Abstract
The reproductive (gametophytic) phase in flowering plants is often highly sensitive to hot or cold temperature stresses, with even a single hot day or cold night sometimes being fatal to reproductive success. This review describes studies of temperature stress on several crop plants, which suggest that pollen development and fertilization may often be the most sensitive reproductive stage. Transcriptome and proteomic studies on several plant species are beginning to identify stress response pathways that function during pollen development. An example is provided here of genotypic differences in the reproductive stress tolerance between two ecotypes of Arabidopsis thaliana Columbia (Col) and Hilversum (Hi-0), when reproducing under conditions of hot days and cold nights. Hi-0 exhibited a more severe reduction in seed set, correlated with a reduction in pollen tube growth potential and tropism defects. Hi-0 thus provides an Arabidopsis model to investigate strategies for improved stress tolerance in pollen. Understanding how different plants cope with stress during reproductive development offers the potential to identify genetic traits that could be manipulated to improve temperature tolerance in selected crop species being cultivated in marginal climates.
Keywords: Cold stress, fertilization, gene expression, heat stress, plant reproduction, pollen, pollen tropism, seed set
Introduction
While temperature and other abiotic stresses are clearly limiting factors for crops cultivated on marginal lands, crop productivity everywhere is often at the mercy of random environmental fluctuations. Current speculation about global climate change is that most agricultural regions will experience more extreme environmental fluctuations (Solomon et al., 2007). Hot or cold temperature stresses can be detrimental to all phases of plant development. As the majority of our food supply is a product of sexual reproduction in flowering plants, understanding how different plants cope with stress during their reproductive (gametophytic) phase is critical to managing the future of agricultural productivity. During the short time surrounding fertilization, even a single hot day or cold night can be fatal to reproductive success for many plant species. Thus, it is important to consider what aspects of plant sexual reproduction may become the ‘weak links’ in agricultural productivity.
While temperature stress has been extensively studied and reviewed (Iba et al., 2002; Yamaguchi-Shinozaki and Shinozaki, 2006; Chinnusamy et al., 2007; Kotak et al., 2007; Wahid et al., 2007), most of the literature emphasizes insights from experimentally accessible tissues, such as leaves and roots. By comparison, studies on sexual reproduction are often more difficult because gamete development and fertilization are complex processes that occur during a short window of time, and are predominantly hidden within tissues of the flower. Nevertheless, sexual reproduction has been long recognized as being highly stress-sensitive, with reproductive stress tolerance often a limiting trait in crop plant productivity (Charles and Harris, 1972; Herrero and Johnson, 1980). This aspect of stress sensitivity was recently reviewed by Barnabás et al. (2008), Hedhly et al. (2008), and Thakur et al. (2010). The objectives of this review are: (i) to provide a short summary of general mechanisms by which high and low temperature stress can impact sexual plant reproduction, (ii) to identify examples in which pollen development and fertilization have been implicated as the most temperature-sensitive aspect of reproductive development, (iii) to describe a previously unpublished example in which an ecotype from Arabidopsis provides a useful model to investigate pollen stress sensitivity, and (iv) to summarize the experimental strategies being used to help identify the genes and pathways involved in how pollen responds to temperature stresses.
General principles of plant responses to temperature stress
Plants have evolved complex mechanisms to cope with daily changes in the environment. At a molecular level, this is illustrated by the thousands of transcriptional changes observed in seedlings, leaves, roots, and pollen as plants reprogramme cellular processes to adapt to hot or cold temperatures (Fowler and Thomashow, 2002; Kreps et al., 2002; Busch et al., 2005; Larkindale and Vierling, 2008; Frank et al., 2009). One developmental programme that provides a very robust stress tolerance is the production of a dehydrated embryo that can remain dormant for long periods of time inside a seed. Similarly, pollen development often includes the production of dehydrated pollen grains, which are also relatively stress tolerant. While these dormancy programmes are used in special circumstances, actively growing plants utilize less extreme measures for dealing with daily changes in the environment. These daily responses to changing environments have been extensively studied in root and leaf tissues, providing a framework of general principles for investigating stress tolerance in all plant cells, including cells involved in reproduction.
The impact of temperature stress is a complex function of intensity, duration, and rate of temperature change (Wahid et al., 2007; Thakur et al., 2010). Both hot and cold stresses can alter multiple aspects of cellular physiology. For example, temperature can dramatically change membrane fluidity, nucleic acid and protein structures, as well as metabolite and osmolyte concentrations (Wang et al., 2003; Howarth, 2005; Chinnusamy et al., 2007). Both hot and cold temperature stresses induce the production of ROS (reactive oxygen species), which at elevated concentrations will result in oxidative damage and, potentially, cell death (Apel and Hirt, 2004).
One of the most striking consequences of temperature stress on photosynthetic tissues is the inhibition of photosynthesis. High temperatures damage the OEC (oxygen evolving complex) of PSII (photosystem II) (Strasser, 1997), reduce Rubisco activity (Law and Crafts-Brandner, 1999), and cause disorganization of the thylakoid membranes (Gounaris et al., 1983). Chilling stress under high light conditions disrupts thylakoid electron transport, reduces Rubisco activity, and induces stomatal closure, which reduces CO2 uptake (Allen and Ort, 2001). With respect to reproductive success, a reduction of photosynthesis ultimately reduces parental resources available for reproduction (Blum et al., 1994; Snider et al., 2009).
While many plants can withstand highly stressful conditions, they nevertheless require adequate time to sense and adapt to new environments. Understanding how plants sense and respond to specific combinations of stress is a challenging area of research (Suzuki and Mittler, 2006). For example, increased levels of ROS can function to trigger transcriptional changes in both hot and cold stress conditions. Hormone production and signalling may also change during temperature stress (Kotak et al., 2007). For example, ABA (abscisic acid), SA (salicylic acid), and ethylene signalling are activated in response to high temperature stress. ABA signalling can also play a significant role in establishing cold tolerance (Thomashow, 1999). In addition, the phytohormones ethylene, GA (gibberellins), and auxin are also thought to be involved in cold stress signalling (Lee et al., 2005).
The final transcriptional responses to hot and cold stress are quite different, indicating that different sets of biophysical and hormonal signals are integrated into a specific response for a given stress or stress combination. For a heat stress, responses include the expression of heat stress transcription factors (HSF), which then trigger an increased expression of many additional stress-related transcripts (Baniwal et al., 2004). One important class of up-regulated genes encodes heat shock proteins (HSP), some of which act as chaperones to stabilize proteins against denaturation (Wang et al., 2004).
Cold stress responses include the up-regulation of cold-specific transcription factors, including CBF/DREB1. These transcription factors then promote the expression of LEA (late-embryogenesis abundant)/COR (cold-regulated) genes (Thomashow, 1999). Many of these target genes are thought to encode proteins that enhance freezing tolerance by putatively stabilizing membranes, or increasing levels of protective osmolytes.
Reproductive tissue responses to temperature stress
Hot and cold temperature stresses have several major effects on reproductive tissues that contribute to poor seed set yield: (i) early or delayed flowering, (ii) asynchrony of male and female reproductive development, (iii) defects in parental tissue, and (iv) defects to male and female gametes. While the focus of this review is on pollen, some brief examples of temperature effects on other aspects of reproductive development will be provided first.
Temperature stress can trigger either early or delayed flowering, depending on the species and other environmental conditions. One important modifier is the photoperiod, which provides seasonal information in which a stress can be interpreted in the appropriate context (Putterill et al., 2004; Craufurd and Wheeler, 2009). Nevertheless, moderate heat stress will often accelerate flowering, which may cause reproduction to occur before plants accumulate adequate resources (i.e. biomass) for allocation to developing seeds. Mechanisms of heat-stimulated bolting and flowering in Arabidopsis (Arabidopsis thaliana) are being uncovered (Balasubramanian et al., 2006; Tonsor et al., 2008). Cold temperatures will typically delay flowering, which may cause seeds to develop later in the growing season under suboptimal temperatures.
Temperature stress can sometimes have different effects on male and female structures, thereby creating asynchrony between male and female reproductive development (Herrero, 2003; Hedhly et al., 2008). In maize (Zea mays), floral asynchrony is a significant problem under conditions of combined stress from heat and water deficit (http://www.agry.purdue.edu/Ext/corn/news/articles.02/WTMDS-0717.html; Barnabás et al., 2008). In addition, high temperature stress can shorten the period of time in which the stigmas in the flowers are receptive to pollen, and thereby decrease the chances for a successful fertilization. For example, the stigmas in peach (Prunus persica L.) at 30 °C lost their ability to support pollen germination after 3 d, whereas at 20 °C they were viable for 8 d (Hedhly et al., 2005).
A third category of temperature stress effects includes defects in the structure and function of parental tissues (i.e. corollas, carpels, and stamens). Heat stress can reduce the number, decrease the size, and cause deformity of floral organs (Takeoka et al., 1991; Morrison and Stewart, 2002). For example, a high frequency of flower abortion in response to low temperatures is well documented for chickpea (Cicer arietinum) (Croser et al., 2003).
There are relatively few examples in which the effects of temperature stress on female reproductive organs have been investigated. For heat stress, ovary abnormalities were observed in wheat (Triticum aestivum, variety ‘Gabo’) (Saini et al., 1983). In Arabidopsis, heat stress reduced the total number of ovules and increased ovule abortion (Whittle et al., 2009). For cold stress, a study on chickpeas showed reduced ovule size, reduced ovule viability, missing embryo sacs, and impaired pistil function in temperature-sensitive cultivars (Srinivasan et al., 1999). In rice (Oryza sativa) and maize, evidence suggests that ovule/female fertility is fairly tolerant of a moderate cold stress (Hayase et al., 1969; Dupuis and Dumas, 1990).
More is known of the effects of temperature stress on male reproductive structures (Barnabás et al., 2008; Thakur et al., 2010). For example, in wheat, heat stress during the period of microspore meiosis can induce tapetum degradation (Saini et al., 1984; Sakata et al., 2000). This degradation of the nutritive tissues of the tapetum leads to pollen sterility. High temperatures cause poor anther dehiscence characterized by tight closure of the locules, which was shown to reduce pollen dispersal in rice and tomato (Solanum lycopersicum) (Matsui and Omasa, 2002; Sato et al., 2002).
Cold temperatures can induce pollen sterility, which may be due to disruption of sugar metabolism in the tapetum, ultimately abolishing starch accumulation (i.e. energy reserves) in the pollen grains (Oliver et al., 2005). Evidence suggests that cold-induced disruption of anther sugar transport and corresponding pollen sterility is signalled by ABA, in part by down-regulating expression of cell wall invertase and monosaccharide transporters (Oliver et al., 2007). Heat stress reduces carbohydrate deposition in pollen grains (Jain et al., 2007). The pre-existing carbohydrate reserves within the pollen grains fuel tube growth, but pollen later switch to using carbohydrates provided by the transmitting tract of the style (Herrero and Arbeloa, 1989). Cotton (Gossypium hirsutum) flowers exposed to moderately high temperatures showed reduced carbohydrate reserves (particularly sucrose) and ATP production in the pistils, which correlated with a heat stress-induced decline in net photosynthesis (Snider et al., 2009).
In the final category of temperature-induced reproductive effects, temperature stress may directly affect the development of male and female gametes. The effects of temperature stress on male gametes are well documented for numerous plant species. Pollen maturation, viability, germination ability, and pollen tube growth can be negatively affected by heat (Dupuis and Dumas, 1990; Peet et al., 1998; Prasad et al., 1999; Aloni et al., 2001; Young et al., 2004). Cold stress can disrupt mitosis I and II, thus preventing rice microspores from maturing into tricellular pollen grains (Sataka and Hayase, 1970). Cold temperatures inhibited pollen germination and shortened pollen tubes in Trifolium repens and chickpea (Jakobsen and Martens, 1994; Srinivasan et al., 1999). The following sections of this review are focused on pollen, first discussing evidence that pollen development and fertilization are often the weak links in reproductive stress tolerance, and then summarizing investigations on the stress response pathways that function in these cells.
Pollen development and function: a weak link in stress tolerance?
In many cases pollen and female gametes can be independently subjected to a temperature stress and subsequently tested for deficiencies in fertilization (Fig. 1). The following three examples illustrate how this experimental strategy was used to implicate pollen as a weak link in stress tolerance. In each example, the mechanistic basis for the stress sensitivity was attributed to different aspects of pollen development.
Fig. 1.
Diagram depicting the reciprocal crossing strategy employed in experiments to determine the reproductive tissue responsible for temperature stress sensitivity. The Pollen Donor Plant is represented by ‘♂’ and the Female Receptor Plant is represented by ‘♀’. The panel at the bottom highlighted in grey represents temperature-stressed male and female contributions. Arrows depict the possible combination of crosses. The dashed line represents temperature-stressed male contributions and the solid line represents the control temperature male contribution.
In the first example, pollen grain maturation in tomato was implicated as the most heat sensitive stage. Peet et al. (1998) treated genetically male-sterile and male-fertile tomato plants with a maximal 12/12 h day/night temperature stress of 32/26 °C or a control temperature of 28/22 °C, and performed reciprocal crosses between complementary reproductive organs of the different temperature regimes (Fig. 1). Female receptor plants treated with heat stress (32/26 °C) and crossed with pollen from plants grown at 28/22 °C exhibited reduced fruit set and a reduced number of seeds per fruit compared with the control (female receptor plants grown at 28/22 °C). Notably, female receptor plants (regardless of growth temperature) produced no fruit when crossed with pollen from plants grown under 32/26 °C heat stress conditions. For all combinations of crosses reported by Peet et al. (1998), heat stress applied to the pollen donor plant before and during pollen release decreased seed number and fruit set more severely than heat stress applied to the developing ovule and to the style after pollen application. This suggests that the effects of temperature stress were the most severe during pollen maturation rather than during pollen germination, tube growth, and fertilization.
In a second example, Young et al. (2004) used Brassica napus (canola) to perform crosses between male donor and female receptor plants exposed to either control conditions or heat stress and they measured reproductive output in terms of seed production. B. napus was subjected to 4 d of high temperatures stress. The daytime heat stress (35 °C) was reached by a gradual temperature increase followed by a gradual decrease to the night temperature (18 °C). Emasculated female plants treated with heat stress and crossed with control pollen had a 37% reduction in seed set compared with the control. However, pollen donor plants treated with heat stress and crossed with control emasculated female plants showed an even more severe reduction in seed set, 88% less than the control. The most acute reduction in seed set resulted from crossing gametes of heat-stressed pollen donor plants with heat-stressed emasculated female receptor plants. The compounding effect of heat stress on both male and female organs resulted in seed set levels 97% less than the control. The proposed site of the male reproductive defect for B. napus was an irreversible reduction in pollen germination, which was observed during an in vitro growth assay of temperature-stressed pollen.
In a third example, Dupuis and Dumas (1990) studied the response of maize male and female reproductive tissues to high and low temperature stresses. Male and female tissues were treated separately with 4 h of control temperature (28 °C) or temperature stress (4 °C or 40 °C) and used for in vitro fertilization with a complementary reproductive organ (Fig. 1). Unlike the seed set reduction manifested in heat-stressed B. napus female organs (Young et al., 2004), no significant difference in fertilization was found between female maize spikelets treated with control (28 °C), cold (4 °C), or heat (40 °C) temperatures and then crossed with control pollen at 28 °C (Dupuis and Dumas, 1990). Fertilization rates from crosses using pollen submitted to cold (4 °C) stress were not significantly different from the control. However, fertilization was unsuccessful when using pollen submitted to heat stress prior to application to the spikelet, suggesting that maize pollen maturation is highly sensitive to heat stress in a manner similar to the tomato example described above. In addition, in vitro fertilization was unsuccessful when pollinated spikelets were treated with 6 h of heat stress immediately following application of pollen grown under control temperatures. This indicates that the heat-stress sensitivity of pollen extends beyond the early events of pollen grain maturation. Moreover, autoradiography of 32P-labelled pollen tubes showed reduced growth through the silk during a heat stress (40 °C). These observations suggested that the pollen tube growth potential was a highly sensitive phase of development.
Taken together, the examples above suggest that when high temperature stress is placed separately upon male and female gametes prior to pollination, pollen is often the most vulnerable. The ability experimentally to evaluate whether the weakest link is the male or female is useful in directing research aimed at improving tolerance. However, it is important to remember that temperature stress is normally experienced simultaneously by both male and female reproductive structures.
Synergistic effects of temperature stress on both male and female reproductive tissues
Synergistic effects of temperature stress on both male and female tissues were observed in systems such as B. napus, tomato, and wheat (Saini et al., 1983; Peet et al., 1998; Young et al., 2004). This is not surprising for several reasons, including the fact that there are dynamic interactions between the growing pollen tube and the cells of the pistil. These dynamic interactions were documented by the observation of both physiological and transcriptional changes in the pollen (Lord, 2003; Qin et al., 2009). For example, Qin et al, (2009) analysed the transcriptome of Arabidopsis pollen tubes that grew through a female pistil and observed a 10% larger transcriptome as compared with in vitro germinated pollen, and identified 383 pollen-expressed transcripts that are specifically responsive to male-to-female contact. Temperature stress to both male and female reproductive organs may have detrimental effects on the cell-to-cell signals that mediate pollen processes from germination to fertilization.
As previously discussed, heat stress can reduce carbohydrates in pollen grains and ATP in stigmatic tissue (Herrero and Arbeloa, 1989; Snider et al., 2009). Thus, the additive effect of high temperature stress to both male and female tissues may also contribute to a reduction in energy available to the growing pollen tube. In reciprocal crosses between a heat-stressed B. napus gamete and a non-stressed partner, the non-stressed partner (male or female) appears to contribute to a partial rescue of gametophytic function (Young et al., 2004), potentially through an increase in available nutrients.
An Arabidopsis model for stress sensitive pollen
By comparison with crop plants, less is known about the effects of temperature stress on pollen development and fertilization in Arabidopsis. To explore the potential of using the Arabidopsis system to study pollen stress, a survey of 20 different Arabidopsis ecotypes was initiated for the effects on reproductive success (i.e. seed set per silique) caused by exposing plants to hot days and cold nights during flowering (Harper laboratory, unpublished results). Of the ecotypes tested, Hilversum (Hi-0) showed the most significant decrease in reproductive success (Fig. 2A, B). By comparison with the Col ecotype, which displayed about a 50% reduction in seed set, most of the Hi-0 siliques failed to produce any seed under the same conditions. For the Hi-0 siliques that did produce a few seed, the positions of those seed were analysed and compared to Col grown under the same stress conditions, as shown in Fig. 2B. In contrast to the Col seed set pattern that showed a uniform distribution throughout the silique, the few Hi-0 seeds were typically clustered in the top half of the silique (relative to the stigma end).
Fig. 2.
Distribution of seed set within a silique showing relative patterns of seed set reduction in Arabidopsis plants subjected to a stress of hot days and cold nights. (A) In comparison to the even distribution of seeds in the Col ecotype, the Hi-0 ecotype showed a seed set clustered towards the stigma end. (B) The percentage seed set in each silique quadrant for stressed plants was determined by comparison to control Col and Hi-0 plants grown with 16 h of light (at 20 °C) and 8 h of dark (at 18 °C), respectively. The hot/cold-stress was implemented by growing plants in a growth chamber with 16 h of light (day) and 8 h dark (night). Plants were subjected a hot/cold-stress regime continuously from the initiation of bolting until silique maturity. This regime involved a gradual shift from a day-time peak hot stress of 40 °C, to a night-time cold stress at –1 °C. Specifically, at daybreak the temperature was shifted over 5 h from –1 °C to a 1 h peak at 40 °C, followed by a drop to 10 °C for 10 h. At the onset of night, the temperature was dropped to –1 °C for 8 h. Error bars represent the ratio of standard errors of the experimental quadrant seed set over the standard errors of the control quadrant seed set.
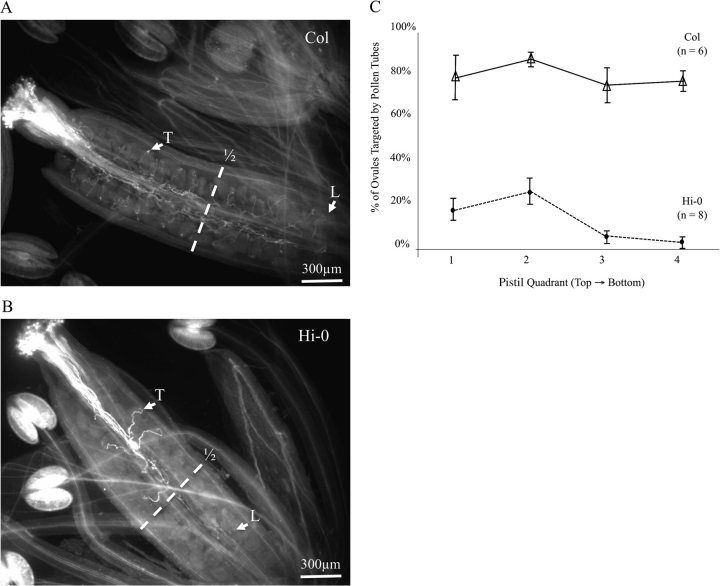
To test whether the top-biased pattern of seed set in Hi-0 was the result of impaired tube growth under hot/cold conditions, pollen tubes were visualized by aniline blue staining (Fig. 3A, B). Consistent with this hypothesis, very few Hi-0 pollen tubes were observed to grow to the bottom half of the pistil (Fig. 3B). For example, an average of 22% of ovules in the top half of the silique and 4.5% of ovules in the bottom half of the silique were targeted by a pollen tube. In contrast to Hi-0, pollen tubes in Col showed a regular distribution of interactions throughout the pistil (Fig. 3A, C), consistent with the development of an evenly distributed seed set (Fig. 2B). For Hi-0 tubes that grew far enough along the transmitting tract to reach an ovule, most were still unable to locate and grow towards an ovule (tropism defect) (Fig. 3B, C).
Fig. 3.
Aniline blue staining of pistils shows Hi-0 pollen tubes with stress-dependent defects in growth potential and tropism. Arabidopsis ecotypes Hi-0 and Col were subjected to a hot/cold stress as described in Fig. 2. Pistils from self-fertilized flowers were stained with aniline blue to reveal the locations of pollen tubes. Flowers of Col and Hi-0 were collected at identical growth stages. (A) Pollen tubes in the Col ecotype showed a uniform targeting of ovules throughout the pistil. B) Pollen tubes in the Hi-0 ecotype were only occasionally observed to target the ovule, and only two pollen tubes extended beyond the first half of the pistil. The pistil halves are indicated by the white dotted line (1/2). The white arrows (designated ‘T’) point to examples of ovules successfully targeted by pollen tubes. The white arrows (designated ‘L’) specify the location of the longest pollen tube in the pistils. Images shown are representative of approximately 6–8 pistils analysed for each ecotype. (C) Distribution of ovules targeted by pollen for Col and Hi-0 Arabidopsis ecotypes grown under hot/cold conditions. Aniline blue-stained pistils were used to determine the percentage of pollen-targeted ovules in each pistil quadrant. Error bars represent the standard error.
Based on the severity of seed set reduction, Hi-0 appears to be more temperature sensitive than Col. In Hi-0, the short growth and tropism deficiencies of the pollen tubes support an hypothesis that Hi-0 pollen tubes are more temperature sensitive than those from Col. However, an alternative hypothesis is that these pollen deficiencies may be an indirect effect of an increased stress sensitivity associated with either (i) defects in the transmitting tract that provide structure or nutritional support for growing tubes or, (ii) defects in the ovules that decrease their ability to attract pollen tubes through tropism signals.
In Col plants subjected to the same stress, the more uniform success of pollen tube growth and fertilization suggests that the more modest 50% reduction in seed set may be due to a different set of stress sensitivities, either in female ovule production or abortion, or early zygotic embryo lethalities. Thus, the observation that the pollen from Hi-0 are potentially hypersensitive to a temperature stress provides a potentially useful model for investigating temperature tolerance mechanisms in Arabidopsis. For example, Hi-0 could be used to test the efficacy of various candidate stress protection genes by quantifying pollen transmission efficiency differences between Hi-0 pollen with or without a candidate stress-tolerance transgene.
Pollen transcriptomics and proteomics: tools for understanding responses to temperature stress
Genome-wide strategies are being used to investigate all aspects of pollen development, including responses to temperature stress (Lee and Lee, 2003; Honys and Twell, 2004; Pina et al., 2005; Frank et al., 2009; Jagadish et al., 2010). In Arabidopsis, expression profiling experiments indicate that pollen development involves approximately half the genome (i.e. more than 14 000 genes) (Borg et al., 2009). Moreover, almost half (600) of the 1350 predicted transcription factors in Arabidopsis are detected at some stage of pollen development (Honys and Twell, 2004). Each stage of pollen development includes dramatic changes in mRNA profiles. For example, a comparison of mRNA between mature dry pollen and growing pollen tubes identified as many as 1600 significant changes (Qin et al., 2009). Some of the genes that are up-regulated during pollen germination and tube growth include calmodulin and calmodulin-like proteins (Wang et al., 2008; Qin et al., 2009), which may be involved in calcium signalling pathways important for tip growth (Malhó et al., 2006) and responses to the environment (Snedden and Fromm, 2001). In addition, genes involved in stress response pathways such as ABA receptors (Park et al., 2009) and components of an ethylene response pathway (e.g. CTR1, ETR1, EIN2) (Lin et al., 2009) are expressed at detectable levels in pollen (Honys and Twell, 2004; Wang et al., 2008; Qin et al., 2009).
The effect of short-term heat stress on mRNA expression profiles in maturing tomato microspores from heat-sensitive and heat-tolerant genotypes was analysed (Frank et al., 2009). Of the two strategies used, the microarray platform utilized a 10 k probe set representing known transcripts, whereas, in the second strategy, the cDNA-AFLP technique identified differentially expressed transcripts without prior knowledge of gene sequences, thus potentially discovering transcripts not represented on the microarray. The majority of genes identified in the cDNA-AFLP experiments showed no homology to known sequences, and represent potential candidates for further study in the tomato pollen heat-stress response.
Among the known genes identified by cDNA-AFLP analysis, calcium dependent protein kinase 2 (CDPK2) was highlighted as a potentially interesting example of an up-regulated gene in heat stressed microspores (Frank et al., 2009). CDPKs are implicated in transducing calcium signals to stimulate pollen tube growth and to mediate tropism (Myers et al., 2009), as well as abiotic stress responses (Ma and Wu, 2007; Zhu et al., 2007). Whether the heat stress induction of CDPK2 (Frank et al., 2009) results in a more active stress tolerance response has yet to be determined.
Microarray analysis also showed many up-regulated genes that are representative of previously characterized temperature stress pathways (Frank et al., 2009). Increased expression of SlAPX3 (a reactive oxygen species scavenger), ethylene responsive genes (including the MBF1 homologue ER24), HSFA2, and HSFA3, and HSP family members were observed in heat-stressed microspores. Moreover, microspores of the heat-tolerant tomato expressed higher levels of HSF2A and LeHSP17.4-CII (a HSP) than the heat-sensitive variety.
Likewise, other studies also illustrate HSP transcription in pollen. During the early stages of maize pollen development HSP90, HSP70, and HSP60 are expressed and the corresponding proteins persist at low levels within the pollen grain (Barnabás et al., 2008). In mature pollen, HSP transcript accumulation in response to high temperature is either extremely low (such as HSP101, HSP70, and HSP18 in maize) or absent (such as HSP70 in B. napus) (Hopf et al., 1992; Young et al., 2004; Barnabás et al., 2008).
In addition, changes in protein profiles are associated with pollen developmental processes and stress responses. In a proteomic study of pollen, a considerable overlap was seen for some of the major proteins present in mature pollen grains and seeds (Grobei et al., 2009). Notably, both proteomes contained high levels of LEA proteins and chaperones, consistent with an expected role of these proteins in conferring relatively stress-tolerant dormant states for both mature pollen grains and seeds.
A second proteomic analysis compared proteins expressed in heat-stressed anthers from three rice varieties with different temperature tolerances (Jagadish et al., 2010). Interestingly, a temperature-tolerant rice genotype (N22) showed a higher accumulation of small heat shock proteins (sHSP) compared with a temperature-sensitive rice genotype (Moroberekan). The moderately-tolerant rice genotype (IR64) showed intermediate sHSP accumulation. The authors speculated that the accumulation of sHSP may confer greater heat tolerance in N22 rice.
Taken together, expression profiling and proteomic studies on multiple plant species supports a perspective that the genetic programme for the male gametophytic life cycle is highly complex, and highly responsive to changes in the environment.
Future perspectives
Impending global climate change, with predicted 1.5–5.8 °C increases in temperatures by 2100, pose threats to agricultural production (Rosenzweig et al., 2001). Crop yields are predicted to decrease approximately 10% for every one-degree increase in temperature (USDA Release no. 0501.09, 2009). Developing nations that already suffer from heat-stress related crop failures are predicted to be especially susceptible to climate change, particularly those located in the sub-Saharan Africa (Fischer et al., 2005).
While climate change is projected to negatively impact future agricultural production, significant temperature-stress-induced yield losses happen right now. In the United States during August 2000, approximately US$4.2 billion in agricultural losses were caused by the combination of high temperature and drought (Mittler, 2006). This example shows how rapidly and severely high temperature can decrease agricultural production, especially in combination with other stresses. An historical example of note was the negative impact on wheat yields due to the high summer temperatures in the former Soviet Union during the summer of 1972 (Battisti and Naylor, 2009). Wheat prices on the international market rose from $60 to $208 per metric tonne, thus provoking fears of political instability in some developing nations (Battisti and Naylor, 2009).
In many cases, crop plants are now cultivated in new regions with different climatic fluctuations. Soybeans (Glycine max) are now widely farmed at higher latitudes, which experience colder temperatures than the plants’ original zone of cultivation in central China (Dong et al., 2004; Funatsuki et al., 2009). In addition, seven million hectares of rice fields are now in regions susceptible to yield-reducing cold damage at the reproductive stage (Oliver et al., 2005). Furthermore, particularly in semi-arid climates, plants can be exposed to the extremes of temperature stresses including hot days and cold nights (Smith and Nowak, 1990). Wide fluctuations in temperatures pose an additional risk to plant reproduction since plants need constantly to switch between hot and cold stress responses.
Both the current and the anticipated reduction in crop yields caused by temperature stress emphasize a need to understand how climatic variations can impact the life cycles of different crop plants. For many food crops, such as corn, tomato, and B. napus, pollen growth and fertilization appear to be particularly sensitive to heat stress. When pollen grains rehydrate, germinate, and begin tip growth, they become one of the most physiologically active plant cells known, growing at extremely rapid rates, with the precise navigation needed to find ovules for fertilization. Fertilization itself requires communication between cells and rapid fundamental changes in cellular programmes. Given these complex physiological and developmental events, it is perhaps not surprising that pollen growth and fertilization might often be found as a weak-link in stress tolerance.
Continued improvements in reproductive stress tolerance are expected to occur through multiple strategies. Interestingly, stress tolerance in vegetative and reproductive tissues is not always correlated (Salem et al., 2007). In vitro pollen growth assays have been considered as a possible screen to identify germplasm with the potential for improved reproductive stress tolerance (Kakani et al., 2002, 2005; Salem et al., 2007; Singh et al., 2008). In cases where pollen tube growth is the weak link in temperature stress tolerance, this simple strategy may be highly rewarding. Furthermore, conventional breeding strategies can take advantage of the strong selection pressures on pollen fitness to select for improved stress tolerance (Hedhly et al., 2008).
The potential also exists to introduce new stress tolerance traits through genetic engineering. For example, a protein identified in lily pollen (LLA23) was expressed in Arabidopsis and successfully used to confer salt and drought tolerance to vegetative tissues (Yang et al., 2005). A similar over-expression of a related gene (OsAsr) from rice also conferred improved cold tolerance in transgenic rice (Kim et al., 2009). Whether these and other proteins can be used to improve stress tolerance for pollen has yet to be tested.
Acknowledgments
This work was supported by grants to JFH from NSF DBI-0420033 to establish reproductive stress assays, NIH 1RO1 GM070813-01 to survey ecotypes for stress tolerance, and NSF MCB-0920624 for the pollen growth assays. Support for the literature research and personnel was provided by all grants, including DOE DE-FG03-94ER20152.
References
- Allen DJ, Ort DR. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends in Plant Science. 2001;6:36–42. doi: 10.1016/s1360-1385(00)01808-2. [DOI] [PubMed] [Google Scholar]
- Aloni B, Peet M, Pharr M, Karni L. The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination. Physiologia Plantarum. 2001;112:505–512. doi: 10.1034/j.1399-3054.2001.1120407.x. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. Plos Genetics. 2006;2:980–989. doi: 10.1371/journal.pgen.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniwal SK, Bharti K, Chan KY, et al. Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. Journal of Biosciences. 2004;29:471–487. doi: 10.1007/BF02712120. [DOI] [PubMed] [Google Scholar]
- Barnabás B, Jager K, Feher A. The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell and Environment. 2008;31:11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Battisti DS, Naylor RL. Historical warnings of future food insecurity with unprecedented seasonal heat. Science. 2009;323:240–244. doi: 10.1126/science.1164363. [DOI] [PubMed] [Google Scholar]
- Blum A, Sinmena B, Mayer J, Golan G, Shpiler L. Stem reserve mobilization supports wheat-grain filling under heat-stress. Australian Journal of Plant Physiology. 1994;21:771–781. [Google Scholar]
- Borg M, Brownfield L, Twell D. Male gametophyte development: a molecular perspective. Journal of Experimental Botany. 2009;60:1465–1478. doi: 10.1093/jxb/ern355. [DOI] [PubMed] [Google Scholar]
- Busch W, Wunderlich M, Schoffl F. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. The Plant Journal. 2005;41:1–14. doi: 10.1111/j.1365-313X.2004.02272.x. [DOI] [PubMed] [Google Scholar]
- Charles WB, Harris RE. Tomato fruit-set at high and low-temperatures. Canadian Journal of Plant Science. 1972;52:497–506. [Google Scholar]
- Chinnusamy V, Zhu J, Zhu JK. Cold stress regulation of gene expression in plants. Trends in Plant Science. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Craufurd PQ, Wheeler TR. Climate change and the flowering time of annual crops. Journal of Experimental Botany. 2009;60:2529–2539. doi: 10.1093/jxb/erp196. [DOI] [PubMed] [Google Scholar]
- Croser JS, Clarke HJ, Siddique KHM, Khan TN. Low-temperature stress: implications for chickpea (Cicer arietinum L.) improvement. Critical Reviews in Plant Sciences. 2003;22:185–219. [Google Scholar]
- Dong YS, Zhao LM, Liu B, Wang ZW, Jin ZQ, Sun H. The genetic diversity of cultivated soybean grown in China. Theoretical and Applied Genetics. 2004;108:931–936. doi: 10.1007/s00122-003-1503-x. [DOI] [PubMed] [Google Scholar]
- Dupuis I, Dumas C. Influence of temperature stress on in vitro fertilization and heat-shock protein-synthesis in maize (Zea mays L.) reproductive tissues. Plant Physiology. 1990;94:665–670. doi: 10.1104/pp.94.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Shah M, Tubiello FN, van Velhuizen H. Socio-economic and climate change impacts on agriculture: an integrated assessment, 1990–2080. Philosophical Transactions of the Royal Society B-Biological Sciences. 2005;360:2067–2083. doi: 10.1098/rstb.2005.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S, Thomashow MF. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. The Plant Cell. 2002;14:1675–1690. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G, Pressman E, Ophir R, Althan L, Shaked R, Freedman M, Shen S, Firon N. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. Journal of Experimental Botany. 2009;60:3891–3908. doi: 10.1093/jxb/erp234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsuki H, Ohnishi S. Recent advances in physiological and genetic studies on chilling tolerance in soybean. Jarq-Japan Agricultural Research Quarterly. 2009;43:95–101. [Google Scholar]
- Gounaris K, Brain ARR, Quinn PJ, Williams WP. Structural reorganization of chloroplast thylakoid membranes in response to heat-stress. Biochimica et Biophysica Acta. 1984;766:198–208. [Google Scholar]
- Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang R, Roschitzki B, Basler K, Ahrens CH, Grossniklaus U. Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Research. 2009;19:1786–1800. doi: 10.1101/gr.089060.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase H, Satake T, Nishiyama I, Ito N. Male sterility caused by cooling treatment at the meiotic stage in rice plants. II. The most sensitive stage to cooling and the fertilising ability of pistils. Proceedings of the Crop Science Society of Japan. 1969;38:706–711. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. The effect of temperature on pollen germination, pollen tube growth, and stigmatic receptivity in peach. Plant Biology. 2005;7:476–483. doi: 10.1055/s-2005-865850. [DOI] [PubMed] [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. Global warming and plant sexual reproduction. Trends in Plant Science. 2008;14:30–36. doi: 10.1016/j.tplants.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Herrero M. Male and female synchrony and the regulation of mating in flowering plants. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2003;358:1019–1024. doi: 10.1098/rstb.2003.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M, Arbeloa A. Influence of the pistil on pollen-tube kinetics in peach (Prunus persica) American Journal of Botany. 1989;76:1441–1447. [Google Scholar]
- Herrero MP, Johnson RR. High-temperature stress and pollen viability of maize. Crop Science. 1980;20:796–800. [Google Scholar]
- Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biology. 2004;5:R85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf N, Plesofskyvig N, Brambl R. The heat-shock response of pollen and other tissues of maize. Plant Molecular Biology. 1992;19:623–630. doi: 10.1007/BF00026788. [DOI] [PubMed] [Google Scholar]
- Howarth CJ. Genetic improvements of tolerance to high temperature. In: Ashraf M, Harris PJC, editors. Abiotic stresses: plant resistance through breeding and molecular approaches. New York: Haworth Press Inc.; 2005. pp. 277–300. [Google Scholar]
- Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annual Review of Plant Biology. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- Jagadish SVK, Muthurajan R, Oane R, Wheeler TR, Heuer S, Bennett J, Craufurd PQ. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.) Journal of Experimental Botany. 2010;61:143–156. doi: 10.1093/jxb/erp289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Prasad PVV, Boote KJ, Hartwell AL, Chourey PS. Effects of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench) Planta. 2007;227:67–79. doi: 10.1007/s00425-007-0595-y. [DOI] [PubMed] [Google Scholar]
- Jakobsen HB, Martens H. Influence of temperature and aging of ovules and pollen on reproductive success in Trifolium repens L. Annals of Botany. 1994;74:493–501. [Google Scholar]
- Kakani VG, Prasad PVV, Craufurd PQ, Wheeler TR. Response of in vitro pollen germination and pollen tube growth of groundnut (Arachis hypogaea L.) genotypes to temperature. Plant, Cell and Environment. 2002;25:1651–1661. [Google Scholar]
- Kakani VG, Reddy KR, Koti S, Wallace TP, Prasad PVV, Reddy VR, Zhao D. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Annals of Botany. 2005;96:59–67. doi: 10.1093/aob/mci149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee SC, Hong SK, An K, An G, Kim SR. Ectopic expression of a cold-responsive OsAsr1 cDNA gives enhanced cold tolerance in transgenic rice plants. Molecules and Cells. 2009;27:449–458. doi: 10.1007/s10059-009-0055-6. [DOI] [PubMed] [Google Scholar]
- Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf KD. Complexity of the heat stress response in plants. Current Opinion in Plant Biology. 2007;10:310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Kreps JA, Wu YJ, Chang HS, Zhu T, Wang X, Harper JF. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiology. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant Physiology. 2008;146:748–761. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiology. 1999;120:173–181. doi: 10.1104/pp.120.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Henderson DA, Zhu JK. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. The Plant Cell. 2005;17:3155–3175. doi: 10.1105/tpc.105.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lee DH. Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiology. 2003;132:517–529. doi: 10.1104/pp.103.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZF, Zhong SL, Grierson D. Recent advances in ethylene research. Journal of Experimental Botany. 2009;60:3311–3336. doi: 10.1093/jxb/erp204. [DOI] [PubMed] [Google Scholar]
- Lord EM. Adhesion and guidance in compatible pollination. Journal of Experimental Botany. 2003;54:47–54. doi: 10.1093/jxb/erg015. [DOI] [PubMed] [Google Scholar]
- Ma SY, Wu WH. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Molecular Biology. 2007;65:511–518. doi: 10.1007/s11103-007-9187-2. [DOI] [PubMed] [Google Scholar]
- Malhó R, Liu Q, Monteiro D, Rato C, Camacho L, Dinis A. Signalling pathways in pollen germination and tube growth. Protoplasma. 2006;228:21–30. doi: 10.1007/s00709-006-0162-6. [DOI] [PubMed] [Google Scholar]
- Matsui T, Omasa K. Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: anther characteristics. Annals of Botany. 2002;89:683–687. doi: 10.1093/aob/mcf112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Abiotic stress, the field environment and stress combination. Trends in Plant Science. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Morrison MJ, Stewart DW. Heat stress during flowering in summer Brassica. Crop Science. 2002;42:797–803. [Google Scholar]
- Myers C, Romanowsky SM, Barron YD, Garg S, Azuse CL, Curran A, Davis RM, Hatton J, Harmon AC, Harper JF. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. The Plant Journal. 2009;59:528–539. doi: 10.1111/j.1365-313X.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- Oliver SN, Dennis ES, Dolferus R. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant and Cell Physiology. 2007;48:1319–1330. doi: 10.1093/pcp/pcm100. [DOI] [PubMed] [Google Scholar]
- Oliver SN, Van Dongen JT, Alfred SC, et al. Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant, Cell and Environment. 2005;28:1534–1551. [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet MM, Sato S, Gardner RG. Comparing heat stress effects on male-fertile and male-sterile tomatoes. Plant, Cell and Environment. 1998;21:225–231. [Google Scholar]
- Pina C, Pinto F, Feijo JA, Becker JD. Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiology. 2005;138:744–756. doi: 10.1104/pp.104.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad PVV, Craufurd PQ, Summerfield RJ. Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Annals of Botany. 1999;84:381–386. [Google Scholar]
- Putterill J, Laurie R, Macknight R. It's time to flower: the genetic control of flowering time. Bioessays. 2004;26:363–373. doi: 10.1002/bies.20021. [DOI] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genetics. 2009 doi: 10.1371/journal.pgen.1000621. 5, e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig CA, Iglesias XB, Yang PR, Epstein E, Chivian E. Climate change and extreme weather events. Implications for food production, plant diseases and pest. Global Change and Human Health. 2001;2:90–104. [Google Scholar]
- Saini HS, Sedgley M, Aspinall D. Effect of heat-stress during floral development on pollen-tube growth and ovary anatomy in wheat (Triticum aestivum L.) Australian Journal of Plant Physiology. 1983;10:137–144. [Google Scholar]
- Saini HS, Sedgley M, Aspinall D. Developmental anatomy in wheat of male-sterility induced by heat-stress, water deficit or abscisic-acid. Australian Journal of Plant Physiology. 1984;11:243–253. [Google Scholar]
- Sakata T, Takahashi H, Nishiyama I, Higashitani A. Effects of high temperature on the development of pollen mother cells and microspores in barley Hordeum vulgare L. Journal of Plant Research. 2000;113:395–402. [Google Scholar]
- Salem MA, Kakani VG, Koti S, Reddy KR. Pollen-based screening of soybean genotypes for high temperatures. Crop Science. 2007;47:219–231. [Google Scholar]
- Sataka T, Hayase H. Male sterility caused by cooling treatment at the young microspore stage in rice plants. V. Estimation of pollen developmental stage and the most sensitive stage to coolness. Proceedings of the Crop Science Society of Japan. 1970;39:468–473. [Google Scholar]
- Sato S, Peet MM, Thomas JF. Determining critical pre- and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. Journal of Experimental Botany. 2002;53:1187–1195. doi: 10.1093/jexbot/53.371.1187. [DOI] [PubMed] [Google Scholar]
- Singh SK, Kakani VG, Brand D, Baldwin B, Reddy KR. Assessment of cold and heat tolerance of winter-grown canola (Brassica napus L.) cultivars by pollen-based parameters. Journal of Agronomy and Crop Science. 2008;194:225–236. [Google Scholar]
- Smith SD, Nowak RS. Ecophysiology of plants in the intermountain lowlands. In: Osmond CB, Pitelka LF, Hidy GM, editors. Plant biology of the basin and range. New York: Springer-Verlag; 1990. pp. 179–241. [Google Scholar]
- Snedden WA, Fromm H. Calmodulin as a versatile calcium signal transducer in plants. New Phytologist. 2001;151:35–66. doi: 10.1046/j.1469-8137.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Snider JL, Oosterhuis DM, Skulman BW, Kawakami EM. Heat stress-induced limitations to reproductive success in. Gossypium hirsutum. Physiologia Plantarum. 2009;137:125–138. doi: 10.1111/j.1399-3054.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- Solomon S, Qin D, Manning M, Marquis M, Averyt K, Tignor MMB, Miller HL, Cheng Z. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. New York: Cambridge University Press; 2007. [Google Scholar]
- Srinivasan A, Saxena NP, Johansen C. Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.): genetic variation in gamete development and function. Field Crops Research. 1999;60:209–222. [Google Scholar]
- Strasser BJ. Donor side capacity of Photosystem II probed by chlorophyll a fluorescence transients. Photosynthesis Research. 1997;52:147–155. [Google Scholar]
- Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: a delicate balance between signalling and destruction. Physiologia Plantarum. 2006;126:45–51. [Google Scholar]
- Takeoka Y, Hiroi K, Kitano H, Wada T. Pistil hyperplasia in rice spikelets as affected by heat-stress. Sexual Plant Reproduction. 1991;4:39–43. [Google Scholar]
- Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H. Cold stress effects on reproductive development in grain crops: an overview. Environmental and Experimental Botany. 2010;67:429–443. [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Tonsor SJ, Scott C, Boumaza I, Liss TR, Brodsky JL, Vierling E. Heat shock protein 101 effects in A. thaliana: genetic variation, fitness and pleiotropy in controlled temperature conditions. Molecular Ecology. 2008;17:1614–1626. doi: 10.1111/j.1365-294X.2008.03690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environmental and Experimental Botany. 2007;61:199–223. [Google Scholar]
- Wang WX, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- Wang WX, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends in Plant Science. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiology. 2008;148:1201–1211. doi: 10.1104/pp.108.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle CA, Otto SP, Johnston MO, Krochko JE. Adaptive epigenetic memory of ancestral temperature regime in. Arabidopsis thaliana. Botany-Botanique. 2009;87:650–657. [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annual Review of Plant Biology. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- Yang CY, Chen YC, Jauh GY, Wang CS. A lily ASR protein involves abscisic acid signalling and confers drought and salt resistance in Arabidopsis. Plant Physiology. 2005;139:836–846. doi: 10.1104/pp.105.065458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LW, Wilen RW, Bonham-Smith PC. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany. 2004;55:485–495. doi: 10.1093/jxb/erh038. [DOI] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. The Plant Cell. 2007;19:3019–3036. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]