0.08 | 0.15 |
| Fruit juice (oz/d) | ||||
| Low risk | 4.6 ± 1.3 | 5.4 ± 1.2 | 4.5 ± 1.3 | 5.8 ± 1.3 |
| High risk | 12.3 ± 1.3* | 8.9 ± 1.3* | 7.2 ± 1.4 | 4.5 ± 1.4 |
| Effect size | 0.79* | 0.37 | 0.28 | 0.13 |
| Power | 0.99 | 0.50 | 0.31 | 0.10 |
| Fruit drink (oz/d) | ||||
| Low risk | 0.95 ± 0.8 | 1.7 ± 0.8 | 3.4 ± 0.8 | 2.9 ± 0.8 |
| High risk | 1.4 ± 0.9 | 2.8 ± 0.9 | 3.8 ± 0.9 | 2.4 ± 0.9 |
| Effect size | 0.06 | 0.18 | 0.06 | 0.09 |
| Power | 0.06 | 0.15 | 0.06 | 0.07 |
| Milk (oz/d) | ||||
| Low risk | 9.2 ± 1.3 | 10.3 ± 1.3 | 9.0 ± 1.3 | 10.4 ± 1.4 |
| High risk | 5.8 ± 1.4** | 8.6 ± 1.4 | 10.4 ± 1.5 | 11.8 ± 1.4 |
| Effect size | 0.33 | 0.18 | 0.14 | 0.13 |
| Power | 0.41 | 0.15 | 0.11 | 0.11 |
| Soda (oz/d) | ||||
| Low risk | 0.9 ± 0.5 | 1.7 ± 0.5 | 1.6 ± 0.5 | 1.2 ± 0.6 |
| High risk | 0.9 ± 0.6 | 1.3 ± 0.6 | 3.0 ± 0.6 | 2.9 ± 0.6* |
| Effect size | 0.01 | 0.10 | 0.32 | 0.40* |
| Power | 0.05 | 0.08 | 0.38 | 0.54 |
| Diet soda (oz/d) | ||||
| Low risk | 0.00 ± 0.2 | 0.00 ± 0.1 | 0.1 ± 0.2 | 0.2 ± 0.2 |
| High risk | 0.05 ± 0.2 | 0.02 ± 0.2 | 0.2 ± 0.2 | 0.5 ± 0.2 |
| Effect size | 0.04 | 0.02 | 0.02 | 0.25 |
| Power | 0.06 | 0.05 | 0.05 | 0.27 |
| Soft drinks and fruit juice (oz/d) | ||||
| Low risk | 6.4 ± 1.5 | 8.8 ± 1.4 | 9.5 ± 1.5 | 10.1 ± 1.5 |
| High risk | 14.5 ± 1.5* | 13.1 ± 1.6* | 14.3 ± 1.7* | 10.1 ± 1.6 |
| Effect size | 0.72* | 0.38* | 0.40* | 0.002 |
| Power | 0.96 | 0.51 | 0.55 | 0.05 |
| Soft drinks (oz/d) | ||||
| Low risk | 1.8 ± 1.0 | 3.5 ± 1.0 | 5.1 ± 1.0 | 4.4 ± 1.0 |
| High risk | 2.3 ± 1.0 | 4.2 ± 1.1 | 7.0 ± 1.1 | 5.7 ± 1.1 |
| Effect size | 0.06 | 0.10 | 0.23 | 0.18 |
| Power | 0.06 | 0.08 | 0.23 | 0.15 |
Means indicated with asterisks are significantly different between risk groups within a given year (P < 0.05);
P = 0.08. Effect sizes indicated with asterisks are based on significant mean differences between risk groups within a given year (P < 0.05).
To test aim two, we conducted a longitudinal analysis relating changes in beverage intake to changes in children’s anthropometric measures. These analyses pooled children from the two risk groups based on preliminary analyses testing whether associations differed between the risk groups. Specifically, for each beverage category, we conducted a series of multiple regression analyses in which BMI z-score or waist circumference was the outcome measure and risk group, total energy intake, calories consumed from the respective beverages, and the beverage intake by risk group interaction were predictors. Across these regression analyses, none of the interaction terms were statistically significant. We therefore pooled the two risk groups for the longitudinal analyses.
The longitudinal analyses followed a modeling procedure similar to the one used by Berkey et al. (33). Specifically, we used a mixed linear model (SAS PROC MIXED) to test whether changes in beverage intakes from ages 3 to 5 years were associated with changes in child BMI z-score and waist circumference (cm) from ages 5 to 6 years using a separate model for each beverage category. We included change in BMI z-score or waist circumference from ages 3 to 5 years and total energy intake from food at age 3 as covariates in the model. When adding total energy intake to the model as a covariate, we excluded all beverages in order to avoid intercorrelation between the predictor variables (34). We identified two children who at various time points qualified as statistical outliers per Tukey criteria (35) (scores exceeded 1.5 times the interquartile range for BMI z-score and waist circumference, respectively). We excluded these children from the longitudinal analysis. We comment in the Results section on how the outcomes changed when all children (including the outliers) were kept in the data set. None of the results from the longitudinal analysis changed with risk group in the model. Finally, in an exploratory analysis, changes in milk intake (kcal) from years 3 to 5 were correlated with changes in soft drink and fruit juice intake (kcal) from years 3 to 5.
Data were analyzed using SPSS software (version 12.0; SPSS, Chicago, IL) and SAS software (version 9.1; SAS Institute, Cary, NC). P values <0.05 were considered statistically significant.
RESULTS
Descriptive analyses
Participant characteristics
Children included 25 boys (13 low risk, 12 high risk) and 20 girls (10 low risk, 10 high risk) at age 3 years, 23 boys (14 low risk, 9 high risk) and 25 girls (13 low risk, 12 high risk) at age 4 years, 21 boys (11 low risk, 10 high risk) and 21 girls (12 low risk, 9 high risk) at age 5 years, and 19 boys (8 low risk, 11 high risk) and 23 girls (14 low risk, 9 high risk) at age 6 years, respectively. The means for BMI z-score at ages 3, 4, 5, and 6 years were −0.4 ± 0.2, 0.1 ± 0.1, 0.1 ± 0.2, and −0.3 ± 0.2 for low-risk children and −0.4 ± 0.3, 0.4 ± 0.3, 0.5 ± 0.4, and 0.2 ± 0.3 for high-risk children, respectively (30). The means for waist circumference at ages 3, 4, 5, and 6 years were 49.6 ± 0.6, 52.4 ± 0.5, 53.6 ± 0.4, and 54.9 ± 0.7 cm for low-risk children and 50.0 ± 0.7, 54.6 ± 1.2, 57.5 ± 1.9, and 60.2 ± 2.2 cm for high-risk children, respectively (30). Only waist circumference at age 6 was significantly different between risk groups (P= 0.03).
Longitudinal analyses of children’s beverage consumption patterns
Table 1 depicts the mean (±s.e.m.) percentage of energy consumed from beverages and mean amount of beverages (oz) consumed daily from beverage categories by risk group and age. We present least square means from the mixed linear model. The table also presents the effect size corresponding to each between-group mean difference at each year, expressed as Cohen’s d, along with the power to detect a statistical difference as determined from the post hoc power analysis.
Percent energy consumed from beverages
There was a significant risk group by time interaction (P = 0.02) and a significant linear risk group by time interaction (P = 0.006), indicating a progressive decrease in energy consumed from beverages from ages 3 to 6 years among high-risk children only. A pairwise follow-up comparison showed that the mean percentage of energy consumed from beverages was significantly greater among high-risk children (26 ± 3%) than low-risk children (20 ± 2%) at age 3 (P = 0.02).
Amount (oz) of fruit juice
There was a significant main effect of risk group (P = 0.01) and a significant main effect of time (P = 0.02). There also was a significant risk group by time interaction (P = 0.002) and a significant linear risk group by time interaction (P = 0.0002), indicating a progressive decrease in fruit juice intake from 3 to 6 years among high-risk children only. A pairwise follow-up comparison showed that fruit juice consumption by high-risk children was significantly greater than that by low-risk children at ages 3 (13 ± 2 oz vs. 5 ± 1 oz; P < 0.0001) and 4 years (9 ± 2 oz vs. 6 ± 1 oz; P = 0.05).
Amount (oz) of fruit drinks
There was a significant main effect of time (P = 0.008) and a significant linear effect of time (P = 0.009) indicating that children’s consumption of fruit drinks increased over time. The risk group by time interaction was nonsignificant (P = 0.70).
Amount (oz) of milk
There was a significant main effect of time (P = 0.01); the main effect of risk group was nonsignificant (P = 0.70). There was a borderline significant risk group by time interaction (P = 0.05) and a significant linear risk group by time interaction (P = 0.009), indicating a progressive increase in milk intake from 3 to 6 years among high-risk children only. There was a trend for children in the high-risk group to have consumed less milk at age 3 than low-risk children (5 ± 1 oz vs. 9 ± 1 oz; P = 0.08).
Amount (oz) of soda
There was a significant main effect of time (P = 0.03); the main effect of risk group was nonsignificant (P = 0.22). There also was a significant linear risk group by time interaction (P = 0.03), indicating a more pronounced increase in soda intake from years 3 to 6 years among high-risk than low-risk children. A pairwise follow-up comparison indicated that the amount of soda consumed by high-risk children was significantly greater than that of low-risk children at age 6 (3 ± 1 oz vs. 1 ± 1 oz; P = 0.04).
Amount (oz) of diet soda
There was a trend for a significant main effect of time (P = 0.08) and a significant linear effect of time (P = 0.02), indicating that consumption of diet soda increased over time in both risk groups. The risk group by time interaction was nonsignificant (P = 0.78).
Amount (oz) of soft drinks
There was a significant main effect of time (P = 0.0001) and a significant linear effect of time (P = 0.0001) indicating that consumption of soft drinks increased over time in both risk groups. The risk group by time interaction was nonsignificant (P = 0.85).
Amount (oz) of soft drinks including fruit juice
There was a significant main effect of risk group (P = 0.01); the main effect of time was nonsignificant (P = 0.52). There also was a significant risk group by time interaction (P = 0.02) and a significant linear risk group by time interaction (P = 0.003), indicating that consumption of soft drinks including fruit juice increased over time in low-risk children while that of high-risk children remained consistently higher, except for age 6. A pairwise follow-up comparison showed that the amount of soft drinks including fruit juice consumed by high-risk children was significantly greater than low-risk children at ages 3 (15 ± 2 oz vs. 7 ± 1 oz; P = 0.0002), 4 (14 ± 2 oz vs. 9 ± 1 oz; P = 0.05), and 5 (15 ± 2 oz vs. 10 ± 1 oz; P= 0.04), respectively.
Power analysis
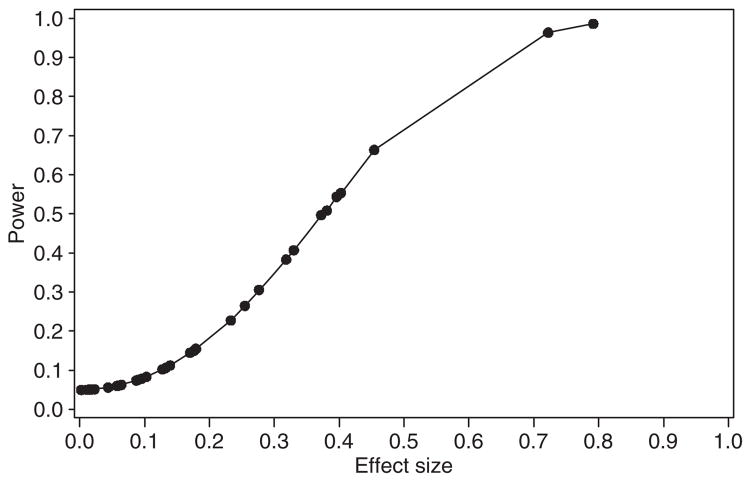
Figure 1 plots all effect sizes from Table 1 and their corresponding power from the post hoc analyses. The effect sizes of the significant findings ranged from d = 0.37 to 0.79. The minimum sample size necessary to replicate all of these significant findings at the 0.05 level of significance with at least 80% power would be 116 subjects per group. These 116 subjects per risk group are based on the smallest effect size (0.37) that provided statistically significant mean differences between groups in this sample. The minimum sample size required to detect smaller effects that did not attain significance in this sample would require larger samples. For example, the median effect sizes of all the nonsignificant effects in our sample was d = 0.12. To detect such an effect with 80% power and α = 0.05 would require a minimum sample size of 1,092 children per risk group.
Figure 1.
Effect sizes and power corresponding to between-group differences found in Table 1.
Longitudinal analyses of 3-year changes in beverage intake in relation to changes in BMI z-score and waist circumference from ages 5 to 6 years
There was no significant association between change in consumption from individual beverage categories and change in BMI z-score across analyses (P > 0.10). Greater increases in calories (β = −0.01; P = 0.02) and percent energy (β = −0.11; P = 0.02) consumed from all beverages and greater increases in calories consumed from milk (β = −0.01; P = 0.04) were inversely related to changes in children’s waist circumference (Table 2). On the other hand, a greater increase in calories consumed from soda (β = 0.04; P = 0.001) was associated with a greater increase in waist circumference. When outliers were included in the analysis, the following predictors became borderline significant: change in total calories consumed from beverages (parameter estimate (β): −0.01; P = 0.06) and change in calories consumed from milk (parameter estimate (β): −0.01; P = 0.055). All other significant outcomes remained unchanged.
Table 2.
Results from the mixed linear model that was used to predict change in waist circumference from ages 5 to 6 years (risk groups combined) when adjusting for change in waist circumference from ages 3 to 5 years and total energy intake at age 3
| Model | Parameter | Parameter estimate (β) | s.e. | P value |
|---|---|---|---|---|
| 1 | Intercept | −4.06 | 2.16 | 0.07 |
| Change in calories consumed from all beverages at ages 3–5 | −0.01 | 0.003 | 0.02 | |
| Change in waist circumference from ages 3–5 | 0.34 | 0.10 | 0.002 | |
| Total energy intake at age 3 | 0.005 | 0.002 | 0.04 | |
| 2 | Intercept | −5.10 | 2.33 | 0.04 |
| Change in percent energy consumed from beverages at ages 3–5 | −0.11 | 0.04 | 0.02 | |
| Change in waist circumference from ages 3–5 | 0.30 | 0.10 | 0.004 | |
| Total energy intake at age 3 | 0.01 | 0.002 | 0.02 | |
| 3 | Intercept | −1.43 | 1.87 | 0.45 |
| Change in calories consumed from milk at ages 3–5 | −0.01 | 0.004 | 0.04 | |
| Change in waist circumference from ages 3–5 | 0.34 | 0.10 | 0.003 | |
| Total energy intake at age 3 | 0.002 | 0.002 | 0.32 | |
| 4 | Intercept | −1.57 | 1.63 | 0.34 |
| Change in calories consumed from soda at ages 3–5 | 0.04 | 0.009 | 0.001 | |
| Change in waist circumference from ages 3–5 | 0.20 | 0.09 | 0.03 | |
| Total energy intake at age 3 | 0.002 | 0.002 | 0.22 |
Relationship between 3-year changes in milk and soft drink/fruit juice consumption by risk group
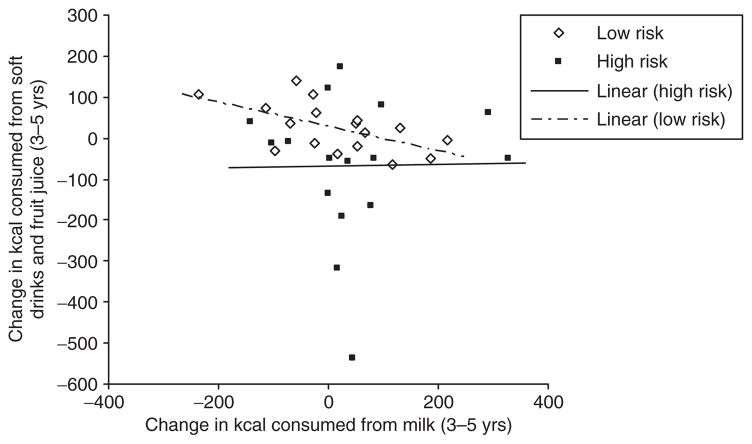
Changes in milk consumption from years 3 to 5 were inversely related with changes in soft drink and fruit juice consumption from years 3 to 5 for low-risk children only (r = −0.58; P = 0.02). There was no significant relationship between these two variables for high-risk children (r = 0.02; P = 0.96) (Figure 2).
Figure 2.
Relationship between change in milk consumption (kcal) from years 3 to 5 and change in soft drink and fruit juice consumption (kcal) from years 3 to 5 by risk group.
DISCUSSION
The main findings of this study were that during early childhood there were distinct differences between high- and low-risk children in their beverage consumption patterns over time. Specifically, at a young age children born at high risk for obesity showed beverage consumption patterns that favored increased intakes of sugar-sweetened beverages such as fruit juice and soft drinks whereas intake of milk was comparatively low. The consumption of milk progressively increased among high-risk children over time; however, this increase did not displace calories consumed from sugar-sweetened beverages and fruit juice.
Increased consumption of fruit juice, soda, and soft drinks have been associated with increased energy intake (36,37), excessive weight gain in children (3,4,9), and child overweight (8,9,38). In this study, we observed a significant linear risk group by time interaction for the amount of fruit juice, soda, and soft drinks including fruit juice that children consumed. Specifically, while fruit juice consumption was relatively stable over time for low-risk children, high-risk children’s intake was significantly higher at ages 3–4, but then declined over time. Similarly, the increase in soda intake over time was more pronounced for high-risk children than for low-risk children. Finally, high-risk children consumed significantly greater amounts of soft drinks (including fruit juice) at ages 3–5 years compared to low-risk children. These results are intriguing in that they point to different beverage consumption patterns between children with a different predisposition to obesity. It is possible that children who were born at high risk for obesity may exhibit a greater preference for these types of beverages by ways of their genetic predisposition (39). It is also possible that families with a history of obesity may create a food home environment, which provides more easy access to sugar-sweetened beverages to their children than do families with no prior history of obesity. Studies using genetically sensitive designs are necessary to address these issues.
While high-risk children consumed more fruit juice and soft drinks at an early age, they also consumed relatively smaller amounts of milk compared to low-risk children (trend for a significant difference at age 3). Over time, however, high-risk children progressively increased their intake of milk to the level of low-risk children. Interestingly, while a greater increase in milk consumption over a 3-year period was inversely correlated with a 3-year change in calories consumed from soft drinks and fruit juice in low-risk children, there was no association among high-risk children. Thus, for low-risk children only, increased milk consumption may have displaced the consumption of soft drinks and fruit juice. For high-risk children, the results suggest that rather than having displaced calories that were consumed from soft drinks and fruit juice, milk intake instead may have added onto the calories consumed from these beverages. Previous studies among children and adolescents suggested that decreases in milk consumption may be due to an increased consumption of soft drinks and juice drinks over time (36,40–42). While these results may speak to a possible displacement of calories consumed from certain beverages, only an experiment which systematically adjusts intake from beverage categories could generate more conclusive answers. Finally, a decrease in milk consumption over a 3-year period predicted an increase in children’s waist circumference from ages 5 to 6 years in the full sample. These results are in line with findings from previous studies that showed that increased milk/dairy intake may have a protective effect on child overweight (18,43–45).
A curious finding from the longitudinal analysis was that change both in the relative (percent) and absolute calorie intake of beverages over time was inversely related to change in child waist circumference. In light of the previous finding of an inverse relationship between milk consumption and change in waist circumference, we attribute this inverse relationship to the inclusion of milk and milk-based beverages in this overall category. When examining the percentage of calories consumed from beverages as milk during ages 3–6 years, the data showed that children, on average, consumed ~47% beverage calories from milk (data not shown). Thus, having milk included in the all beverages category may explain the inverse relationship between changes in calories consumed from all beverages and changes in waist circumference. It is interesting to note that in the longitudinal analysis there were significant findings only for changes in waist circumference and not for changes in BMI z-score. It is not clear as to why changes in beverage consumption patterns may differentially affect abdominally accumulated fat (i.e., central adiposity) but not the sum of fat-free mass and fat mass. It has been shown that the central distribution of body fat has a major genetic component (46,47).
It is noteworthy that fruit juice intake of high-risk children exceeded recommendations put forward by the American Academy of Pediatrics and the US Department of Agriculture 2005 Dietary Guidelines which recommend limiting fruit juice consumption to 4–6 oz per day for children ages 1–6 years. When examining the mean intakes of fruit juice in this study, it becomes clear that the children born at high risk for obesity exceeded this recommended intake during ages 3–5 years. The current recommendation for milk intake for children 2–8 years per the US Department of Agriculture Dietary Guidelines and the American Dietetic Association amounts to two servings (approximately two cups) per day. Children in both risk groups thus consumed less than the recommended amount of milk during all 4 years. Children in our study (groups combined and intakes averaged across 4 years) also consumed overall smaller amounts of milk (9.2 oz vs. 12.32 oz) and more fruit juice (6.8 oz vs. 4.7 oz) per day compared to intakes of children in a nationally representative sample (37).
The strengths of this study include (i) the unique sample of children born at high risk and low risk of obesity who were studied prospectively and (ii) the use of weighed food records as opposed to visual estimates. There are several ways in which this study should be extended. First, the results from this study are limited to the relatively narrowly defined study sample, namely healthy, full-term, white children, and may therefore not be generalizable to children as a whole. It thus would be desirable to examine beverages consumption patterns in a larger cohort of children who are ethnically diverse and show greater variability in their weight status. The relatively small sample size in this study may have also prevented us from finding more significant between-group differences in beverage consumption patterns as well as a greater number of significant associations with longitudinal anthropometric measures. Second, it would be desirable to obtain intake data over a longer period of time to determine children’s habitual diet and to complement the self-reported intake data by laboratory measures. Third, it is possible that parents’ education level and children’s physical activity habits may have affected children’s beverage consumption patterns. These data were not readily available for the years under study but should be examined in future investigations. Finally, the present designs, unlike a classic twin design or other genetically sensitive designs, could not formally test the respective influence of genetic and environmental influences on beverage intake patterns.
In conclusion, this study showed that children who were born at high risk for obesity showed increased intakes of fruit juice and soft drinks and lower intakes of milk and milk-based beverages during early childhood compared to low-risk children. For the group as whole, greater increases in soda consumption from ages 3 to 5 years was associated with greater increases in child waist circumference during the subsequent year whereas a decrease in milk consumption was associated with greater increases in child waist circumference during the subsequent year. Among low-risk children only, increases in milk consumption from ages 3 to 5 years were negatively associated with calories consumed from soft drinks and fruit juice. Future research should examine whether an increase in intake in one beverage category displaces intake from another beverage category or whether these increases are additive.
Acknowledgments
This research was supported by the National Institutes of Health Grant DK068899, the General Clinical Research Center (Grant RR 00240), and the Nutrition Center of the Children’s Hospital of Philadelphia.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27:205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 5.Pereira MA. The possible role of sugar-sweetened beverages in obesity etiology: a review of the evidence. Int J Obes. 2006;30:S28–S36. [Google Scholar]
- 6.Bachman cm, Baranowski T, Nicklas TA. Is there an association between sweetened beverages and adiposity? Nutr Rev. 2006;64:153–174. doi: 10.1111/j.1753-4887.2006.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 7.Dennison BA, Rockwell HL, Baker SL. Excess fruit juice consumption by preschool-aged children is associated with short stature and obesity. Pediatrics. 1997;99:15–22. [PubMed] [Google Scholar]
- 8.Dennison BA, Rockwell HL, Nichols MJ, Jenkins P. Children’s growth parameters vary by type of fruit juice consumed. J Am Coll Nutr. 1999;18:346–352. doi: 10.1080/07315724.1999.10718874. [DOI] [PubMed] [Google Scholar]
- 9.Faith MS, Dennison BA, Edmunds LS, Stratton HH. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066–2075. doi: 10.1542/peds.2006-1117. [DOI] [PubMed] [Google Scholar]
- 10.Alexy U, Sichert-Hellert W, Kersting M, Manz F, Schöch G. Fruit juice consumption and the prevalence of obesity and short stature in german preschool children: results of the DONALD Study. Dortmund Nutritional and Anthropometrical Longitudinally Designed. J Pediatr Gastroenterol Nutr. 1999;29:343–349. doi: 10.1097/00005176-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Skinner JD, Carruth BR. A longitudinal study of children’s juice intake and growth: the juice controversy revisited. J Am Diet Assoc. 2001;101:432–437. doi: 10.1016/S0002-8223(01)00111-0. [DOI] [PubMed] [Google Scholar]
- 12.Skinner JD, Carruth BR, Moran J, 3rd, Houck K, Coletta F. Fruit juice intake is not related to children’s growth. Pediatrics. 1999;103:58–64. doi: 10.1542/peds.103.1.58. [DOI] [PubMed] [Google Scholar]
- 13.Newby PK, Peterson KE, Berkey CS, et al. Beverage consumption is not associated with changes in weight and body mass index among low-income preschool children in North Dakota. J Am Diet Assoc. 2004;104:1086–1094. doi: 10.1016/j.jada.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Lappe JM, Rafferty KA, Davies KM, Lypaczewski G. Girls on a high-calcium diet gain weight at the same rate as girls on a normal diet: a pilot study. J Am Diet Assoc. 2004;104:1361–1367. doi: 10.1016/j.jada.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Venti CA, Tataranni PA, Salbe AD. Lack of relationship between calcium intake and body size in an obesity-prone population. J Am Diet Assoc. 2005;105:1401–1407. doi: 10.1016/j.jada.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Barba G, Troiano E, Russo P, Venezia A, Siani A. Inverse association between body mass and frequency of milk consumption in children. Br J Nutr. 2005;93:15–19. doi: 10.1079/bjn20041300. [DOI] [PubMed] [Google Scholar]
- 17.Moreira P, Padez C, Mourao I, Rosado V. Dietary calcium and body mass index in Portuguese children. Eur J Clin Nutr. 2005;59:861–867. doi: 10.1038/sj.ejcn.1602147. [DOI] [PubMed] [Google Scholar]
- 18.Skinner JD, Bounds W, Carruth BR, Ziegler P. Longitudinal calcium intake is negatively related to children’s body fat indexes. J Am Diet Assoc. 2003;103:1626–1631. doi: 10.1016/j.jada.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Berkey CS, Rockett HR, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Arch Pediatr Adolesc Med. 2005;159:543–550. doi: 10.1001/archpedi.159.6.543. [DOI] [PubMed] [Google Scholar]
- 20.de Castro JM. A twin study of genetic and environmental influences on the intake of fluids and beverages. Physiol Behav. 1993;54:677–687. doi: 10.1016/0031-9384(93)90076-r. [DOI] [PubMed] [Google Scholar]
- 21.Wardle J, Guthrie C, Sanderson S, Birch L, Plomin R. Food and activity preferences in children of lean and obese parents. Int J Obes Relat Metab Disord. 2001;25:971–977. doi: 10.1038/sj.ijo.0801661. [DOI] [PubMed] [Google Scholar]
- 22.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42:963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 23.Faith MS, Berkowitz RI, Stallings VA, et al. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obes Res. 2006;14:131–138. doi: 10.1038/oby.2006.16. [DOI] [PubMed] [Google Scholar]
- 24.Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, not energy output, is a determinant of body size in infants. Am J Clin Nutr. 1999;69:524–530. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- 25.Faith MS, Berkowitz RI, Stallings VA, et al. Parental feeding attitudes and styles and child body mass index: prospective analysis of a gene-environment interaction. Pediatrics. 2004;114:e429–e436. doi: 10.1542/peds.2003-1075-L. [DOI] [PubMed] [Google Scholar]
- 26.Berkowitz RI, Stallings VA, Maislin G, Stunkard AJ. Growth of children at high risk of obesity during the first 6 y of life: implications for prevention. Am J Clin Nutr. 2005;81:140–146. doi: 10.1093/ajcn/81.1.140. [DOI] [PubMed] [Google Scholar]
- 27.Stunkard AJ, Berkowitz RI, Schoeller D, Maislin G, Stallings VA. Predictors of body size in the first 2 y of life: a high-risk study of human obesity. Int J Obes Relat Metab Disord. 2004;28:503–513. doi: 10.1038/sj.ijo.0802517. [DOI] [PubMed] [Google Scholar]
- 28.Callaway CW, Chumlea WC, Bouchard C, et al. Circumferences. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, IL: 1988. pp. 39–54. [Google Scholar]
- 29.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 30.Kral TV, Berkowitz RI, Stunkard AJ, et al. Dietary energy density increases during early childhood irrespective of familial predisposition to obesity: results from a prospective cohort study. Int J Obes. 2007;31:1061–1067. doi: 10.1038/sj.ijo.0803551. [DOI] [PubMed] [Google Scholar]
- 31.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2. SAS Institute; Cary, NC: 2006. [Google Scholar]
- 32.Cohen J. Statistical Power Analyses for the Behavioral Sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 33.Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12:778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis For The Behavioral Sciences. 3. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. [Google Scholar]
- 35.Tukey J. Exploratory Data Analysis. Addison-Wesley Publishing; Reading, MA: 1977. [Google Scholar]
- 36.Harnack L, Stang J, Story M. Soft drink consumption among US children and adolescents: nutritional consequences. J Am Diet Assoc. 1999;99:436–441. doi: 10.1016/S0002-8223(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor TM, Yang SJ, Nicklas TA. Beverage intake among preschool children and its effect on weight status. Pediatrics. 2006;118:e1010–e1018. doi: 10.1542/peds.2005-2348. [DOI] [PubMed] [Google Scholar]
- 38.Dennison BA. Fruit juice consumption by infants and children: a review. J Am Coll Nutr. 1996;15:4S–11S. doi: 10.1080/07315724.1996.10720475. [DOI] [PubMed] [Google Scholar]
- 39.de Castro JM. Genes and environment have gender-independent influences on the eating and drinking of free-living humans. Physiol Behav. 1998;63:385–395. doi: 10.1016/s0031-9384(97)00455-1. [DOI] [PubMed] [Google Scholar]
- 40.Rampersaud GC, Bailey LB, Kauwell GP. National survey beverage consumption data for children and adolescents indicate the need to encourage a shift toward more nutritive beverages. J Am Diet Assoc. 2003;103:97–100. doi: 10.1053/jada.2003.50006. [DOI] [PubMed] [Google Scholar]
- 41.Fisher JO, Mitchell DC, Smiciklas-Wright H, Mannino ML, Birch LL. Meeting calcium recommendations during middle childhood reflects mother-daughter beverage choices and predicts bone mineral status. Am J Clin Nutr. 2004;79:698–706. doi: 10.1093/ajcn/79.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher J, Mitchell D, Smiciklas-Wright H, Birch L. Maternal milk consumption predicts the tradeoff between milk and soft drinks in young girls’ diets. J Nutr. 2001;131:246–250. doi: 10.1093/jn/131.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanasescu M, Ferris AM, Himmelgreen DA, Rodriguez N, Pérez-Escamilla R. Biobehavioral factors are associated with obesity in Puerto Rican children. J Nutr. 2000;130:1734–1742. doi: 10.1093/jn/130.7.1734. [DOI] [PubMed] [Google Scholar]
- 44.Novotny R, Daida YG, Acharya S, Grove JS, Vogt TM. Dairy intake is associated with lower body fat and soda intake with greater weight in adolescent girls. J Nutr. 2004;134:1905–1909. doi: 10.1093/jn/134.8.1905. [DOI] [PubMed] [Google Scholar]
- 45.Carruth BR, Skinner JD. The role of dietary calcium and other nutrients in moderating body fat in preschool children. Int J Obes Relat Metab Disord. 2001;25:559–566. doi: 10.1038/sj.ijo.0801562. [DOI] [PubMed] [Google Scholar]
- 46.Dietz WH. Childhood weight affects adult morbidity and mortality. J Nutr. 1998;128:411S–414S. doi: 10.1093/jn/128.2.411S. [DOI] [PubMed] [Google Scholar]
- 47.Carey DG, Nguyen TV, Campbell LV, Chisholm DJ, Kelly P. Genetic influences on central abdominal fat: a twin study. Int J Obes Relat Metab Disord. 1996;20:722–726. [PubMed] [Google Scholar]