Abstract
Objective
The preferred amino acids in the proteolytic sites have been considered to be similar between caspase-1 and caspase-9, which do not support their differential functions in inflammatory pyroptosis and apoptosis. We attempted to solve this problem.
Methods
We analyzed the flanking 20 amino acid residues in the cleavage sites in 34 caspase-1 and 11 capase-9 experimentally identified substrates.
Results
This study has made the following findings: first, we verified that caspase-1 and caspase-9 shared 100% aspartic acid in the P1 position. However, the structures in the cleavage sites of most caspase-1 substrates are different from that of caspase-9 substrates in the following three aspects, a) the amino acid residues with the statistically high frequencies; b) the hydrophobic amino acid occurrence frequencies; and c) the charged amino acid occurrence frequencies; second, the amino acid pairs P1-P1′ are different; third, our identified cleavage site patterns are useful in the prediction for the 91.4% cleavage sites of 35 new caspase-1 substrates.
Conclusion
Since most caspase-1 substrates are involved in vascular function, inflammation and atherogenesis, our novel structural patterns for the caspases’ substrates are significant in developing new diagnostics and therapeutics.
Keywords: inflammatory cell death (pyroptosis), apoptosis, inflammation, caspase-1, caspase-9
INTRODUCTION
A new form of cell death, pyroptosis, or caspase 1-dependent cell death, is inflammatory and is triggered by various pathological stimuli, both endogenous stimuli such as stroke, heart attack or cancer, and exogenous ones including bacterial and viral infections1, 2, 3. The recognition of pathogen-associated molecular patterns’ (PAMPs) by PAMP-receptor families4 leads to activation of caspase-1 (EC 3.4.22.36, a proinflammatory caspase), and subsequent proteolytic conversion of proinflammatory cytokines, interleukin-1β (IL-1β) and IL-18 from their precursors pro-IL-1β and pro-IL-18, respectively. Activation of pro-caspase-1 to form active caspase-1 is mediated by a cytosolic protein complex, termed inflammasome4. Therefore, PAMP-Rs-caspase-1-IL-1β pathway becomes an essential mechanism for sensing stimuli and initiating inflammation and pyroptosis. In contrast, in the activation of apoptosis, another form of cell death, the initiator caspases’ activation platforms, such as death initiation signal complex (DISC) and apoptosome, sense pro-apoptotic stimuli and activate either death receptor/DISC-activated caspase-8 or mitochondrion/cytochrome c/apoptosome-activated caspase-9 pathway, respectively5, 6. Activation of caspase-9 results in apoptosis without inflammation. Since different cell death pathways require cellular substrates, therefore, the substrates cleaved by these two types of caspases have to be different.
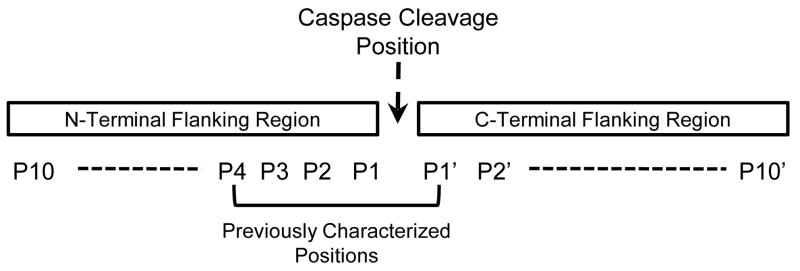
The name caspase, derived from cysteine-dependent aspartate specific protease, suggests its stringent specificity for cleaving substrates containing aspartic acid (Asp or D in abbreviation). The seminar work has identified the preferred amino acid residues with the Schechter and Berger’s enzymatic cleavage nomenclature (Fig. 1)7, P4-P3-P2-P1, in the cleavage sites of caspases’ substrates as bulky X-Glu-X-Asp (X-E-X-D)8, 9. These short sequences are very similar among the proteolytic cleavage sites cleaved by different caspases10. However, the pathophysiological relevance of the amino acid preferences of caspases cleavage sites is limited, which could not explain the functional differences of caspases. The preferences of amino acid residues in the cleavage sites used by experimentally verified intracellular caspase-1 substrates remain to be analyzed. In addition, another important question also remains to be identified is that whether the flanking regions extended outside of P4-P1 motif in the natural caspase-1 substrates have any preferred amino acids. Therefore, we hypothesized that, caspase-1 cleaves different set of protein substrates from that of caspase-9, which is accomplished by preferring certain amino acid residues in the P10…P1-P1′…P10′ regions. Our results indicate that most extended cleavage sites of caspase-1 substrates are different from that of the extended cleavage sites of caspase-9 substrates.
Figure 1. The position nomenclature for the caspases’ cleavage sites and the flow chart of this study.
The position nomenclature for the caspases’ cleavage sites. The Schechter and Berger’s enzymatic cleavage nomenclature is used in reference to the amino acid positions in the N-terminal and C-terminal flanking regions, relative to the caspases’ cleavage sites. The amino acid preferences in the positions P4-P1′ have been previously characterized with synthetic peptide library screening, as marked.
MATERIALS AND METHODS
1. The experimentally verified cleavage sites of natural caspase-1 substrates and natural caspase-9 substrates
As we reported11, a data mining strategy (suppl. Fig. 1) was adopted to analyze the amino acid occurrence frequencies in the flanking regions around the cleavage sites of experimentally identified human caspase-1 and caspase-9 substrates, according to the Schechter and Berger’s enzymatic cleavage nomenclature (Fig. 1)7. The protein sequences of caspases’ substrates were obtained from the protein database of the NIH/NCBI (http://www.ncbi.nlm.nih.gov/sites/entrez) as we reported12.
2. Amino acid occurrence frequencies in 30 randomly selected human proteins
Using a web based protein sequence software (http://www.expasy.ch/tools/protscale.html), the confidential intervals of the amino acid occurrence frequencies in the 30 randomly selected human house keeping gene proteins13 were generated by calculating the mean ± 1.96 × the standard deviation. If the amino acid occurrence frequency of a given amino acid in the caspases’ cleavage sites was larger than the upper limit of the confidential intervals (the mean ± 1.96 × the standard deviations) of the 30 proteins, the amino acid frequency in the position was statistically significant.
3. Prediction of the potential caspases’ cleavage sites in experimentally verified caspases’ substrates and potential caspases’ substrates
The caspases’ cleavage sites in the experimentally identified caspase-1 substrates and other proteins were predicted by analyzing caspases’ cleavage sites in protein substrates using the caspase-1 cleavage site consensus sequence generated in this study and a web based protein alignment software (http://imed.med.ucm.es/PVS/).
4. Statistical analysis
The statistical analyses were performed using the functions of t test, confidential intervals and the chi-square in Microsoft Office Excel14.
EXPERIMENTAL RESULTS
1. The amino acid occurrence frequencies in the flanking regions P10…P1-P1′-P10′ of natural caspase-1 substrates are different from that of natural caspase-9 substrates
To analyze the amino acid occurrence frequencies of the cleavage sites of caspase-1 substrates, we collected 34 experimentally characterized caspase-1 cleavage sites in the 23 human proteins (Table 1A). In addition, for comparison, we also collected 11 experimentally identified caspase-9 cleavage sites in the six human proteins (Table 1B).
Table 1. The experimentally identified caspase-1 substrates and caspase-9 substrates.
The experimentally identified caspase-1 substrates and caspase-9 substrates are presented with the cleavage site position P1 of caspase, the NCBI protein database accession number, cellular function of the protein and the reference of the substrates.
| Table 1A. Experimentally Identified Substrates of Caspase-1 with Characterized Cleavage Site | ||||
|---|---|---|---|---|
| Substrate | Cleavage Position (P1 amino acid) | NCBI/Protein Database (GI) Accession No. | Cellular Function | Reference |
| caspase-1 | ||||
| IL-1β - cut 1 | 27 | 28317372 | Mediator of Inflammation | PMID: 10872455 |
| IL-1β - cut 2 | 116 | 28317372 | Mediator of Inflammation | PMID: 10872455 |
| IL-18 | 36 | 4504653 | Stimulation of INF-γ | PMID: 10872455 |
| β-actin - cut 1 | 11 | 46397333 | Cytoskeleton | PMID: 8700913 |
| β-actin - cut 2 | 244 | 46397333 | Cytoskeleton | PMID: 8700913 |
| GADPH | 189 | 31645 | Glycolysis | PMID: 17959595 |
| HnRNP A2-cut 1 | 49 | 500638 | Translation | PMID: 17273173 |
| HnRNP A2-cut 2 | 55 | 500638 | Translation | PMID: 17273173 |
| HnRNP A2-cut 3 | 76 | 500638 | Translation | PMID: 17273173 |
| Hsp90 | 259 | 306891 | Chaperone | PMID: 17273173 |
| Calpastatin - cut 1 | 137 | 303599 | Calpain Inhibitor | PMID: 9705209 |
| Calpastatin - cut 2 | 216 | 303599 | Calpain Inhibitor | PMID: 9705209 |
| Calpastatin - cut 3 | 417 | 303599 | Calpain Inhibitor | PMID: 9705209 |
| PPAR-γ | 64 | 116284373 | Transcription Factor | PMID: 18497737 |
| Nedd4 | 237 | 32172435 | Ubiquitin-protein Ligase | PMID: 9593687 |
| Parkin | 126 | 3063388 | E3 ubiquitin ligase component | PMID: 12692130 |
| Ataxin-3 - cut 1 | 241 | 14149093 | Polyglutamine disease protein | PMID: 15140190 |
| Ataxin-3 - cut 2 | 244 | 14149093 | Polyglutamine disease protein | PMID: 15140190 |
| Ataxin-3 - cut 3 | 248 | 14149093 | Polyglutamine disease protein | PMID: 15140190 |
| BCL-XL | 61 | 510901 | Anti-apoptotic protein | PMID: 9435230 |
| PARP | 214 | 116283598 | DNA Repair | PMID: 7642516 |
| TF AP-2α | 19 | 4507441 | Transcription Factor | PMID: 11438643 |
| MAP-Tau Isoform 2 | 421 | 6754638 | Stablization of Microtubules | PMID: 12888622 |
| PSEN1 | 341 | 15079861 | Regulation of APP | PMID: 10069390 |
| PSEN2 | 329 | 13623517 | Regulation of APP | PMID: 10069390 |
| Pyrin | 330 | 2407316 | Mediterranean fever protein | PMID: 18577712 |
| LMNA Isoform 2 | 230 | 5031875 | Nuclear Membrane | PMID: 8978814 |
| PLA2G4A | 459 | 56202754 | Phospholipid Hydrolysis | PMID: 9875225 |
| SPTAN1 | 1185 | 55663122 | Cytoskeleton | PMID: 9894612 |
| IL1F7 | 20 | 20127524 | Cytokine | PMID: 12096920 |
| Caspase-1 - cut 1 | 103 | 266321 | Pyroptosis | PMID: 7721861 |
| Caspase-1 - cut 2 | 119 | 266321 | Pyroptosis | PMID: 7721861 |
| Caspase-1 - cut 3 | 297 | 266321 | Pyroptosis | PMID: 7721861 |
| Caspase-1 - cut 4 | 316 | 266321 | Pyroptosis | PMID: 7721861 |
| Table 1B. Experimentally Identified Substrates of Caspase-9 with Characterized Cleavage Site | ||||
|---|---|---|---|---|
| Substrate | Cleavage Position (P1 amino acid) | NCBI/Protein Database (GI) Accession No. | Cellular Function | Reference |
| Caspase-9 | ||||
| proCaspase 3 - cut 1 | 28 | 77416852 | Apoptosis | PMID: 15274128 |
| proCaspase 3 - cut 2 | 175 | 77416852 | Apoptosis | PMID: 15274128 |
| proCaspase 7 - cut 1 | 23 | 1730092 | Apoptosis | PMID: 15274128 |
| proCaspase 7 - cut 2 | 198 | 1730092 | Apoptosis | PMID: 15274128 |
| proCaspase 7 - cut 3 | 206 | 1730092 | Apoptosis | PMID: 15274128 |
| Vimentin - cut 1 | 85 | 62414289 | Cytoskeleton | PMID: 11514563 |
| Vimentin - cut 2 | 259 | 62414289 | Cytoskeleton | PMID: 11514563 |
| Vimentin - cut 3 | 429 | 62414289 | Cytoskeleton | PMID: 11514563 |
| DCC | 1290 | 1169233 | Transmembrane receptor | PMID: 11248093 |
| RB1 | 270 | 132164 | Tumor suppressor | PMID: 15735701 |
| Raf-1 | 279 | 125651 | Signal Transduction | PMID: 15674327 |
In order to identify any amino acid residues having the higher frequencies with statistical significance than the normal amino acid occurrence frequencies, we generated the confidential intervals of amino acid occurrence frequency for each amino acid by calculating 12,467 amino acid positions in the 30 randomly selected human house keeping proteins13 (Suppl. Table 1). The results showed that the mean amino acid occurrence frequencies derived by analyzing 1490 human proteins15 were all within the confidential intervals that we generated (p >0.05), suggesting that the confidential intervals of amino acid occurrence frequencies for each amino acid in Suppl. Table 1 are statistically unbiased representation of human proteins, and thus can be used in this study.
The significant group contained the amino acid residues with a frequency higher than the confidential interval of corresponding amino acid (p < 0.05). The non-significant group contained the amino acid residues with a frequency within the confidential interval in Suppl. Table 1 (p > 0.05). As expected, the P1 position of natural caspase-1 substrates had 100% stringently conserved amino acid Asp (Tables 2A and B). The positions of caspase-1 substrates P10, P7, P5, P2′, P4′, P6′, P7′, P8′, P9′ and P10′ had lower percentages of amino acids (≤40%) in the significant group. The positions of caspase-1 substrates P9, P8, P6, P4, P3, P2, P1, P1′, P3′ and P5′ had higher percentages of amino acids (>40%) in the significant group. Similarly, the P1 position of caspase-9 substrates had 100% stringently conserved Asp. In contrast to caspase-1 substrates, none of the positions in caspase-9 substrates had lower percentages of amino acids in the significant group (≤40%), suggesting that caspase-9 can cleave fewer substrates with more specialized cellular function than caspase-1. We then compared the percentages of amino acids in the P10-P10′ positions in the significant group of caspase-1 substrates to that of caspase-9 substrates. As shown in Table 2C, the percentages of amino acids in the positions P7, P5, P4, P3, P2′, P6′, P7′, P8′, P9′, and P10′ in the significant group of caspase-1 substrates were statistically different from that of caspase-9 substrates.
Table 2. The amino acid occurrence frequencies in the flanking 20 amino acid positions P10-P10′ around the cleavage sites of human caspase-1 and caspase-9 substrates.
A. The amino acid occurrence frequencies in the flanking 20 amino acid positions P10-P10′ around the cleavage sites of human caspase-1 substrates. By comparing to the confidential intervals of human amino acid occurrence frequencies generated in supplemental Table 1, the amino acid residues occurred in the flanking 20 amino acid positions P10-P10′ around the cleavage sites of human caspase-1 are classified into the significant section with the frequencies higher than the upper limit of the confidential intervals (p<0.05) and the non-significant section with the frequencies in the range of the confidential intervals (p>0.05). B. The amino acid occurrence frequencies in the flanking 20 amino acid positions P10-P10′ around the cleavage sites of human caspase-9 substrates. By comparing to the confidential intervals of human amino acid occurrence frequencies generated in supplemental Table 1, the amino acid residues occurred in the flanking 20 amino acid positions P10-P10′ around the cleavage sites of human caspase-9 are classified into the significant section with the frequencies higher than the upper limit of the confidential intervals (p<0.05) and the non-significant section with the frequencies in the range of the confidential intervals (p>0.05). C. The amino acid occurrence frequencies in the flanking 20 amino acid positions P10-P10′ around the cleavage sites of human caspase-1 and caspase-9 substrates. The amino acid occurrence frequencies in the significant sections with the frequencies higher than the confidential intervals of each amino acid are presented in the left two columns. The hydrophobic amino acid occurrence frequencies in the flanking 20 amino acid positions P10-P10′ around the cleavage sites are presented in the central two columns. The charged amino acid occurrence frequencies in the flanking 20 amino acid positions P10-P10′ around the cleavage sites are presented in the right two columns. The features of amino acids are marked with different fonts, acidic = italicized; basic = underlined; hydrophobic = bolded; and neutral = normal font.
| Table 2A. Significant Amino Acids in Experimentally Identified Caspase-1 Cleavage Sites | ||
|---|---|---|
| Position | Significant a | Non-significantb |
| P10 | D14.7 Y5.9 | S8.8 K8.8 E8.8 A8.8 V5.9 T5.9 P5.9 N5.9 L5.9 I5.9 F5.9 R2.9 |
| P9 | S17.6 G14.7 D11.8 W2.9 | P8.8 L8.8 E8.8 T5.9 I5.9 Y2.9 M2.9 K2.9 H2.9 F2.9 |
| P8 | D20.6 E14.7 T11.8 W2.9 | G8.8 A8.8 R5.9 P5.9 Y2.9 V2.9 N2.9 M2.9 L2.9 K2.9 F2.9 |
| P7 | M11.8 D11.8 T8.8 | A11.8 G8.8 V5.9 S5.9 Q5.9 N5.9 I5.9 E5.9 R2.9 P2.9 L2.9 K2.9 |
| P6 | E20.6 S17.6 V11.8 D8.8 W5.9 C5.9 | P5.9 G5.9 Y2.9 T2.9 R2.9 L2.9 K2.9 A2.9 |
| P5 | E14.7 I8.8 D8.8 W2.9 | A11.8 I8.8 V5.9 R5.9 Q5.9 G5.9 Y2.9 T2.9 S2.9 P2.9 N2.9 M2.9 F2.9 |
| P4 | D23.5 A14.7 W5.9 Y5.9 | L11.8 V8.8 K5.9 F5.9 E5.9 Q2.9 N2.9 H2.9 C2.9 |
| P3 | E32.4 V14.7 M11.8 Q8.8 | L14.7 S5.9 T2.9 R2.9 H2.9 F2.9 |
| P2 | A14.7 V14.7 T8.8 H5.9 | S8.8 R8.8 Q5.9 P5.9 K5.9 I5.9 E5.9 D5.9 Y2.9 |
| P1 | D100 | |
| P1′ | S29.4 G14.7 N11.8 Y5.9 | A11.8 L5.9 E5.9 P2.9 M2.9 F2.9 D2.9 C2.9 |
| P2′ | P20.6 Q8.8 | V8.8 A8.8 T5.9 R5.9 K5.9 I5.9 G5.9 F5.9 E5.9 S2.9 L2.9 H2.9 C2.9 |
| P3′ | G17.6 S14.7 D8.8 C5.9 | K11.8 A11.8 V8.8 Q5.9 Y2.9 T2.9 R2.9 M2.9 L2.9 |
| P4′ | K14.7 D8.8 M5.9 | G11.8 V8.8 E8.8 A8.8 Q5.9 T2.9 S2.9 R2.9 N2.9 L2.9 I2.9 H2.9 F2.9 C2.9 |
| P5′ | L17.6 S14.7 M5.9 W2.9 | P11.8 R8.8 A8.8 V2.9 T2.9 N2.9 I2.9 G2.9 E2.9 D2.9 C2.9 |
| P6′ | T8.8 H5.9 | E11.8 S8.8 P8.8 L8.8 K8.8 G8.8 R5.9 Q5.9 I5.9 A5.9 D2.9 C2.9 |
| P7′ | W5.9 | A11.8 S8.8 R8.8 P8.8 L8.8 K8.8 N5.9 G5.9 Y2.9 T2.9 Q2.9 I2.9 H2.9 F2.9 E2.9 D2.9 C2.9 |
| P8′ | A17.6 | S11.8 K11.8 R8.8 Q5.9 P5.9 L5.9 E5.9 D5.9 V2.9 T2.9 N2.9 H2.9 G2.9 F2.9 C2.9 |
| P9′ | S17.6 M5.9 | E11.8 P8.8 K8.8 G8.8 T5.9 L5.9 F5.9 D5.9 A5.9 R2.9 Q2.9 I2.9 |
| P10′ | Q8.8 | S11.8 G11.8 E11.8 R8.8 A8.8 P5.9 L5.9 I5.9 Y2.9 V2.9 T2.9 K2.9 H2.9 F2.9 D2.9 |
| Table 2B. Significant Amino Acids in Experimentally Identified Caspase-9 Cleavage Sites | ||
|---|---|---|
| Position | Significanta | Not Significantb |
| P10 | T18.2 A18.2 Q9.1 | S9.1 R9.1 L9.1 K9.1 G9.1 E9.1 |
| P9 | E27.3 D18.2 V18.2 I9.1 | R9.1 P9.1 L9.1 |
| P8 | I18.2 S18.2 L18.2 N9.1 Q9.1 | V9.1 R9.1 P9.1 |
| P7 | D27.3 H18.2 A18.2 L18.2 F9.1 | G9.1 |
| P6 | P18.2 C9.1 N9.1 D9.1 | V9.1 S9.1 R9.1 L9.1 K9.1 |
| P5 | Q27.3 E18.2 G18.2 T9.1 I9.1 | S9.1 R9.1 |
| P4 | I27.3 D18.2 L18.2 M9.1 N9.1 T9.1 | E9.1 |
| P3 | S36.4 D18.2 E18.2 N9.1 Q9.1 I9.1 | |
| P2 | V36.4 T18.2 M9.1 N9.1 | L9.1 E9.1 A9.1 |
| P1 | D100 | |
| P1′ | S36.4 A27.3 T9.1 F9.1 | V9.1 R9.1 |
| P2′ | G36.4 S18.2 N9.1 I9.1 | R9.1 L9.1 K9.1 |
| P3′ | P36.4 I18.2 F9.1 | V9.1 R9.1 L9.1 K9.1 |
| P4′ | I18.2 D18.2 S18.2 | R9.1 P9.1 L9.1 G9.1 A9.1 |
| P5′ | D27.3 H9.1 Y9.1 N9.1 | V9.1 R9.1 L9.1 E9.1 A9.1 |
| P6′ | D36.4 S18.2 | V9.1 L9.1 K9.1 G9.1 A9.1 |
| P7′ | T27.3 I18.2 M9.1 N9.1 | S9.1 R9.1 L9.1 E9.1 |
| P8′ | S27.3 A18.2 C9.1 H9.1 N9.1 F9.1 D9.1 | P9.1 |
| P9′ | A27.3 V18.2 C9.1 Y9.1 T9.1 Q9.1 | S9.1 K9.1 |
| P10′ | E27.3 K18.2 S18.2 H9.1 N9.1 | P9.1 L9.1 |
| Table 2C. Comparison of Amino Acids in the Cleavage Sites of Caspase-1 substrates and Caspase-9 Substrates | ||||||
|---|---|---|---|---|---|---|
| a Significant AA’s Percentage | b Hydrophobic AA’s Percentage | c Charged AA’s Percentage | ||||
| Position | Casp-1 | Casp-9 | Casp-1 | Casp-9 | Casp-1 | Casp-9 |
| P10 | 20.6 | 45.5 | 38.3 | 36.4 | 35.2 | 27.3 |
| P9 | 47.0 | 72.8 | 46.9 | 45.5 | 26.4 | 54.6 |
| P8 | 50.0 | 72.8 | 38.0 | 54.6 | 44.1* | 9.1* |
| P7 | 32.4* | 91* | 50.0 | 54.6 | 23.5 | 45.5 |
| P6 | 70.6 | 45.5 | 41.2 | 45.5 | 35.2 | 27.3 |
| P5 | 35.2* | 81.9* | 44.0 | 27.3 | 29.4 | 27.3 |
| P4 | 50* | 91* | 50.0 | 54.6 | 38.2 | 27.3 |
| P3 | 67.7* | 100* | 44.1* | 9.1* | 38.2 | 36.4 |
| P2 | 44.1 | 72.8 | 41.2 | 63.7 | 32.4 | 9.1 |
| P1 | 100.0 | 100.0 | 0.0 | 0.0 | 100 | 100 |
| P1′ | 61.8 | 81.9 | 44.0 | 45.5 | 8.8 | 9.1 |
| P2′ | 29.4* | 72.8* | 61.7 | 54.6 | 20.6 | 18.2 |
| P3′ | 47.0 | 63.7 | 49.9 | 81.9 | 23.5 | 18.2 |
| P4′ | 29.4 | 54.6 | 46.9 | 54.6 | 38.1 | 27.3 |
| P5′ | 41.1 | 54.6 | 58.6 | 27.3 | 14.6* | 54.6* |
| P6′ | 14.7* | 54.6* | 41.1 | 36.4 | 35.3 | 45.5 |
| P7′ | 5.9* | 63.7* | 49.9 | 36.4 | 26.3 | 18.2 |
| P8′ | 17.6* | 91* | 41.0 | 45.5 | 35.3 | 18.2 |
| P9′ | 23.5* | 81.9* | 44.1 | 54.6 | 29.4 | 9.1 |
| P10′ | 8.8* | 81.9* | 44.1 | 18.2 | 29.3 | 54.6 |
Frequencies of AAs statistically higher than the background frequencies (P<0.05).
Frequencies of AAs within 1.96×SD confidence intervals.
The amino acid features are indicated by different fonts: Acidic – italicized; Basic – underlined; Hydrophobic – bolded; Neutral - normal text
Significant AA’s total percentage
Hydrophobic percentage
Charged percentage
and underlined fonts indicate the position has statistical difference between caspase-1 and capase-9 substrates (P<0.05)
In addition, we examined the hydrophobic amino acid occurrence frequencies in these positions (Table 2C). The positions of caspase-1 substrates P7, P4, P2′, P3′, P5′ and P7′ had higher percentages of hydrophobic amino acids (≥50%). In comparison, the positions of caspase-9 substrates P8, P7, P4, P2, P2′, P3, P4′, and P9′ had higher percentages of hydrophobic amino acids (≥50%). The percentages of amino acids in the position P3 in the hydrophobic amino acid group of caspase-1 substrates were statistically different from that of caspase-9 substrates.
Furthermore, we examined the charged amino acid occurrence frequencies in these positions (Table 2C). The position of caspase-1 substrates P1 had higher percentages of charged amino acids (≥50%). In comparison, the positions of caspase-9 substrates P9, P1, P5′ and P10′ had higher percentages of charged amino acids (≥50%). The percentages of amino acids in the positions P8 and P5′ in the charged amino acid group of caspase-1 substrates were statistically different from that of caspase-9 substrates.
2. The amino acid pairs in the cleavage sites P1-P1′ of caspase-1 substrates are, in as high as 55.9%, different from that of caspase-9 substrates
We compared occurrence frequencies of the amino acid pairs in the positions P1-P1′ of caspases’ substrates11, since they are primary structural features for the enzyme recognition and cleavage7. As shown in Suppl. Table 2, the three amino acid pairs in the cleavage sites P1-P1′ of caspase-1 substrates were shared 44.1% with that of caspase-9 substrates, including the amino acid pairs D–S, D-A and D–F. However, the amino acid pairs in the cleavage sites of caspase-1 were, in the rates as high as 55.9%, different from that of caspase-9 substrates. Therefore, these results suggest that the amino acid pairs used in the cleavage sites of caspase-1 substrates are different from that of caspase-9 substrates.
3. The extended cleavage site patterns P10-P10′ of caspase-1 substrates can be used in the prediction of cleavage sites for caspase-1 substrates
A recent report identified 41 new substrates of caspase-1, which characterized only one cleavage site in details (glyceraldehyde-3-phosphate dehydrogenase, GAPDH) and left the cleavage sites in the rest of 40 caspase-1 substrates unidentified16. Of note, out of the other 40 substrates, four substrates including pro-caspase-1, β-actin, HnRNP A2, and HSP90 were previously characterized and listed in Table 1A. The calnexin’s cleavage site was predicted using a recently reported prediction method (not shown) (http://us.expasy.org/tools/peptidecutter/)17. The uncharacterized cleavage sites of 35 substrates out of the 40 substrates could not be predicted by the existing algorithms, which were included in our studies. We hypothesized that the cleavage site patterns recognized in this study may be used in the prediction for the cleavage sites of caspase-1 substrates. To demonstrate the proof of principle, we used the amino acid sequences with the highest occurrence frequencies in all the P10-P10′ positions (Table 2A) for the initial prediction of caspase-1 cleavage sites including Asp in the P1 position. In the following fine tuning (Table 2A), the matches with other amino acids in the significant amino acid group and with amino acids in the non-significant amino acid group were also considered. In Table 3, our analysis of 35 caspase-1 substrates, with previously uncharacterized cleavage sites, showed that the P1 position of 32 cleavage sites (91.4%) out of 35 caspase-1 substrates were precisely predicted whereas the cleavage sites in the remaining 3 caspase-1 substrates (8.6%) were not predicted (Table 3A). The 60 predicted cleavage sites had more than 2 amino acid matches with ones from the significant amino acid group in the P10-P10′ positions of casapse-1 substrates (Table 2A). One substrate, adenyl cyclase, had nine amino acid matches with ones from the significant amino acid group in the positions. In addition, there were amino acid matches with the non-significant amino acids (Table 2A), which made the total amino acid matches up to 20 amino acids in the P10-P10′ region in four substrates (ARP3, SET translocation, calreticulin precursor and PHAP1/April). Of note, future experiments are required to verify the prediction of these cleavage sites of caspase-1. Taken together, the new cleavage site patterns can be used for prediction of the cleavage sites of caspase-1 substrates.
Table 3. Prediction of caspase-1 cleavage sites in the experimentally identified 35 caspase-1 substrates.
A. Prediction of the cleavage site of caspase-1 substrates. The predictions of caspase-1 cleavage sites in the experimentally identified caspase-1 substrates, in which the cleavage sites have not been mapped, are presented in Table 3A, which includes six columns: (1) substrate name; (2) the NCBI protein database accession number; (3) the cleavage site position P1 of caspase; (4) the numbers of the amino acid matches between the target sequences and the best cleavage site patterns; (5) the numbers of the amino acid matches between the target sequences and the cleavage site patterns including other amino acids in the significant section of the cleavage site patterns; and (6) the total numbers of the amino acid matches between the target sequences and the cleavage site patterns with (a) best cleavage site patterns, (b) other amino acids in the significant section of the cleavage site patterns, and (c) other amino acids in the insignificant section of the cleavage site patterns. The 25 cleavage sites from 20 caspase-1 substrates have been predicted with the minimal 4 amino acid matches with the amino acid residues in the significant section of the cleavage site patterns, which are presented in the upper portion of the table. The cleavage sites from three caspase-1 substrates have not been predicted with the current threshold of two amino acid matches with the amino acid residues in the significant section of the cleavage site patterns, which are presented in the lower portion of the table. B. Summary of predicted caspase-1 substrate cleavage site. The prediction is classified into two groups: no match and matched, based on a criterion that whether or not the numbers of the amino acid matches between the target sequences and the best cleavage site patterns are larger or equal to two. The summary presented in the Table 3B includes four columns: (1) group; (2) criterion; (3) match hit; and (4) percentage of the hits in the groups.
| A. Prediction of the cleavage site of caspase-1 substrates | |||||
|---|---|---|---|---|---|
| Substrate | NCBI/Protein Database (GI) Accession No. | Cleavage Position (P1 amino acid) | Best Sequence Matches | Other Significant Matches | Total Matches (X/20) |
| β-tubulin | 4580988 | 355 | 2 | 2 | 15 |
| 31 | 4 | 2 | 16 | ||
| ARP3 | 5031573 | 172 | 2 | 4 | 20 |
| F-actin α-1 | 5453597 | 114 | 7 | 2 | 17 |
| 10 | 3 | 3 | 16 | ||
| 125 | 2 | 1 | 16 | ||
| Aldolase A | 28614 | 141 | 2 | 3 | 17 |
| TIP | 136066 | 56 | 2 | 1 | 14 |
| 36 | 2 | 0 | 17 | ||
| 225 | 3 | 1 | 15 | ||
| α-enolase | 4503571 | 265 | 4 | 2 | 19 |
| 91 | 2 | 1 | 16 | ||
| 383 | 3 | 2 | 18 | ||
| Pyruvate kinase | 35505 | 369 | 3 | 2 | 14 |
| L-Lactate Dehydrogenase | 13786848 | 166 | 2 | 1 | 14 |
| ATP synthase | 32189394 | 365 | 3 | 1 | 16 |
| Malate dehydrogenase | 5174539 | 177 | 2 | 0 | 14 |
| 314 | 3 | 1 | 16 | ||
| 33 | 3 | 2 | 16 | ||
| Adenylate kinase 2 | 7524346 | 132 | 5 | 0 | 18 |
| Adenyl-cyclase | 5453595 | 33 | 9 | 1 | 17 |
| Catalase | 179950 | 52 | 3 | 2 | 17 |
| Peroxiredoxin 6 | 3318842 | 123 | 4 | 4 | 19 |
| Carbonic anhydrase II | 179780 | 110 | 4 | 2 | 16 |
| Glyoxalase I | 6573422 | 23 | 4 | 1 | 15 |
| Rnh1 | 15029922 | 182 | 4 | 2 | 18 |
| 390 | 2 | 1 | 14 | ||
| EEF1A1 | 48735185 | 368 | 4 | 1 | 18 |
| Tu elongation factor | 704416 | 126 | 3 | 2 | 17 |
| 132 | 3 | 4 | 19 | ||
| Ribosomal protein S9 | 550023 | 26 | 4 | 0 | 13 |
| 95 | 3 | 1 | 16 | ||
| Proteosome α7 subunit | 4092058 | 185 | 4 | 2 | 19 |
| SET translocation | 4506891 | 263 | 6 | 2 | 20 |
| 150 | 3 | 2 | 16 | ||
| 202 | 4 | 3 | 18 | ||
| 260 | 4 | 4 | 18 | ||
| PPPIA | 190281 | 255 | 3 | 2 | 15 |
| GRB2 | 4504111 | 33 | 2 | 0 | 13 |
| 14 | 3 | 1 | 17 | ||
| Rac2 | 4506381 | 65 | 2 | 2 | 16 |
| 150 | 4 | 1 | 18 | ||
| 124 | 5 | 0 | 17 | ||
| Rab GDI | 285975 | 183 | 4 | 1 | 19 |
| Rho GDI beta | 56676393 | 19 | 6 | 3 | 18 |
| 182 | 3 | 2 | 12 | ||
| Calreticulin precursor | 4757900 | 397 | 7 | 2 | 20 |
| 237 | 3 | 0 | 16 | ||
| 362 | 3 | 0 | 17 | ||
| Cyclophilin B | 118090 | 183 | 4 | 1 | 16 |
| 117 | 5 | 0 | 14 | ||
| Chloride ch 1 | 4588526 | 226 | 2 | 1 | 18 |
| Annexin IV | 189617 | 284 | 4 | 2 | 17 |
| 304 | 3 | 2 | 14 | ||
| 65 | 2 | 3 | 17 | ||
| 163 | 3 | 1 | 14 | ||
| 176 | 3 | 3 | 18 | ||
| PHAP1/April | 1498227 | 182 | 7 | 2 | 19 |
| 159 | 3 | 1 | 17 | ||
| 171 | 4 | 2 | 20 | ||
| RAB7 | 1174149 | - | - | - | - |
| HSP27 | 662841 | - | - | - | - |
| ER protein 29 | 5803013 | - | - | - | - |
| B. Summary of predicted caspase-1 substrate cleavage site | |||
|---|---|---|---|
| Number of: | Hits | Percentage | |
| No Match | 0–1 | 3 | 4.8% |
| Matched | 2+ | 60 | 95.2% |
DISCUSSION
Current understanding of the specificity of caspases in the P4-P1 positions9, 18, 8 has been employed in the various aspects: (1) as substrate reporter reagents; (2) as inhibitors; and (3) as experimental therapeutic agents for stroke and neurodegenerative diseases19, 20. However, these tetrapeptide-based approaches have a problem of a significant overlap between caspases’ consensus sequences since caspases are promiscuous on these sequences10. This problem has hampered the characterization of functional differences between caspase-1-dependent inflammatory pyroptosis and caspase-9-dependent non-inflammatory apoptosis. To solve the problem, we analyzed the amino acid residues in the extended cleavage sites in the experimentally identified caspase-1 substrates and caspase-9 substrates. This study has made the following findings: first, caspase-1 and caspase-9 shared 100% stringently conserved Asp in the P1 position. However, the structures in most extended cleavage sites of caspase-1 substrates are different from that of caspase-9 substrates in the following three aspects: a) the cleavage site positions having amino acid residues with the statistically high frequencies; b) the positions with hydrophobic amino acid occurrence frequencies; and c) the positions with charged amino acid occurrence frequencies; second, the amino acid pairs P1-P1′ used in the cleavage sites of caspase-1 substrates are different from that of caspase-9 substrates; third, new cleavage site patterns may be used in the prediction of the uncharacterized cleavage sites of caspase-1 substrates. We have predicted the precise P1 position of 32 cleavage sites (91.4%) out of 35 experimentally identified caspase-1 substrates with previously uncharacterized cleavage sites16, suggesting that our new cleavage site patterns have improved current caspases’ cleavage site prediction methods. Our new cleavage patterns of caspase-1 could be used in the various aspects: (1) as substrate reporter reagents; (2) as inhibitors; and (3) as experimental therapeutic agents but with the accuracy and efficacy potentially much higher than the tetrapeptide-based compounds19, 20. In addition, our results in defining the cleavage site differences between caspase-1 substrates and caspase-9 substrates may also reflect the potential differences between caspase-1 enzymatic active site and the counterpart of caspase-910.
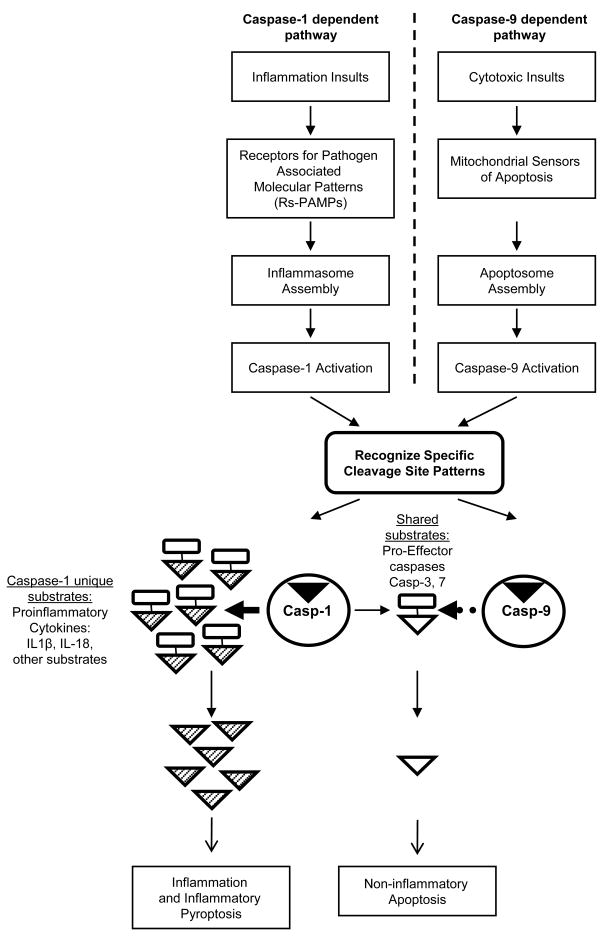
Increasing evidence suggests that caspases have important functions to regulate cell proliferation, differentiation, and migration in addition to cell death5. In contrast to 11 substrates identified for caspase-9, up to 70 caspase-1 substrates have been reported. Functions of caspase-1, in addition to regulate inflammatory pyroptosis (maturation of proinflammatory cytokines IL-1β and IL-18, activation of caspase-321 and caspase-722), have also been identified including protein translation, ubiquitination-proteasome degradation, DNA repair, stabilization of cytoskeleton and glycolysis16, etc (Table 1A). One unique function of caspase-1 substrates constitutes orchestrated caspase-1 dependent inflammatory pyroptosis phenotype. Indeed, we also found that most caspase-1 substrates play a role in vascular inflammation, function and atherogenesis (Suppl. Table 3). Although caspase-1 also activates effector caspase-321 and caspase-722 that are traditionally regarded as caspase-9 substrates (Table 2A), relative celerity of intracellular activation of caspase-3 and caspase-7 by caspase-1 are predicted to be slower than caspase-9 because of competition between pro-casapses-3 and -7 and many other proinflammatory caspase-1 substrates for caspase-1 cleavage. In addition, initiation of casapse-1 activation and inflammation is dependent on activation of NF-κB pathway3. However, activation of NF-κB also leads to inhibition of cell death by upregulating anti-apoptotic proteins such as Bcl-xL23. Therefore, comparing to apoptosis, caspase-1 dependent cell death has two unique features: a) it is slow in activating effector caspases-3 and 7; b) the cell survival mechanism associated with activation of NF-κB pathway serves as an additional “brake” for cell death aspect of pyroptosis. Our new working model in Fig. 2 emphasizes structural differences in the extended cleavage sites between caspase-1 and caspase-9. Our results on the extended cleavage sites of caspase-1 and caspase-9 are significant in developing new detection tools, diagnostics and therapeutics for the pathology that caspases are involved.
Figure 2.
The working model supported by our results presented in this paper.
Supplementary Material
Acknowledgments
This work was partially supported by the National Institutes of Health Grants HL094451 (X.-F. Yang) and HL67033, HL82774, and HL77288 (HW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Yang XF. Factors regulating apoptosis and homeostasis of CD4+CD25highFOXP3+ regulatory T cells are new therapeutic targets. Front Biosci. 2008;13:1472–1499. doi: 10.2741/2775. [DOI] [PubMed] [Google Scholar]
- 3.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 6.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 7.Schechter I, Berger A. On the size of the active site in proteases. I Papain. Biochemical and biophysical research communications. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 9.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 10.Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ. 2007;14:66–72. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- 11.Yang XF, Mirkovic D, Zhang S, Zhang QE, Yan Y, Xiong Z, Yang F, Chen IH, Li L, Wang H. Processing sites are different in the generation of HLA-A2.1-restricted, T cell reactive tumor antigen epitopes and viral epitopes. Int J Immunopathol Pharmacol. 2006;19:853–870. doi: 10.1177/039463200601900415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenberg E, Levanon EY. Human housekeeping genes are compact. Trends Genet. 2003;19:362–365. doi: 10.1016/S0168-9525(03)00140-9. [DOI] [PubMed] [Google Scholar]
- 14.Rosner B. Hypothesis testing: one-sample inference. In: Rosner B, editor. Fundamentals of Biostatistics. Australia, Canada, Mexico, Singapore, Spain, United Kingdom, United States: Duxbury; 2000. pp. 211–271. [Google Scholar]
- 15.Wada K, Wada Y, Ishibashi F, Gojobori T, Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1992;20 (Suppl):2111–2118. doi: 10.1093/nar/20.suppl.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 17.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker JM, editor. The Proteomics Protocols Handbook. Humana Press; 2005. pp. 571–607. [Google Scholar]
- 18.Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 19.Schulz JB, Weller M, Moskowitz MA. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol. 1999;45:421–429. doi: 10.1002/1531-8249(199904)45:4<421::aid-ana2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 21.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 22.Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Nunez G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basak S, Hoffmann A. Crosstalk via the NF-kappaB signaling system. Cytokine & growth factor reviews. 2008;19:187–197. doi: 10.1016/j.cytogfr.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.