Abstract
The Drosophila leg imaginal disc provides a paradigm with which to understand the fundamental developmental mechanisms that generate an intricate appendage structure. Leg formation depends on the subdivision of the leg proximodistal (PD) axis into broad domains by the leg gap genes. The leg gap genes act combinatorially to initiate the expression of the Notch ligands Delta (Dl) and Serrate (Ser) in a segmental pattern. Dl and Ser induce the expression of a set of transcriptional regulators along the segment border, which mediate leg segment growth and joint morphogenesis. Here we show that Lines accumulates in nuclei in the presumptive tarsus and the inter-joints of proximal leg segments and governs the formation of these structures by destabilizing the nuclear protein Bowl. Across the presumptive tarsus, lines modulates the opposing expression landscapes of the leg gap gene dachshund (dac) and the tarsal PD genes, bric-a-brac 2 (bab), apterous (ap) and BarH1 (Bar). In this manner, lines inhibits proximal tarsal fates and promotes medial and distal tarsal fates. Across proximal leg segments, lines antagonizes bowl to promote Dl expression by relief-of-repression. In turn, Dl signals asymmetrically to stabilize Bowl in adjacent distal cells. Bowl, then, acts cell-autonomously, together with one or more redundant factors, to repress Dl expression. Together, lines and bowl act as a binary switch to generate a stable Notch signaling interface between Dl-expressing cells and adjacent distal cell. lines plays analogous roles in developing antennae, which are serially homologous to legs, suggesting evolutionarily conserved roles for lines in ventral appendage formation.
Keywords: proximodistal patterning, segmental patterning, tarsus, Notch signaling, odd-skipped family genes, dAP-2, dachshund, bric-a-brac, apterous, Bar
INTRODUCTION
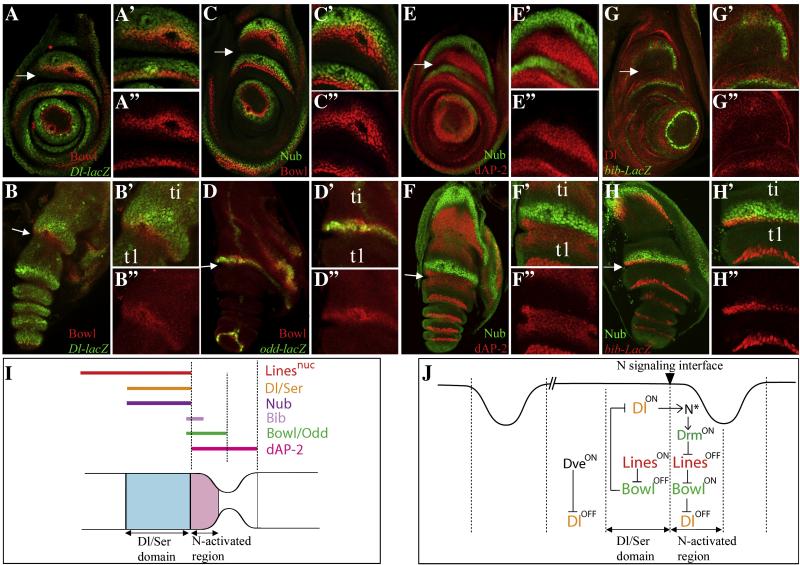
The Drosophila leg imaginal disc provides a tractable system with which to investigate the molecular mechanisms and regulatory logic of limb development (Galindo and Couso, 2000; Kojima, 2004). The leg primordium originates in the embryonic surface ectoderm as a cluster of approximately 20-30 cells, which subsequently invaginates to form a flattened epithelial disc. During larval development the disc proliferates rapidly to generate a concentrically folded epithelial layer composed of approximately 20,000 cells. During these stages the disc is progressively subdivided into six “true” segments independently movable by muscle: the coxa (co), trochanter (tr), femur (fe), tibia (ti), tarsus (tr), and pretarsus (pt). The tarsus is further subdivided into five nonmusculated subsegments (Fig. 1A)(Cohen, 1993; Fristrom, 1993).
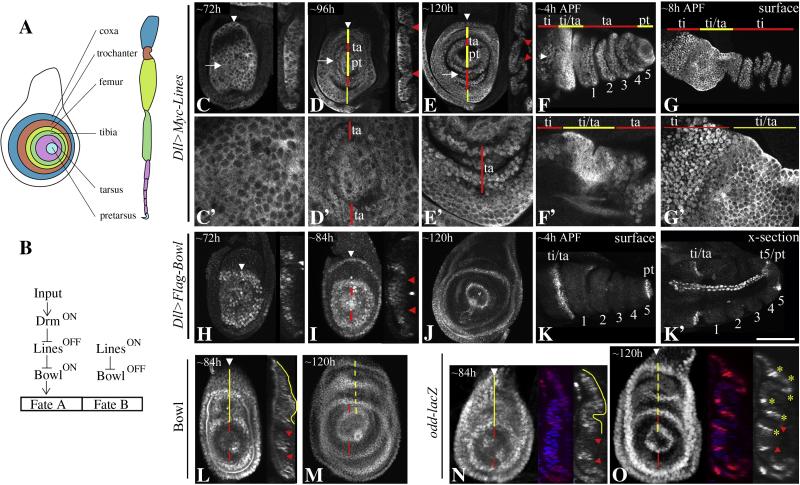
Figure 1.
The dynamics of Lines and Bowl distribution during leg disc development (A) A cartoon depicting the PD subdivisions of a mature leg disc and an adult leg. (B) The interactions linking Drm, Lines and Bowl in embryonic patterning. (C-G’) Dll>Myc-Lines; red bars and arrowheads indicate regions where Myc-Lines was nuclear, yellow bars areas where Lines was cytoplasmic. (H-L) Dll>Flag-Bowl; Red bars and arrowheads point to regions where Flag-Bowl was downregulated. White arrowheads in C-F, H-I point to plane of Z-sections shown in corresponding insets. White arrows in C-E point to magnified regions shown in corresponding lower panels. (C-C’) ~72h; Myc-Lines was broadly cytoplasmic. (D-D’) ~96h; Myc-Lines accumulated in nuclei in the central fold in the emerging tarsus (ta). (E) ~120; Myc-Lines was nuclear in the expanding tarsus and cytoplasmic in the pretarsus (pt). (F-F’) ~4h After Pupal Formation (APF) and (G-G’) ~8h APF; in everting discs, Myc-Lines was nuclear in the tarsus and in the inter-joint region of the tibia (ti), and cytoplasmic in the pretarsus, and in the tarsal/tibial joint. (H) ~72h; Bowl was broadly nuclear. (I) ~84h; Bowl was downreglated in the nascent central fold. (J) ~120h; Bowl was stabilized in nuclei in three rings that correspond to the presumptive pretarsa/tarsal, tarsal/tibial and tibial/femural joints. (K-K’) ~4h APF; Bowl was stable in the proximal half of the t5/pretarsal and the tibial/t1 joints. (L-M) Bowl. (N-O) odd-lacZ. (L) ~84h; Bowl was broadly expressed at early stages of leg disc development. Yellow bars and lines highlight the broad uninterrupted pattern of Bowl accumulation in proximal cells. Red bars and arrowheads point to the nascent tarsus where Bowl was downregulated. (M) ~120h; Yellow bar highlight the accumulation of Bowl is six rings that correspond to the Notch activated region of true leg segments. Red bars point to the tarsal region. (N) ~84h and (O) ~120h; an odd-lacZ reporter was expressed in a similar pattern to Bowl. (N) The odd-lacZ reporter was broadly expressed at early stages. (O) At later stages, the odd-lacZ reporter was maintained in 6 rings that correspond to the Notch activated region of each true leg segment as indicated with yellow bars and asterisks. Scale bar= 30μm in C, H, L, N, O, 40μm in F, K-K’, M, 50μm in D, E, H, I, 80μm in G-G’.
Leg development depends on the subdivision of the anteroposterior (AP), dorsoventral (DV) and proximodistal (PD) axes of the leg primordium into progressively smaller domains. Many of the genes and pathways that establish these axes have been identified. However, it remains unclear how new PD domains are added to the growing leg during development, how the leg PD axis is progressively subdivided into a series of segments, and how segments acquire their unique size and morphological features.
The early limb field is established during embryogenesis in the surface ectoderm and is subdivided into a proximal domain expressing homothorax (hth) and a distal domain expressing Dll (Cohen et al. 1989; Abu-Shaar & Mann 1998; Wu & Cohen 1999). hth and Dll code for conserved homeobox proteins. The further elaboration of the PD axis is mediate by the morphogens Decapentaplegic (Dpp) and Wingless (Wg) that emanate from dorsal and ventral sources along the AP compartment boundary (Basler and Struhl, 1994; Campbell et al., 1993; Diaz-Benjumea et al., 1994). Wg and Dpp cooperate to induce dac expression between the Dll and hth domains at an intermediate PD position (Lecuit and Cohen, 1997; Abu-Shaar & Mann 1998). dac codes for a pioneer nuclear protein (Mardon et al., 1994). Additionally, wg and dpp cooperate to establish a secondary pattern-organizing center at the distal tip of the leg. Ligands that emanate from this organizer activate the Epidermal Growth Factor receptor (EGF receptor) pathway to control the expression of the tarsal PD genes in a graded manner (Campbell, 2002; Galindo et al., 2002). While hth, dac and Dll respectively control the formation of broad proximal, medial and distal domains along the leg PD axis (Abu-Shaar and Mann, 1998; Azpiazu and Morata, 2002; Campbell and Tomlinson, 1998; Cohen et al., 1993; Cohen et al., 1989; Gorfinkiel et al., 1997; Mardon et al., 1994; Wu and Cohen, 1999), the tarsal PD genes act locally to fine-pattern the tarsal region.
Distinct combinations of leg gap genes and tarsal PD genes initiate the expression of Dl and Ser across each prospective leg segment either directly or indirectly (Rauskolb, 2001). At the end of larval development, Dl and Ser are expressed at the distal end of each prospective leg segment and signal asymmetrically to distal cells to control the expression of downstream target genes that mediate leg segment growth and joint morphogenesis (Bishop et al., 1999; de Celis et al., 1998; Mishra et al., 2001; Rauskolb and Irvine, 1999). Downstream of Notch are the Drosophila transcriptional activator protein 2 (dAP-2), nubbin (nub) and the odd-skipped family genes drumstick (drm), odd-skipped (odd), bowl, and sister of odd and bowl (sob). dAP-2 mediates growth of all the leg segments and the formation of all the joints (Kerber et al., 2001; Monge et al., 2001). nub encodes a POU domain protein whose role in leg development is yet to be elucidated. nub hypomorphs develop shortened and gnarled legs indicating that nub contributes to the growth and patterning of leg segments (Cifuentes and Garcia-Bellido, 1997). The odd-skipped family genes, drm, odd, sob and bowl, share a highly conserved zinc finger domain (Coulter et al., 1990; Green et al., 2002; Hart et al., 1996; Wang and Coulter, 1996) and are induced by Notch signaling along the borders of true leg segments (de Celis Ibeas and Bray, 2003; Hao et al., 2003). The nuclear protein Lines acts reciprocally to Drm and Bowl in patterning embryonic and larval structures (Bras-Pereira C, 2006; Green et al., 2002; Hatini et al., 2005; Johansen et al., 2003; Nusinow et al., 2008). Lines destabilizes Bowl by binding to the Bowl protein, while Drm stabilizes Bowl by binding and restricting Lines to the cytoplasm (Green et al., 2002; Hatini et al., 2005). The analysis of lines and bowl function in several tissues have led to a model whereby the two genes act as a binary switch to subdivide a field of cells into adjacent domains (Fig. 1B). bowl had been reported to specify distal and proximal tarsal fates and to inhibit medial tarsal fates. bowl had also been reported to mediate the morphogenesis of true joints but its role in this process had not been investigated (de Celis Ibeas and Bray, 2003). Given the relationship between lines and bowl in other tissues, we sought to understand how lines might complement the activity of bowl in patterning the tarsus, how lines and bowl might contribute to the patterning, growth and morphogenesis of true leg segments, and whether lines and bowl might play a general role in ventral appendage development.
We find that Lines is broadly cytoplasmic and thus inactive while Bowl is broadly nuclear and thus active throughout the leg disc at early stages. The progressive segmental subdivision of the leg disc correlates with the segmental accumulation of Lines in nuclei and a corresponding segmental destabilization of the Bowl protein. Across the emerging tarsus, Lines accumulates in nuclei where it modulates the opposing expression landscapes of dac and the tarsal PD genes. In this manner, Lines inhibits proximal tarsal fate and promotes medial and distal tarsal fates. Across emerging proximal segments, lines promotes Dl expression by destabilizing Bowl using a relief-of-repression mechanisms. Dl, then, acts to maintain Bowl expression in adjacent distal cells. In turn, bowl, together with one or more redundant factors, acts cell-autonomously to repress Dl expression in the Notch-activated region. This regulatory feedback mechanism generates a stable Notch signaling interface at segment borders. Our results lead us to extend and revise previous models of tarsal and segmental patterning. Finally, we uncovered analogous roles for lines in developing antennae, which are serially homologous to legs, revealing fundamental roles for lines in ventral appendage formation.
MATERIAL AND METHODS
Genetics and fly strains
UAS-N[rk111]Δ34 (C. Rauskolb), UAS-Dl, UAS-Myc-Lines (8) (weak insertion), UAS-Lines (9.2) (strong insertion), UAS-LinesRNAi (16801, VDRC), UAS-BowlRNAi (3775, VDRC), UAS-DrmEst (2.1) and UAS-Flag-Bowl (29) were expressed in clones using a combination of the FLP/FRT and the UAS/GAL4 techniques (Pignoni and Zipursky, 1997), or in restricted domains using the GAL4/UAS technique (Brand and Perrimon, 1993) with ptc-GAL4; UAS-GFP, Dll-GAL4md23, bab-GAL4Agal4-2, bab-GAL4Agal4-5, rn-GAL4GAL4-5, rn-GAL4GAL4-DeltaS and dac-GAL4P7d23, Klu-GAL4G410. The lines2f, linesG2, drm3, oddrk111, and bowl1 alleles were used to generate mutant clones using the FLP/FRT (Xu and Rubin, 1993), or the MARCM techniques (Lee and Luo, 2001). The following FRT-carrying chromosomes were used: w; 42DFRT Ubi-GFP/CyO (B. Edgar), y w hs-FLP122, Tub-GAL4, UAS-GFP; 42DFRT Tub-Gal80 hs-CD2, y+/CyO (G. Struhl); y w hs-FLP122, Tub-GAL4, UAS-GFP; Tub-Gal80 40AFRT/CyO; y w hs-FLP122; 42DFRT linesG2/CyO; bowl1 FRT40A/CyO. The 42DFRT Tub-Gal80 CD2, y+ chromosome was used to identify lines mutant clones in adult flies. The following reporters were used: Bar-lacZB-H2P058, ap-lacZUG62, odd-lacZrk111, Dl-lacZ and bib-lacZ4163.
Immunofluorescence and imaging
Eggs were collected in a drop of live yeast on grape agar plates and aged at 25°C for 60h, 72h, 84h, 96h or 120h to examine the dynamic distribution of Myc-Lines and Flag-Bowl. Discs were fixed and stained according to standard protocols using rabbit anti-Bowl (S. Bray), rabbit anti-dAP-2 (D1; P. Mitchell), mouse anti-Nub (2DAb7; S. Cohen), mouse anti-Dll (S. Cohen), guinea pig anti-Hth (R. Mann), rabbit anti-β-galactosidase (Cappel), rabbit anti-BarH1 (T. Kojima), rat anti-Bab2 (A. Laski), mouse anti-Dac, mouse anti-Dl and rat anti-Ci (DSHB), rat anti-C15 and rat anti-Al (G. Campbell) and guinea pig anti-dLim1 (J. Botas). Secondary antibodies conjugated to the Cy2, Cy3 and Cy5 fluorophores (Jackson ImmunoResearch) were used at 1:150. Nuclei were stained with Hoechst 33258 (Molecular Probes). Stained discs were scanned using a Zeiss LSM510 confocal microscope in multi-tracking mode. Adult legs were imaged using a Zeiss Axioscope 2+ and reconstructed using compositeZP. Images were assembled and adjusted using Adobe Photoshop CS3.
RESULTS
Lines accumulates in nuclei across the emerging tarsal primordium and the inter-joints of proximal leg segments
The activities of the Lines and Bowl proteins are regulated by post-translational mechanisms. Lines accumulates in nuclei where it is active and in the cytoplasm where it is repressed. Reciprocally, Bowl is unstable where Lines is active and stable where Lines is repressed (Hatini et al., 2003; Hatini et. al, 2005; Nusinow et al., 2008). To understand how lines contributes to leg development, we first examined the dynamics of Lines distribution relative to Bowl in developing leg imaginal discs. To do the analysis, we expressed a weak Myc-Lines transgene (UAS-Myc-Lines 8) and separately a weak Flag-Bowl transgene (UAS-Flag-Bowl 29) in a broad central domain using the Dll-GAL4 driver and examined the dynamic subcellular distribution of the tagged proteins. The ectopic expression of Myc-Lines and Flag-Bowl did not alter the morphology of adult legs suggesting that the tagged proteins were regulated by post-translational mechanisms that regulate the abundance and distribution of the endogenous proteins. At the early third larval stages, Lines was cytoplasmic, and thus inactive, in a broad central domain (Fig. 1C-C’). By the mid-third instar, Myc-Lines was detected in nuclei in the central fold in the emerging tarsal primordium (Fig. 1D-D’). At later stages, Myc-Lines remained nuclear in the tarsus (Fig. 1E-F’). In addition, Myc-Lines accumulated in nuclei in emerging inter-joints of proximal leg segments, and remained enriched in the cytoplasm in the pretarsus and presumptive true joints (e.g. ti/ta joint; Fig. 1F-G’). Expression of Myc-Lines with dac-GAL4 across the intermediate region of the leg disc revealed nuclear accumulation of Myc-Lines across the inter-joints of the tibia, femur and trochanter and cytoplasmic accumulation across true joints (Fig. S1C and data not shown). klumpfuss (klu)-GAL4 is expressed across the tarsus, the inter-joint of each leg segment and a narrow stripe immediately distal to the segment border. Expression of Myc-Lines with klu-GAL4 revealed a nuclear accumulation of Lines across the tarsus and across the inter-joint of each leg segments and a cytoplasmic accumulation in a narrow stripe immediately distal to the segment border (Fig S1B). In contrast, lines transcripts were detected broadly and at a uniform level consistent with the notion that lines is primarily regulated by post-translational mechanisms (Fig. S1A). The accumulation of Myc-Lines in nuclei coincided with the formation of the tarsus and each proximal leg segment suggesting an important role for lines in leg segmentation.
Lines destabilizes Bowl across the growing tarsus and the inter-joints of proximal leg segments
The Flag-Bowl protein was enriched in nuclei in a roughly complementary pattern to Myc-Lines. By the early third instar, Flag-Bowl was detected in nuclei in a broad central domain where Lines was cytoplasmic (Fig. 1H). At the mid-third instar, Bowl was downregulated in a circumferential domain in the nascent central fold (Fig. 1I) where Lines accumulated in nuclei. At later stages, Flag-Bowl was stable in the proximal half of true joints where Lines was cytoplasmic (Fig. 1J-K’). However, Flag-Bowl was unstable across the tarsal field and across the growing inter-joints of proximal segments where Lines was nuclear. Flag-Bowl was also unstable in the pretarsus and in the distal half of true joints where Lines was cytoplasmic suggesting that additional mechanisms modulate the activities of Lines and Bowl in these regions.
Analysis of Bowl accumulation with an antibody that recognizes the endogenous protein revealed a broad accumulation of Bowl at early stages (data not shown). This was followed by the loss of Bowl in a circular domain across the nascent tarsus and subsequently across each proximal leg segment (Fig. 1L, M, respectively). The spatiotemporal expression pattern of an odd-lacZ reporter was similar to that of Bowl (Fig. 1N-O). Collectively, these findings are consistent with a model whereby the formation of leg segments is dependent on the segmental repression of odd-skipped related genes and the coincidental segmental activation of Lines.
The roughly complementary distributions of Lines and Bowl suggested that lines destabilizes Bowl across the tarsal primordium and the inter-joints of true segments. Consistent with this idea, Bowl was stabilized cell-autonomously in lines mutant clones that were induced in inter-joint territories (Fig 5D-D”) (Hatini et al., 2005), and was destabilized in the Ptc domain or in FLP-out clones expressing a strong UAS-lines transgene (see Fig. S2A-A’ in supplementary material and data not shown). During embryogenesis, Drm stabilizes Bowl by inhibiting Lines (Hatini et al., 2005). Similarly, Bowl was stabilized ectopically across presumptive inter-joints in cells expressing drm with Ptc-GAL4 (Fig 5G-G’). However, Bowl was stable in drm mutant clones (Fig S2B-B”) suggesting that drm acts with other redundant factor/s to stabilize Bowl across presumptive joints. The dynamic pattern of Lines and Bowl distribution was largely complementary suggesting reciprocal roles for lines and bowl in tarsal and segmental patterning.
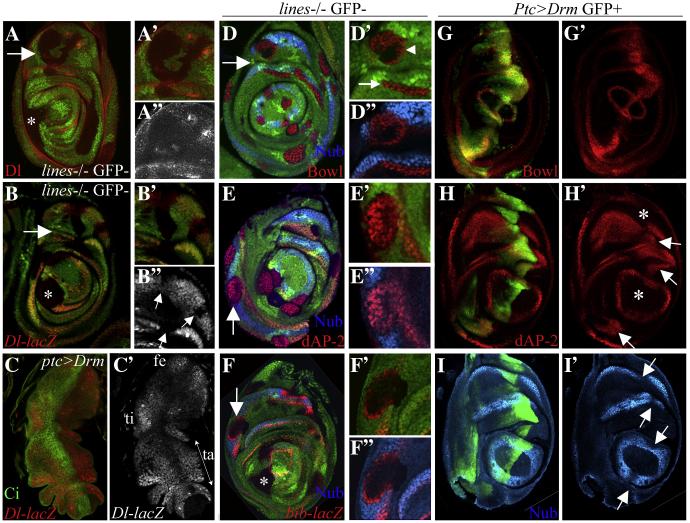
Figure 5.
lines maintains Dl expression across inter-joint territories and inhibits formation of ectopic segment borders
(A-B”, D-F”) lines FLP/FRT clones marked by the absence of GFP. (C-C’, G-I”) ptc>Drm; the ptc domains is marked by high Ci levels in C, and by GFP in G-I. Arrows in A-B and D-F point to magnified areas shown in insets. Asterisk in A, B and F indicates tarsal clones. (A) Dl and (B) Dl-lacZ were downregulated in lines mutant clones generated across the tarsus and across proximal leg segments. (C) Dl-lacZ was downregulated in drm-expressing cells. (D) Bowl was ectopically expressed and Nub was repressed in all the lines mutant clones. (E) Nub was repressed and dAP-2 was ectopically expressed in most of the lines mutant clones that were induced in the Nub domain. (F) The ring pattern of bib-lacZ expression was disrupted in lines mutant tarsal clones (asterisk). In addition, bib-lacZ accumulated ectopically in lines mutant clones that were induced in the Nub domain along clone borders. (G-G’) Bowl, and (H-H’) dAP-2 were ectopically expressed and (I-I’) Nub was repressed (arrows) in Ptc>drm expressing cells. dAP-2 was not induced in the pretarsus and was only weakly induced distal to the tibia in Ptc>drm expressing cell (asterisks).
lines promotes the growth and patterning of the tarsus and the proximal leg segments
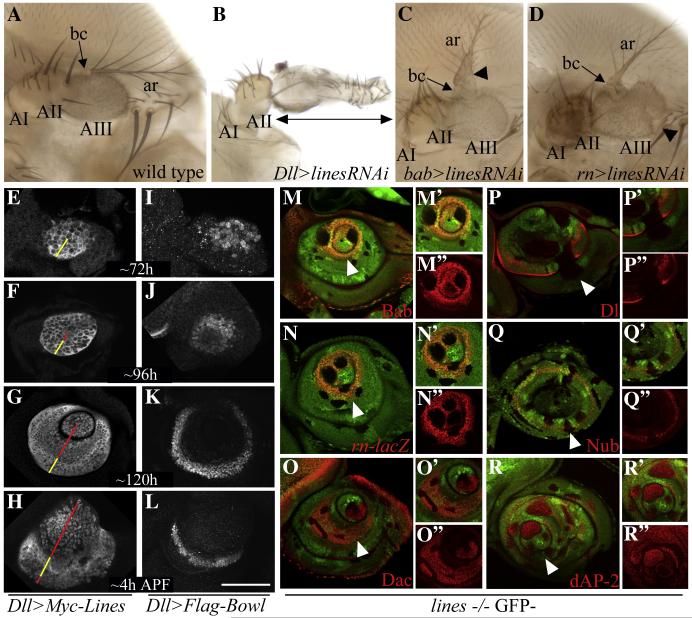
To test the contribution of lines to the formation of the tarsus, we expressed a UAS-linesRNAi transgene using the tarsal-specific bab-GAL4 and rn-GAL4 drivers, and the more broadly expressed Dll-GAL4 and dac-GAL4 drivers. We also removed lines function using the FLP/FRT and the MARCM techniques in genetically marked clones (Golic, 1991; Xu and Rubin, 1993; Lee and Luo, 2001). Subsequently, we analyzed the resulting phenotypes in adult legs. We focused the analysis on male prothoracic legs in which the proximal tarsal segment 1 (t1) is decorated at its base with a row of darkly pigmented sex comb (sc) bristles (Fig. 2A). Expression of linesRNAi with bab-Gal4, rn-GAL4 and Dll-GAL4 resulted in a phenotypic series in which progressively more distal tarsal segments were fused with t1 and differentiated sex comb bristles indicating that distal tarsal cells assumed t1 identity (Fig. 2B-E compare to wild type in Fig. 2A). Expression of linesRNAi with Dll-GAL4 led to the most severe tarsal phenotype-- the fusion of all the tarsal segments and the differentiation of sex comb bristles along the fused tarsus (Fig. 2E).
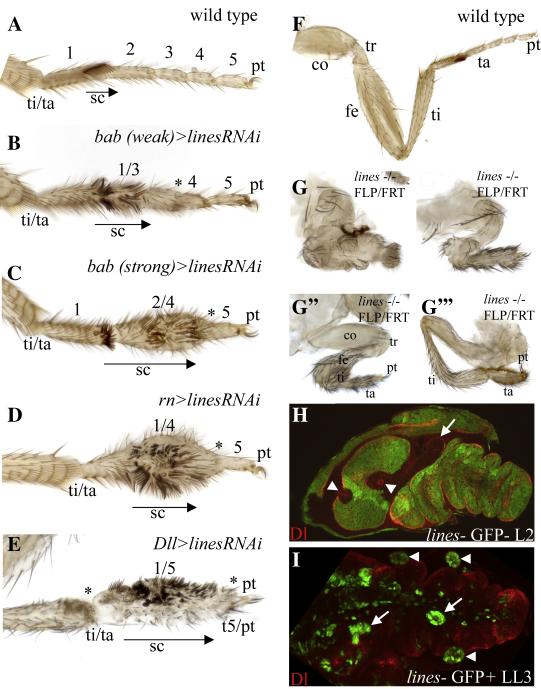
Figure 2.
The loss of lines function disrupts the formation of the tarsus and the growth and morphogenesis of true leg segments
(A-E) Tarsi of adult prothoracic legs; ti/ta- tibial/tarsal joint, 1-5- tarsal subsegments t1-t5, pt-pretarsus, sc- sex comb. Arrows indicate the scope of the region that differentiates sex comb bristles and assumes t1 identity, *-fused joint. (A) Wild type; the tarsus is subdivided into five jointed subsegments. The pretarsus forms the claw. (B) bab (weak)>linesRNAi, (C) bab (strong)>linesRNAi and (D) rn>linesRNAi (strong). Broad expression of linesRNAi across the tarsal primordium with various GAL4 drivers led to a phenotypic series in which progressively more distal segments fused with t1 and differentiated sex comb bristles indicating assumed t1 identity. (E) Dll>linesRNAi; most severe phenotype. t1-t5 were fused and sex comb bristles differentiated along the fused tarsus. In addition, the ti/ta and t5/pt joints were malformed and associated with internal necrotic vesicles. Bristle orientation in the tibia was randomized. (F) Wild type. (G-G”) Legs with lines MARCM clones induced at the second instar caused deep invaginations and a severe reduction in the growth of the tarsus and the proximal leg segments. (G”’) Clones induced at the early third instar led to reduced growth and segmentation of the tarsus and the bending of true leg segments. Images in F and G-G’” are shown at equal magnification. (H) lines FLP/FRT clones induced at second instar (48-72h after egg laying) were recovered in the tarsus and in proximal segments. However, the clones disrupted the shape and size of leg segments in a cell autonomous manner and blocked the formation of the segment border as reflected by the loss of Dl expression. Arrow points to tibial clone that caused a reduction in tibial size and loss of Dl expression. Arrowhead point to two smaller clones that appeared to sort out from surrounding cells. (I) lines MARCM clone induced at the late third instar (96-120 AEL); the clones formed round vesicles with smooth borders that segregated from the DP and accumulated both below (arrows) and above (arrows) the surface epithelium.
The expression of linesRNAi with dac-GAL4 along the intermediate region of the leg disc led to a severe decrease in size of true segments and loss of true joints (Fig. S1E-E”, compare to wild type in S1D-D”; asterisks mark fused joints). These phenotypes suggested additional roles for lines in controlling the growth of proximal segments and the formation of true joints. In addition, cuticle decorated with bristles was replaced with naked cuticle and numerous vesicles were detected under the basal surface of the epithelium within the leg shaft (Fig. S1E-E”).
To explore the role of lines in leg development in further detail we examined the effect of lines mutant clones on leg development. lines mutant clones induced at the second instar led to the shortening and fusion of all the proximal segments and the formation of deep invaginations in the cuticle (Fig. 2G-G”). Clones that were induced at the early third instar led to the shortening of the tarsus and the fusion of tarsal segments (Fig. 2G”’). However, lines mutant clones marked by the loss of a yellow+ transgene in a yellow− background were not recovered suggesting that the lines mutant cells failed to contribute to inter-joint tissue or to differentiate inter-segmental yellow− bristles consistent with the phenotype of the dac>linesRNAi legs described above. We occasionally observed out-pocketing of epithelial tissue decorated with smooth cuticle at inter-joint territories suggesting that at least a subset of the lines mutant clones survived to adult stages, sorted out from the epithelium and failed to differentiate bristles (Fig. S1F, arrow indicates a positively marked lines MARCM clone). The over-expression of lines in cell clones led to the accumulation of melanotic tissue near true leg joints (Fig. S1G). Altogether, these loss- and gain-of-function phenotypes implicated lines in the formation, growth and patterning of each leg segment.
Analysis of clone recovery and sorting behavior in developing imaginal discs revealed that the lines mutant clones induced at the second instar were recovered at a similar rate to control clones. However, these clones appeared to cause a reduction in the size of proximal leg segments (Fig. 1H, arrow points to a tibial clones that caused a cell autonomous reduction in tibial size). Similarly, clones that were induced at the early and third larval stages survived to late larval and early pupal stages and their morphology was dependent on their position across both tarsal and proximal leg segments. Clones that were induced in the native Bowl domain assumed a normal elongated shape comparable to the morphology of control clones (Fig 5D’, arrow). In contrast, clones that were induced adjacent to the endogenous Bowl domain assumed an abnormal rounded shape with smooth borders and thus sorted apart from surrounding wild type cells (Fig 5D’, arrowhead). Similarly, lines MARCM clones induced at the third instar and analyzed at early pupal stages either extruded inwards from the basal surface of the epithelium into the disc lumen (Fig. 2I, arrows), or outwards from the apical surface (Fig. 2I, arrowheads). Below we examined the lines loss- and gain-of-function phenotypes using molecular markers to delineate the pathways in which lines acts.
lines specifies distal and medial tarsal fates and inhibits the specification of proximal tarsal fates
de-Celis Ibeas & Bray (2003) had previously reported that bowl promotes proximal and distal tarsal fates and inhibits medial tarsal fates. If lines acts reciprocally to bowl, lines should promote medial tarsal fates and repress proximal and distal tarsal fates. To test this prediction, we examined the expression of the leg gap gene dac and the tarsal PD genes, bab2 and BarH1 (referred to as bab and Bar) in Dll>linesRNAi leg discs. These genes are expressed in broad overlapping PD domains across the tarsal region, and their expression pattern roughly corresponds to the regions affected by their absence. dac mediates the formation of the femur, tibia and the three proximal tarsomeres (Mardon et al., 1994). bab 1 and 2 mediate the formation of tarsal segments 2 to 5 (Couderc et al., 2002; Godt et al., 1993; St Pierre et al., 2002) while BarH1 and 2 (Bar) mediate the formation of tarsal segments 3-5 (Kojima et al., 2000). We found distal expansion of Dac, loss of Bab, and distal retraction of Bar expression in these discs (Fig. 3D-F” compare to wild type in A-C”). The coordinated changes in expression of Dac and the tarsal PD genes corresponded nicely to the patterning defects seen in adult Dll>linesRNAi legs (Fig. 2E). To confirm these results, we examined the expression of these genes in lines mutant clones induced at the second instar using the FLP/FRT technique. We found that Bab was lost in all the lines mutant clones (Hatini et al., 2005), and Bar was downregulated in the proximal region of the Bar domain (Fig. 3G-G” and H-H”, respectively). The tarsal PD gene ap is expressed in tarsal segment 4 and controls that proper development of this segment (Kojima et al., 2000). Similar to the regulation of bab and bar by lines, the expression of an ap-lacZ reporter was repressed in drm-expressing clones in which the activity of lines was inhibited (Fig. S3A). Conversely, Dac was ectopically expressed in tarsal clones that were induced between the native Dac domain and the central fold (Fig. 3I-I”).
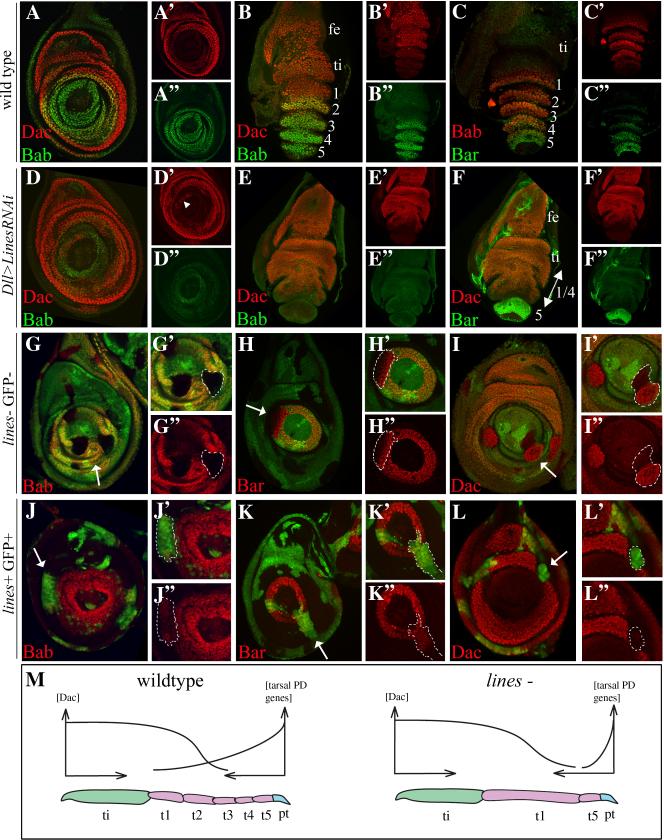
Figure 3.
lines specifies distal and medial tarsal fates and inhibits the specification of proximal tarsal fate
(A-C”) Wild type. (D-F”) Dll>linesRNAi. (G-I”) lines FLP/FRT clones. (J-L”) lines expressing FLP-out clones. Boundaries of selected clones were outlined by dashes for ease of identification. Arrows in G-L point to magnified areas shown in insets. (A-B”) Dac and Bab and (C-C”) Bab and Bar are expressed in broad nested domains. (A-B”) Dac levels are high in the tibia, t1 and t2 and lower in t3. (A-C”) Bab expression is high in t4 and t3 and progressively lower in t2 and t1; (C-C”) Bar is high in t5 and progressively lower in t4 and t3; (D-F”) Dll>linesRNAi, (D-D”) third instar and (E-F”) everting discs; (D-F”) Dac expression expanded distally. Note ectopic Dac in distal cells in D (arrowhead); (D-E”) Bab was lost; (F-F”) Bar was retracted distally. Similarly, in lines mutant clones (G-G”) Bab was lost, and (H-H”) Bar was downregulated in the proximal region of the Bar domain. (I-I”) Dac was ectopically expressed between the Dac domain and the central fold in lines expressing clones, (J-J”) Bab and (K-K”) Bar were ectopically expressed adjacent and near their respective domains, and (L-L”) Dac was repressed in the proximal region of the Dac domain. (M) A model depicting the contribution of dac, the tarsal PD genes and lines to tarsal patterning and segmentation. lines represses dac expression and promotes expression of tarsal PD genes proximal to the central fold, which marks the boundary between the pretarsus and tarsal segment 5 and the remaining proximal tarsomeres. See text for further detail.
To determine if lines was sufficient to influence tarsal patterning, we examined the expression of these proteins in genetically marked clones expressing a strong UAS-lines transgene. We found ectopic expression of Bab, Bar and ap-lacZ in clones that were induced near their respective expression domains (Fig. 3J-J”, K-K” and Fig. S3B-C, respectively). Conversely, we found repression of Dac in clones spanning the distal region of the Dac domain (Fig. 3L-L”).
Finally, we found that bowl mediated the lines clonal phenotypes because Bab was maintained in lines bowlRNAi clones (Fig. S2C). Thus, contrary to our expectation, we found that lines promotes the specification of both distal (marked by ap and Bar) and medial (marked by Bab) tarsal fates and inhibits the specification of proximal tarsal fate (marked by Dac). We therefore propose an extension, and a partial revision, to the model proposed by de-Celis Ibeas & Bray (2003) whereby bowl promotes the specification of proximal tarsal fates only, and lines antagonizes bowl across the emerging tarsal primordium, proximal to the central fold, to allow expression of tarsal PD genes by a gradient of EGF receptor signaling that emanate from the distal tip of the leg disc (see Fig. 3M for a schematic depiction of the model).
To determine if lines contributes to the primary subdivision of the leg PD axis, we also examined the expression of Dll and Hth in lines mutant clones. We found no change in expression of Dll in the clones indicating that lines acts either downstream or in parallel to Dll to pattern the tarsus (Fig. S4A). Similarly, we found no change in expression of Hth indicating that lines is not involved in PD patterning of the disc periphery (Fig. S4B). Finally, we also examined the expression of the pretarsal proteins dLim1, C15 and Aristaless (Al) (Campbell, 2005; Campbell et al., 1993; Kojima et al., 2005; Lilly et al., 1999; Schneitz et al., 1993; Tsuji et al., 2000), whose expression is dependent on high levels of EGFR signaling (Campbell, 2002; Galindo et al., 2002). We detected relatively normal levels of these proteins in the clones indicating that lines does not affect the expression of EGFR ligands or the activation of EGF receptor signaling (see Fig S4C, and data not shown).
lines maintains Dl expression across proximal leg segments to maintain a stable Notch signaling interface at leg segment borders
In the absence of lines function true leg segments were reduced in size and joints were lost as shown above (Fig. 2G-G” and S1E-E”). We hypothesized that, similar to its segment polarity role in embryos lines could participate in patterning each true leg segment. A key step in segmental patterning is the initiation of Dl and Ser expression across each leg segment and the establishment of a stable border between Dl/Ser expressing cells and adjacent distal cells. Dl and Ser signal across this border to induce expression of target genes, which further mediate segmental growth and joint formation. In addition, these targets participate in a negative feedback regulation to repress Dl and Ser expression cell-autonomously to stabilize the segment border (Ciechanska et al., 2007; Shirai et al., 2007). Notch pathway activation promotes Bowl accumulation in the Notch-activated region (Campbell, 2005; de Celis Ibeas and Bray, 2003). In turn, Bowl may act to repress Dl and Ser cell-autonomously to stabilize the segment border. Reciprocally, lines may antagonize bowl to maintain Dl and Ser expression in proximally adjacent cells by relief-of-repression (see Fig. 4J for a model). If this model is correct, the accumulation of Bowl in lines mutant clones that form within the Dl/Ser domain could lead to the repression of Notch ligands in the clones. Dl and Ser produced by surrounding wild type cells may then be permitted to induce expression of Notch targets in the clones.
Figure 4.
Expression of Dl and Notch targets defines multiple domains across true leg segments (A, C, E, G) Late third instar. (B, D, F, H) Everting pupal legs. Leg discs were double labeled to map domains of gene expression. Dl, Dl-lacZ, dAP-2 and bib-lacZ were detected in all the leg segments, while Bowl, odd-lacZ and Nub were detected in true leg segments only. (A-B”) Bowl was detected in the proximal half of true joints distal to the Dl-lacZ domain in a partially overlapping domain. (C-C”) Bowl was detected distal to Nub in a partially overlapping domain. (D-D”) odd-lacZ was coexpressed with Bowl in the proximal half of joint constrictions. (E-F”) dAP-2 was detected distally adjacent to the Nub domain in a broad region that spanned the joint constriction. (G-H”) bib-lacZ was detected along the segment border distal to Dl (G-G”) and Nub (H-H’) in a partially overlapping domain. (I) A cartoon depicting the expression of Dl and Notch targets relative to the tibial/tarsal joint in everting legs. (J) A model depicting the interactions that regulate Dl expression across true leg segments. dve represses Dl expression proximal to Dl-expressing cells and Bowl together with one or more redundant factor represses Dl expression in the Notch activated region. Lines antagonizes Bowl to maintain Dl expression in proximal cells. Together, lines and bowl act as a binary switch to maintain a stable Notch-signaling interface at the distal end of each leg segment.
To address this hypothesis, we first examined the relative expression of the Notch ligand Dl and the Notch targets Bowl, Odd, Nub, dAP-2, and Bib in wild type leg discs at late stages of leg development. While Dl, dAP-2 and Bib are expressed across both tarsal and true leg segments, Bowl, Odd and Nub are only expressed across true leg segments (Fig. 4). At late third instar and in everting leg discs, a Dl-lacZ reporter was detected at the distal end of leg segments and Bowl was detected in a distal and a slightly overlapping domain in the proximal half of presumptive joints (Fig. 4A-B). Similarly, an odd-lacZ reporter was co-expressed with Bowl in this region (Fig. 4D) (de Celis Ibeas and Bray, 2003). The expression pattern of Nub and dAP-2 differed significantly from that of Bowl. Similar to Dl-lacZ, Nub was expressed proximal to Bowl in a partially overlapping domain (Fig. 4C). By the late third larval stage, Nub and dAP-2 were detected in adjacent non-overlapping domains (Fig. 4E). In everting legs, Nub was detected in a narrow domain just proximal to the presumptive joint (e.g. ti/ta joint), and dAP-2 was broadly expressed across the joint constriction (Fig. 4F). Bib, a member of the aquaporin family of channel proteins, is required for the reception of the Notch signal and is upregulated in Notch activated cells (Doherty et al., 1997; Rao et al., 1990). A bib-lacZ reporter was upregulated at the distal edge of Dl and Nub expressing cells along the segment border (Fig. 4G-H”). Thus, at late larval and early pupal stages, Bowl (and Odd), dAP-2, Bib, and Nub are each expressed in a different domain relative to the segment border (see Fig. 4I for a schematic depiction of the segment border). The co-expression of Nub and Dl is surprising given that Nub is a Notch target (Rauskolb and Irvine, 1999) and the Dl/Ser expressing-cells are believed to be refractory to Notch signaling. The expression of bib-lacZ along the segment border appears to mark cells that respond to Notch signaling. Bowl and dAP-2 are both detected across several cell diameters distal to the Notch signaling interface suggesting that their expression at a distance from this interface is maintained by autoregulatory mechanisms.
To determine if lines contributes to the formation of segment borders (see Fig. 4J for a model), we examined the expression of the Dl ligand and a Dl-lacZ reporter in lines mutant clones. The expression of Dl and Dl-lacZ was either lost or reduced in lines mutant clones in both tarsal and non-tarsal segments (Fig. 5A-A” and B-B”, respectively), which reflects the dual role that lines plays in tarsal and segmental patterning. The downregulation of Dl and Dl-lacZ expression in tarsal clones (indicated in asterisks in Fig. 5A-B) results from changes in expression of Dac and the tarsal PD genes, which mediate tarsal segmentation. The loss of Dl expression in proximal clones could reflect a second role for lines in maintaining Dl expression and thus a stable Notch signaling interface across true leg segments.
To further test this hypothesis, we examined the expression of the Notch targets, Bowl, Nub, dAP-2 and Bib in lines mutant clones. We detected downregulation of Nub in clones that spanned the endogenous Nub domain (Fig. 5D-F”). Reciprocally, we detected ectopic cell-autonomous induction of Bowl in all the clones (Fig. 5D-D”). Similarly, we detected ectopic dAP-2 expression in most clones that were induced in the Nub domain (Fig. 5E-E”). The bib gene is induced in a narrow stripe along the segment border in the Notch activated region as shown above (Fig. 4G-H”). We detected perturbation of bib expression in tarsal clones (asterisks in Fig. 5F points to a tarsal clone) reflecting the role lines plays in tarsal segmentation as discussed above. In addition, we detected ectopic bib expression along the borders of lines mutant clones that were induced in the Nub domain of true segments (Fig. 5F’-F”). We propose that the loss of Dl expression in the lines mutant clones permitted Dl produced by surrounding wild type cells to induce expression of Notch targets in the clones. These results further support the idea that lines controls the formation of segment borders.
To determine if bowl mediates the lines clonal phenotype, we ectopically expressed drm with Ptc-GAL4 to block Lines and stabilize Bowl (Fig. 5G-G’). We found that drm was sufficient to downregulate the expression of Dl-lacZ and Nub (Fig. 5C-C’ and I-I’, respectively) and to promote the expression of dAP-2 (Fig. 5H-H’) across inter-joints. To test this idea directly, we examined Dl expression in lines bowlRNAi clones. We found no change in Dl expression in the clones indicating that bowl represses Dl expression and lines inhibits bowl to promote Dl expression by a relief-of-repression mechanism (Fig S2D).
If bowl represses Dl expression in the Notch-activated region, the loss of bowl function or the ectopic expression of lines should disrupt the formation of the segment border. To test this idea, we examined the expression of dAP-2 and Nub in bowl MARCM clones and in lines FLP-out clones. We found no change in expression of these markers in most clones. However, in a small number of clones we detected downregulation of dAP-2 and upregulation of Nub along the segment border (Fig. S2E-E”, F-F”, respectively, and data not shown). These changes in gene expression suggest that bowl contributes to the formation of a stable segment border although it is not absolutely essential. The low incidence of these phenotypes suggests that bowl acts redundantly with one or more factors, possibly odd and/or sob, to stabilize the segment border. The drmP2 deficiency removes drm, sob and odd and approximately 30 other genes. We therefore attempted to generate drmP2 mutant clones to determine if the three genes act redundantly to stabilize the segment border. However, we failed to recover drmP2 clones in either a wild type or a Minute background (data not shown). In addition, we generated MARCM bowl oddRNAi clones but observed no changes in expression of dAP-2 or Nub in these clones (data not shown). Thus, additional work will be required to identify the combination of factors that act redundantly to repress Dl expression in the Notch activated region.
lines organizes PD and segmental patterning in developing antennal imaginal discs
The Drosophila antennae are serially homologous to legs and have been considered to evolve from an ancestral leg-like appendage by the activity of homeotic and field-specific selector genes (Schneuwly et al., 1987; Shubin et al., 1997; Casares and Mann, 1998; Casares and Mann, 2001). To determine if lines is generally required to mediate ventral appendage development, we examined the dynamic sequence of Lines distribution relative to Bowl in developing antennae, and the contribution of lines to antennal PD and segmental patterning.
The antennal appendage is composed of the proximal AI-AIII segments, the basal cylinder (bc) and the distal arista (ar) (Fig. 6A). At the early third instar, a Myc-Lines transgene expressed with Dll-GAL4 was enriched in the cytoplasm in a broad central domain (Fig. 6E). By the mid-third instar, Myc-Lines appeared in nuclei at the distal tip of the antenna and in the cytoplasm in surrounding proximal cells (red bar in Fig. 6F). At late larval and early pupal stages, Myc-Lines was nuclear in a broader distal region corresponding to the presumptive AIII, the basal cylinder and the arista, and cytoplasmic in the adjacent proximal region (Fig. 6G and H, respectively). While Lines accumulated in nuclei at the distal tip of the antenna (Fig. 6H), it accumulated in the cytoplasm at the distal tip of the leg (Fig. 1D) revealing a variation in the regulation of Lines between legs and antennae or the lack of equivalent tissue in antennae. We also expressed UAS-Flag-Bowl with Dll-GAL4 to examine the pattern of Bowl stabilization relative to Myc-Lines. By the early third instar, Bowl was nuclear at the distal region of the disc where Lines was cytoplasmic (Fig. 6I). At later stages, Flag-Bowl was lost from the distal tip, but was nuclear in surrounding proximal cells where Lines was cytoplasmic (Fig. 6J-L). Similarly, the Bowl protein was broadly nuclear at early stages (data not shown). At later stages, Bowl was lost from a circular domain at the distal tip of the antenna (Fig S5A) and subsequently in three rings corresponding to the inter-joints of antennal segments AI-AIII (Fig. S5B).
Figure 6.
lines contributes to antennal PD and segmental patterning
(A-D) Adult antennae. (E-H) Dll>Myc-Lines; yellow and red bars mark regions where Lines was enriched in the cytoplasm and nucleus, respectively. (I-L) Dll>Flag-Bowl. (M-R’) lines mutant FLP/FRT clones marked by the absence of GFP and stained for PD (M-O”) and segmental markers (P-R”). (A) Adult antennae consist of the AI-AIII segments, the basal cylinder (bc) and the arista (ar). (B) Dll>linesRNAi; AIII and the arista were replaced with an elongated tubular structure (double arrow) that was poorly differentiated. (C) bab>linesRNAi; the basal cylinder (arrow) and the aristal stalk (arrowhead) were expanded, malformed and poorly differentiated. (D) rn>linesRNAi; AIII and the basal cylinder were malformed. (E) ~72h; Myc-Lines was cytoplasmic in a broad central domain; (F) ~96h; a new domain where Lines accumulates in nuclei emerged at the distal tip. (G-H) ~120h and ~4h APF, respectively; this domain expanded to encompass the presumptive AIII, basal cylinder and the arista. Myc-Lines also appeared in nuclei in emerging antennal segments. (I-L) Bowl accumulated in a reciprocal pattern to Lines. (I)~72h; Bowl was nuclear in a broad distal domain where Lines was cytoplasmic. (J) ~96h; Bowl was unstable in the distal tip of the antenna where Lines was nuclear. (K-L) ~120h and ~4h APF; Bowl was stabilized in presumptive AII/AIII joints. The endogenous Bowl protein is detected in two concentric rings that correspond to the AI/AII and AII/AIII antennal joints (not shown). (M) Bab and (N) rn-lacZ were lost in lines mutant clones. (O) Dac was ectopically expressed distal to the Dac domain and was lost in the endogenous Dac domain. (P) Dl, and (Q) Nub were downregulated and (R) dAP-2 was ectopically expressed in most of the lines mutant clone. Scale bar= 20μm in E, I, 50μm in F, J, H, L, 75μm in G, K.
To investigate the contribution of lines to antennal PD patterning, we expressed a linesRNAi transgene with Dll-GAL4 and found that AIII, the basal cylinder and the arista were replaced with a poorly differentiated tubular structure (Fig. 6B). The expression of linesRNAi with bab-GAL4 led to poor differentiation and expansion of the basal cylinder and the aristal stalk (Fig. 6C). The expression of linesRNAi with rn-GAL4 distorted the morphology of AIII and led to a poor differentiation of the basal cylinder (Fig. 6D). The observed morphological malformations were restricted to the regions where each driver was expressed. lines mutant clones induced at the second instar led to similar malformations in adult antennae (not shown). Thus, lines patterns a broad distal domain in which it localizes to nuclei. To further explore the role of lines in antennal PD patterning, we examined the expression of Bab, rn-LacZ and Dac in lines mutant clones. We detected loss of Bab and rn-LacZ (Fig. 6M-M” and N-N”, respectively) and ectopic Dac expression in lines mutant clones that were induced distal to the endogenous Dac domain (Fig 6O-O”). rn was also lost in lines mutant clones induced in the leg imaginal disc (data not shown). We also detected repression of Dac in the endogenous Dac domain (Fig 6O-O”) revealing a dual role for lines in repressing Dac expression distally and maintaining its expression medially. In the leg, lines does not maintain Dac expression medially, revealing a variation between the function of lines in legs and antennae. Similarly, we detected a near complete loss of Bab expression and upregulation of Dac expression near the distal tip in Dll>linesRNAi antennal discs (data not shown). To analyze the role of lines in patterning proximal antennal segments, we examined the expression of Dl, Nub and dAP-2 in lines mutant clones. We detected downregulation of Dl and Nub and ectopic dAP-2 expression in the clones across both distal and proximal antennal segments (Fig 6P-P”, Q-Q”, R-R”, respectively). We infer that lines maintains Dl expression to generate a stable Notch signaling interface across each antennal segment. Our data suggests that lines plays analogous roles in both leg and antennal development. However, we also find significant variations in the regulation and function of lines between legs and antennae that may have contributed to the evolution of distinct ventral appendage morphologies.
DISCUSSION
We assign two crucial roles for lines in patterning the tarsal PD axis and in patterning true leg segments. Lines accumulates in nuclei in the emerging tarsal primordium where it antagonizes bowl to specify medial and distal tarsal fates and inhibit proximal tarsal fates. In addition, Lines accumulates in nuclei across inter-joints of true leg segments where it antagonizes bowl to promote Dl expression. We provide evidence from misexpression analysis and genetic epistasis to suggest that bowl together with one or more redundant factors, possibly odd and sob, acts reciprocally to lines to repress Dl expression in the Notch-activated region in order to maintain a stable Notch signaling interface between Dl-expressing cells and adjacent distal cells. Finally, we assign analogous roles for lines in patterning the antennal imaginal disc. We propose central roles for lines in mediating leg and antennal segmentation and consider a possible evolutionarily conserved role for odd-skipped genes in arthropod and vertebrate limb development.
lines modulates the expression levels of the leg gap gene dac and the tarsal PD genes bab, ap and bar to mediate tarsal patterning and segmentation
At the mid third larval stage, Lines accumulates in nuclei in a circumferential domain in the emerging tarsal primordium (Fig. 1D-E). In lines deficient legs, tarsal segments 1-5 were fused and distal cells assumed proximal t1 identity (Fig. 2E) indicating that lines specifies medial and distal tarsal fates. The leg gap gene dac and the tarsal PD genes mediate tarsal patterning and segmentation. Dac is distributed in a modest proximal to distal gradient with high levels in the tibia, t1 and t2 and lower levels in t3 (Fig. 3A-B) (Mardon et al., 1994). The tarsal PD genes are distributed in a modest distal to proximal gradient (Fig. 3A-C) (Couderc et al., 2002; Godt et al., 1993; Kojima et al., 2000; St Pierre et al., 2002). The graded expression of the tarsal PD genes is established by a gradient of EGFR signaling generated by the secretion of EGF receptor ligands from the distal tip of the leg (Campbell, 2002; Galindo et al., 2002). Reciprocal cross-regulatory interactions between these genes further refine their expression domains. We find that lines is both necessary and sufficient to repress dac expression and to promote the expression of the tarsal PD genes bab, ap and bar across the tarsal primordium. By expressing a linesRNAi transgene with various GAL4 drivers, we obtained a phenotypic series that reveals a higher sensitivity of t2 and progressively lower sensitivities of t3 and t4 towards transformation into t1 (Fig. 2B-E) indicating that the region most sensitive to fate transformation is where the opposing expression landscapes of dac and the tarsal PD genes intersect. We, thus, infer that lines modulates these expression landscapes to establish cell type diversity across the tarsal field and to mediate tarsal segmentation (see Fig. 3M for a model). bowl has been proposed to repress ap expression to subdivide the distal limb field into smaller domains (Campbell, 2005). However, the observation that Bowl and Ap are expressed several cell diameters apart from one another makes it unlikely for Bowl to directly repress ap expression and inconsistent with this model. We favor an alternative model whereby Bowl acts to generally repress the expression of the tarsal PD genes at early stages, whereas Lines destabilizes Bowl at later stages to permit expression of the tarsal PD genes by relief-of-repression. This model is consistent with the dynamic expression pattern of Lines and Bowl in the emerging tarsal field and with the general role that lines plays in regulating the expression of tarsal PD genes as reported in this study.
lines deficient legs develop a simple tarsus that resembles the unsegmented tarsus of primitive arthropods (Snodgrass, 1935). It is therefore conceivable that bowl mediates the formation of the ancestral unsegmented form of the tarsus, a function reflected in the stabilization of Bowl in a broad domain at early stages. The activation of Lines within the nascent tarsus may reflect a more recent evolutionary change that enabled the formation of additional tarsal segments found in higher arthropods. Phylogenetic comparisons of the regulation and function of lines and odd-skipped genes in tarsal patterning will be required to evaluate this model.
lines and odd-skipped related genes may act as a binary switch to maintain a stable Notch signaling interface at segment borders
In lines deficient legs, the proximal leg segments were severely reduced in size and joints were lost (Fig. 2G-G” and Fig S1E-E”). A key step in the formation of leg segments is the initiation of Dl and Ser expression across each leg segment and the generation of a Notch signaling interface between the Dl/Ser domain and the adjacent distal domain (Bishop et al., 1999; de Celis et al., 1998; Mishra et al., 2001; Rauskolb and Irvine, 1999). The formation of a stable segment border depends on multiple levels of control. The gene defective proventriculus (dve) represses Dl expression in the proximal part of each leg segment (Ciechanska et al., 2007), while the glycosyl transferase fringe (fng) can modulate binding between Notch and its ligands in the Dl/Ser domain or proximal to it (Fleming et al., 1997; Panin et al., 1997). In addition, Dl and Ser can autonomously inhibit Notch activation within the Dl/Ser domain (de Celis and Bray, 1997; Doherty et al., 1996; Klein et al., 1997; Micchelli et al., 1997). Finally, Notch targets, such as dAP-2, can repress Dl and Ser expression in the Notch activated region to stabilize the segment border using negative feedback regulation (Ciechanska et al., 2007; Shirai et al., 2007).
Our studies provide evidence that lines and odd-skipped related genes drm and bowl participate in a gene regulatory network to generate the segment border. Following the activation of Dl proximal to the presumptive joint, Dl signals to adjacent distal cells to activate drm (and one or more redundant factors, possibly odd and/or sob) (Hao et al., 2003). Drm, in turn, acts cell autonomously to inhibit Lines thereby allowing Bowl to accumulate in the distal region of each true leg segment. Bowl, then, functions to repress Dl expression in this region. Reciprocally, Lines antagonizes Bowl to maintain Dl expression in adjacent proximal cells. Together, lines and bowl (and one or more redundant factors) act to generate and maintain a stable Notch signaling interface between Dl-expressing cells and adjacent distal cells (see Fig 4J for a model). Factors induced along this interface are believed to mediate the growth of leg segments and the morphogenesis of leg joints.
In early leg discs, Bowl and odd are broadly expressed. The formation of leg segments correlates with the segmental downregulation of Bowl and odd expression and the coincidental accumulation of Lines in nuclei. It is plausible that the leg gap gene hth, dac and Dll act combinatorially to repress the expression of the odd-skipped genes and to initiate the segmental expression of Dl and Ser using a relief-of-repression mechanism. We provide evidence that Dl, Ser and odd-skipped genes, in turn, regulate each other’s expression to stabilize and maintain the segment border. This model predicts that cis-regulatory modules in the odd-skipped related genes drm, sob and/or odd are configured to respond to the repressive activities of certain combinations of the leg gap proteins, while cis-regulatory modules in Dl and Ser are configured to respond to the repressive activities of Odd-skipped proteins.
While the morphogenesis of tarsal joints depends on Jun Kinase (JNK)-reaper-dependent apoptosis (Manjón et al., 2007), the mechanisms that generate true joints are not known. odd-skipped family genes have been proposed to initiate the cytoskeletal rearrangements that mediate leg joint morphogenesis at the border between leg segments by controlling the expression of putative cytoskeletal regulators (Hao et al., 2003). Our findings suggest instead that odd-skipped related genes influence epithelial morphology rather indirectly by stabilizing the Notch signaling interface at segment borders (Fig. 5). Our findings, however, do not exclude the possibility that odd-skipped related genes might act in a parallel pathway to control joint morphogenesis. It is conceivable that the special mechanical properties of the interface between Bowl-expressing cells and the adjacent distal cells buckle the epithelium along this border to initiate joint morphogenesis. While Bowl accumulates in the proximal half of true joints (Fig 1K’, 4B-B”, D-D”), dAP-2 is expressed uniformly across prospective joints (Fig. 4F-F”). dAP-2 may therefore act to modulate cell adhesion and cytoskeletal dynamics to remodel the topology of prospective joints. The phenotype of dAP-2 mutant legs is consistent with such a role (Ciechanska et al. 2007; Kerber et al., 2001; Monge et al., 2001). Understanding how segmental patterning is coordinated with epithelial morphogenesis will necessitate the identification of the genes that directly regulate epithelial morphogenesis.
During antennal development, bowl inhibits the formation of ectopic antennae by repressing wg expression in dorsal cells (Bras-Pereira & Casares, 2008). Loss of bowl function, however, does not cause defects in antennal PD patterning or in antennal segmentation. Given that lines affects both antennal PD patterning and antennal segmentation (Fig. 6) and given the relationship between lines and bowl in other tissues, we would predict that bowl acts together with one or more redundant factors reciprocally to lines to pattern the antennal PD axis and mediate antennal segmentation.
Evolutionary perspective
The antennae, feeding appendages, legs and genitalia are very different in structure and function but have been considered to diverge from an ancestral ventral appendage on the basis of comparative anatomical and molecular studies (Shubin et al., 1997). The analogous contribution of lines to leg and antennal development suggests an evolutionary conserved role for lines in ventral appendage formation. The variations in the regulation and function of lines and odd-skipped genes between legs and antennae may have contributed to the morphological diversity of these appendages. It is further intriguing to consider the possibility that Odd-skipped related (Osr) genes had been deployed in the common ancestor of arthropods and vertebrates, and had evolved to perform analogous roles in limb development in both phyla. Consistent with this idea, at early stages, vertebrate Osr1 and Osr2 are expressed in broad PD domains across the nascent limb bud, while at later stages their expression shifts to sites of synovial joint formation (Stricker et al., 2006). The striking similarity in the dynamic expression of odd-skipped family genes in flies and vertebrate limb development may reflect a remarkable case of convergent evolution or a common origin in developmental pathways that are now deployed in both phyla. Comparative phylogenetic studies will be required to explore this question.
Supplementary Material
Figure S1: The expression, distribution and function of lines across intermediate leg segments
(A) Analysis of lines expression using in situ hybridization; lines was expressed ubiquitously and at uniform levels in developing leg imaginal discs. (B-C) However, the subcellular distribution of the Lines protein was modulated. Red bars, regions where Myc-Lines accumulates in nuclei; yellow bar, regions where Myc-Lines accumulates in the cytoplasm. (B) klu>myc-lines; klu-GAL4 is expressed across each inter-joint and in a distal stripe along true segment borders. Lines accumulated in nuclei across the tarsus and the inter-joint of each proximal leg segment (red bars) and in the cytoplasm in a thin stripe adjacent to the segment border (yellow bars). (C) dac-GAL4 is expressed in an intermediate region of the leg in the presumptive femur, tibia and three proximal tarsi. Lines was nuclear across tarsal segments and across the inter-joint of the tibia and femur (red bars and data not shown). In contrast, Lines was enriched in the cytoplasm in the ti/ta and the fe/ti joints (yellow bars). (D) Wild type; the tr/fe (D’), fe/ti and ti/t1 (D”) form flexible joints in adult leg. (E) dac>linesRNAi; the tr/fe (E’), fe/ti and ti/t1 (E”) were fused (asterisks indicate fused joints). Leg segments differentiated smooth cuticle and numerous vesicles were detected basal to the surface epithelium. In addition tarsal segments 1 to 3 were also fused (not shown). (F) lines MARCM clones induced at 96-120h; GFP-positive clones sorted out from the surface epithelium and formed lateral outgrowths (arrow). (G) lines FLP-out clones induced at 96-120h caused accumulation of melanotic tissue adjacent to true joints (arrow).
Figure S2: The regulation and function of bowl in PD and segmental patterning
Ectopic expression of lines with ptc-GAL4 (A) strongly destabilized the Bowl protein in a cell autonomous manner. (B) Bowl was stable in drm mutant clones indicating that drm may act with other redundant factors to stabilize Bowl. Bab expression was lost in lines mutant clones (Fig. 3A), and was ectopically expressed proximal to the Bab domain in FLP out clones expressing a bowlRNAi transgene (not shown). (C) However, Bab levels were normal in lines MARCM clones expressing the bowlRNAi transgene indicating that bowl mediates the lines clonal phenotypes in the tarsus. (D) Dl expression was lost in lines mutant clones (Fig 5A). However, Dl expression was normal in MARCM lines bowlRNAi clones indicating that bowl represses Dl expression in lines mutant clones. Expression of the segmental markers dAP-2 and Nub was mostly normal in bowl mutant FLP/FRT clones. In a small subset of clones, dAP-2 was repressed (E) and Nub was ectopically expressed (F). The low incidence of these phenotypes suggests that bowl acts with other redundant factors to stabilize the segment border.
Figure S3: lines is both necessary and sufficient to promote ap expression
(A) Ectopic drm expression, an inhibitor of lines, if FLP-out clones led to a cell autonomous repression of ap-lacZ expression (arrowhead). (B-C)Ectopic lines expression led to a cell-autonomous induction of ap-lacZ expression in tarsal clones. Note ectopic ap-lacZ expression both proximal and distal (arrows) to the ap-lacZ expression domain.
Figure S4: The role of lines in PD patterning is limited to the tarsal region
(A-C) lines FLP/FRT clones marked by the absence of GFP and stained for (A) Dll, (B) Hth and (C) the pretarsal marker dLim1. Expression of these proteins was unaffected by the absence of lines function. dLim1 is also expressed in a ring pattern in a subset of proximal true leg segments. We note that dLim1 was ectopically expressed in a subset of lines mutant clones across proximal leg segment (small arrow in C). This response may reflect the second role that lines plays in patterning proximal leg segments.
Figure S5: The pattern of Bowl accumulation correlates with the formation of antennal segments
(A) At early stages, Bowl accumulated broadly in the disc proper of the antennal disc and in part of the PE (data not shown). At later stages, Bowl was downregulated in a central circular domain. (B) Subsequently, Bowl was downregulated in a segmental pattern in proximal cells. Arrowheads mark the plane of confocal cross sections shown in insets.
ACKNOWLEDGMENTS
We thank J. Botas, S. Bray, G. Campbell, K. Irvine, T. Kojima, A. Laski, R. Mann, P. Mitchell, C. Rauskolb, G. Struhl, the Bloomington Stock Center, the Vienna Drosophila Research Center (VDRC), and the Developmental Studies Hybridoma Bank for generous gifts of fly stocks and antibodies, and P. Juo, S. DelSignore and D. Nusinow for comments on the manuscript We also thank anonymous reviewers for invaluable insight and suggestions. This work was supported by a grant from the NIH to V.H. (R01GM06806).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–30. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- Azpiazu N, Morata G. Distinct functions of homothorax in leg development in Drosophila. Mech Dev. 2002;119:55–67. doi: 10.1016/s0925-4773(02)00295-2. [DOI] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Bishop SA, Klein T, Arias AM, Couso JP. Composite signalling from Serrate and Delta establishes leg segments in Drosophila through Notch. Development. 1999;126:2993–3003. doi: 10.1242/dev.126.13.2993. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bras-Pereira C, B. J, Casares F. Odd-skipped genes specify the signaling center that triggers retinogenesis in Drosophila. Development. 2006;132:4145–4149. doi: 10.1242/dev.02593. [DOI] [PubMed] [Google Scholar]
- Campbell G. Distalization of the Drosophila leg by graded EGF-receptor activity. Nature. 2002;418:781–5. doi: 10.1038/nature00971. [DOI] [PubMed] [Google Scholar]
- Campbell G. Regulation of gene expression in the distal region of the Drosophila leg by the Hox11 homolog, C15. Dev Biol. 2005;278:607–18. doi: 10.1016/j.ydbio.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125:4483–93. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- Campbell G, Weaver T, Tomlinson A. Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell. 1993;74:1113–23. doi: 10.1016/0092-8674(93)90732-6. [DOI] [PubMed] [Google Scholar]
- Casares F, Mann RS. Control of antennal versus leg development in Drosophila. Nature. 1998;392:723–6. doi: 10.1038/33706. [DOI] [PubMed] [Google Scholar]
- Casares F, Mann RS. The ground state of the ventral appendage in Drosophila. Science. 2001;293:1477–80. doi: 10.1126/science.1062542. [DOI] [PubMed] [Google Scholar]
- Ciechanska E, Dansereau DA, Svendsen PC, Heslip TR, Brook WJ. dAP-2 and defective proventriculus regulate Serrate and Delta expression in the tarsus of Drosophila melanogaster. Genome. 2007;50:693–705. doi: 10.1139/g07-043. [DOI] [PubMed] [Google Scholar]
- Cifuentes FJ, Garcia-Bellido A. Proximo-distal specification in the wing disc of Drosophila by the nubbin gene. Proc Natl Acad Sci U S A. 1997;94:11405–10. doi: 10.1073/pnas.94.21.11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Imaginal disc development. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. vol. II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 747–842. [Google Scholar]
- Cohen SM, Brönner G, Küttner F, Jürgens G, Jäckle H. Distal-less encodes a homoeodomain protein required for limb development in drosophila. Nature. 1989;338:432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- Couderc JL, Godt D, Zollman S, Chen J, Li M, Tiong S, Cramton SE, Sahut-Barnola I, Laski FA. The bric a brac locus consists of two paralogous genes encoding BTB/POZ domain proteins and acts as a homeotic and morphogenetic regulator of imaginal development in Drosophila. Development. 2002;129:2419–33. doi: 10.1242/dev.129.10.2419. [DOI] [PubMed] [Google Scholar]
- Coulter DE, Swaykus EA, Beran-Koehn MA, Goldberg D, Wieschaus E, Schedl P. Molecular analysis of odd-skipped a zinc finger encoding segmentation gene with a novel pair-rule expression pattern. EMBO (Eur. Mol. dBiol. Organ.) J. 1990;8:3795–3804. doi: 10.1002/j.1460-2075.1990.tb07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis Ibeas JM, Bray SJ. Bowl is required downstream of Notch for elaboration of distal limb patterning. Development. 2003;130:5943–52. doi: 10.1242/dev.00833. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Bray S. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development. 1997;124:3241–51. doi: 10.1242/dev.124.17.3241. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Tyler DM, de Celis J, Bray SJ. Notch signalling mediates segmentation of the Drosophila leg. Development. 1998;125:4617–26. doi: 10.1242/dev.125.23.4617. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372:175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN. Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 1996;10:421–34. doi: 10.1101/gad.10.4.421. [DOI] [PubMed] [Google Scholar]
- Doherty D, Jan LY, Jan YN. The Drosophila neurogenic gene big brain, which encodes a membrane-associated protein, acts cell autonomously and can act synergistically with Notch and Delta. Development. 1997;124:3881–93. doi: 10.1242/dev.124.19.3881. [DOI] [PubMed] [Google Scholar]
- Fleming RJ, Gu Y, Hukriede NA. Serrate-mediated activation of Notch is specifically blocked by the product of the gene fringe in the dorsal compartment of the Drosophila wing imaginal disc. Development. 1997;124:2973–81. doi: 10.1242/dev.124.15.2973. [DOI] [PubMed] [Google Scholar]
- Fristrom D, Fristrom JW. The Development of Drosophila melanogaster. vol. II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1993. The metamorphic development of the adult epidermis; pp. 843–897. [Google Scholar]
- Galindo MI, Bishop SA, Greig S, Couso JP. Leg patterning driven by proximal-distal interactions and EGFR signaling. Science. 2002;297:256–9. doi: 10.1126/science.1072311. [DOI] [PubMed] [Google Scholar]
- Galindo MI, Couso JP. Intercalation of cell fates during tarsal development in Drosophila. BioEssays. 2000;22:777–80. doi: 10.1002/1521-1878(200009)22:9<777::AID-BIES1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Godt D, Couderc JL, Cramton SE, Laski FA. Pattern formation in the limbs of Drosophila: bric a brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119:799–812. doi: 10.1242/dev.119.3.799. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Morata G, Guerrero I. The homeobox gene Distal-less induces ventral appendage development in Drosophila. Genes Dev. 1997;11:2259–71. doi: 10.1101/gad.11.17.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RB, Hatini V, Johansen KA, Liu XJ, Lengyel JA. Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development. 2002;129:3645–56. doi: 10.1242/dev.129.15.3645. [DOI] [PubMed] [Google Scholar]
- Hao I, Green RB, Dunaevsky O, Lengyel JA, Rauskolb C. The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev Biol. 2003;263:282–95. doi: 10.1016/j.ydbio.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Hart MC, Wang L, Coulter DE. Comparison of the structure and expression of odd-skipped and two related genes that encode a new family of zinc finger proteins in Drosophila. Genetics. 1996;144:171–82. doi: 10.1093/genetics/144.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V, Bokor P, Goto-Mandeville R, DiNardo S. Tissue- and stage-specific modulation of Wingless signaling by the segment polarity gene lines. Genes Dev. 2000;14:1364–76. [PMC free article] [PubMed] [Google Scholar]
- Hatini V, Green RB, Lengyel JA, Bray SJ, Dinardo S. The Drumstick/Lines/Bowl regulatory pathway links antagonistic Hedgehog and Wingless signaling inputs to epidermal cell differentiation. Genes Dev. 2005;19:709–18. doi: 10.1101/gad.1268005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen KA, Green RB, Iwaki DD, Hernandez JB, Lengyel JA. The Drm-Bowl-Lin relief-of-repression hierarchy controls fore- and hindgut patterning and morphogenesis. Mech Dev. 2003;120:1139–51. doi: 10.1016/j.mod.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Kerber B, Monge I, Mueller M, Mitchell PJ, Cohen SM. The AP-2 transcription factor is required for joint formation and cell survival in Drosophila leg development. Development. 2001;128:1231–8. doi: 10.1242/dev.128.8.1231. [DOI] [PubMed] [Google Scholar]
- Klein T, Brennan K, Arias AM. An intrinsic dominant negative activity of serrate that is modulated during wing development in Drosophila. Dev Biol. 1997;189:123–34. doi: 10.1006/dbio.1997.8564. [DOI] [PubMed] [Google Scholar]
- Kojima T. The mechanism of Drosophila leg development along the proximodistal axis. Dev Growth Differ. 2004;46:115–29. doi: 10.1111/j.1440-169X.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Kojima T, Sato M, Saigo K. Formation and specification of distal leg segments in Drosophila by dual Bar homeobox genes, BarH1 and BarH2. Development. 2000;127:769–78. doi: 10.1242/dev.127.4.769. [DOI] [PubMed] [Google Scholar]
- Kojima T, Tsuji T, Saigo K. A concerted action of a paired-type homeobox gene, aristaless, and a homolog of Hox11/tlx homeobox gene, clawless, is essential for the distal tip development of the Drosophila leg. Dev Biol. 2005;279:434–45. doi: 10.1016/j.ydbio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Proximal-distal axis formation in the Drosophila leg. Nature. 1997;388:139–45. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Lilly B, O’Keefe DD, Thomas JB, Botas J. The LIM homeodomain protein dLim1 defines a subclass of neurons within the embryonic ventral nerve cord of Drosophila. Mech Dev. 1999;88:195–205. doi: 10.1016/s0925-4773(99)00189-6. [DOI] [PubMed] [Google Scholar]
- Manjón C, Sánchez-Herrero E, Suzanne M. Sharp boundaries of Dpp signalling trigger local cell death required for Drosophila leg morphogenesis. Nat Cell Biol. 2007;9:57–63. doi: 10.1038/ncb1518. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–86. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Rulifson EJ, Blair SS. The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development. 1997;124:1485–95. doi: 10.1242/dev.124.8.1485. [DOI] [PubMed] [Google Scholar]
- Mishra A, Agrawal N, Banerjee S, Sardesai D, Dalal JS, Bhojwani J, Sinha P. Spatial regulation of DELTA expression mediates NOTCH signalling for segmentation of Drosophila legs. Mech Dev. 2001;105:115–27. doi: 10.1016/s0925-4773(01)00387-2. [DOI] [PubMed] [Google Scholar]
- Monge I, Krishnamurthy R, Sims D, Hirth F, Spengler M, Kammermeier L, Reichert H, Mitchell PJ. Drosophila transcription factor AP-2 in proboscis, leg and brain central complex development. Development. 2001;128:1239–52. doi: 10.1242/dev.128.8.1239. [DOI] [PubMed] [Google Scholar]
- Nusinow D, Greenberg L, Hatini V. Reciprocal roles for bowl and lines in specifying the peripodial epithelium and the disc proper of the Drosophila wing primordium. Development. 2008;135:3031–41. doi: 10.1242/dev.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panin VM, Papayannopoulos V, Wilson R, Irvine KD. Fringe modulates Notch-ligand interactions. Nature. 1997;387:908–12. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by decapentaplegic. Development. 1997;124:271–8. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Rao Y, Jan LY, Jan YN. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990;345:163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- Rauskolb C. The establishment of segmentation in the Drosophila leg. Development. 2001;128:4511–21. doi: 10.1242/dev.128.22.4511. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Irvine KD. Notch-mediated segmentation and growth control of the Drosophila leg. Dev Biol. 1999;210:339–50. doi: 10.1006/dbio.1999.9273. [DOI] [PubMed] [Google Scholar]
- Schneitz K, Spielmann P, Noll M. Molecular genetics of aristaless, a prd-type homeo box gene involved in the morphogenesis of proximal and distal pattern elements in a subset of appendages in Drosophila. Genes Dev. 1993;7:114–29. doi: 10.1101/gad.7.1.114. [DOI] [PubMed] [Google Scholar]
- Schneuwly S, Klemenz R, Gehring WJ. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. Nature. 1987;325:816–8. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Shirai T, Yorimitsu T, Kiritooshi N, Matsuzaki F, Nakagoshi H. Notch signaling relieves the joint-suppressive activity of Defective proventriculus in the Drosophila leg. Dev Biol. 2007;312:147–56. doi: 10.1016/j.ydbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388:639–48. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- Snodgrass . Principles of Insect Morphology. McGraw-Hill; New York: 1935. [Google Scholar]
- St Pierre SE, Galindo MI, Couso JP, Thor S. Control of Drosophila imaginal disc development by rotund and roughened eye: differentially expressed transcripts of the same gene encoding functionally distinct zinc finger proteins. Development. 2002;129:1273–81. doi: 10.1242/dev.129.5.1273. [DOI] [PubMed] [Google Scholar]
- Stricker S, Brieske N, Haupt J, Mundlos S. Comparative expression pattern of Odd-skipped related genes Osr1 and Osr2 in chick embryonic development. Gene Expr Patterns. 2006;6:826–34. doi: 10.1016/j.modgep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Sato A, Hiratani I, Taira M, Saigo K, Kojima T. Requirements of Lim1, a Drosophila LIM-homeobox gene, for normal leg and antennal development. Development. 2000;127:4315–23. doi: 10.1242/dev.127.20.4315. [DOI] [PubMed] [Google Scholar]
- Wang L, Coulter DE. bowel, an odd-skipped homolog, functions in the terminal pathway during Drosophila embryogenesis. Embo J. 1996;15:3182–96. abs.html. [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen SM. Proximodistal axis formation in the Drosophila leg: subdivision into proximal and distal domains by Homothorax and Distal-less. Development. 1999;126:109–17. doi: 10.1242/dev.126.1.109. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: The expression, distribution and function of lines across intermediate leg segments
(A) Analysis of lines expression using in situ hybridization; lines was expressed ubiquitously and at uniform levels in developing leg imaginal discs. (B-C) However, the subcellular distribution of the Lines protein was modulated. Red bars, regions where Myc-Lines accumulates in nuclei; yellow bar, regions where Myc-Lines accumulates in the cytoplasm. (B) klu>myc-lines; klu-GAL4 is expressed across each inter-joint and in a distal stripe along true segment borders. Lines accumulated in nuclei across the tarsus and the inter-joint of each proximal leg segment (red bars) and in the cytoplasm in a thin stripe adjacent to the segment border (yellow bars). (C) dac-GAL4 is expressed in an intermediate region of the leg in the presumptive femur, tibia and three proximal tarsi. Lines was nuclear across tarsal segments and across the inter-joint of the tibia and femur (red bars and data not shown). In contrast, Lines was enriched in the cytoplasm in the ti/ta and the fe/ti joints (yellow bars). (D) Wild type; the tr/fe (D’), fe/ti and ti/t1 (D”) form flexible joints in adult leg. (E) dac>linesRNAi; the tr/fe (E’), fe/ti and ti/t1 (E”) were fused (asterisks indicate fused joints). Leg segments differentiated smooth cuticle and numerous vesicles were detected basal to the surface epithelium. In addition tarsal segments 1 to 3 were also fused (not shown). (F) lines MARCM clones induced at 96-120h; GFP-positive clones sorted out from the surface epithelium and formed lateral outgrowths (arrow). (G) lines FLP-out clones induced at 96-120h caused accumulation of melanotic tissue adjacent to true joints (arrow).
Figure S2: The regulation and function of bowl in PD and segmental patterning
Ectopic expression of lines with ptc-GAL4 (A) strongly destabilized the Bowl protein in a cell autonomous manner. (B) Bowl was stable in drm mutant clones indicating that drm may act with other redundant factors to stabilize Bowl. Bab expression was lost in lines mutant clones (Fig. 3A), and was ectopically expressed proximal to the Bab domain in FLP out clones expressing a bowlRNAi transgene (not shown). (C) However, Bab levels were normal in lines MARCM clones expressing the bowlRNAi transgene indicating that bowl mediates the lines clonal phenotypes in the tarsus. (D) Dl expression was lost in lines mutant clones (Fig 5A). However, Dl expression was normal in MARCM lines bowlRNAi clones indicating that bowl represses Dl expression in lines mutant clones. Expression of the segmental markers dAP-2 and Nub was mostly normal in bowl mutant FLP/FRT clones. In a small subset of clones, dAP-2 was repressed (E) and Nub was ectopically expressed (F). The low incidence of these phenotypes suggests that bowl acts with other redundant factors to stabilize the segment border.
Figure S3: lines is both necessary and sufficient to promote ap expression
(A) Ectopic drm expression, an inhibitor of lines, if FLP-out clones led to a cell autonomous repression of ap-lacZ expression (arrowhead). (B-C)Ectopic lines expression led to a cell-autonomous induction of ap-lacZ expression in tarsal clones. Note ectopic ap-lacZ expression both proximal and distal (arrows) to the ap-lacZ expression domain.
Figure S4: The role of lines in PD patterning is limited to the tarsal region
(A-C) lines FLP/FRT clones marked by the absence of GFP and stained for (A) Dll, (B) Hth and (C) the pretarsal marker dLim1. Expression of these proteins was unaffected by the absence of lines function. dLim1 is also expressed in a ring pattern in a subset of proximal true leg segments. We note that dLim1 was ectopically expressed in a subset of lines mutant clones across proximal leg segment (small arrow in C). This response may reflect the second role that lines plays in patterning proximal leg segments.
Figure S5: The pattern of Bowl accumulation correlates with the formation of antennal segments
(A) At early stages, Bowl accumulated broadly in the disc proper of the antennal disc and in part of the PE (data not shown). At later stages, Bowl was downregulated in a central circular domain. (B) Subsequently, Bowl was downregulated in a segmental pattern in proximal cells. Arrowheads mark the plane of confocal cross sections shown in insets.