Abstract
Escherichia coli ribonucleotide reductase (RNR), an α2β2 complex, catalyzes the conversion of nucleoside 5′-diphosphate substrates (S) to 2′-deoxynucleoside 5′-diphosphates. α2 houses the active site for nucleotide reduction and the binding site for allosteric effectors (E). β2 contains the essential diferric tyrosyl radical (Y122•) cofactor which, in the presence of S and E, oxidizes C439 in α to a thiyl radical, C439•, to initiate nucleotide reduction. This oxidation occurs over 35 Å and is proposed to involve a specific pathway: Y122•→W48→Y356 in β2 to Y731→Y730→C439 in α2. 3-Aminotyrosine (NH2Y) has been site specifically incorporated at residues 730 and 731, and formation of the aminotyrosyl radical (NH2Y•) has been examined by stopped-flow (SF) UV-vis and EPR spectroscopies. To examine the pathway dependence of radical propagation, the double-mutant complexes Y356F–β2:Y731NH2Y-α2, Y356F–β2:Y730NH2Y–α2, and wt-β2:Y731F/Y730NH2Y–α2, in which the non-oxidizable F acts as a pathway block, were studied by SF and EPR spectroscopies. In all cases, no NH2Y• was detected. To study off-pathway oxidation, Y413, located 5 Å from Y730 and Y731 but not implicated in long-range oxidation, was examined. Evidence for NH2Y413• was sought in three complexes: wt-β2:Y413NH2Y-α2 (a), wt-β2:Y731F/Y413NH2Y–α2 (b), and Y356F-β2:Y413NH2Y–α2 (c). With (a), NH2Y• was formed with a rate constant of 25-30% and an amplitude of 25% that observed for its formation at residues 731 and 730. With (b), the rate constant for NH2Y• formation was 0.2-0.3% that observed at 731 and 730, and with (c), no NH2Y• was observed. These studies suggest the evolution of an optimized pathway of conserved Ys in the oxidation of C439.
Long-range electron transfer (ET) (1) is prevalent in biology and plays a central role in many processes including respiration (2), nitrogen fixation (3), and the photosynthetic reaction centers (4). Studies over many decades have established that ET occurs rapidly and efficiently between metal clusters that are spaced 10 to 15 Å apart (5) and that the process involves electron tunneling as described by the Marcus-Levich equation (6). The importance of the medium, the intervening polypeptide structural motifs, has been studied extensively in model proteins and biological systems (1, 7). The protein fold clearly plays a central role in lowering the reorganization energy of the biological ET reaction. It is also clear that ET kinetics can be regulated by the dynamics of conformational changes, especially across protein-protein interfaces (8, 9).
The discovery of the central role of a tyrosyl radical (Y•) in the class Ia ribonucleotide reductase (10) and the O2-evolving complex of photosystem II (11) led to the realization that ET processes over long distances are not limited to metal clusters. Long-range ET can also involve the aromatic amino acids tryptophan and tyrosine, the oxidations of which require loss of both an electron and a proton (12). Ribonucleotide reductase (RNR), which catalyzes the conversion of nucleotides to deoxynucleotides (13), has served as the paradigm for long-range proton-coupled electron transfer (PCET) with the tyrosyl radical (Y•) in the β subunit mediating the oxidation of a cysteine in the α subunit 35 Å removed (Figure 1) (12, 14). The present paper describes the use of 3-aminotyrosine (NH2Y), site specifically incorporated into the α subunit of RNR (15), to examine the pathway dependence, and the importance of transient intermediates, proposed for this oxidation.
FIGURE 1.
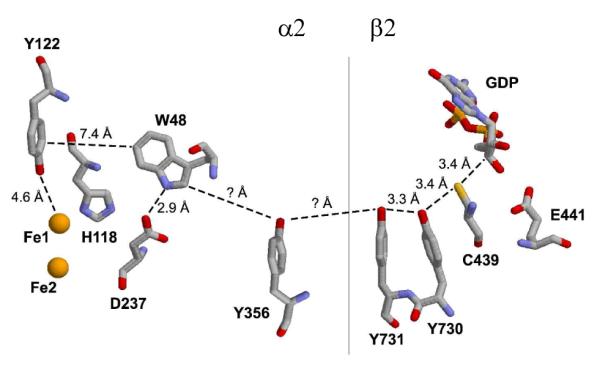
The proposed radical propagation pathway within an α:β pair of E. coli RNR. Y356, Y731, and Y730 have been shown to be redox-active during radical transfer and their positions within the complex have been determined structurally (14) and/or experimentally (35). Y356 is located in the structurally disordered C-terminal tail of β (44), and thus its location in the complex is not known.
Tyrosyl radicals (Y•) and tryptophan radicals (W•) have been studied in three different contexts indirectly related to radical propagation in RNRs. In the area of model proteins and peptides, oxidizable amino acids have been positioned between electron donor/acceptor pairs. Studies of the ET process in the resulting systems have demonstrated rate accelerations associated with a hopping model requiring formation of a transient intermediate (16, 17). These “simple” systems have allowed detailed mechanistic analyses and provide precedent for the multiple hopping steps proposed for RNR.
Y and W radicals have been observed in heme and non-heme iron dependent proteins, in which a reactive metal-based intermediate generated during catalysis is, in the absence of substrate, reduced by ET from an aromatic residue(s) in the vicinity of the metallocofactor (18-21). In general, low amounts of multiple radical species are generated on a slow (second) time scale. These systems are starting to define the mechanisms and consequences of aberrant oxidations and provide a model for the phenotype of an off-pathway oxidation in RNR.
Finally, transient aromatic amino acid radicals have been studied in enzymes such as cytochrome c peroxidase and prostaglandin synthase, in which metallocofactors are responsible for the oxidation of aromatic amino acids over short distances in the presence of substrate (22, 23). Only ribonucleotide reductase and photolyase (PL) however are believed to utilize multiple, transient aromatic amino acid radical intermediates in long-range ET. PL, as isolated, contains a flavin cofactor in the semiquinone form (FADH•) that needs to be reduced to its active form (FADH−) prior to catalysis (24). Time-resolved kinetic studies suggest that the reduction occurs by a hopping mechanism involving three conserved Ws over a 15 Å distance (25). To obtain insight into the pathway dependence of reduction of FADH•, the non oxidizable amino acid phenylalanine (F) was incorporated in place of each of the Ws and re-analyzed by ultrafast kinetics (26-28). The measurements on the wild-type and mutant PLs support a model in which 3 Ws act as a wire to the flavin cofactor.
The class Ia ribonucleotide reductases are the only known enzymes whose physiological function requires long-range oxidation, thought to occur in a pathway-dependent fashion through a series of aromatic amino acids (Figure 1). This provocative mechanism was first proposed by Uhlin and Eklund on the basis of a docking model of the α2:β2 complex and on the strict conservation of the residues in primary sequence alignments (14). Mutagenesis studies in which each of the residues was replaced individually by F demonstrated their importance in catalysis, structure, or both (29-32). However, only with the advent of technology for the site-specific incorporation of unnatural amino acids into α and β has the involvement of the three tyrosines (356 in β2, 731 and 730 in α2, Figure 1) and the distance of the individual oxidation “steps” been examined (15, 33-36). We have demonstrated recently the site-specific incorporation of 3-aminotyrosine (NH2Y) into the α and β subunits at each of these positions. SF UV-vis and EPR studies revealed that NH2Y• is generated in a nucleotide-dependent manner with multiphasic kinetics, and that the rates of formation are maximized when substrate and effectors are bound (15, 37). In addition, our studies indicated that NH2Y-αs are capable of supporting nucleotide reduction, suggesting that the observed radical may be an intermediate on the proposed reaction pathway. From these collective studies, we have proposed that radical propagation occurs via orthogonal PCET in the β2 subunit, with the proton transferring off-pathway to an amino acid acceptor or to solvent, and via co-linear PCET in the α2 subunit, with both proton and electron transferring between the same donor/acceptor pair (12, 15).
Despite the appeal of a conserved PCET pathway, evidence in support of transient radical intermediates in RNR has been challenging to obtain because the reaction is rate-limited by conformational changes gated by binding of substrates and effectors to α (38). In previous efforts to induce light-mediated turnover on a photopeptide:α2 RNR complex, we have observed deoxynucleotide formation and transient formation of a Y• within the peptide, but due to conformational gating, we have thus far been unable to detect transient intermediates within α (39, 40).
NH2Y is easier to oxidize than Y by 190 mV at pH 7 (15). From a thermodynamic perspective, the detection of NH2Y• in NH2Y-αs may not be entirely surprising. Thus the pathway dependence of NH2Y• formation is important to establish (Figure 1). To provide further support for a defined pathway, F has been incorporated in place of each Y in the pathway to function as a block of NH2Y• formation. Incubation of Y356F-β2:NH2Y731-α2, Y356F-β2:NH2Y730-α2, or wt-β2:Y731F/NH2Y730-α2 with CDP/ATP completely blocks NH2Y oxidation, supporting the importance of a specific pathway. As a further test of the pathway dependence, the residue Y413 was selected for site specific “off-pathway” NH2Y incorporation due to its proximity to Y731 and Y730 in α. Analogous studies were conducted with wt-β2:Y413NH2Y-α2, wt-β2:Y731F/Y413NH2Y-α2, and Y356F-β2:Y413NH2Y-α2 in which evidence for NH2Y• formation was sought by SF and EPR spectroscopy. The results of these experiments establish that functional long-range PCET in RNR occurs via an optimized pathway of amino acids spanning >35 Å across the α:β interface.
MATERIALS AND METHODS
Materials
The expression and purification of wt-α2 (2500 nmol/min/mg), wt-β2 (1.2 Y122•/dimer, 7600 nmol/min/mg), Y731NH2Y-α2 (150 nmol/min/mg), Y730NH2Y-α2 (110 nmol/min/mg), His-Y356F-β2 (1.0-1.2 Y122•/dimer, <1 nmol/min/mg), and Y730F-α2 (<25 nmol/min/mg) were conducted as previously described (15, 41). His-Y356F-β2 will be referred to as “Y356F-β2” henceforth. E. coli thioredoxin (TR, 40 units/mg) and thioredoxin reductase (TRR, 1400 units/mg) were isolated as previously described (41). [5-3H]-CDP was purchased from ViTrax (Placentia, CA).
Generation of pTrc-nrdA-TAG730TTT731, pTrc-nrdA-TAG413 and pTrc-nrdA-TAG413TTT731
The QuikChange Kit (Stratagene) was used according to manufacturer’s instructions to generate pTrc nrdA-TAG730TTT731, pTrc-nrdA-TAG413 and pTrc-nrdA-TAG413TTT731. To generate pTrc-nrdA-TAG730TTT731, the template, pTrc-nrdA-TAG730, (15) was amplified in the presence of forward (5′-TTC GGG GTC AAA ACA CTG TAG TTT CAG AAC ACC CGT GAC GGC GCT-3′) and reverse (5′-AGC GCC GTC ACG GGT GTT CTG AAA CTA CAG TGT TTT GAC CCC GAA-3′) primers to insert a TTT codon (Phe) at residue 731. To generate pTrc-nrdA-TAG413, the template, pTrc-nrdA-wt, was amplified in the presence of forward (5′-GCG TCT ACC GGT CGT ATC TAG ATT CAG AAC GTT GAC CAC TGC-3′) and reverse (5′-GCA GTG GTC AAC GTT CTG AAT CTA GAT ACG ACC GGT AGA CGC-3′) primers to insert the amber stop codon (TAG) at residue 413. pTrc-nrdA-TAG413TTT731 was then generated from pTrc-nrdA-TAG413, with forward (5′-C GGG GTC AAA ACA CTG TAT TTT CAG AAC ACC CGT GAC G-3′) and reverse (5′-C GTC ACG GGT GTT CTG AAA ATA CAG TGT TTT GAC CCC G-3′) primers to insert a TTT codon (Phe) at residue 731. For each plasmid, the mutation(s) was confirmed by sequencing the entire gene at the MIT Biopolymers Laboratory.
Expression, purification, and activity determination of Y731F/Y730NH2Y-α2
Y731F/Y730NH2Y-α2 was expressed, purified, and pre-reduced as described for Y730NH2Y-α2 and Y731NH2Y-α2 (15). Specific activity was determined by the radioactive RNR assay (33). Assays were conducted with mutant α2 and wt β2 at concentrations of 0.5 μM and 2.5 μM, respectively, in 50 mM HEPES, 15 mM MgSO4, 1 mM EDTA, pH 7.6 (assay buffer) at 25 °C. [5-3H]-CDP had a specific activity of 1450 cpm/nmol. The reaction product was dephosphorylated by treatment with alkaline phosphatase and the resulting dC was quantitated by the method of Steeper and Steuart (33).
Expression, purification, and activity determination of Y413NH2Y-α2 and Y731F/Y413NH2Y-α2
Y413NH2Y-α2 and Y731F/Y413NH2Y-α2 were expressed and purified as described for Y730NH2Y-α2 and Y731NH2Y-α2 (15). However, an additional step using a Poros HQ/20 FPLC anion exchange column (Applied Biosystems, 1.6 × 10 cm, 20 mL) was required to obtain protein of >95% purity judged by SDS-PAGE analysis. The column was equilibrated in 50 mM Tris, 1 mM EDTA, and 5 mM DTT, pH 7.6 and was loaded with 15-20 mg of Y413NH2Y–β2 or Y731F/Y730NH2Y-α2. The column was washed with one column volume of equilibration buffer at a flow rate of 4 mL/min, then eluted with a linear gradient of 100 to 500 mM NaCl (60 mL × 60 mL) in the same buffer at the same flow rate. In the case of Y731F/Y730NH2Y-α2, the protein was chromatographed twice. Subsequent to the FPLC purification step, the protein was pre-reduced according to the standard procedure (15).
The activities of Y413NH2Y-α2 and Y731F/Y413NH2Y-α2 were determined using the spectrophotometric (41) and/or radioactive assays (33), with minor modifications. Mutant α2s (0.1 or 0.2 μM) were assayed in the presence of a 5-fold excess of β2 (0.5 μM or 1.0 μM) in assay buffer at 25 °C. [5-3H]-CDP had activity between 2700 and 4800 cpm/nmol.
Determination of the Kd for Y413NH2Y-α2 and β2 interaction
The Kd between Y413NH2Y-α2 and wt β2 was determined using Y730F-α2 as a competitive inhibitor of nucleotide reduction (29, 42). Y413NH2Y-α2 (0.7 μM) and β2 (0.35 μM) were incubated with CDP (1 mM), ATP (1.6 mM), TR (50 μM), TRR (1 μM), and NADPH (0.2 mM) in assay buffer. Inhibition of RNR activity (as measured by decrease in NADPH consumption) was determined in the presence of increasing Y730F-α2 (0.1 μM to 3 μM). The data analysis for this procedure typically relies on the mutant α2 of unknown Kd acting as the inhibitor of nucleotide reduction (36). The analysis was modified, as the Kd of the active Y413NH2Y-α2 is not known, whereas the Kd of the inhibitor (Y730F-α2) had been previously determined under conditions in which the Ki and Kd of the inhibitor are equivalent (30). The Kd for the Y413NH2Y-α2:wt-β2 complex was approximated and the experimentally determined relative activities were then used to extrapolate the Kd for the Y730F-α2:wt-β2 complex. The Kd for Y413NH2Y-α2 was refined by iterative fitting of the experimental data until the fit yielded a Kd for Y730F-α2 that was in good agreement with the literature value.
Reaction of NH2Y-α2s with Y356F-β2 monitored by EPR spectroscopy
Pre reduced Y730NH2Y-α2 or Y731NH2Y-α2 (15 μM, final concentration) was mixed with Y356F-β2 (15 μM, 1 Y•/β2), and CDP/ATP (1 mM/3 mM, respectively) in assay buffer at 25 °C. The reactions were quenched at 20 s or 2 min by hand in liquid N2. The reaction of Y413NH2Y-α2 and Y356F-β2 was conducted similarly, except the final protein concentration was 50 μM. EPR spectra were recorded at 77 K on a Brüker ESP-300 X-band spectrometer equipped with a quartz finger dewar containing liquid N2 in the Department of Chemistry Instrumentation Facility. EPR parameters were as follows: microwave frequency=9.34 GHz, power=30 μW, modulation amplitude=1.5 G, modulation frequency=100 kHz, time constant=5.12 ms, scan time=41.9 s. EPR spin quantitation was carried out in WinEPR (Brüker) using CuII as a standard (43). EPR subtractions were conducted with an in-house, Excel-based program using the spectrum of Y122• from either wt β2 or a wt-β2:mutant-α2 complex as a reference.
Reaction of Y356F-β2 with NH2Y-α2s monitored by SF UV-vis spectroscopy
SF kinetics were performed on an Applied Photophysics DX 17MV instrument equipped with the Pro-Data upgrade. Pre-reduced Y730NH2Y-α2 (or Y731NH2Y-α2) and ATP were mixed with Y356F-β2 and CDP to yield final concentrations of 8 μM, 3 mM, 8 μM and 1 mM, respectively, in assay buffer at 25 °C. The concentrations of Y122•, NH2Y731• and NH2Y730• were monitored at 410 nm (ε = 3,700 M−1 cm−1), 320 nm (ε = 11,000 M−1cm−1) and 325 nm (ε = 10,500 M−1 cm−1), respectively, using PMT detection. In each experiment, 5–7 traces were averaged and kinetic parameters obtained by curve fitting using OriginPro or Kaleidagraph software. Iterative rounds of fitting were carried out until both the residual plot and the r2 correlation value were optimized.
Reaction of Y731F/Y730NH2Y-α2, Y413NH2Y-α2, and Y731F/Y413NH2Y-α2 with wt β2 monitored by EPR and SF UV-vis spectroscopies
Y731F/Y730NH2Y-α2, Y413NH2Y-α2, or Y731F/Y413NH2Y-α2 was reacted with wt β2 in the presence of CDP/ATP at 25 °C as described for NH2Y-α2s above. For the EPR and SF experiments, α2 and β2 concentrations were 50 μM and 5 μM, respectively. The EPR reactions were hand quenched at 40 s. The EPR spectrum of a putative NH2Y413• was obtained subsequent to subtraction of the spectrum of Y122• (in the wt-β2:mutant-α2 complex) as described above.
RESULTS
Reaction of Y730 NH2Y-α2 (or Y731NH2Y–α2) with Y356F-β2 monitored by EPR and SF spectroscopies
It was previously demonstrated that reaction of either Y730NH2Y-α2 or Y731NH2Y-α2 with wt-β2, CDP, and ATP resulted in the loss of Y122• (λmax = 410 nm, gav of 2.0047) concomitant with formation of a new radical species, assigned as NH2Y• on the basis of its absorbance and EPR spectroscopic features (λmax = 320-325 nm, gav of 2.0043) (15). In this paper, experiments with Y356F-β2, in which the radical propagation pathway is disrupted with a Y to F mutation, were conducted to establish that trapping of NH2Y• at residues 730 and 731 is the result of the participation of these residues in radical transfer, rather than the consequence of introducing a pathway-independent thermodynamic trap into α2. If formation of NH2Y730• or NH2Y731• requires a redox-active Y356 residue, then no NH2Y• should be observed. Reactions were carried out with CDP/ATP, Y356F-β2 and Y730 NH2Y-α2 (or Y731NH2Y–α2) and quenched at 77 K after 20 s and 2 min. Analysis of the EPR spectra, subsequent to subtraction of the spectrum of the resting Y122• at time zero gave no evidence of NH2Y• at either time point for reactions with Y730NH2Y-α2 or with Y731NH2Y–α2 (Figure S1). In addition, spin quantitation of the signal at time zero in comparison to times 20 s and 2 min revealed no significant loss of spin. A control with non tagged Y356F-β2 gave the same result as that observed with His-Y356F-β2, demonstrating that the His-tag does not interfere with radical formation. Oxidation of Y356 is thus a prerequisite for hole migration into the α2 subunit.
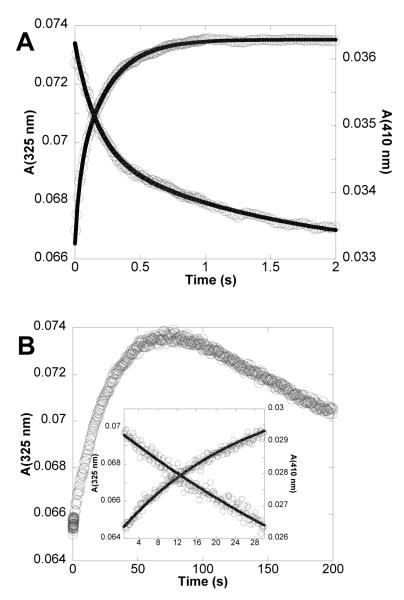
To determine if NH2Y• was formed transiently, the reactions of NH2Y-α2s with Y356F-β2, CDP, and ATP were monitored by SF UV-vis spectroscopy at 325 nm (λmax of NH2Y730•) or 320 nm (λmax of NH2Y731•) and at 410 nm (λmax of Y122•). The results for Y730NH2Y-α2 with Y356F-β2 are shown in Figure 2, and for Y731NH2Y-α2 with Y356F-β2 in Figure S2. A small increase at 325 (320 nm) corresponding to ~2 % of total initial Y122• was observed. However, these spectral changes were not correlated with the expected decrease in absorbance at 410 nm due to loss of the Y•. In fact, a small increase in this wavelength was observed (Figures 2 & Figure S2, inset). The lack of correspondence between the spectral changes at 325 (or 320) nm and 410 nm indicates these features are not related to NH2Y• formation. The nature of these changes is not understood at present, but similar changes have been observed previously under different reaction conditions (37) and may be related to minor structural changes of the Y122• diferric cluster upon α2:β2 complex formation (Seyedsayamdost, Minnihan, and Stubbe, unpublished results). The SF UV-vis and EPR spectroscopic data together indicate that no NH2Y• is formed at residues 730 or 731 with Y356F-β2, with our lower limit of detection approximated as less than 2% (by SF UV-vis) and less than 3% (by EPR) of the total initial Y122•. These data support the conclusion that a redox-active residue at 356 is essential for radical transfer into α2.
FIGURE 2.
SF UV-vis spectroscopy of the reaction of Y356F-β2 with Y730NH2Y-α2 in the presence of CDP/ATP. The reaction was monitored at 325 nm (trace A, λmax of NH2Y730•) and at 410 nm (trace B, λmax of Y122•). Insets show magnified views of the initial 0.5 s of each trace.
Reaction of Y731F/Y730NH2Y-α2 with wt-β2 monitored by EPR and SF spectroscopies
To assess the role of residue Y731 in the radical transfer chain, the double mutant Y731F/Y730NH2Y-α2 was isolated, characterized, and examined for NH2Y• formation. The protein was purified to homogeneity and its specific activity measured to be 36 nmol/min/mg. This low activity, approximately 1.4% that of the wild-type enzyme, is likely associated with co-purifying endogenous α2, rather than activity inherent to the mutant protein. This result is in agreement with the activity of Y731F-α2 (26 nmol/min/mg) and contrasts to activities measured in Y730NH2Y-α2 or Y731NH2Y-α2 (110-150 nmol/min/mg) (15).
Y731F/Y730NH2Y-α2 and ATP were mixed with wt-β2 and CDP and the reaction examined by EPR and SF spectroscopy. With EPR, the reactions were quenched at 40 s or 2 min and analyzed as described above. No NH2Y• formation was detected in either case and the total spin relative to starting Y122• remained unchanged (Figure S3). The SF UV-vis experiments gave results similar to those observed with Y356F-β2 at both 325 nm and 410 nm (Figure S3). Thus formation of NH2Y730• also requires a redox active Y731 for radical propagation.
Expression, purification, and characterization of Y413NH2Y-α2 and Y731F/Y413NH2Y -α2
As an additional test of the pathway dependence of NH2Y• formation at Y730 and Y731, NH2Y was site-specifically incorporated at a Y in α2 that is thought not to participate in the radical transfer pathway (Figure 1). Residue Y413 was selected, as the X-ray structure of E. coli α2 (14) reveals distances of 5.0 Å or 5.2 Å between the phenolic oxygen of Y413 and that of Y730 or Y731, respectively (Figure 3). Thus, Y413 is a distance from Y730 and Y731 that is reasonable for ET between these residues. Y731 and Y730 are separated by 3.3 Å in the same structure. Alignment of 142 primary sequences of class Ia/b α2s reveals that while 413 is not conserved, it is always an aromatic amino acid. Y, F, and W occupy this position 65%, 21%, and 14% of the time, respectively. Thus, the proteins Y413NH2Y-α2 and Y731F/Y413NH2Y-α2 were expressed and purified to be examined for NH2Y• formation.
FIGURE 3.
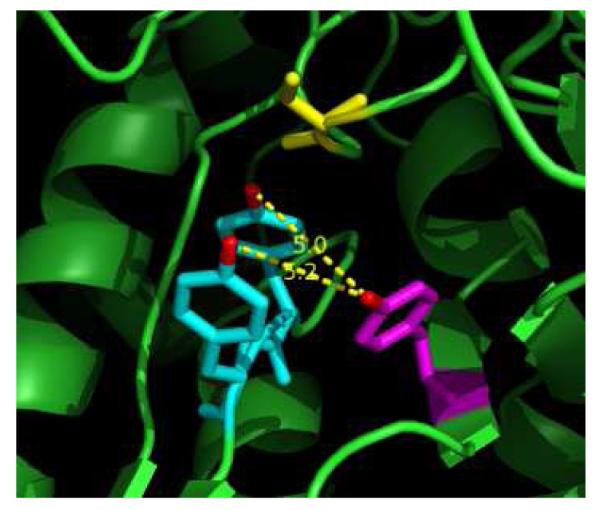
Location of Y413 (magenta) in relation to Y731 (blue), Y730 (blue), and C439 (yellow) of the radical propagation pathway within the α subunit. Y413 is located 5.0 and 5.2 Å from Y730 and Y731, respectively, with distances measured between phenolic oxygens (red). The figure was generated as a PyMol (www.pymol.org) rendition of PDB ID: 1RLR (14).
Y413NH2Y-α2 and Y731F/Y413NH2Y-α2 were expressed in a fashion analogous to other NH2Y-substituted α2s. Their purification, however, proved more difficult than for previous NH2Y-sustituted α2s. Protein of high purity (as judged by SDS-PAGE) was eventually isolated via anion-exchange FPLC with an optimized salt gradient, but at the expense of overall protein yield (Figure S4).
The activities of the purified mutants were determined by the spectrophotometric and/or radioactive RNR assays. The specific activity of Y413NH2Y-α2 was 46% of wt α2 (~1200 nmol/min/mg), while that of Y731F/Y413NH2Y-α2 was 1.9% of wt α2 (~48 nmol/min/mg). The activity of Y731F/Y413NH2Y-α2 is similar to that of Y731F/Y730NH2Y-α2, and is likely associated with endogenous E. coli α2 that co-purified with the recombinantly expressed mutant protein.
The reduction in activity of Y413NH2Y-α2 relative to wt α2, the proximity of Y413 to the putative α2:β2 interface, and the difficulty encountered in purification of the related mutants provided the impetus to determine the Kd for the interaction between this mutant and β2 using a modified version of the competitive inhibition assay developed by Climent et al. (29, 36, 42). Data analysis was modified relative to the previously published method (36) to accommodate for the condition that the active species, rather than the inhibitor, was also the species of unknown Kd (Figure S5). Analysis by this method afforded a Kd of 0.05 μM, similar to the value of 0.06 μM for wt-α2:β2 in the presence of CDP and ATP (Hassan and Stubbe, unpublished results). This finding, in conjunction with the activity of Y413NH2Y-α2, suggests that Y413NH2Y-α2 is folded and that binding between subunits is minimally perturbed. However, attempts to crystallize Y413NH2Y-α2 under conditions optimized for wt-α2 and successfully used to crystallize other NH2Y-α2 mutants failed to provide crystals of Y413NH2Y-α2, suggesting that mutation of Y413 produces some effect on protein stability or solubility (Uhlin and Stubbe, unpublished results).
Reactions of Y413NH2Y-α2 or Y731F/Y413NH2Y-α2 with wt-β2, and Y413NH2Y-α2 with Y356F-β2 monitored by EPR spectroscopy
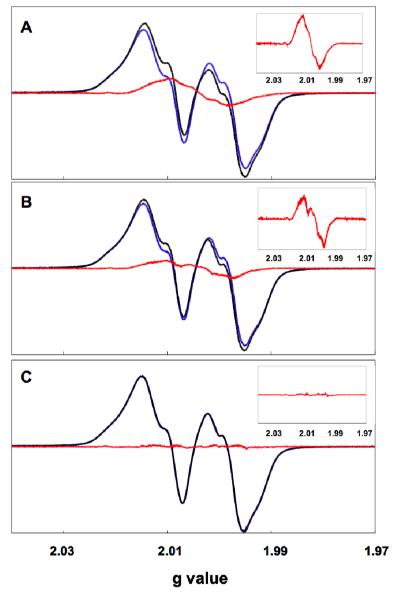
EPR experiments were carried out as described above by mixing Y413NH2Y-α2 (or Y731F/Y413NH2Y-α2) with CDP, ATP and wt-β2, or Y413NH2Y-α2 with CDP, ATP, and Y356F-β2. Each reaction was quenched at 40 s. The resulting spectra, subsequent to subtraction of the resting Y122• in the mutant α2:β2 complex, are shown in Figure 4, and the spin quantitation of the resulting NH2Y• is given in Table 1. In the reactions of Y413NH2Y-α2 and Y731F/Y413NH2Y-α2 with wt-β2 (Figure 4a and 4b, respectively), the difference EPR spectrum reveals features characteristic of an NH2Y•. For the reaction with Y413NH2Y-α2, the signal attributed to NH2Y• accounts for 12% of the total spin. Differences in the spectral features relative to those previously reported NH2Y730• and NH2Y731• cannot be assigned at this stage, but perhaps report on differences in the dihedral angles of the Cβ-protons on NH2Y at position 413 versus those at positions 730 and 731. For the reaction with Y731F/Y413NH2Y-α2, the signal attributed to NH2Y• accounts for 7% of the total spin. The magnitude of the signal in both cases is substantially lower than the “on pathway” radicals, which have been shown by EPR to constitute 53% and 45% of the total spin at the same time point when NH2Y is incorporated at 730 or 731, respectively (15). The difference EPR spectrum from the reaction of Y413NH2Y-α2 with Y356F-β2 with CDP/ATP quenched at 40 s reveals no detectable NH2Y• (Figure 4c).
FIGURE 4.
Formation of NH2Y413• monitored by EPR spectroscopy. In each case, CDP and ATP were mixed at 25 °C with (A) wt β2 and Y413NH2Y-α2, (B) wt β2 and Y731F/Y413NH2Y-α2, and (C) Y356F-β2 and Y413NH2Y-α2 and quenched at 77 K after 40 s. For each reaction, the residual Y122• signal (blue) was subtracted from the composite reaction spectrum (black) to give a putative NH2Y413• (red). The inset gives an expanded view of the difference spectrum, which in the case of (A) and (B) corresponds to the putative NH2Y413•.
TABLE 1.
Characterization of NH2Y• formation in α:β complexes with various on- and off-pathway mutations
| β:α complex | % NH2Y• (EPR)a |
% NH2Y• (SF UV-vis)a |
first phase kobs (s−1) |
second phase kobs (s−1) |
|---|---|---|---|---|
| wt-β2:Y730NH2Y-α2b | 53 | 39 | 12.8 | 2.5 |
| wt-β2:Y731NH2Y-α2b | 45 | 35 | 19.2 | 2.7 |
| Y356F-β2:Y730NH2Y-α2 | <3% c | <2% c | -- | -- |
| Y356F-β2:Y731NH2Y-α2 | <3% | <2% | -- | -- |
| wt-β2:Y731F/Y730NH2Y-α2 | <3% | <2% | -- | -- |
| wt-β2:Y413NH2Y-α2 | 12 | 8 | 4.8d | 0.5 |
| wt-β2:Y731F/Y413NH2Y-α2 | 7 | 12 | 0.04 | -- |
| Y356F-β2:Y413NH2Y-α2 | <3% | NDe | -- | -- |
Y122• converted to NH2Y•, as a percent of the starting [Y122•].
Originally reported in reference 15.
No conversion detected within our lower limit of detection, approximated to be 3% of the total initial Y122• by EPR and 2% by SF UV-vis spectroscopy.
Taken as the average of the rate constants measured at 325 nm and 410 nm.
Not determined.
Reactions of Y413NH2Y-α2 or Y731F/Y413NH2Y-α2 with wt-β2 monitored by SF UV-vis spectroscopy
Given the results of the EPR experiments above, the kinetics of formation of the NH2Y413• were assessed for both Y413NH2Y-α2 and Y731F/Y413NH2Y-α2 using SF UV-vis spectroscopy. The results with Y413NH2Y-α2 and Y731F/Y413NH2Y-α2 are shown in Figure 5a and 5b, respectively, and are summarized in Table 1. For the reaction with Y413NH2Y-α2 monitored at 325 nm, the observed absorbance increase may be fit to biphasic kinetics with rate constants of 26.4 s−1 and 4.2 s−1. These phases correspond to 30% and 70%, respectively, of the total absorbance increase observed at 325 nm over the reaction course. The faster phase is not correlated, however, with a decrease at 410 nm, and thus is not associated with NH2Y• formation.
FIGURE 5.
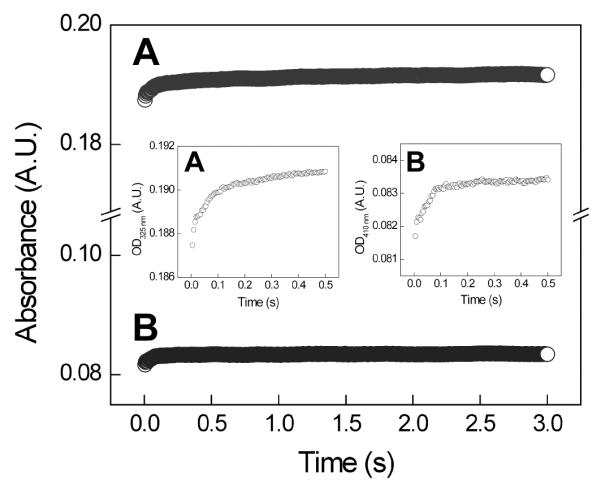
Reaction of wt β2, CDP, ATP and (A) Y413NH2Y-α2 or (B) Y731F/Y413NH2Y-α2 monitored by SF UV-vis spectroscopy. The reaction was monitored for an increase at 325 nm (λmax of NH2Y413•) and a decrease at 410 nm (λmax of Y122•). The reaction with Y413NH2Y-α2 (A) was complete within 2 seconds. The reaction with Y731F/Y413NH2Y-α2 was monitored over 200 s (B), with the first 30 s expanded in the inset. Exponential fits to the data are indicated by solid black lines.
The A410 nm decrease can be fit to biphasic kinetics with rate constants of 5.3 s−1 410 nm and 0.5 s−1, corresponding to 47% and 53% of the total absorbance change at 410 nm during the reaction. The rate constant of 5.3 s−1 agrees well with the rate constant of 4.2 s−1 at 325 nm, and together suggest the formation of NH2Y413•. Using the extinction coefficients of NH2Y• and Y•, the extent of formation of NH2Y413• was determined to be 8% of the starting Y122•. This is substantially lower than the amount of NH2Y• observed in SF UV-vis studies with NH2Y at 730 (39%) and 731 (35%) under analogous reaction conditions (15). While a rate constant of 4-5 s−1 is fast enough to be involved in production of dNDPs, the collective data on the three Y413 mutant complexes suggest NH2Y413• formation is off-pathway and we propose that it is not functionally relevant to catalysis.
For the reaction of Y731F/Y413NH2Y-α2, a slow increase in A325 nm was observed concomitant with a loss of absorbance at 410 nm (Figure 5b). The first 30 s of data collected at both wavelengths were fit to a monoexponential function with a rate constant of 0.04 s−1. The SF data suggest that 12% of the starting Y122• is converted to NH2Y413• over the course of 30 s. The slow rate of radical formation, 320 to 475-fold slower than NH2Y• formation at 730 (13 s−1) or 731 (19 s−1), suggest off-pathway oxidation.
DISCUSSION
The rate and extent of NH2Y• formation in the presence of the β subunit and the S/E pair CDP/ATP (when NH2Y has been site-specifically incorporated into α at residue 730, 731, or 413) may be used as indicators of the participation of a specific residue in a defined pathway for C439 oxidation in RNR (Figure 1). We have previously established that formation of NH2Y• is biphasic, giving rise to rate constants and amplitudes (as a percent of initial Y122•) for NH2Y• formation of 19 s−1(24%) and 2.7 s−1 (11%) at 731, and 13 s−1 (20%) and 2.5 s−1 (19%) at 730 (15). We have analyzed the kinetics of NH2Y• formation with all S/E pairs and argue that the slower rate constant is kinetically competent for deoxynucleotide formation (Seyedsayamdost, Minnihan, and Stubbe, unpublished results). An unexpected observation of our initial studies with the 730- and 731-substituted proteins was the ability of these NH2Y-α2s to catalyze deoxynucleotide formation, suggesting that NH2Y• at either position may be an intermediate on the pathway or may replace Y122• as a radical initiator.
In our current studies, replacement of Y with the non-oxidizable F in the pathway at positions 356 in β or in 731 in β, and incorporation of NH2Y within the pathway after the block, resulted in no detectable NH2Y• by EPR analysis at 20 s or 2 min (Table 1). Since the EPR signal is always a composite of Y• and NH2Y•, a subtraction of the Y• signal is required for spin quantitation of the two species. The broadness of the Y• signal relative to the NH2Y• makes this subtraction straightforward, with the lower limit of detection of an NH2Y• estimated to be 3% of the total spin. It should be noted that cleaner subtractions are achieved when Y122• in an α:β complex is used as the reference, as the hyperfine interactions of Y122• in β appear to be subtly affected by complex formation with α.
To establish that NH2Y• is not generated transiently in the presence of a Y to F mutation, SF UV-vis was conducted and the reaction was monitored at 325 (320) nm and 410 nm. An increase in the former absorption is associated with NH2Y• formation at 730 (731) and a loss of the latter with Y• loss. Monitoring a reaction at 325 nm is complicated by features of the diferric cluster which also absorb in this region. Thus, we have assumed transient features observed by SF are related to NH2Y• formation only if the rate constant and amplitude increase of the 325 nm feature parallels the rate constant and amplitude decrease of the 410 nm feature. As indicated in Figure 2, in all experiments described herein in which the pathway has been “blocked,” a small rise phase (approximately 2% of the Y•) has been observed at 325 nm. This rise is rapid (corresponding to a rate constant >20 s−1), has no corresponding decrease at 410 nm, and is attributed to a small change associated with the diferric Y• cofactor. In the current analysis, this feature is not considered further. Thus, the SF data are in agreement with the EPR results, indicating that a redox-active Y at 356 and 731 is a prerequisite for NH2Y• formation.
As noted above, at pH 7.0 NH2Y is easier to oxidize than Y by 190 mV and thus could potentially be oxidized in an off-pathway process. To test this proposal, studies were conducted in which NH2Y was incorporated in place of a residue proximal to the proposed pathway. Y413 (Figure 3) is located within 5 Å of both Y731 and Y730, a distance feasible for a single oxidation “hop,” and thus became the target of our off-pathway studies. Three experiments were carried out using mutants in which NH2Y was site-specifically incorporated at position 413.
In the first, NH2Y was incorporated at residue 413 while keeping all other on-pathway residues in tact. In two additional experiments, a pathway block was introduced in the form of a Y to F mutation at either 356 or 731. When the single mutant, Y413NH2Y-α2, was reacted with wt-β2, CDP, and ATP, SF experiments revealed formation of NH2Y413• with a rate constant of 4-5 s−1, corresponding to an 8% conversion of the initial Y122• to NH2Y413• (Table 1). EPR analysis of the same reaction after 40 s was in agreement with the SF results, indicating the formation of a NH2Y• species accounting for 12% of the total spin (Figure 4a). The differences in the percent conversion of Y122• to NH2Y413• as measured by SF and EPR may be indicative of the error inherent to the two methods in quantitation of low levels of radical species. Alternately, the differences may arise from the 10-fold difference in protein concentration used in the two experiments (38). As argued above, the rate constant and accumulation of NH2Y• is indicative of the relevance of that specific residue to catalysis in RNR. The rate constant of 4.2 s−1 for NH2Y413• formation is slower by a factor of 4 and the amplitude is reduced by 75% relative to NH2Y• formation at 731. This result suggests that NH2Y at position 413 can be oxidized in an off-pathway fashion by either Y731 or by Y356. With F incorporated at 731 and NH2Y at 413, NH2Y• is still observed. However, a fit of the SF data for the first 30 s gave a rate constant of 0.04 s−1 and an amplitude of 12%. EPR spin quantitation of the same reaction after 40 s indicated 7% of the total spin was associated with the putative NH2Y• (Figure 4b). Finally, with F at 356 and NH2Y at 413, no NH2Y• was detected (Figure 4c). Thus the oxidation at 413 appears to occur predominantly through Y356. The very slow rate constant for NH2Y413• formation with F at 731 (~0.2% that of NH2Y• formation at 731, and ~2% of the steady state-rate constant for dCDP formation) and the complete absence of NH2Y413• with F at 356 suggest that radical formation at 413 in the NH2Y-mutants results from off-pathway ET.
It is experimentally challenging to determine directly whether NH2Y413• can participate as a chemically- and kinetically-competent intermediate in C439 oxidation. However, strong evidence that ET through 413 does not constitute a viable alternate radical pathway comes from the observation that no NH2Y• is generated in the reaction with Y731F/Y730NH2Y-α2. Y413 cannot serve as a bypass to a block at 731, and thus cannot support the oxidation of Y at 730 required for C439• formation and subsequent nucleotide reduction. The NH2Y413• observed in experiments with Y413NH2Y-α2 or Y731F/Y413NH2Y-α2 thus results from an off-pathway oxidation and is nonfunctional.
Many examples of off-pathway oxidation exist in the literature for heme- and non-heme iron-dependent systems. Likewise, evidence for off-pathway oxidation has been reported previously in the class I E. coli RNR (19-21, 41). The phenotypes are usually slow rate constants for radical formation and low amplitudes of new radical species. Thus the experiments presented herein using F and NH2Y as an oxidation block and trap, respectively, support the original proposal of Uhlin and Eklund for long-range oxidation of C439 in α via a conserved pathway of defined aromatic amino acids.
Supplementary Material
ABBREVIATIONS
- α
ribonucleotide reductase large subunit
- ATP
adenosine 5′-triphosphate
- β
ribonucleotide reductase small subunit
- C•
thiyl radical
- CDP
cytidine 5′-diphosphate
- dC
2′-deoxycytidine
- DTT
dithiothreitol
- E
effector
- EPR
electron paramagnetic resonance
- ET
electron transfer
- FPLC
fast protein liquid chromatography
- NH2Y•
3-aminotyrosyl radical
- PCET
proton-coupled electron transfer
- PL
photolyase
- RNR
ribonucleotide reductase
- S
substrate
- SF
stopped-flow
- TR
thioredoxin
- TRR
thioredoxin reductase
- W•
tryptophan radical
- wt
wild-type
- Y•
tyrosyl radical
Footnotes
SUPPORTING INFORMATION AVAILABLE Stopped-flow traces, EPR reaction spectra and corresponding subtractions, and SDS-PAGE of purified Y413NH2Y-α2 are included, as indicated in the article text. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by the NIH grant GM29595 (to J.S.). E.C.M. was supported by a Koch Graduate Fellowship.
REFERENCES
- 1.Gray HB, Winkler JR. Long-range electron transfer. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3534–3539. doi: 10.1073/pnas.0408029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkhovskaya ML, Belevich N, Euro L, Wikstrom M, Verkhovsky MI. Real-time electron transfer in respiratory complex I. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3763–3767. doi: 10.1073/pnas.0711249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Igarashi RY, Seefeldt LC. Nitrogen fixation: the mechanism of the Mo-dependent nitrogenase. Crit. Rev. Biochem. Mol. Biol. 2003;38:351–384. doi: 10.1080/10409230391036766. [DOI] [PubMed] [Google Scholar]
- 4.Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- 5.Page CC, Moser CC, Chen X, Dutton PL. Natural engineering principles of electron tunnelling in biological oxidation-reduction. Nature. 1999;402:47–52. doi: 10.1038/46972. [DOI] [PubMed] [Google Scholar]
- 6.Marcus RA, Sutin N. Nature of biological electron transfer. Biochim. Biophys. Acta. 1985;811:265–322. [Google Scholar]
- 7.Langen R, Chang IJ, Germanas JP, Richards JH, Winkler JR, Gray HB. Electron tunneling in proteins: coupling through a beta strand. Science. 1995;268:1733–1735. doi: 10.1126/science.7792598. [DOI] [PubMed] [Google Scholar]
- 8.Osyczka A, Moser CC, Daldal F, Dutton PL. Reversible redox energy coupling in electron transfer chains. Nature. 2004;427:607–612. doi: 10.1038/nature02242. [DOI] [PubMed] [Google Scholar]
- 9.Xiong P, Nocek JM, Griffin AK, Wang J, Hoffman BM. Electrostatic redesign of the [myoglobin, cytochrome b5] interface to create a well-defined docked complex with rapid interprotein electron transfer. J. Am. Chem. Soc. 2009;131:6938–6939. doi: 10.1021/ja902131d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöberg BM, Reichard P. Nature of the free radical in ribonucleotide reductase from Escherichia coli. J Biol Chem. 1977;252:536–541. [PubMed] [Google Scholar]
- 11.Barry BA, Babcock GT. Tyrosine radicals are involved in the photosynthetic oxygen-evolving system. Proc. Natl. Acad. Sci. U.S.A. 1987;84:7099–7103. doi: 10.1073/pnas.84.20.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stubbe J, Nocera DG, Yee CS, Chang MCY. Radical initiation in the class I ribonucleotide reductase: long-range proton-coupled electron transfer? Chem. Rev. 2003;103:2167–2201. doi: 10.1021/cr020421u. [DOI] [PubMed] [Google Scholar]
- 13.Stubbe J, van der Donk WA. Protein radicals in enzyme catalysis. Chem. Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- 14.Uhlin U, Eklund H. Structure of ribonucleotide reductase protein R1. Nature. 1994;370:533–539. doi: 10.1038/370533a0. [DOI] [PubMed] [Google Scholar]
- 15.Seyedsayamdost MR, Xie J, Chan CT, Schultz PG, Stubbe J. Site-specific insertion of 3-aminotyrosine into subunit α2 of E. coli ribonucleotide reductase: direct evidence for involvement of Y730 and Y731 in radical propagation. J. Am. Chem. Soc. 2007;129:15060–15071. doi: 10.1021/ja076043y. [DOI] [PubMed] [Google Scholar]
- 16.Shih C, Museth AK, Abrahamsson M, Blanco-Rodriguez AM, Di Bilio AJ, Sudhamsu J, Crane BR, Ronayne KL, Towrie M, Vlcek A, Jr., Richards JH, Winkler JR, Gray HB. Tryptophan-accelerated electron flow through proteins. Science. 2008;320:1760–1762. doi: 10.1126/science.1158241. [DOI] [PubMed] [Google Scholar]
- 17.Cordes M, Kottgen A, Jasper C, Jacques O, Boudebous H, Giese B. Influence of amino acid side chains on long-distance electron transfer in peptides: electron hopping via “stepping stones”. Angew. Chem. Int. Ed. Engl. 2008;47:3461–3463. doi: 10.1002/anie.200705588. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharjee S, Deterding LJ, Jiang J, Bonini MG, Tomer KB, Ramirez DC, Mason RP. Electron transfer between a tyrosyl radical and a cysteine residue in hemoproteins: spin trapping analysis. J. Am. Chem. Soc. 2007;129:13493–13501. doi: 10.1021/ja073349w. [DOI] [PubMed] [Google Scholar]
- 19.Lendzian F. Structure and interactions of amino acid radicals in class I ribonucleotide reductase studied by ENDOR and high-field EPR spectroscopy. Biochim. Biophys. Acta. 2005;1707:67–90. doi: 10.1016/j.bbabio.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Sahlin M, Lassmann G, Pötsch S, Slaby A, Sjöberg BM, Gräslund A. Tryptophan radicals formed by iron/oxygen reaction with Escherichia coli ribonucleotide reductase protein R2 mutant Y122F. J. Biol. Chem. 1994;269:11699–11702. [PubMed] [Google Scholar]
- 21.Sahlin M, Lassmann G, Pötsch S, Sjöberg BM, Gräslund A. Transient free radicals in iron/oxygen reconstitution of mutant protein R2 Y122F. Possible participants in electron transfer chains in ribonucleotide reductase. J. Biol. Chem. 1995;270:12361–12372. doi: 10.1074/jbc.270.21.12361. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury K, Sundaramoorthy M, Hickman A, Yonetani T, Woehl E, Dunn MF, Poulos TL. Role of the proximal ligand in peroxidase catalysis. Crystallographic, kinetic, and spectral studies of cytochrome c peroxidase proximal ligand mutants. J. Biol. Chem. 1994;269:20239–20249. [PubMed] [Google Scholar]
- 23.Karthein R, Dietz R, Nastainczyk W, Ruf HH. Higher oxidation states of prostaglandin H synthase. EPR study of a transient tyrosyl radical in the enzyme during the peroxidase reaction. Eur. J. Biochem. 1988;171:313–320. doi: 10.1111/j.1432-1033.1988.tb13792.x. [DOI] [PubMed] [Google Scholar]
- 24.Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 2003;103:2203–2237. doi: 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- 25.Aubert C, Vos MH, Mathis P, Eker AP, Brettel K. Intraprotein radical transfer during photoactivation of DNA photolyase. Nature. 2000;405:586–590. doi: 10.1038/35014644. [DOI] [PubMed] [Google Scholar]
- 26.Byrdin M, Eker AP, Vos MH, Brettel K. Dissection of the triple tryptophan electron transfer chain in Escherichia coli DNA photolyase: Trp382 is the primary donor in photoactivation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8676–8681. doi: 10.1073/pnas.1531645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukacs A, Eker AP, Byrdin M, Villette S, Pan J, Brettel K, Vos MH. Role of the middle residue in the triple tryptophan electron transfer chain of DNA photolyase: ultrafast spectroscopy of a Trp-->Phe mutant. J. Phys. Chem. B. 2006;110:15654–15658. doi: 10.1021/jp063686b. [DOI] [PubMed] [Google Scholar]
- 28.Lukacs A, Eker AP, Byrdin M, Brettel K, Vos MH. Electron hopping through the 15 Å triple tryptophan molecular wire in DNA photolyase occurs within 30 ps. J. Am. Chem. Soc. 2008;130:14394–14395. doi: 10.1021/ja805261m. [DOI] [PubMed] [Google Scholar]
- 29.Climent I, Sjöberg BM, Huang CY. Site-directed mutagenesis and deletion of the carboxyl terminus of Escherichia coli ribonucleotide reductase protein R2. Effects on catalytic activity and subunit interaction. Biochemistry. 1992;31:4801–4807. doi: 10.1021/bi00135a009. [DOI] [PubMed] [Google Scholar]
- 30.Ekberg M, Sahlin M, Eriksson M, Sjöberg B-M. Two conserved tyrosine residues in protein R1 participate in an intermolecular electron transfer in ribonucleotide reductase. J. Biol. Chem. 1996;271:20655–20659. doi: 10.1074/jbc.271.34.20655. [DOI] [PubMed] [Google Scholar]
- 31.Rova U, Goodtzova K, Ingemarson R, Behravan G, Gräslund A, Thelander L. Evidence by site-directed mutagenesis supports long-range electron transfer in mouse ribonucleotide reductase. Biochemistry. 1995;34:4267–4275. doi: 10.1021/bi00013a016. [DOI] [PubMed] [Google Scholar]
- 32.Rova U, Adrait A, Pötsch S, Gräslund A, Thelander L. Evidence by mutagenesis that Tyr(370) of the mouse ribonucleotide reductase R2 protein is the connecting link in the intersubunit radical transfer pathway. J. Biol. Chem. 1999;274:23746–23751. doi: 10.1074/jbc.274.34.23746. [DOI] [PubMed] [Google Scholar]
- 33.Seyedsayamdost MR, Yee CS, Reece SY, Nocera DG, Stubbe J. pH rate profiles of FnY356-R2s ( n = 2, 3, 4) in Escherichia coli ribonucleotide reductase: evidence that Y356 is a redox-active amino acid along the radical propagation pathway. J. Am. Chem. Soc. 2006;128:1562–1568. doi: 10.1021/ja055927j. [DOI] [PubMed] [Google Scholar]
- 34.Seyedsayamdost MR, Stubbe J. Site-specific replacement of Y356 with 3,4-dihydroxyphenylalanine in the β2 subunit of E. coli ribonucleotide reductase. J. Am. Chem. Soc. 2006;128:2522–2523. doi: 10.1021/ja057776q. [DOI] [PubMed] [Google Scholar]
- 35.Seyedsayamdost MR, Chan CT, Mugnaini V, Stubbe J, Bennati M. PELDOR spectroscopy with DOPA-β2 and NH2Y-α2s: distance measurements between residues involved in the radical propagation pathway of E. coli ribonucleotide reductase. J. Am. Chem. Soc. 2007;129:15748–15749. doi: 10.1021/ja076459b. [DOI] [PubMed] [Google Scholar]
- 36.Seyedsayamdost MR, Stubbe J. Forward and reverse electron transfer with the Y356DOPA-β2 heterodimer of E. coli ribonucleotide reductase. J. Am. Chem. Soc. 2007;129:2226–2227. doi: 10.1021/ja0685607. [DOI] [PubMed] [Google Scholar]
- 37.Seyedsayamdost MR. Ph.D. Thesis. Massachusetts Institute of Technology; Cambridge, MA: 2007. [Google Scholar]
- 38.Ge J, Yu G, Ator MA, Stubbe J. Pre-steady-state and steady-state kinetic analysis of E. coli class I ribonucleotide reductase. Biochemistry. 2003;42:10071–10083. doi: 10.1021/bi034374r. [DOI] [PubMed] [Google Scholar]
- 39.Reece SY, Seyedsayamdost MR, Stubbe J, Nocera DG. Photoactive peptides for light-initiated tyrosyl radical generation and transport into ribonucleotide reductase. J. Am. Chem. Soc. 2007;129:8500–8509. doi: 10.1021/ja0704434. [DOI] [PubMed] [Google Scholar]
- 40.Reece SY, Seyedsayamdost MR, Stubbe J, Nocera DG. Direct observation of a transient tyrosine radical competent for initiating turnover in a photochemical ribonucleotide reductase. J. Am. Chem. Soc. 2007;129:13828–13830. doi: 10.1021/ja074452o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yee CS, Seyedsayamdost MR, Chang MCY, Nocera DG, Stubbe J. Generation of the R2 subunit of ribonucleotide reductase by intein chemistry: insertion of 3-nitrotyrosine at residue 356 as a probe of the radical initiation process. Biochemistry. 2003;42:14541–14552. doi: 10.1021/bi0352365. [DOI] [PubMed] [Google Scholar]
- 42.Climent I, Sjöberg BM, Huang CY. Carboxyl-terminal peptides as probes for Escherichia coli ribonucleotide reductase subunit interaction: kinetic analysis of inhibition studies. Biochemistry. 1991;30:5164–5171. doi: 10.1021/bi00235a008. [DOI] [PubMed] [Google Scholar]
- 43.Palmer G. Electron paramagnetic resonance. Methods Enymol. 1967;10:595–610. [Google Scholar]
- 44.Nordlund P, Sjöberg BM, Eklund H. Three-dimensional structure of the free radical protein of ribonucleotide reductase. Nature. 1990;345:593–598. doi: 10.1038/345593a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.