Abstract
Selective breeding of rats exhibiting differences in novelty-induced locomotion revealed that this trait predicts several differences in emotional behavior. Bred High Responders (bHRs) show exaggerated novelty-induced locomotion, aggression, and psychostimulant self-administration, compared to bred Low Responders (bLRs), which are inhibited and prone to anxiety- and depression-like behavior. Our breeding studies highlight the heritability of the bHR/bLR phenotypes, although environmental factors like maternal care also shape some aspects of these traits. We previously reported that HR vs. LR mothers act differently, but it was unclear whether their behaviors were genetically driven or influenced by their pups. The present study (a) used cross-fostering to evaluate whether the bHR/bLR maternal styles are inherent to mothers and/or are modulated by pups; and (b) assessed oxytocin and oxytocin receptor mRNA expression to examine possible underpinnings of bHR/bLR maternal differences. While bHR dams exhibited less maternal behavior than bLRs during the dark/active phase, they were very attentive to pups during the light phase, spending greater time passive nursing and in contact with pups compared to bLRs. Cross-fostering only subtly changed bHR and bLR dams’ behavior, suggesting that their distinct maternal styles are largely inherent to the mothers. We also found elevated oxytocin mRNA levels in the supraoptic nucleus of the hypothalamus in bHR versus bLR dams, which may play some role in driving their behavior differences. Overall these studies shed light on the interplay between the genetics of mothers and infants in driving differences in maternal style.
Keywords: HR pup, LR pup; novelty-seeking, High Responder; Low Responder; cross-fostering; maternal behavior; oxytocin
INTRODUCTION
Rats, like all organisms, display a wide variety of behavioral and physiological responses when placed in a new or stressful situation. Such individual differences in environmental or emotional reactivity in humans influence personality and temperament, and may ultimately place certain people at risk for developing stress-induced pathology, including depression, anxiety and a host other psychiatric and addictive disorders (Ball et al., 2005). We have developed selectively-bred rat lines which capture two extremes of environmental or emotional reactivity (Stead et al., 2006a). When placed in a novel environment, selectively-bred High Responder (bHR) rats exhibit a high level of exploratory activity, while bred Low Responder (bLR) animals are very inhibited, showing much less exploration. Several studies show that this single behavioral trait (high- versus low-novelty exploration) strongly predicts a number of behavioral features, including anxiety (Kabbaj et al., 2000; Mallo et al., 2007; Stead et al., 2006a; White et al., 2007), depression (Mallo et al., 2007; Orr et al., 2008), aggression (Abraham et al., 2006), impulsivity (Flagel et al., 2010), and drug-taking behavior (Davis et al., 2008; Hooks et al., 1991; Piazza et al., 1989; Piazza et al., 1991a). We have generally observed that bLR rats exhibit a highly anxious/depressive-like syndrome and are sensitive to the negative effects of chronic stress (e.g. prenatal stress (Clinton et al., 2008) and chronic mild stress (Stedenfeld et al., 2009)), while bHRs are prone to be aggressive, impulsive, and self-administer drugs of abuse.
Numerous studies have identified a series of neurobiological factors that may underlie the marked bHR/bLR phenotypic differences (Ballaz et al., 2007; Cecchi et al., 2007; Hooks et al., 1994a; Hooks et al., 1994b; Kabbaj, 2004; Kabbaj et al., 2000; Piazza et al., 1991b; Rosario and Abercrombie, 1999). For instance, differences in drug- and reward-seeking in commercially-available (i.e. non-selectively-bred) HR/LR rats are likely associated with their distinct dopaminergic circuits (Hooks et al., 1994a; Hooks et al., 1994b; Lucas et al., 1998; Piazza et al., 1991b), although such differences may also stem from their distinct hypothalamic-pituitary-adrenal (HPA) axis reactivity (Maccari et al., 1991; Piazza et al., 1991a; Rouge-Pont et al., 1998). Other work using Affymetrix microarrays identified numerous putative gene expression differences in the hippocampus of commercially-purchased HR/LR rats both basally and following psychosocial stress, highlighting differences in a range of molecules involved in intracellular signal transduction pathways, neuroplasticity, and neurogenesis (Kabbaj et al., 2004). In addition, our selective breeding of the bHR/bLR lines has shown the high level of heritability of the bHR/bLR phenotypes, with the traits being highly predictable based on parental phenotype (Stead et al., 2006a). Thus, there appears to be a strong genetic component that underlies the emergence of the bHR and bLR phenotypes.
A vast literature illustrates the profound impact of early life experience on emotional and neuroendocrine stress reactivity (Arnold and Siviy, 2002; Denenberg et al., 1967; Hofer, 1973; Ladd et al., 2000; Levine, 1962; Levine et al., 1957; Levine et al., 1967; Russell, 1973; Sanchez et al., 2001; Zarrow et al., 1972). The first 3 postnatal weeks represent a critical developmental period for rodent neural systems, evident in radical changes in behavior, neuroendocrine response, synaptic connectivity, and global neural gene expression (Altman and Sudarshan, 1975; Card et al., 2005; Eilam and Golani, 1988; Rinaman et al., 2000; Sapolsky and Meaney, 1986; Smart and Dobbing, 1972; Stead et al., 2006b; Wiedenmayer and Barr, 1998). From P4–P14, rodents exhibit a ‘stress hypo-responsive period’ (SHRP) characterized by a reduced capacity to secrete corticosterone in response to stressful stimuli, which protects the developing brain against the deleterious effects of elevated glucocorticoids. Maternal behavior is critical for maintaining the SHRP period as well as a variety of other physiological processes in developing infant rats (Levine, 2001). Thus, it is perhaps not surprising that stress-induced perturbations of mother-pup interactions, or even naturally occurring variation in mothering-styles, can markedly impact neurodevelopment and subsequent behavior (Fleming et al., 1999; Francis et al., 1996; Meaney and Szyf, 2005). Given the broad behavioral and neuroendocrine differences between bHR/bLR (as well as commercially-available HR/LR) animals, we have been interested in studying whether these distinct phenotypes are shaped by early life experience, specifically in terms of the quality and/or quantity of maternal care that they receive in the first postnatal weeks. We recently demonstrated that commercially-purchased HR-LR dams display marked differences in maternal styles during the first two postpartum weeks, with LR dams spending more time licking, grooming, and arched-back nursing their pups compared to HR dams (Clinton et al., 2007). In the present study, we hypothesized 1) that the selectively-bred bHR and bLR dams would exhibit similar maternal behavior differences; 2) that bHR and bLR dams would show neurobiological differences that may contribute to their maternal behavior differences; and 3) that cross-fostering studies would reveal whether the bHR/bLR maternal styles are inherent to the mothers themselves, or driven by the phenotype of the pups that they are raising.
MATERIALS & METHODS
Animals
bHR and bLR animals were acquired from our in-house breeding colony where we have maintained the bHR/bLR lines for many generations. We recently published a description of our breeding strategy and initial behavioral characterization of the bHR and bLR lines (Stead et al., 2006a). Rats were housed in 43×21.5×25.5-cm polycarbonate cages (Nalgene, 24×45×20) throughout the studies. The rooms were kept under constant temperature (25±2 °C) and lighting conditions. Mating pairs as well as dams and litters during lactation were housed on a 14:10 light–dark cycle (lights on at 6:00 a.m.). Food and tap water were available ad libitum. All experiments were conducted in accordance with the National Institute of Health (NIH) guidelines on laboratory animal use and care, dictated by the National Research Council in 1996, and all procedures were approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC).
Mating bHR-bLR animals
bHR and bLR females were paired for 14 days with bHR and bLR males, respectively. Mating pairs were kept on the 14:10 light–dark cycle as this has been shown to promote regular estrous cycles and fertility (Everett and Sawyer, 1949; Ying et al., 1973). Conception was verified by the presence of a vaginal plug. Pregnant females were individually housed on the calculated eighteenth day of gestation, and litters were culled to 12 healthy pups (6 males, 6 females) on the day of birth (P0). After the initial handling of cages at birth, the mothers and litters were not disturbed in order to minimize disruption of mother–pup interactions, except for weekly cage change. Pups were weaned on postnatal day 21 and grouped 4 animals per cage according to sex, with water and food available ad libitum.
Monitoring maternal behavior in bHR/bLR dams
bHR dams and litters (N=12) and bLR dams and litters (N=12) from the 14th generation of our colony were observed from postnatal day 1 to 14 using a protocol similar to that used by Myers and colleagues (Myers et al., 1989). Each cage was observed twice daily – once during the light phase (at approximately 2:00 p.m.) and once during the dark phase (at approximately 10:00 p.m.). Each observation period (lasting 45 min to 1 hour) consisted of a series of 10 5-sec “snapshot” observations for each cage, which were taken approximately 5 min apart. During a “snapshot” observation, a checklist was used to note which behaviors were being observed. The behaviors noted were: 1) mother in or out of nest; 2) mother in contact with any pups; 3) mother in contact with more than half of the litter; 4) mother licking or grooming a pup; 5) mother transporting a pup; 6) mother manipulating non-nest bedding; 7) mother manipulating nest bedding; 8) mother eating; 9) mother drinking; 10) mother self-grooming; 11) mother rearing; 12) mother resting away from litter; 13) mother passive nursing pups; 14) mother arched-back nursing pups. Passive nursing was defined as the mother lying on her side or back and nursing any number of pups. Arched-back nursing was classified as the mother arched over any number of nursing pups with her legs extended. By the end of the 14 observation days, each cage had accumulated 280 observations (10 observations per session × 2 sessions each day × 14 total days).
In situ hybridization studies in brains of bHR and bLR dams
bHR and bLR dams (N=8 per group) from the 12th generation of our selectively-bred lines were sacrificed by rapid decapitation between 8:00 AM and 10:00 AM on postpartum day 8, a timepoint when we previously observed marked maternal behavior differences in commercially-purchased HR/LR dams (Clinton et al., 2007). Brains were removed, snap frozen, stored at −80 °C, and later cryostat sectioned at 12 µm. Sections were taken at 240 µm intervals from the tip of the frontal cortex through the midbrain, and then prepared for in situ hybridization as previously described (Clinton et al., 2008). Briefly, sections were fixed in 4% paraformaldehyde at room temperature for 1 h. The slides were then washed three times in room temperature 2 × SSC (300mM NaCl/30mM sodium citrate, pH 7.2), 5 min each wash. Next, the slides were placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1 M), pH 8.0, for 10 min at room temperature, rinsed in distilled water, and dehydrated through graded ethanol washes (50%, 75%, 85%, 95%, and 100%). After air-drying, the sections were hybridized with a 35S-labeled cRNA probe for oxytocin or the oxytocin receptor. The probes were labeled in a reaction mixture consisting of 1 µg of linearized plasmid, 1 × transcription buffer (Epicenter Technologies, Madison, WI), 125 µCi of 35S-labeled-UTP, 125 µCi of 35S-CTP, 150 µM ATP and GTP, 12.5mM dithiothreitol, 0.5 ml of RNase inhibitor, and 1.5 µl of T3 RNA polymerase. The reactions were incubated for 120 min at 37 °C, and then 1 µl of DNAse (RNAse free) was added to the reaction to incubate for another 15 min at room temperature. The labeled probes were purified using Micro Bio-Spin P-30 Tris Spin Columns (Bio-Rad Laboratories), then diluted in hybridization buffer (containing 50% formamide, 10% dextran sulfate, 3 × SSC, 50mM sodium phosphate buffer, pH 7.4, 1 × Denhardt’s solution, 0.1 mg/ml yeast tRNA, and 10mM dithiothreitol) to yield 106 dpm/70 µl. A cover slip with 70 µl of diluted riboprobe was placed on each slide. Slides were placed in a humidified box with filter paper saturated with 50% formamide buffer, and incubated overnight at 55 °C. Following hybridization, coverslips were removed and the slides were washed in room temperature 2 × SSC twice for 5 min each, and then incubated for 1 h in RNaseA (200 µg/ml in 10mM Tris buffer containing 0.5M NaCl, pH 8) at 37 °C. The slides were then washed in increasingly stringent SSC solutions: 2 ×, 1 ×, and 0.5 × for 5 min each at room temperature, followed by incubation for 1 h in 0.1 × SSC at 65 °C. Finally, slides were rinsed in distilled water and dehydrated through graded ethanol washes, air-dried, and apposed to Kodak XAR film (Eastman Kodak, Rochester, NY) for 7 d. Autoradiograms were digitized using a ScanMaker 1000XL Pro (Microtek, Carson, CA) with LaserSoft Imaging software (AG, Kiel, Germany). Digitized images were analyzed using Scion Image Beta 3b for PC. For the oxytocin probe, optical density measurements were taken for 5 different hypothalamic brain regions: 1) supraoptic nucleus (SON); 2) paraventricular nucleus (PVN); 3) anterior commisural nucleus (ACN); 4) nucleus circularis (NC); 5) accessory nuclei (AN). Anatomical regions were identified on the basis of previous work (Burbach et al., 1987; Ju et al., 1986). For the oxytocin receptor probe, optical density measurements were taken for cingulate cortex, prelimbic cortex, dorsal peduncular, nucleus accumbens, septum, bed nucleus of stria terminalis (BNST), and medial preoptic area (MPOA) from the left and right sides of the brain. Regions were chosen based on oxytocin receptor expression and areas known to be involved in maternal behavior (Numan, 1988; Numan, 1994; Numan and Sheehan, 1997). For each animal, we analyzed 8–10 sections spaced 240 µm apart across the rostro-caudal extent of the hypothalamus. The person analyzing the autoradiograms was blind as to which group each slide belonged. Optical density measurements were corrected for background, and then multiplied by the area sampled to produce an integrated density measurement. These data were averaged to produce one data point for each brain region analyzed per animal, and then group averages were calculated and compared statistically.
Monitoring maternal behavior in bHR/bLR dams with cross-fostered litters
In order to evaluate whether the observed bHR/bLR maternal behavior differences were inherent to the dams themselves, or at least in part driven by the characteristics of the bHR versus bLR pups that they raised, we utilized a cross-fostering paradigm and then monitored the dams’ maternal behavior as described above. This study used bHR/bLR dams and litters from the 15th generation of our colony. Within 24 hours of parturition, litters were culled to 12 pups per dam (6 male, 6 female), and then assigned to one of three maternal care conditions. Litters were either (a) returned to their biological mother, (b) placed with a dam of the same bHR/bLR phenotype, or (c) cross-fostered to a dam of the opposite bHR/bLR phenotype (N=6–8 dams/litters per phenotype for each of the three conditions). For example, a bHR litter might have been returned to its biological bHR mother, placed with another bHR foster mother, or cross-fostered to a bLR mother. From this point, litters were cared for by their assigned mother until they were separated at weaning. For the first two weeks after birth (P1–P14) dams and litters were observed using the same procedure described above.
Statistical Analysis
Maternal behavior observations were analyzed by calculating for each animal the average number of observations for each behavior observed within either the light or dark phase for the first versus second postpartum week. Group averages were then compared statistically (e.g. bHR versus bLR). Since our data showed a normal distribution and homogeneity of variances, we proceeded to analyze it via ANOVA. Two-way ANOVA (bHR/bLR phenotype × postpartum week) was used to determine differences in the first behavioral study. The cross-fostering maternal behavior data was analyzed using two-way ANOVA (maternal bHR/bLR phenotype × foster group). Data was separately analyzed for the light vs. dark phase or first vs. second postnatal week. ANOVAs were followed by Fisher's post-hoc comparisons when necessary. In situ hybridization data analysis was carried out separately for each region and probe, using one-way ANOVA, with bHR/bLR phenotype as the independent variable. Data were analyzed using Statview 5.0.1 for Windows, and for all tests α=0.05.
RESULTS
Maternal Behavior in bHR/bLR Dams
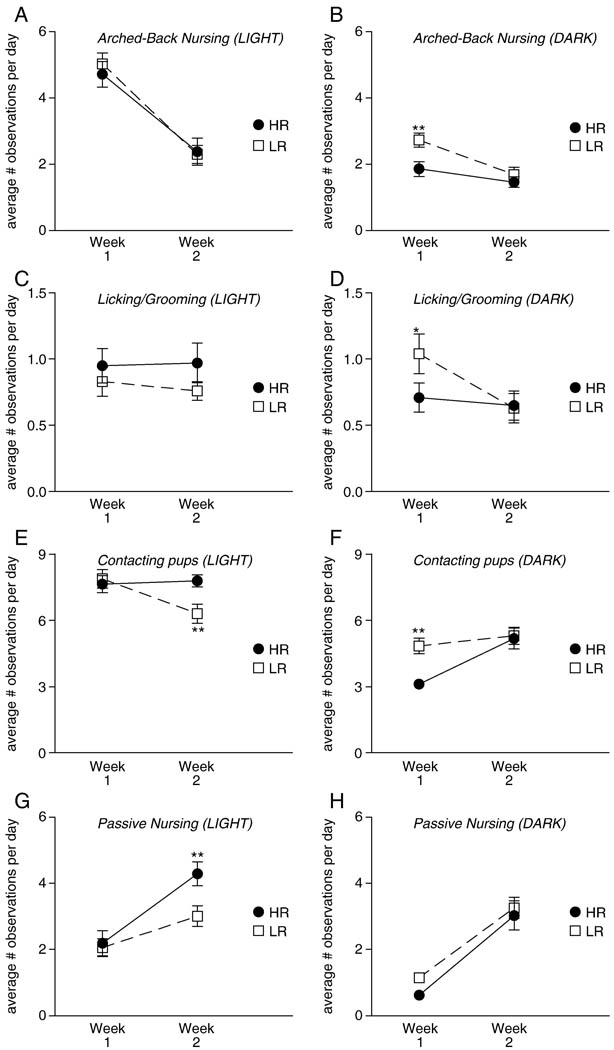
Figure 1 shows the amount of arched-back nursing, licking/grooming, pup contact, and passive nursing that bHR and bLR dams exhibited during the first two postpartum weeks. In general, bLR dams showed a higher degree of active maternal behavior than bHR dams, particularly during the dark (active) phase during the first week of life.
Figure 1.
Arched-back nursing, licking/grooming, pup contact and passive nursing in bHR versus bLR dams during the first two postpartum weeks. Graphs depict the average number of behavioral observations for postpartum week one versus two, with data shown separately for the light (left column) and dark (right column) phases. (A–B) All mothers exhibited more arched back nursing during postpartum week one versus two. During the dark phase, bLR mothers spent more time arched-back nursing their pups compared to bHRs during the first postnatal week. (C–D) bLR mothers also spent more time licking/grooming their pups compared to bHRs, although this effect was only observed during the dark phase and during the first postnatal week. (E–F) bLR and bHR dams displayed differences in the time spent in contact with their pups. bLR mothers spent more time in contact with pups during the first postpartum week compared to bHRs, although this was only apparent in the dark phase. On the other hand, bHR mothers spent more time with their pups compared to bLRs during the second postpartum week, although this effect was only observed during the light phase. (G–H) Overall both groups exhibited more passive nursing during second versus first postpartum week, although, bHR dams exhibited more passive nursing during the light phase of the second postpartum week compared to bLR mothers. * indicates p<0.05; ** indicates p<0.01
For arched-back nursing during the light phase, there was a main effect of postpartum week (p<0.0001), with all mothers engaging in more arched-back nursing during the first week compared to the second (Figure 1A). There was no effect of bHR/bLR phenotype and no phenotype × postpartum week interaction. For arched-back nursing during the dark phase, there was a main effect of phenotype (p<0.01), postpartum week (p<0.001), and phenotype × postpartum week interaction (p<0.05). Similar to what was observed during the light phase, both groups of mothers displayed more arched-back nursing during the first versus second postpartum week. Overall bLR mothers spent more time arched-back nursing their pups during the dark phase during the first postpartum week compared to bHR dams (Figure 1B). Also, given the significant effect of postpartum week, and similar to what was observed during the light phase, both groups of mothers displayed more arched-back nursing during the dark phase of the first versus second postpartum week.
For licking/grooming behavior during the light phase, there were no significant effects of bHR/bLR phenotype, postpartum week, or phenotype × postpartum week interaction (Figure 1C). However, for licking/grooming behavior during the dark phase, there was a main effect of postpartum week (p<0.05), with dams generally showing more licking/grooming during the first versus second postpartum weeks. There was also a significant phenotype × postpartum interaction (p<0.05) as bLR mothers exhibited more licking/grooming behavior than bHRs during the first week (Figure 1D).
For the amount of pup contact during the light phase, there was a significant main effect of postpartum week (p<0.05), with mothers generally having greater contact with their pups during the first versus second postpartum week. While there was no main effect of bHR/bLR phenotype, there was a significant phenotype × postpartum week interaction (p<0.01), with bHR mothers, somewhat surprisingly, spending more time in contact with their pups during the light phase of the second postpartum week compared to bLR mothers (Figure 1E). For amount of pup contact during the dark phase, analysis showed a main effect of phenotype (p<0.05), postpartum week (p<0.01), and a significant phenotype × postpartum week interaction (p<0.05). Mothers overall engaged in more pup contact during the second versus first postpartum week during the dark phase. However, during the dark phase of the first postpartum week, bLR dams contacted their pups more than bHR mothers (Figure 1F).
By contrast, to these other maternal behaviors, passive nursing was more prominent in the bHR dams compared to bLR mothers, especially during the light (low activity) phase during the second week of life (Figure 1G). Thus, for passive nursing observed during the light phase, there was a main effect of bHR/bLR phenotype (p<0.05), postpartum week (p<0.0001), and a significant phenotype × postpartum week interaction (p<0.05). bHR dams engaged in more passive nursing compared to bLRs during the second postpartum week (Figure 1G). Overall, both groups of mothers exhibited more passive nursing during the second versus first postpartum week, likely because the pups become more mobile during this time. For passive nursing during the dark phase, there was a significant main effect of postpartum week (p<0.0001), since all mothers displayed more passive nursing the second versus first postpartum week (Figure 1H), but there was no effect of phenotype and no phenotype × postpartum week interaction.
Other Behaviors in bHR/bLR Dams
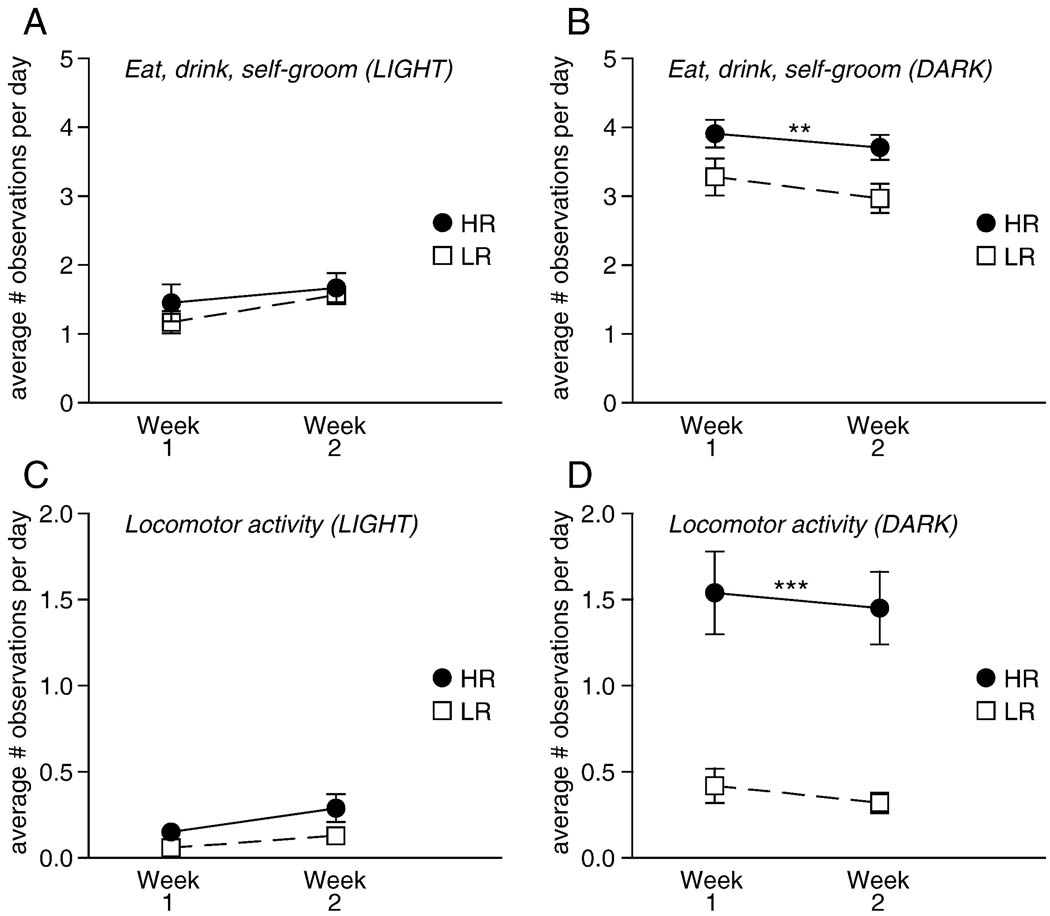
Self-directed behaviors (a combination of eating, drinking, and self-grooming) and locomotor activity (a combination of running and rearing activity) were also analyzed in bHR/bLR dams during the light and dark phases of the first two postpartum weeks (Figure 2). For self-directed behaviors (eating/drinking/self-grooming) observed during the light phase, there was a main effect of postpartum week (p<0.01) as all dams engaged in more of these self-directed behaviors during the second versus first postpartum week (Figure 2A). There was no effect of phenotype and no significant phenotype × postpartum week interaction. During the dark phase, there was a main effect of phenotype (p<0.01) and postpartum week (p<0.01), but no phenotype × postpartum week interaction. bHR mothers overall engaged in much more self-directed behavior compared to bLRs (Figure 2B). For locomotor activity during light phase, there was a main effect of postpartum week (p<0.05) with all mothers generally being more active during the second versus first week, but no significant effect of phenotype and no phenotype × postpartum week interaction (Figure 2C). During the dark phase there was a main effect of phenotype (p<0.0001) with bHR mothers displaying much more nighttime locomotion compared to bLRs (Figure 2D). There was no significant effect of postpartum week and no phenotype × postpartum week interaction.
Figure 2.
Self-directed behaviors (eating, drinking, self-grooming) and locomotor activity (rearing and running) in bHR versus bLR dams during the first two postpartum weeks. Graphs depict the average number of behavioral observations for postpartum week one versus two, with data shown separately for the light (left column) and dark (right column) phases. (A–B) bHR dams spent more time eating, drinking and self-grooming compared to bLRs, although these differences were only apparent during the dark phase. (C–D) bHR dams were also much more active compared to bLRs, showing much greater locomotor activity than bLR dams during the dark phase. *** indicates p<0.0001; ** indicates p<0.01
Oxytocin and Oxytocin Receptor mRNA expression in bHR versus bLR dams
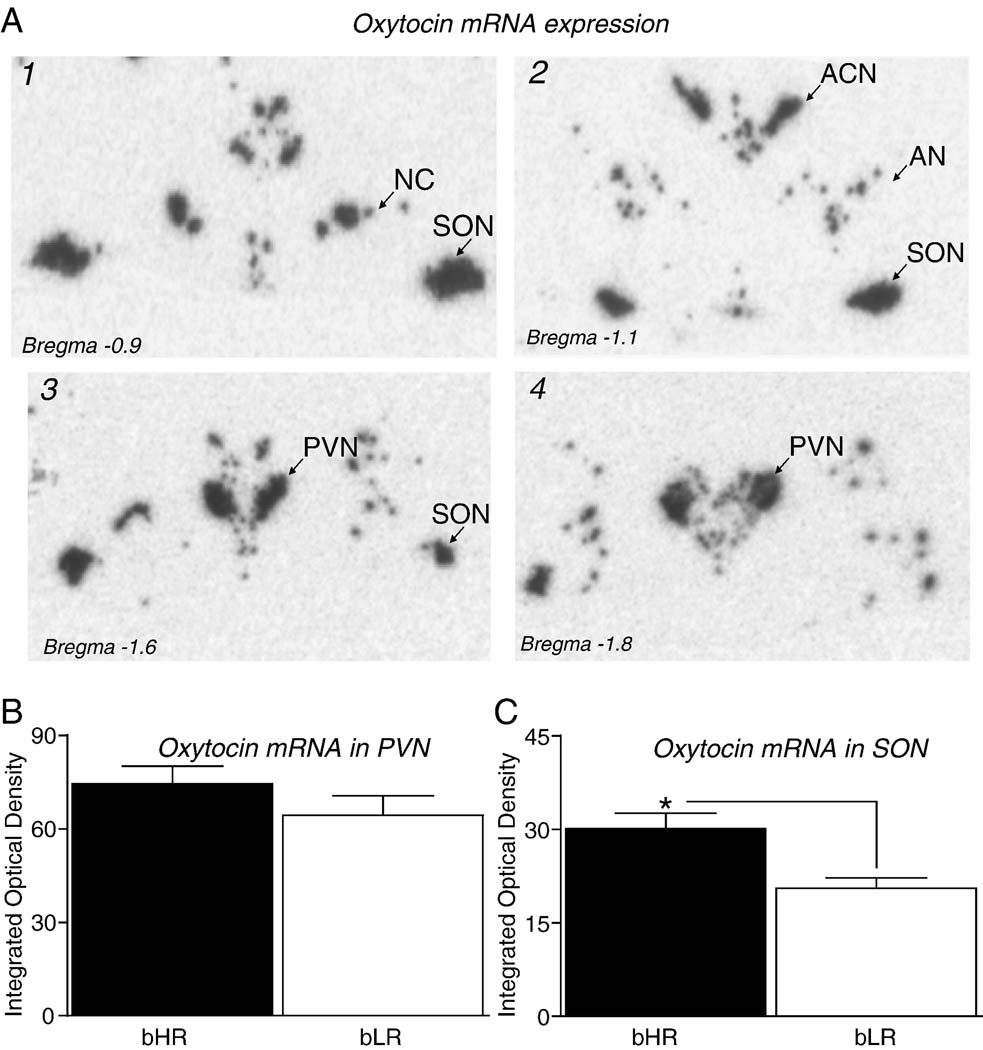
Integrated optical density (IOD) measurements for oxytocin mRNA were analyzed from sections through the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus from bHR and bLR dams sacrificed on the morning of postpartum day 8 (Figure 3A). While there was no main effect of bHR/bLR phenotype in the PVN (Figure 3B), there was a main effect of phenotype in the SON (p<0.05), since bHR mothers showed increased oxytocin mRNA expression compared to bLR mothers (Figure 3C). We also measured oxytocin mRNA levels in the anterior commisural nucleus (ACN), nucleus circularis (NC) and accessory nuclei (AN) of the hypothalamus, however there were no effects of phenotype. Oxytocin receptor mRNA levels were analyzed within several brain regions implicated in maternal behavior, including the septum, MPOA, cingulate cortex, prelimbic cortex, nucleus accumbens and bed nucleus of the stria terminalis. Data are summarized in Table 1. There were no significant bHR/bLR differences in oxytocin receptor mRNA levels in any of these regions.
Figure 3.
Oxytocin mRNA expression in bHR versus bLR dams on postnatal day 8. (A) Oxytocin mRNA expression was measured in the paraventricular nucleus of the hypothalamus (PVN), the supraoptic nucleus (SON), the anterior commissural nucleus (ACN), nucleus circularis (NC), and accessory nuclei (AN) of the hypothalamus. The four panels (A1–A4) show representative anatomical levels where these nuclei were located and analyzed. Each panel displays an autoradiogram taken of an X-ray film exposed for 24 hours after in situ hybridization with antisense riboprobe against rat oxytocin mRNA. (B) bHR and bLR dams expressed similar levels of oxytocin mRNA in the PVN. (C) However, in the SON, bHR dams expressed higher levels of oxytocin mRNA compared to bLRs. bHR and bLR dams expressed similar oxytocin mRNA levels in the ACN, NC, and AN (data not shown). * indicates p<0.05
Table 1.
Oxytocin receptor mRNA expression in bHR vs. bLR dams.
| bHR |
bLR |
|||
|---|---|---|---|---|
| Brain region | Mean IOD | sem | Mean IOD | sem |
| Acc | 13.71 | 0.99 | 14.02 | 1.49 |
| BNST | 12.78 | 1.24 | 12.62 | 1.18 |
| Cg | 18.99 | 1.38 | 19.85 | 1.48 |
| DP | 24.41 | 1.03 | 24.67 | 2.54 |
| MPOA | 14.16 | 1.12 | 16.48 | 2.29 |
| PrL | 24.16 | 1.44 | 23.75 | 1.90 |
| Septum | 14.11 | 0.98 | 13.89 | 1.11 |
Gene expression levels represented as average integrated optical density (IOD) ± standard error (sem).
Acc — Nucleus accumbens; BNST — bed nucleus of the stria terminalis; Cg — cingulate; DP — Dorsal peduncular cortex; MPOA — Medial preoptic area; PrL — Prelimbic cortex.
Effects of Cross-Fostering on bHR versus bLR Maternal Behavior
We repeated the maternal behavior observation paradigm in bHR and bLR dams, but used a cross-fostering paradigm to evaluate whether the mothers’ behavior was dependent upon the phenotype (bHR or bLR) of the litter that they raised. In general, the results replicated the earlier findings of greater active maternal behavior on the part of the bLR dams, specifically during the dark/active phase, and these effects were mostly present no matter what litter the bLR dams were raising. Cross-fostering subtly shifted maternal behavior in both bHR and bLR mothers, but the most marked effect was that bHR foster pups elicited greater amounts of passive nursing from both bHR and bLR mothers compared to their comparison groups.
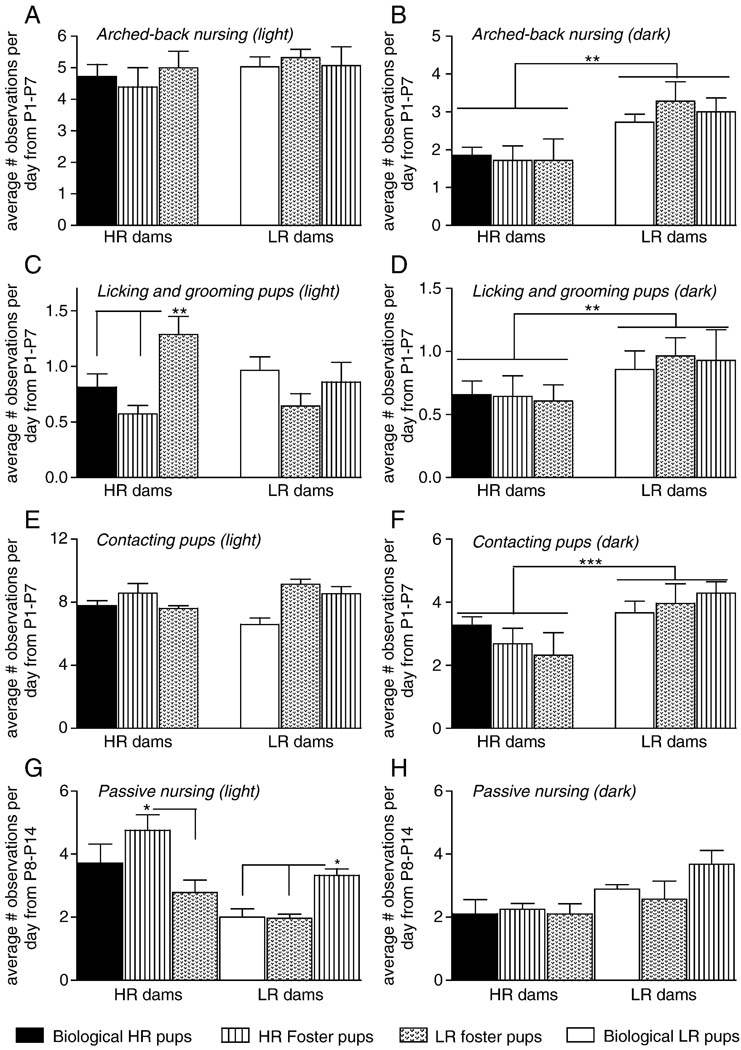
Figure 4 shows maternal behavior for bHR and bLR dams caring for either their biological pups, foster pups of the same bHR/bLR classification, or foster pups of the opposite bHR/bLR classification. Data for arched-back nursing (Figure 4A–B), licking/grooming (Figure 4C–D), and pup contact (Figure 4E–F) represent average behavioral scores during the first postnatal week since this was the timeframe when bHR/bLR differences were found in the previous study. Data for passive nursing (Figure 4G–H), on the other hand, represent average passive nursing scores during the second postnatal week since this was the timeframe when bHR/bLR differences were found in the previous study.
Figure 4.
Effect of cross-fostering on arched-back nursing, licking/grooming, pup contact, and passive nursing in bHR versus bLR dams. Graphs depict the average number of behavioral observations over either the first postnatal week (A–F) or second postnatal week (G–H) for each experimental group. Data are presented separately for the light (left column) versus dark (right column) phases. Within 24 hours of parturition, litters were assigned to one of three maternal care conditions and were either (a) returned to their biological mother, (b) placed with a dam of the same bHR/bLR phenotype, or (c) cross-fostered to a dam of the opposite bHR/bLR phenotype. (A–B) bLR dams spent more time arched-back nursing compared to bHR dams regardless of the phenotype of the litter that they raised, although this effect was only observed during the dark phase. (C–D) Similarly, bLR dams spent more time licking/grooming their pups compared to bHR dams regardless of the phenotype of the litter that they raised, although this effect was only observed during the dark phase. Interestingly, bHR dams spent more time licking/grooming bLR foster pups compared to bHR pups during the light phase. (E–F) bLR dams spent more time in contact with their pups compared to bHR dams regardless of the phenotype of the litter that they raised, although this effect was only observed during the dark phase. (G–H) bHR dams overall displayed more passive nursing behavior compared to bLR mothers. Interestingly, though, bHR foster pups elicited the greatest amount of passive nursing relative to their comparison groups; bHR foster pups received more passive nursing from bHR mothers compared to bLR foster pups. Similarly, bHR foster pups elicted greater passive nursing from bLR mothers relative to bLR pups. P1–7=postnatal days 1–7; P8–14=postnatal days 8–14; *** indicates p<0.0001; ** indicates p<0.01
For arched-back nursing during the light phase, we found no significant effects of maternal phenotype or foster group and no maternal phenotype × foster group interaction (Figure 4A). However, during the dark phase, there was a main effect of maternal phenotype (p<0.01) as bLR dams displayed greater amounts of arched-back nursing compared to bHR dams, regardless of the type of litter that they raised (Figure 4B). There were no significant effects of foster group and no interaction of maternal phenotype × foster group.
For licking/grooming behavior during the light phase, there were no significant effects of maternal phenotype or foster group, but there was a maternal phenotype × foster group interaction (p<0.05) (Figure 4C). Post hoc analysis revealed that this effect was due to bHR foster mothers spending more time licking/grooming bLR pups compared to bHR pups during the light phase (p<0.01). bLR dams, on the other hand, displayed the same amount of licking/grooming regardless of the phenotype of the litter they raised. In the dark phase there was a main effect of maternal phenotype (p<0.01) as bLR dams demonstrated increased arched-back nursing compared to bHR dams regardless of the phenotype of the litter that they raised (Figure 4D). There were no significant effects of foster group and no interaction of maternal phenotype × foster group.
For the amount of pup contact during the light phase, there was no effect of maternal phenotype or foster group, and no maternal phenotype × foster group interaction (Figure 4E). During the dark phase, there was a main effect of maternal phenotype (p<0.001), with bLR dams engaging in more pup contact than bHRs regardless of the phenotype of the litter that they raised (Figure 4F). There were no significant effects of foster group and no interaction of maternal phenotype × foster group.
For passive nursing during the light phase there was a main effect of maternal phenotype (p<0.05), and foster group (p<0.01). Overall, bHR mothers spent more time passive nursing their pups compared to bLR dams (p<0.05), although this effect was mostly carried by bHR dams passive nursing of either bHR biological pups or bHR foster pups (Figure 4G). For the effect of foster group, post-hoc analysis showed that bHR foster pups received more passive nursing compared to other groups (p<0.05), with both bHR and bLR mothers showing increased passive nursing specifically of bHR foster pups relative to the other groups (p<0.05, Figure 5G). There was no significant maternal phenotype × foster group interaction. During the dark phase there were no significant effects of maternal phenotype or foster group, and no maternal phenotype × foster group interaction (Figure 5H).
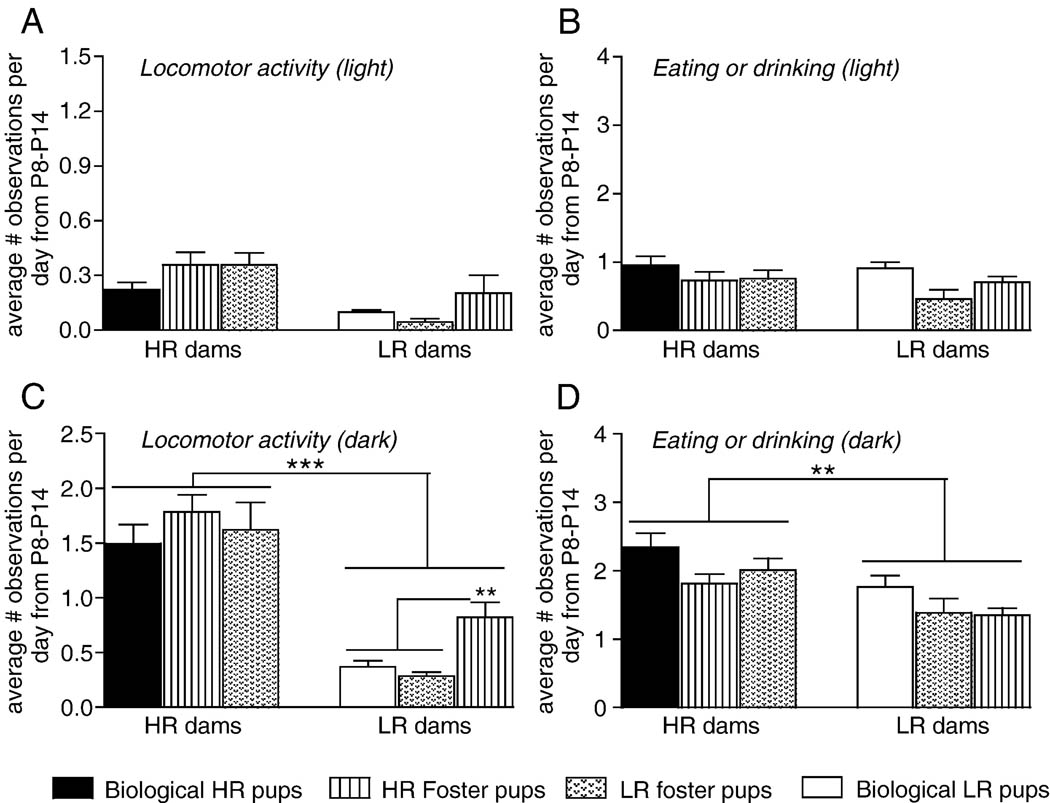
Figure 5.
Effect of cross-fostering on locomotor activity and self-directed behaviors in bHR versus bLR dams. Graphs depict the average number of behavioral observations over the second postnatal week for each experimental group. Data are presented separately for the light (left column) versus dark (right column) phases. The cross-fostering paradigm was conducted as described in the text and Figure 4. (A) All groups showed similarly low levels of locomotor activity during the light phase, and (B) also showed low levels of self-directed behaviors (eating, drinking, and self-grooming) during the light phase. (C) As before, bHR dams were much more active than bLR dams during the dark phase. Interestingly, however, bLR mothers that were fostering bHR pups were significantly more active than bLR dams that raised bLR litters. (D) bHR dams also spent more time engaged in self-directed behaviors than bLRs during the dark phase, but these differences were not affected by the phenotype of the litter that mothers’ raised. P8–14=postnatal days 8–14; *** indicates p<0.0001; ** indicates p<0.01
Effects of Cross-Fostering on Other Behaviors in bHR/bLR Dams
We also determined whether cross-fostering impacted locomotor activity (running and rearing) and self-directed behaviors (eating, drinking, self-grooming) in bHR/bLR dams (Figure 5). Data represent average behavior scores during the second postnatal week. For locomotor activity during the light phase there were no effects of maternal phenotype, foster group and no significant maternal phenotype × foster group interaction (Figure 5A). During the dark phase, there was an effect of maternal phenotype (p<0.0001) with bHR dams overall showing increased locomotion compared to bLRs (Figure 5C). Interestingly there was also a significant maternal phenotype × foster group interaction ( p<0.05), as bLR mothers that raised bHR foster pups were more active than those rearing either biological or foster bLR pups (p<0.01; Figure 5C).
For self-directed behavior during the light phase there were no effects of maternal phenotype, foster group, and no maternal phenotype × foster group interaction (Figure 5B). In the dark phase there was a significant main effect of maternal phenotype (p<0.01), as bHR dams overall spent more time performing self-directed behavior compared to bLR mothers (Figure 5D). There was no effect of foster group and no maternal phenotype × foster group interaction.
DISCUSSION
Although our earlier work demonstrated distinct maternal styles in commercially-purchased HR versus LR dams, it was unclear whether those behavior differences were driven by the genetics of the mothers themselves, or rather influenced by the behavior of the pups that they raised. Therefore, the current study used cross-fostering to examine whether the bHR and bLR maternal styles are inherent to mothers and/or are modulated by pup behavior, and also assessed oxytocin and oxytocin receptor mRNA expression to examine possible neurobiological underpinnings of bHR/bLR maternal differences. Consistent with our earlier findings, bHR dams exhibited less maternal behavior than bLRs during the dark/active phase, although they were very attentive to pups during the light phase, spending greater time passive nursing and in contact with pups compared to bLRs. Cross-fostering subtly influenced bHR and bLR dams’ behavior, leading bHR mothers to show more licking/grooming behavior of bLR foster pups, and leading both bHR and bLR mothers to exhibit greater passive nursing specifically of bHR foster pups. Thus, overall these data suggest that most aspects of the bHR versus bLR maternal styles are driven by innate qualities of the mothers themselves rather than by the pups that they are caring for.
Maternal behavior differences in bHR/bLR dams
Our earlier and present studies both show that commercially-purchased HR/LR and bHR/bLR mothers, respectively, interact differently with their offspring, with LR/bLR mothers being generally more attentive to their pups compared to HR/bHR mothers. During the first two postpartum weeks, bLR dams spent more time contacting their pups, arched-back nursing, and licking/groom their pups compared to bHR mothers, while bHR mothers spent much more time engaged in locomotor activity or self-directed behaviors (e.g. eating, drinking, self-grooming) compared to bLRs. Interestingly we found that during the second postpartum week, bHR mothers spent more time passive nursing compared to bLR mothers. Passive nursing was generally increased during the second versus first postnatal week, most likely due to all pups’ increased mobility. Since passive nursing is at least partly pup-initiated, requiring the pups to seek out and nurse from the resting dam (Galler and Propert, 1981), our data suggest that bHR pups may more actively approach their mother, thus leading to increased passive nursing.
Oxytocin receptor and oxytocin mRNA expression in bHR/bLR dams
To begin to examine possible neurobiological differences in bHR versus bLR mothers that may account for their behavioral differences, we performed in situ hybridization experiments to measure the levels of oxytocin mRNA levels in the hypothalamus and oxytocin receptor mRNA expression in several brain areas known to regulate maternal behavior, including the MPOA, BNST, lateral septum, nucleus accumbens, and amygdala (Fleming and Anderson, 1987; Fleming and Korsmit, 1996; Fleming et al., 1994; Fleming and Walsh, 1994; Numan, 1974; Numan, 1988; Numan et al., 1985). Previous work by Meaney and colleagues uncovered differences in oxytocin receptor binding and estrogen receptor α expression in the MPOA, lateral septum, and the central nucleus of the amygdala in dams exhibiting natural differences in licking/grooming and arched-back nursing of their pups (Champagne et al., 2001; Champagne et al., 2003; Francis et al., 2000). We therefore hypothesized that the bHR/bLR maternal behavior differences may also stem from underlying differences in oxytocin and/or oxytocin receptors. Somewhat surprisingly, we did not observe any bHR/bLR differences in oxytocin receptor mRNA expression within the several brain regions that were examined. However, since we measured oxytocin receptor mRNA levels as opposed to receptor binding, the possibility remains that bHR/bLR differences may exist at the protein level, or that the two groups may exhibit differences in estrogen receptor expression, which was not studied here.
We also assessed oxytocin mRNA levels in several hypothalamic nuclei, including the PVN, SON, ACN, NC, and AN. By and large, bHR and bLR dams expressed similar levels of oxytocin mRNA across these nuclei. Interestingly, though, we found higher levels of oxytocin mRNA in the SON of bHR versus bLR mothers. Abundant evidence shows how the brain of maternally behaving animals is altered as a result of her maternal behavior as well as the pups’ behavior towards her (e.g. amount of suckling) (Modney and Hatton, 1994). Specifically, several reports document how lactation/suckling impacts the physiology and morphology of neurons in the SON, with increased suckling and milk-letdown leading to increased firing and dendritic bundling (Modney and Hatton, 1994; Richard et al., 1991). Considering our observation of increased passive nursing in bHR mothers, particularly during the second postpartum week when we evaluated oxytocin mRNA levels in bHR/bLR dams, it is tempting to speculate that their elevated SON oxytocin mRNA levels may be associated with increased passive nursing (with both perhaps stimulated by the increased demand of the bHR pups). However, one argument against this possibility relates to the fact that the passive nursing lactating posture is one of the least effective postures for milk let down (Lonstein et al., 1998). Future studies will further investigate these findings by examining circulating oxytocin blood levels in bHR/bLR dams and also beginning to examine possible morphological differences in the SON of bHR/bLR mothers.
Impact of cross-fostering on bHR/bLR mothers’ behavior
Our final experiment utilized a cross-fostering paradigm to determine whether the bHR/bLR maternal styles were completely inherent to the mothers themselves or in part driven by the phenotype of the pups that they were raising. We found that bLR mothers consistently exhibited more arched-back nursing, licking/grooming, and pup-contact compared to bHR mothers during the dark phase, regardless of the type of litter that they cared for. Interestingly, bHR foster mothers acted somewhat differently during the light phase based on the phenotype of litter they cared for, with bHR dams spending more time licking/grooming bLR foster pups compared to bHR foster pups. This finding is particularly interesting and perhaps a bit unexpected since, in general, our other data suggest that bHR pups may be more demanding of maternal attention/care compared to bLR pups, and prior studies show that more demanding pups tend to elicit greater maternal care (Brouette-Lahlou et al., 1992; Pereira and Ferreira, 2006; Stern, 1997). The effect is unlikely to be simply a non-specific of foster care, since bHR mothers do not show increased licking/grooming of bHR foster pups. Future studies should more closely examine bHR and bLR pups’ behavior under basal conditions as well as under these cross-foster conditions (e.g. by measuring levels of ultrasonic vocalization, locomotor activity, suckling attempts), to determine whether the pups exhibit behavioral differences that might stimulate certain maternal responses.
The pups’ phenotype also influenced passive nursing behavior, although this effect occurred in both bHR and bLR dams. While bHR mothers generally spent more time engaged in passive nursing compared to bLR mothers (similar to our earlier study), we found that both bHR and bLR mothers caring for bHR foster pups exhibited the greatest amount of passive nursing compared to the other groups. These data lend additional support to the notion that bHR pups may be more persistent in seeking out their mother and demanding additional passive nursing compared to bLR pups. It would be especially interesting to look at oxytocin mRNA expression in the SON of these cross-foster study mothers. Based on the earlier findings of increased oxytocin mRNA expression in the SON of bHR mothers, one might predict that bHR/bLR mothers raising bHR litters and therefore performing more passive nursing might also exhibit higher levels of oxytocin mRNA in the SON compared to mothers raising bLR litters.
In addition to asking how cross-fostering impacts bHR and bLR mothers’ behavior, it is also of great interest to determine how the bHR versus bLR maternal style impinges on their offspring’s adult phenotype. This is particularly true considering the fact that Meaney et al. have reported that cross-fostering between the High- and Low-Licking/Grooming and Arched-Back Nursing mothers completely reverses the behavioral and neuroendocrine phenotypes of their adult offspring (Francis et al., 1999). In an earlier study, we performed the cross-fostering paradigm and evaluated its effects on locomotor response to novelty and anxiety-like behavior in bHR and bLR offspring (Stead et al., 2006a). Cross-fostering had no detectable impact on the locomotor response to novelty of adult offspring, with bHR males and females consistently showing much higher novelty-induced activity regardless of the mother that raised them compared to all bLR animals (Stead et al., 2006a). Cross-fostering modestly affected anxiety-like behavior, particularly in the bLR offspring. We found a generally positive effect of foster care on bLRs, with bLR rats fostered by bHR or bLR mothers showing less anxiety-like behavior compared to LRs raised by biological mothers (Stead et al., 2006a). Previous rodent studies have reported that foster care/adoption can positively influence stress reactivity and reverse the negative effects typically incurred by prenatal stress (Maccari et al., 1995), so a similar mechanism may explain the positive effect of foster care/adoption on LRs’ typically high level of anxiety-like behavior. It is important to note that the relatively subtle effects of cross-fostering on the selectively-bred bHR/bLR offspring are likely due to the strong genetic component that underlies their phenotypes. It is conceivable that the distinct bHR versus bLR maternal styles could play some greater role in shaping behavior of offspring that naturally express a more intermediate (i.e. less extreme bHR or bLR) phenotype. In fact, preliminary studies in our laboratory show that cross-breeding the bHR and bLR lines produces animals with an intermediate behavioral phenotype that is influenced by maternal care (Bedrosian et al., 2008). Future studies will continue to evaluate the effects of cross-fostering on neuroendocrine stress reactivity and other behavioral measures (i.e. aggression, impulsivity, drug-taking behavior) in the bHR/bLR as well as more intermediate phenotype animals.
Conclusions
We previously reported that individual differences in novelty-seeking predict differences in maternal style, with low novelty-seeking females (commercially-purchased LRs) generally exhibiting greater maternal care towards their offspring compared to high novelty-seekers (commercially purchased HRs). The present study confirms these maternal style differences in selectively-bred bHR and bLR mothers, demonstrating again that bLR dams are generally more attentive to their pups during the first two postnatal weeks compared to bHR mothers. These differences do not appear to be related to bHR/bLR differences in oxytocin receptor levels, although we did find differential expression of oxytocin mRNA in the SON, with bHR mothers expressing higher levels compared to bLR mothers. Finally, a cross-fostering study demonstrated that several aspects of the bHR/bLR maternal styles are inherent to the mothers themselves, as bLRs were generally more maternal toward their pups than bHRs regardless of the bHR/bLR phenotype of the litter that they raised. The pups’ phenotype affected passive nursing in both bHR and bLR dams, suggesting that bHR pups may be more demanding and elicit different behavior from their mothers compared to bLR pups. Future studies will explore how such differences in pup behavior may impact bHR/bLR mothers at a behavioral, neural, and hormonal level, and continue to explore how bHR/bLR variations in maternal care ultimately influence emotional reactivity in their offspring.
ACKNOWLEDGEMENTS
This work was supported by the Office of Naval Research N00014-02-1-0879, NIDA 5P01DA021633-02 (HA and SJW), L'Oréal USA Fellowship for Women in Science and NIMH 1K99MH085859 (SMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abraham A, Clinton SM, Watson SJ, Akil H. Individual Differences in Novelty-Seeking Correlate with Differences in Aggressive Behavior. Atlanta, GA: Society for Neuroscience 36th Annual Meeting. 2006
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Animal Behavior. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Arnold JL, Siviy SM. Effects of neonatal handling and maternal separation on rough-and-tumble play in the rat. Developmental Psychobiology. 2002;41:205–215. doi: 10.1002/dev.10069. [DOI] [PubMed] [Google Scholar]
- Ball SA, Cobb-Richardson P, Connolly AJ, Bujosa CT, O'Neall TW. Substance abuse and personality disorders in homeless drop-in center clients: symptom severity and psychotherapy retention in a randomized clinical trial. Compr Psychiatry. 2005;46:371–379. doi: 10.1016/j.comppsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ballaz SJ, Akil H, Watson SJ. Analysis of 5-HT6 and 5-HT7 receptor gene expression in rats showing differences in novelty-seeking behavior. Neuroscience. 2007;147:428–438. doi: 10.1016/j.neuroscience.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Clinton SM, Abraham AD, Watson SJ, Akil H. High- and Low-Novelty Seeking Females Exhibit Different Maternal Styles: Impact of Cross-fostering on Offspring’s Behavioral Phenotype. Society for Neuroscience. 2008 [Google Scholar]
- Brouette-Lahlou I, Vernet-Maury E, Vigouroux M. Role of pups' ultrasonic calls in a particular maternal behavior in Wistar rat: pups' anogenital licking. Behav Brain Res. 1992;50:147–154. doi: 10.1016/s0166-4328(05)80296-7. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Voorhuis TA, van Tol HH, Ivell R. In situ hybridization of oxytocin messenger RNA: macroscopic distribution and quantitation in rat hypothalamic cell groups. Biochem Biophys Res Commun. 1987;145:10–14. doi: 10.1016/0006-291x(87)91280-0. [DOI] [PubMed] [Google Scholar]
- Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. Journal of Neuroscience. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H. Beta1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology. 2007;32:589–599. doi: 10.1038/sj.npp.1301140. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the USA. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Clinton S, Miller S, Watson SJ, Akil H. Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology. 2008;33:162–177. doi: 10.1016/j.psyneuen.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Vazquez DM, Kabbaj M, Kabbaj MH, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm Behav. 2007;51:655–664. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg VH, Brumaghim JT, Haltmeyer GC, Zarrow MX. Increased adrenocortical activity in the neonatal rat following handling. Endocrinology. 1967;81:1047–1052. doi: 10.1210/endo-81-5-1047. [DOI] [PubMed] [Google Scholar]
- Eilam D, Golani I. The ontogeny of exploratory behavior in the house rat (Rattus rattus): the mobility gradient. Developmental Psychobiology. 1988;21:679–710. doi: 10.1002/dev.420210707. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. A neural timing factor in the mechanism by which progesterone advances ovulation in the cyclic rat. Endocrinology. 1949;45:581–595. doi: 10.1210/endo-45-6-581. illust. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Anderson V. Affect and nurturance: mechanisms mediating maternal behavior in two female mammals. Progress in Neuropsychopharmacology and Biological Psychiatry. 1987;11:121–127. doi: 10.1016/0278-5846(87)90049-2. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M. Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behavioral Neuroscience. 1996;110:567–582. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- Fleming AS, O'Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neuroscience and Biobehavioral Reviews. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behavioral Neuroscience. 1994;108:724–734. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Walsh C. Neuropsychology of maternal behavior in the rat: c-fos expression during mother-litter interactions. Psychoneuroendocrinology. 1994;19:429–443. doi: 10.1016/0306-4530(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, LaPlante P, Weaver S, Seckl JR, Meaney MJ. The role of early environmental events in regulating neuroendocrine development. Moms, pups, stress, and glucocorticoid receptors. Annals of the New York Academy of Science. 1996;794:136–152. doi: 10.1111/j.1749-6632.1996.tb32517.x. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Galler JR, Propert KJ. Maternal behavior following rehabilitation of rats with intergenerational malnutrition. 2. Contribution of mothers and pups to deficits in lactation-related behaviors. Journal of Nutrition. 1981;111:1337–1342. doi: 10.1093/jn/111.8.1337. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Maternal separation affects infant rats' behavior. Behavioral Biology. 1973;9:629–633. doi: 10.1016/s0091-6773(73)80057-4. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacology Biochemistry and Behavior. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Jr, Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. Journal of Neuroscience. 1994a;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Sorg BA, Kalivas PW. The relationship between MRNA levels and the locomotor response to novelty. Brain Research. 1994b;663:312–316. doi: 10.1016/0006-8993(94)91278-5. [DOI] [PubMed] [Google Scholar]
- Ju G, Liu S, Tao J. Projections from the hypothalamus and its adjacent areas to the posterior pituitary in the rat. Neuroscience. 1986;19:803–828. doi: 10.1016/0306-4522(86)90300-3. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Neurobiological bases of individual differences in emotional and stress responsiveness: high responders-low responders model. Archives of Neurology. 2004;61:1009–1012. doi: 10.1001/archneur.61.7.1009. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. Journal of Neuroscience. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Evans S, Watson SJ, Akil H. The search for the neurobiological basis of vulnerability to drug abuse: using microarrays to investigate the role of stress and individual differences. Neuropharmacology. 2004;47 Suppl 1:111–122. doi: 10.1016/j.neuropharm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in Brain Research. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S, Alpert M, Lewis GW. Infantile experience and the maturation of the pituitary adrenal axis. Science. 1957;126:1347. doi: 10.1126/science.126.3287.1347. [DOI] [PubMed] [Google Scholar]
- Levine S, Haltmeyer GC, Karas GG, Denenberg VH. Physiological and Behavioral Effects of Infantile Stimulation. Physiology and Behavior. 1967;2:55–59. [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Angulo JA, Le Moal M, McEwen BS, Piazza PV. Neurochemical characterization of individual vulnerability to addictive drugs in rats. Eur J Neurosci. 1998;10:3153–3163. doi: 10.1046/j.1460-9568.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Deminiere JM, Angelucci L, Simon H, Le Moal M. Hippocampal type I and type II corticosteroid receptor affinities are reduced in rats predisposed to develop amphetamine self-administration. Brain Research. 1991;548:305–309. doi: 10.1016/0006-8993(91)91137-p. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. Journal of Neuroscience. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo T, Alttoa A, Koiv K, Tonissaar M, Eller M, Harro J. Rats with persistently low or high exploratory activity: behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behavioral Brain Research. 2007;177:269–281. doi: 10.1016/j.bbr.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neuroscience. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Modney BK, Hatton GI. Maternal behaviors: evidence that they feed back to alter brain morphology and function. Acta Paediatr Suppl. 1994;397:29–32. doi: 10.1111/j.1651-2227.1994.tb13262.x. [DOI] [PubMed] [Google Scholar]
- Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Maternal behavior of SHR rats and its relationship to offspring blood pressures. Dev Psychobiol. 1989;22:29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- Numan M. Medial preoptic area and maternal behavior in the female rat. Journal of Comparative and Physiological Psychology. 1974;87:746–759. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Numan M. A neural circuitry analysis of maternal behavior in the rat. Acta Paediatr Suppl. 1994;397:19–28. doi: 10.1111/j.1651-2227.1994.tb13261.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Morrell JI, Pfaff DW. Anatomical identification of neurons in selected brain regions associated with maternal behavior deficits induced by knife cuts of the lateral hypothalamus in rats. Journal of Comparative Neurology. 1985;237:552–564. doi: 10.1002/cne.902370411. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann N Y Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Orr H, Clinton SM, Abraham AD, Bedrosian TA, Watson SJ, Akil H. Low Novelty-Seeking Rats Exhibit Exaggerated Anxiety- and Depression-Like Behavior Compared to High Novelty-Seekers: Impact of Chronic Paxil Treatment. Washington, D.C: Society for Neuroscience; 2008. [Google Scholar]
- Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175:139–148. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proceedings of the National Academy of Sciences of the USA. 1991a;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research. 1991b;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Richard P, Moos F, Freund-Mercier MJ. Central effects of oxytocin. Physiol Rev. 1991;71:331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Levitt P, Card JP. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. Journal of Neuroscience. 2000;20:2731–2741. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario LA, Abercrombie ED. Individual differences in behavioral reactivity: correlation with stress-induced norepinephrine efflux in the hippocampus of Sprague-Dawley rats. Brain Research Bulletin. 1999;48:595–602. doi: 10.1016/s0361-9230(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stressinduced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Russell PA. Effects of maternal separation and maternal disturbance on offspring growth and behavior in rats. Journal of General Psychology. 1973;88:127–133. doi: 10.1080/00221309.1973.9920718. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Developmental Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Smart JL, Dobbing J. Vulnerability of developing brain. IV. Passive avoidance behavior in young rats following maternal undernutrition. Developmental Psychobiology. 1972;5:129–135. doi: 10.1002/dev.420050206. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton SM, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective Breeding for Divergence in Novelty-seeking Traits: Heritability and Enrichment in Spontaneous Anxiety-related Behaviors. Behavioral Genetics. 2006a;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, Watson SJ, Akil H. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. Journal of Neuroscience. 2006b;26:345–353. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-Seeking Behavior Predicts Vulnerability to Depression and Associated Changes in Cardiovascular Function in a Rodent Model Society for Biological Psychiatry. Vancouver, B.C. Canada: 2009. [Google Scholar]
- Stern JM. Offspring-induced nurturance: animal-human parallels. Developmental Psychobiology. 1997;31:19–37. doi: 10.1002/(sici)1098-2302(199707)31:1<19::aid-dev3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Locomotor response to novelty as a predictor of reactivity to aversive stimuli in the rat. Brain Research. 2007;1149:141–148. doi: 10.1016/j.brainres.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Ontogeny of defensive behavior and analgesia in rat pups exposed to an adult male rat. Physiology and Behavior. 1998;63:261–269. doi: 10.1016/s0031-9384(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Ying SY, Gove S, Fang VS, Greep RO. Ovulation in postpartum rats. Endocrinology. 1973;92:108–116. doi: 10.1210/endo-92-1-108. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Campbell PS, Denenberg VH. Handling in infancy: increased levels of the hypothalamic corticotropin releasing factor (CRF) following exposure to a novel situation. Proc Soc Exp Biol Med. 1972;141:356–358. doi: 10.3181/00379727-141-36776. [DOI] [PubMed] [Google Scholar]