Abstract
The authors performed a matched case-control study (1982–2007) nested in a prospective cohort of 22,071 US men to determine the prevalence of chronic diseases of aging in those with newly diagnosed cancer. They matched one control by age to each of 5,622 men who developed cancer over the 25 years of follow-up, as of the date of cancer diagnosis. A modified Charlson score was calculated that reflected comorbidities prior to the matching date, and the authors used conditional logistic regression to determine the odds ratios of various diseases. No substantial differences were found between the scores of cases and controls overall, by cancer subtype, or by age at diagnosis. Overall, men who developed cancer were less likely to have had hypercholesterolemia (odds ratio (OR) = 0.79, 95% confidence interval (CI): 0.72, 0.87) or coronary artery disease (OR = 0.85, 95% CI: 0.77, 0.96). Compared with controls, men with cancers for which there is routine screening had fewer diseases, whereas those with smoking-related cancers had more. Prostate cancer was inversely associated with both coronary artery disease (OR = 0.72, 95% CI: 0.62, 0.84) and diabetes (OR = 0.72, 95% CI: 0.58, 0.89). Overall, men who developed cancer had no more comorbidity or frequent history of chronic disease than their age-matched controls.
Keywords: aging, case-control studies, geriatrics, neoplasms, risk
Chronic conditions that manifest primarily later in life, such as heart disease, cancer, and dementia, account for the majority of deaths in developed countries and are rapidly becoming the leading cause of mortality worldwide (1). An important feature of diseases in older adults is that they are often not independent of each other but may cluster together because of similar risk factors and pathophysiology. Alternatively, vulnerability to one illness can actually protect against the development of others. A clear understanding of the way in which major diseases are related is critical to developing disease prevention programs and estimating their impact on populations (2). Few investigators have explored the relation between cancer and the risk of nonmalignant disease. Although some studies find cancer patients to have a greater risk of chronic illness compared with controls (3, 4), others find a similar (5) or decreased (6) risk.
Associations between cancer and other conditions could be causal, such as that between colon cancer and anemia, or could be mediated by shared risk factors, such as smoking. They may reflect differences in health behaviors or socioeconomic status. Finally, biologic factors that cause vulnerability to cancer might increase or decrease susceptibility to other pathologies. Because cancer is so strongly linked to aging, it might be positively correlated with “age-dependent” diseases such as heart failure and dementia (7). On the other hand, the decreased propensity for apoptosis found in some cancer patients might provide relative protection against conditions such as neurodegeneration (8–10). To better understand the relation between cancer and other chronic diseases of older adults, we performed a case-control study nested within a large prospective cohort of men with more than 25 years of follow-up, confirmed cancer outcomes, and detailed information on health behaviors and cancer risk factors.
MATERIALS AND METHODS
The Physicians’ Health Study is a completed, randomized trial of aspirin (325 mg every other day) and beta-carotene (50 mg on alternate days) for the primary prevention of cardiovascular disease and cancer in 22,071 US male physicians. All participants provided written informed consent, and the trial was approved by the institutional review board of the Brigham and Women's Hospital. The study design and findings have been published previously (11, 12). At study entry in 1982, participants were between 40 and 84 years of age and had no history of cardiovascular disease, cancer (with the exception of nonmelanoma skin cancer), or other serious illnesses; 92.2% identified their race as white. Baseline information was self-reported and was collected by a mailed questionnaire that asked about many cardiovascular and cancer risk factors as well as lifestyle variables. Participants reported twice in the first year and yearly thereafter. Posttrial follow-up is ongoing (13). Follow-up information through March 30, 2007, was used in this analysis.
Ascertainment of cancer cases and controls
Nonfatal cases of cancer were reported by participants and fatal cases by family members or next of kin. Medical records were obtained for all cancers (excluding nonmelanomatous skin cancers). Cases of cancer and deaths were confirmed by review of medical records by the Physicians’ Health Study endpoints committee, which included 2 internists, a cardiologist, and a neurologist. Review of pathology reports was required for confirmation of reported malignancies. Only confirmed events were used for this analysis.
Controls were selected from the study population by using an incidence-density sampling approach (14, 15). We first matched to each case all potential controls who were alive, cancer free, and the same age (exact) at the time of cancer diagnosis in the case. A control for each case was then randomly chosen from the pool of potential controls. Individuals could not serve as a control more than once. If a participant was cancer free at the matching date but then later developed cancer, he or she was allowed to serve as a control only if the date of cancer diagnosis was 5 years or more after the date of cancer in the matched case (to avoid subclinical cancer in controls). However, a control could die from noncancer causes anytime after the index date. We assigned as the index date the date of cancer diagnosis of the case to the matched control.
We defined smoking-related malignancies as including oropharynx, larynx, esophagus, lung, kidney, and bladder cancers. Prostate cancer, colorectal cancer, and melanoma were considered cancers for which routine screening is recommended.
Comorbidity score
We calculated a comorbidity score for each case and control as of the index date using a modified version of the Charlson Comorbidity Index (16) published previously (17). Because Physicians’ Health Study participants were apparently healthy at baseline, the score reflected total comorbidities accumulated between study entry and the index date. Data on comorbidity were self-reported by the physician participants on follow-up questionnaires. Myocardial infarction and cerebrovascular disease were study endpoints and thus were validated by medical record review, but self-report of other diagnoses was not validated. We used all disease categories in the original Charlson Index with the exception of cancer (Table 1). Because we could not determine disease severity, we categorized all liver disease as mild and all renal disease as moderate to severe, as in other modifications (18). We also created a separate “age-related comorbidity” score composed exclusively of conditions strongly associated with older age, as shown in Table 1. Because the diagnosis of dementia was not directly ascertained but self-reported, there were fewer cases than anticipated in our cohort.
Table 1.
Definition of Comorbidity Scores
| Modified Charlson Comorbidity Indexa |
Age-related Disease Score |
||
| Condition | No. of Points | Condition | No. of Points |
| Myocardial infarction | 1 | Coronary artery disease | 1 |
| Congestive heart failure | 1 | Congestive heart failure | 1 |
| Peripheral vascular disease | 1 | Stroke | 1 |
| Cerebrovascular disease | 1 | Peripheral vascular disease | 1 |
| Dementia | 1 | Diabetes | 1 |
| Chronic pulmonary disease | 1 | Major psychiatric disease | 1 |
| Connective tissue disease | 1 | Osteoarthritis | 1 |
| Peptic ulcer disease | 1 | Dementia | 1 |
| Liver disease | 1 | Parkinson's disease | 1 |
| Diabetes | 1 | Cataracts | 1 |
| Hemiplegia | 2 | Macular degeneration | 1 |
| Renal disease | 2 | ||
| Diabetes (end-organ damage) | 2 | ||
| HIV infection/AIDS | 2 | ||
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Excluding cancer.
Statistical analysis
We created separate age-matched case-control sets for overall cancer, individual cancers, cancers localized or metastatic at presentation, smoking-related cancers, screened cancers, and nonscreened cancers. We calculated the mean Charlson score as of the index date among cases and controls for each set, and we compared them by using Student's t tests. To investigate differences in comorbidity between cases and controls by age at cancer onset, we performed comparisons by age group (40–64, 65–79 and ≥80 years). We compared separate age-related comorbidity scores for cases and controls aged 80 years or older at the index date. We used conditional logistic regression for age-matched pairs to determine the odds ratio of specific conditions in cases versus controls at the time of matching. Models were adjusted for the following potential confounders: smoking (ever vs. never), alcohol use (daily vs. less than daily), body mass index (<25 kg/m2 vs. ≥25 kg/m2), and exercise to sweat (>monthly vs. ≤once a month). All statistical calculations were performed by using SAS statistical software, version 9.1 (SAS Institute, Inc., Cary, North Carolina). All P values are 2-tailed, and we considered P < 0.05 significant.
RESULTS
We confirmed 5,622 incident cancers over the 25-year follow-up period and matched an equal number of controls to them. The mean age at randomization was 57.1 years for both groups, and the mean age at cancer diagnosis in cases was 70.3 years. Men who developed cancer were more likely than controls to be current smokers (13.4% vs. 10.1%), daily alcohol users (29.4% vs. 26.2%), and overweight (45.3% vs. 42.4%) at study baseline.
At cancer diagnosis, mean modified Charlson scores were not substantially different between cases and controls, either overall or by cancer subtype (Table 2). Men with prostate cancer, colorectal cancer, melanoma, and lymphoma had slightly lower scores compared with controls, whereas those with lung, pancreas, and bladder cancer had higher scores. Pancreas cancer was associated with the most comorbidity of all tumor types (mean score = 1.25), followed by bladder cancer (mean score = 1.12), and lung cancer (mean score = 1.06). Men with smoking-related cancers had higher mean comorbidity scores (1.05) than controls did (0.98). When we limited this analysis to participants with a healthy lifestyle profile (never smokers, less-than-daily drinkers, not obese), cases actually had a lower comorbidity score (0.93) than controls did (1.05).
Table 2.
Modified Charlson Comorbidity Scores at the Time of Cancer Diagnosis of US Cases and Matched Controls in the Physicians’ Health Study, 1982–2007
| Cancer Type | No. of Cases | No. of Controls | Mean Charlson Scorea (Range) | P Valueb | |
| Cases | Controls | ||||
| All cancers | 5,622 | 5,622 | 0.97 (0–8) | 0.96 (0–9) | 0.63 |
| Prostate | 2,661 | 2,661 | 0.96 (0–7) | 1.01 (0–8) | 0.16 |
| Colorectal | 565 | 565 | 0.91 (0–5) | 0.94 (0–5) | 0.67 |
| Lymphoma | 367 | 367 | 0.99 (0–7) | 1.05 (0–5) | 0.48 |
| Lung | 345 | 345 | 1.06 (0–7) | 1.02 (0–6) | 0.68 |
| Melanoma | 329 | 329 | 0.92 (0–5) | 1.00 (0–8) | 0.41 |
| Bladder | 179 | 179 | 1.12 (0–5) | 0.95 (0–5) | 0.16 |
| Pancreas | 129 | 129 | 1.25 (0–7) | 1.04 (0–7) | 0.20 |
| Localized at presentation | 4,632 | 4,632 | 0.98 (0–8) | 0.96 (0–9) | 0.40 |
| Metastatic at presentation | 987 | 987 | 0.90 (0–7) | 0.94 (0–6) | 0.41 |
| Cancers with screeningc | 3,555 | 3,555 | 0.95 (0–7) | 0.99 (0–8) | 0.14 |
| Cancers without screening | 2,067 | 2,067 | 0.99 (0–8) | 0.94 (0–7) | 0.15 |
| Smoking-relatedd | 952 | 952 | 1.05 (0–7) | 0.98 (0–8) | 0.17 |
Charlson score does not include cancer.
P values from Student's t test.
Prostate, colorectal, melanoma.
Oropharynx, larynx, esophagus, lung, kidney, bladder.
Mean Charlson scores for the entire cohort increased substantially across age groups, from 0.62 (standard deviation, 0.86) for men aged 40–64 years to 1.40 (standard deviation, 1.28) for men aged 80 years or older. However, scores between cases and controls were not meaningfully different in any age group, for any of the tumor types (Web Table 1; this information is described in the first of 3 supplementary tables referred to as “Web Table” in the text and posted on the Journal’s Web site (http://aje.oupjournals.org/)). When we compared scores of age-related comorbidity between men aged 80 years or older at index, there was again no substantial difference between groups (Table 3).
Table 3.
Age-related Condition Scores at the Time of Cancer Diagnosis of US Cases and Matched Controls Aged 80 Years or Older at the Index Date in the Physicians’ Health Study, 1982–2007
| Cancer Type | No. of Cases | No. of Controls | Mean Age-associated Comorbidity Scorea (Range) | P Valueb | |
| Cases | Controls | ||||
| All cancers | 786 | 786 | 1.82 (0–6) | 1.89 (0–7) | 0.30 |
| Prostate | 293 | 293 | 1.72 (0–6) | 1.78 (0–5) | 0.48 |
| Colorectal | 103 | 103 | 1.80 (0–5) | 1.92 (0–5) | 0.44 |
| Lymphoma | 65 | 65 | 2.00 (0–5) | 1.83 (0–6) | 0.45 |
| Lung | 56 | 56 | 2.14 (0–6) | 2.21 (0–6) | 0.79 |
| Urinary | 49 | 49 | 1.92 (0–4) | 1.80 (0–4) | 0.58 |
| Smoking-relatedc | 152 | 152 | 2.07 (0–6) | 1.99 (0–6) | 0.61 |
| Cancers with screeningd | 437 | 437 | 1.78 (0–6) | 1.83 (0–6) | 0.51 |
| Metastatic at presentation | 175 | 175 | 1.93 (0–6) | 1.93 (0–7) | 1.00 |
Includes the following age-related conditions: coronary artery disease, congestive heart failure, stroke, peripheral vascular disease, diabetes, major psychiatric disease, arthritis, Parkinson's disease, dementia, cataracts, and macular degeneration.
P value from Student's t test.
Oropharynx, larynx, esophagus, lung, kidney, bladder.
Prostate, colorectal, melanoma.
Men with overall cancer were significantly less likely than controls to have reported high cholesterol (odds ratio (OR) = 0.79, 95% confidence interval (CI): 0.72, 0.87) and coronary artery disease (OR = 0.85, 95% CI: 0.77, 0.96). Figure 1 (also refer to Web Table 2) displays the odds ratios of various comorbid conditions in cases at the time of cancer diagnosis, by tumor type. Many comorbid conditions found in cases at diagnosis, such as bone disease, liver disease, and anemia, are expected sequelae of cancer. Men with screening-related cancers had substantially less non-cancer-related comorbidity than controls did. Prostate cancer was associated with a decreased odds of coronary artery disease (OR = 0.72, 95% CI: 0.62, 0.84), congestive heart failure (OR = 0.84, 95% CI: 0.61, 1.15), stroke (OR = 0.75, 95% CI: 0.55, 1.04), and diabetes (OR = 0.72, 95% CI: 0.58, 0.89). Bone disease (OR = 1.30, 95% CI: 0.97, 1.74) was the only condition associated with increased risk. Similarly, men with colorectal cancer, compared with controls, had lower odds of high cholesterol (OR = 0.63, 95% CI: 0.45, 0.88), congestive heart failure (OR = 0.78, 95% CI: 0.42, 1.47), stroke (OR = 0.53, 95% CI: 0.27, 1.04), and peripheral vascular disease (OR = 0.81, 95% CI: 0.47, 1.39). However, they were at increased risk of liver disease (OR = 1.34, 95% CI: 0.86, 2.08) and anemia (OR = 4.73, 95% CI: 1.77, 12.61).
Figure 1.
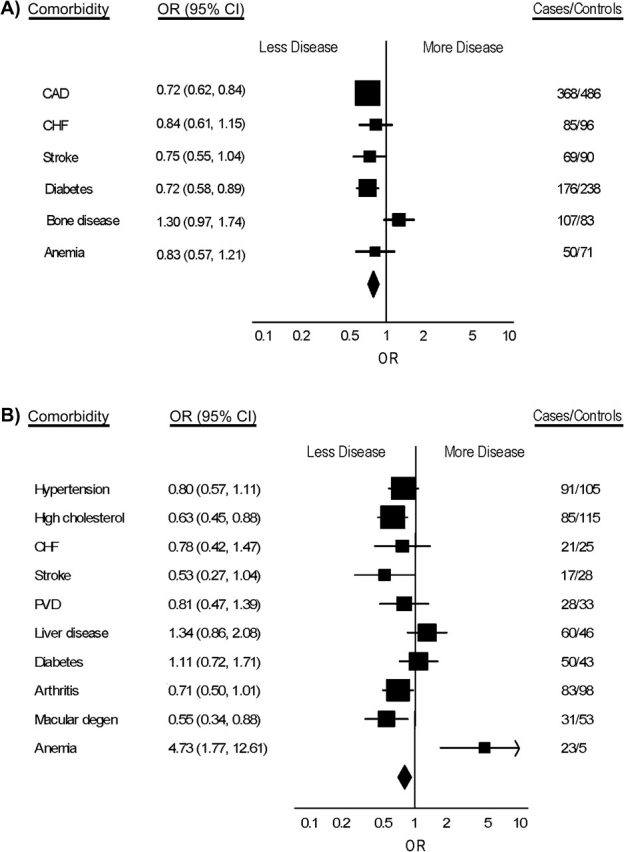

Odds ratios (ORs) and 95% confidence intervals (CIs) for selected comorbidities at the time of cancer diagnosis of US cases and controls in the Physicians’ Health Study, 1982–2007. A) Prostate cancer; B) colorectal cancer; C) lung cancer; D) bladder cancer. Squares: odds ratios for individual comorbidities; lines: 95% confidence intervals; diamond: pooled estimate. The final column shows number of cases and number of controls with the selected comorbidities. CAD, coronary artery disease; CHF, congestive heart failure; degen, degeneration; PVD, peripheral vascular disease.
In contrast, men with cancers for which there is no routine screening and men with smoking-related cancers had an increased odds of many comorbidities compared with controls. Those with lung cancer had an increased risk of stroke (OR = 1.38, 95% CI: 0.58, 2.27), peripheral vascular disease (OR = 1.20, 95% CI: 0.61, 2.36), pulmonary disease (OR = 1.89, 95% CI: 1.22, 2.94), bone disease (OR = 1.83, 95% CI: 0.81, 4.18), and anemia (OR = 1.54, 95% CI: 0.41, 5.78). Table 4 displays the most common comorbidities for each of the major tumor types.
Table 4.
Most Common Self-reported Conditions, and Percentages of US Men Affected at the Time of Cancer Diagnosis, by Tumor Type, in the Physicians’ Health Study, 1982–2007
| Prostate (N = 2,662) | Colorectal (N = 565) | Lymphoma (N = 367) | Lung (N = 345) | Bladder (N = 179) | Pancreas (N = 129) |
| Cataracts, 31.7% | Cataracts, 32.2% | Cataracts, 31.6% | Cataracts, 31.3% | Cataracts, 35.8% | Cataracts, 38.8% |
| High cholesterol, 26.5% | Pulmonary disease, 18.9% | Pulmonary disease, 21.0% | Pulmonary disease, 28.4% | Coronary artery disease/hypertension, 20.1% | Arthritis, 24.8% |
| Pulmonary disease, 21.1% | Hypertension, 16.1% | Hypertension, 20.4% | Coronary artery disease, 20.6% | Arthritis/high cholesterol, 19.6% | High cholesterol, 24.0% |
| Arthritis, 20.3% | High cholesterol, 15.0% | Arthritis, 16.9% | High cholesterol, 17.4% | Pulmonary disease, 18.4% | Diabetes, 21.7% |
| Hypertension, 18.8% | Coronary artery disease/arthritis, 14.7% | High cholesterol, 16.6% | Hypertension, 16.2% | Macular degeneration, diabetes 8.9% | Hypertension, 20.2% |
Figure 2 shows age-related comorbidities in participants aged 80 years or older at the index date (also refer to Web Table 3). Men with prostate cancer had a decreased risk of coronary artery disease (OR = 0.61, 95% CI: 0.41, 0.92). Most other major tumor types were associated with a decreased risk of coronary artery disease and congestive heart failure, although results did not reach statistical significance.
Figure 2.
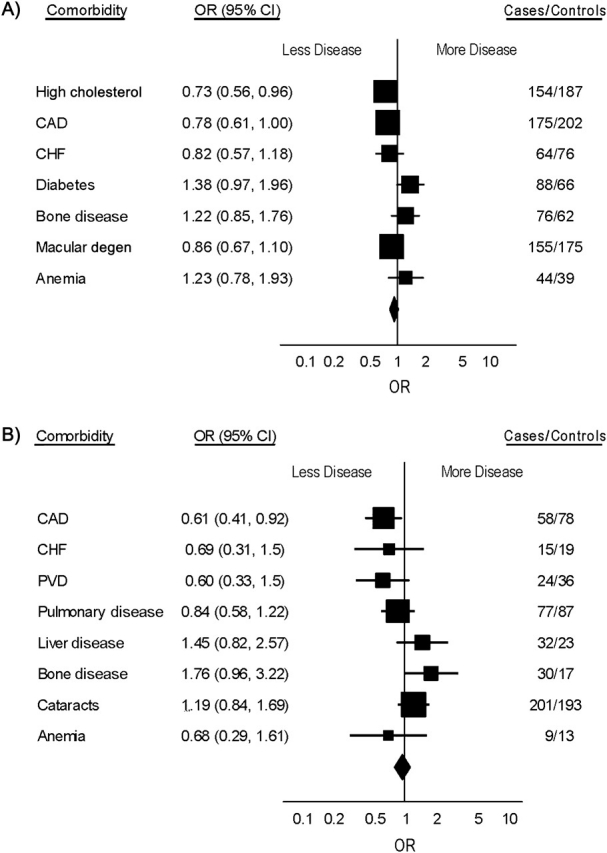

Odds ratios (ORs) and 95% confidence intervals (CIs) for selected comorbidities in US cancer patients and controls aged 80 years or older in the Physicians’ Health Study, 1982–2007. A) Overall cancer; B) prostate cancer; C) colorectal cancer; D) smoking-related cancer. Squares: odds ratios for individual comorbidities; lines: 95% confidence intervals; diamond: pooled estimate. The final column shows number of cases and number of controls with the selected comorbidities. CAD, coronary artery disease; CHF, congestive heart failure; degen, degeneration; PVD, peripheral vascular disease.
DISCUSSION
In this prospective cohort of initially healthy male physicians, we found no substantial difference between the comorbidity scores of newly diagnosed cancer patients and their age-matched controls. This finding was true for both screened and unscreened cancers, regardless of patient age. Age-related conditions for which cancer patients had an increased prevalence seemed to primarily be sequelae of the underlying tumor or the result of common risk factors. The relation between cancer and nonmalignant diseases varied substantially by tumor type. Patients with cancers for which screening is largely available had fewer comorbid conditions than controls at diagnosis, whereas men with smoking-related or nonscreened cancers had more. Men who developed cancer had no more frequent history of age-related diseases than their matched controls. We found a substantially decreased prevalence of coronary artery disease and hypercholesterolemia among men with cancer. There was some suggestion that men who developed cancer at or beyond age 80 years had less cardiovascular disease and congestive heart failure.
Our findings were most similar to those of a US Department of Veterans Affairs health care system study of 194,797 older cancer patients and reference subjects in which the only difference in comorbidity was a lower rate of dementia among those with cancer (5). In contrast, a study of 126,685 Medicare patients found people with a history of cancer to have substantially higher rates of 10 of 12 comorbid conditions, including coronary artery disease, congestive heart failure, stroke, arthritis, and diabetes (4). Of note, this cohort was of low socioeconomic status, with higher rates of disease in general. Finally, in a small study of cancer patients aged 70 years or older, those with cancer had less comorbidity and higher functional status compared with controls (6).
The nature of our cohort reduced the influence of some important potential confounders such as differences in socioeconomic background, screening practices, and health behaviors. Our health-conscious participants had lower rates of current smoking, alcohol use, and obesity than a general population. In addition, the men in our cohort are relatively long-lived, yielding a large number of participants over 80 years of age (19). If a diagnosis of cancer were linked to other chronic diseases of aging on the basis of shared biologic factors, we would expect to see such an association in our cohort. Of the positive associations we found, some were clearly causal, such as the strong 3-fold risk of prior diabetes in men with pancreas cancer. Some cancers, such as lung, bladder, and pancreas, were certainly associated with a wide range of comorbid diseases; however, when we adjusted for smoking, alcohol use, and obesity, the associations disappeared, suggesting confounding by common risk factors. Although some cancer may occur in the context of the syndromes of frailty or “early” aging, our findings suggest that, in the majority of cases, a diagnosis of cancer does not cluster with other diseases strongly linked to aging.
In fact, we found a number of negative associations between cancer and other conditions. Men with prostate cancer were healthier than their age-matched controls at the time of diagnosis, and they had significantly less cardiovascular disease and diabetes. This finding most likely represents residual confounding because men who choose to undergo cancer screening may have better health and more healthy behaviors than those who do not. However, there was a decreased prevalence of prior hypercholesterolemia and coronary artery disease in cancer patients overall and a lower prevalence of prior stroke associated with many tumor types as well.
Prior work has demonstrated negative correlations between cancer and coronary heart disease, stroke, and neurodegenerative diseases (2). In the Physicians’ Health Study, we found a negative association between a history of cancer and the risk of Parkinson's disease (17, 20, 21). Others have found a decreased risk of cancer for patients with Alzheimer's disease and lower rates of Alzheimer's in cancer survivors (22, 23). Finally, in a prospective cohort, individuals with mutations of the p53 gene, which confer less vigorous apoptosis, were predisposed to cancer but had a substantially decreased risk of cardiovascular disease and other chronic diseases of aging, leading to a 41% overall increase in longevity (24). Taken together, these data raise the possibility that vulnerability to cancer might actually protect against aging and its diseases, perhaps because of differences in apoptosis (8, 10, 25). If this possibility is true, the potential gain in life expectancy from cancer eradication might be substantially greater than previously estimated (2).
Our study has several strengths, including its large number of participants and outcome events, prospective design, and well-defined population with a long follow-up. Cancer diagnoses were confirmed by medical record review, and we were able to examine a large number of age-related conditions. The case-control design allowed us to examine associations between cancer and other conditions not influenced by cancer treatment.
A number of limitations must also be considered. First, comorbid conditions were self-reported by the physician participants, creating the possibility of misclassification. However, validation studies in the Physicians’ Health Study have found good correlation between self-reported diagnoses of hypercholesterolemia and hypertension with measured serum low density lipoprotein cholesterol and blood pressure (26). Our study cohort was composed primarily of white male physicians and may not be representative of other populations. We had no information on geriatric syndromes or measures of cognitive and physical function, all important indicators of age-related decline. We looked at only prevalence and not severity of disease, which might also be influenced by a cancer diagnosis.
In conclusion, positive associations between individual cancers and age-related comorbidities in this cohort of health-conscious physicians seemed to be directly caused by the tumor, explained by shared risk factors for disease, or to reflect the timing of diagnosis due to cancer screening. We found no suggestion that men who develop cancer have a higher prevalence of diseases of old age. Whether susceptibility to cancer may decrease the risk of conditions mediated by apoptosis such as heart disease and neurodegeneration is an intriguing question and should be a focus of future research.
Supplementary Material
Acknowledgments
Author affiliations: Division of Aging, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (Jane A. Driver, J. Michael Gaziano, Tobias Kurth); Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (J. Michael Gaziano, Tobias Kurth); Department of Oncology, Dana-Farber Cancer Research Institute, Harvard Medical School, Boston, Massachusetts (Rachel Yung); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Tobias Kurth); Geriatric Research, Education and Clinical Center, Boston, Massachusetts (Jane A. Driver); Massachusetts Veterans Epidemiology Research Information Center, VA Boston Healthcare System, Harvard Medical School, Boston, Massachusetts (J. Michael Gaziano); INSERM Unit 708 - Neuroepidemiology, Paris, France (Tobias Kurth); and Faculty of Medicine, Pierre et Marie Curie University, Paris, France (Tobias Kurth).
This work was supported by a grant from the Hartford Foundation's Center of Excellence in Geriatric Medicine at Harvard Medical School and the Eleanor and Miles Shore/Harvard Medical School Scholars in Medicine Program. The Physicians’ Health Study is supported by grants from the National Cancer Institute (CA-34944, CA-40360, and CA-097193) and grants to the National Heart, Lung, and Blood Institute (HL-26490 and HL-34595), Bethesda, Maryland.
The authors thank the staff of the Physician's Health Study and the 22,071 dedicated physicians who have made this project possible.
Dr. Driver has received research grants from the Hartford Foundation, the Parkinson's Disease Foundation (New York, New York), and Harvard Medical School and is the recipient of a Career Development Award from the Veterans Administration (Washington, DC). Dr. Gaziano has received investigator-initiated research grants from the Department of Veterans Affairs (Washington, DC), National Institutes of Health (Bethesda, Maryland), VeroScience LLC (Tiverton, Rhode Island), and Amgen Inc. (Washington, DC); has served as a consultant for and has received honoraria from Bayer AG (Leverkusen, Germany); and has served as an expert witness for Merck & Co., Inc. (Whitehouse Station, New Jersey). Dr. Kurth has received investigator-initiated research funds from the National Institutes of Health, McNeil Consumer & Specialty Pharmaceuticals (Fort Washington, Pennsylvania), Merck & Co., Inc., and Wyeth Consumer Healthcare (Mountain View, California; he is a consultant to i3 Drug Safety (Minneapolis, Minnesota) and World Health Information Science Consultants, LLC (Newton, Massachusetts); and he has received honoraria from Genzyme Corporation (Cambridge, Massachusetts), Merck & Co., Inc., and Pfizer Inc. (New York, New York) for educational lectures.
Glossary
Abbreviations
- CI
confidence interval
- OR
odds ratio
References
- 1.The top 10 causes of death. Geneva, Switzerland: World Health Organization; 2008. (Fact sheet 310). ( http://www.who.int/mediacentre/factsheets/fs310_2008.pdf) [Google Scholar]
- 2.Yashin AI, Ukraintseva SV, Akushevich IV, et al. Trade-off between cancer and aging: what role do other diseases play? Evidence from experimental and human population studies. Mech Ageing Dev. 2009;130(1-2):98–104. doi: 10.1016/j.mad.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 4.Smith AW, Reeve BB, Bellizzi KM, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29(4):41–56. [PMC free article] [PubMed] [Google Scholar]
- 5.Zeber JE, Copeland LA, Hosek BJ, et al. Cancer rates, medical comorbidities, and treatment modalities in the oldest patients. Crit Rev Oncol Hematol. 2008;67(3):237–242. doi: 10.1016/j.critrevonc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Repetto L, Venturino A, Vercelli M, et al. Performance status and comorbidity in elderly cancer patients compared with young patients with neoplasia and elderly patients without neoplastic conditions. Cancer. 1998;82(4):760–765. [PubMed] [Google Scholar]
- 7.Brody JA, Schneider EL. Diseases and disorders of aging: an hypothesis. J Chronic Dis. 1986;39(11):871–876. doi: 10.1016/0021-9681(86)90035-4. [DOI] [PubMed] [Google Scholar]
- 8.Behrens MI, Lendon C, Roe CM. A common biological mechanism in cancer and Alzheimer's disease? Curr Alzheimer Res. 2009;6(3):196–204. doi: 10.2174/156720509788486608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ørsted DD, Bojesen SE, Tybjaerg-Hansen A, et al. Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival, and risk of cancer in the general population. J Exp Med. 2007;204(6):1295–1301. doi: 10.1084/jem.20062476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West AB, Dawson VL, Dawson TM. To die or grow: Parkinson's disease and cancer. Trends Neurosci. 2005;28(7):348–352. doi: 10.1016/j.tins.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321(3):129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 12.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 13.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II—a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10(2):125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 14.Lubin JH, Gail MH. Biased selection of controls for case-control analyses of cohort studies. Biometrics. 1984;40(1):63–75. [PubMed] [Google Scholar]
- 15.Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004;61(12) doi: 10.1136/oem.2004.014472. e59. (doi:10.1136/oem.2004.014472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Driver JA, Kurth T, Buring JE, et al. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. 2008;70(16 pt 2):1423–1430. doi: 10.1212/01.wnl.0000310414.85144.ee. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein LB, Samsa GP, Matchar DB, et al. Charlson Index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004;35(8):1941–1945. doi: 10.1161/01.STR.0000135225.80898.1c. [DOI] [PubMed] [Google Scholar]
- 19.Yates LB, Djoussé L, Kurth T, et al. Exceptional longevity in men: modifiable factors associated with survival and function to age 90 years. Arch Intern Med. 2008;168(3):284–290. doi: 10.1001/archinternmed.2007.77. [DOI] [PubMed] [Google Scholar]
- 20.Driver JA, Kurth T, Buring JE, et al. Prospective case-control study of nonfatal cancer preceding the diagnosis of Parkinson's disease. Cancer Causes Control. 2007;18(7):705–711. doi: 10.1007/s10552-007-9005-9. [DOI] [PubMed] [Google Scholar]
- 21.Driver JA, Logroscino G, Buring JE, et al. A prospective cohort study of cancer incidence following the diagnosis of Parkinson's disease. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1260–1265. doi: 10.1158/1055-9965.EPI-07-0038. [DOI] [PubMed] [Google Scholar]
- 22.Roe CM, Behrens MI, Xiong C, et al. Alzheimer disease and cancer. Neurology. 2005;64(5):895–898. doi: 10.1212/01.WNL.0000152889.94785.51. [DOI] [PubMed] [Google Scholar]
- 23.Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology. 2010;74(2):106–112. doi: 10.1212/WNL.0b013e3181c91873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Heemst D, Mooijaart SP, Beekman M, et al. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp Gerontol. 2005;40(1-2):11–15. doi: 10.1016/j.exger.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Staropoli JF. Tumorigenesis and neurodegeneration: two sides of the same coin? Bioessays. 2008;30(8):719–727. doi: 10.1002/bies.20784. [DOI] [PubMed] [Google Scholar]
- 26.Camargo CA, Jr, Hennekens CH, Gaziano JM, et al. Prospective study of moderate alcohol consumption and mortality in US male physicians. Arch Intern Med. 1997;157(1):79–85. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


