Abstract
These are exciting times for the biomedical sciences, in general, and, in particular, for those who strive to understand the origins of complex human diseases, as we begin to focus with increasing precision on disease mechanisms at the cellular and molecular levels. Armed with the high-through-put technologies of the Post-Genomic Era, we now face the challenge of understanding biological systems at the level of their complex integration, and this will truly bring meaning to the concept of Systems Biology.
This commentary is intended to provide an overview of the evolution of thought concerning the function and dysfunction of the vascular endothelium, with a particular focus on the central role(s) of hemodynamic forces as pathophysiologic stimuli in complex cardiovascular diseases such as atherosclerosis. Starting with the observation that the earliest lesions of atherosclerosis (in human subjects and various animal models) show a predilection for certain vascular geometries, the roles of distinct biomechanical forces (e.g., laminar versus disturbed flows) as stimuli for human endothelial gene expression have been defined, utilizing a combination of bioengineering technologies and genome-wide transcriptional profiling. This interdisciplinary experimental approach has revealed the coordinated regulation of genetic programs in the human endothelial cell that is involved in “vasoprotection” (e.g., resistance to atherogenic risk factors), and appears to be under the control of specific transcription factors. I hypothesize that the latter represents “critical regulatory nodes” in the homeostatic network of vascular endothelium, which are important in the maintenance of normal cardiovascular function. Better understanding of the stimuli and consequences of endothelial dysfunction, hopefully, will point the way to earlier diagnosis, more effective therapies, and ultimately the prevention of cardiovascular disease.
Non-adaptive interactions of cellular and macromolecular components of circulating blood with the arterial wall play an important role in the pathogenesis of atherosclerosis and coronary thrombosis. Increasing evidence indicates that alterations in the functional properties of the vascular endothelial lining—endothelial dysfunction—may underlie certain of these pathophysiologic interactions and thus contribute to the initiation, progression and clinical complications of atherosclerotic vascular disease. Although involvement of endothelium in the atherosclerotic process has been recognized since the time of Virchow (in the mid-1880's) (1), many relevant aspects of its biology and pathobiology have been recognized only recently. Indeed, a comprehensive monograph published in 1954, entitled “Endothelium: Its Development, Morphology, Function and Pathology” by Dr. Rudolf Altschul, Professor of Histology at the University of Saskatchewan, was a meager 124 pages in length (excluding the Bibliography and Index), and devoted only 20 pages to “Functions of the Endothelium” (2). In contrast, in the calendar year 2009, more than 3,300 publications dealing with vascular endothelium appeared in print (3).
We now appreciate that vascular endothelium, the single-cell thick lining of the circulatory system, is in fact a vital organ, whose health is essential to normal cardiovascular physiology and whose dysfunction can be a critical factor in the pathogenesis of cardiovascular disease. It has been my laboratory's working concept that the vascular endothelium is a dynamically mutable interface, whose structural and unctional properties are responsive to a variety of stimuli, both local and systemic, and further, that its phenotypic modulation to a dysfunctional state can constitute a pathogenic risk factor for vascular diseases. In the arterial wall, certain consequences of endothelial dysfunction are directly related to the pathogenesis of atherosclerosis and its complications (4). These consequences include altered vascular reactivity and vasospasm, altered intimal permeability to lipoproteins, enhanced mononuclear leukocyte recruitment and intimal accumulation as foam cells, altered vascular cell growth regulation and survival (e.g., decreased endothelial regeneration, increased smooth muscle proliferation, enhanced susceptibility to apoptosis), and altered hemostatic/fibrinolytic balances (favoring thrombin generation, and platelet and fibrin deposition). Pathophysiologic stimuli of arterial endothelial dysfunction that are especially relevant to atherogenesis include activation by cytokines and bacterial products, infection by bacteria, viruses and other pathogens, accumulation of advanced glycation end-products (AGEs), non-enzymatically generated in diabetes and aging, chronic exposure to hypercholesterolemia and/or hyperhomocysteinemia, and deposition of oxidized lipoproteins and their components (e.g., lysophosphatidylcholine) within the vessel wall (4–6). In addition to these biochemical stimuli of endothelial dysfunction, it has become increasingly clear that distinct biomechanical forces generated by the pulsatile flow of blood through the branched arterial vasculature can also influence the structure and function of endothelial cells, in particular through the regulation of fundamental genetic programs involved in vascular homeostasis (7, 8).
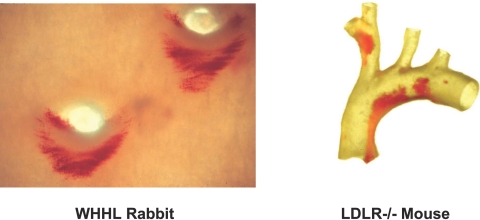
The possibility that hemodynamic forces can act directly as pathophysiologic stimuli for endothelial dysfunction provides a conceptual rationale for the long-standing observation that the earliest lesions of atherosclerosis, in human subjects and various animal models, characteristically develop in a non-random pattern, the geometry of which correlates with branch-points and other regions of altered blood flow (Figure 1) (9–11). The following sections outline studies conducted by my research group that focused on the molecular mechanisms involved in the regulation of endothelial gene expression by biomechanical forces, and highlight the new insights they have provided into vascular homeostasis, in general, and specifically, endothelial dysfunction in the context of atherogenesis.
Fig. 1.
Non-random Distribution of Early Lesions of Atherosclerosis Corresponding to Vascular Geometries Associated with Disturbed Flow. Aortas from two experimental animal models of atherosclerosis, stained with Oil-red O, exhibit intimal lipid accumulation in regions associated with disturbed blood flows. Left panel, Watanabe Heritable Hyperlipidemic (WHHL) Rabbit: en face view of thoracic aorta showing outflow tracts of two intercostal arteries; Right panel, LDL-Receptor-deficient Mouse: transilluminated view of aortic arch and major branches. (Courtesy of Dr. Myron Cybulsky).
HEMODYNAMICS AND VESSEL WALL PATHOBIOLOGY
The pulsatile flow of blood through the branched tubular array of the arterial vasculature generates various types of hemodynamic forces—wall shear stresses, hydrostatic pressures and cyclic strains—that can impact vessel wall biology and pathobiology. As the cellular layer in direct contact with the flowing blood, the endothelium, in particular, bears the frictional forces (wall shear stresses) imparted by the flow of this viscous fluid. Blood flow patterns can vary in complexity from the relatively uniform (time-averaged), well-developed laminar flow that is associated with the unbranched tubular portions of medium-sized muscular arteries, to the complex disturbed flow patterns, involving flow separation, recirculation and reattachment, that generate significant temporal and spatial gradients of wall shear stresses over relatively short distances. Interestingly, the latter disturbed flows typically occur near branch points, bifurcations and major curvatures- arterial geometries typically associated with the early appearance and subsequent progression of atherosclerotic lesions (Figure 1).
A number of in vivo observations suggest that the forces generated by blood flow can alter endothelial structure function. These include the demonstration of increased macromolecular permeability and lipoprotein accumulation in the intima, endothelial cell damage and repair, inducible endothelial-leukocyte adhesion molecule expression (in particular, VCAM-1 or the “athero-ELAM), and mononuclear leukocyte recruitment near branch points and bifurcations, as well as the occurrence of ellipsoidal endothelial cell shape and axial alignment with the primary flow vector in laminar flow regions, and the disruption of this orderly pattern in regions of disturbed flow (5, 11–13). Evidence of the direct action of hemodynamic forces, and in particular wall shear stresses, on endothelial cell structure and function has come primarily from in vitro studies, in which cultured monolayers of human and animal vascular endothelial cells have been subjected to defined fluid mechanical stimulation under well controlled experimental conditions. Utilizing a modified cone and plate viscometer in the early 1980's (14), my group (in collaboration with the Fluid Mechanical Laboratory at the Massachusetts institute of Technology) demonstrated that unidirectional steady laminar shear stresses could induce time- and force-dependent changes in cell shape and alignment in cultured endothelial monolayers, which were reversible upon the cessation of flow (15, 16). These shear-induced changes were accompanied by reorganization of different components of the endothelial cytoskeleton mimicking the morphologic architecture of arterial endothelial cells observed in vivo. Further studies by my group and several others went on to document a variety of changes in the metabolic and synthetic activities of endothelial cells in response to biomechanical stimulation, including the production of prostacyclin, nitric oxide, growth factors (including the platelet-derived growth factor, PDGF), adhesion molecules (including ICAM-1), coagulation and fibrinolytic factors, extracellular matrix components, and vasoactive mediators (17, 18). Some of the more acute changes involved the regulation of rate-limiting enzymes via signaling pathways and/or substrate availability; however, in the case of more delayed, sustained responses, de novo protein synthesis appeared to be modulated at the level of gene transcription. Using the promoter of the human PDGF-B gene as a model, studies by my group defined the first “shear-stress-response element (SSRE)”, a 6 base-pair core binding sequence, GAGACC, which appeared to be necessary and sufficient for the shear stress inducibility of the PDGF-B gene in human endothelial cells (19). This work established a new paradigm for “activation” of endothelial gene expression, in which biomechanical forces per se could act as both positive and negative regulators of endothelial gene transcription and ultimately impact vessel wall (patho)biology. The biomechanical regulation of genes in endothelial cells (and other cell types) is now a widely accepted phenomenon, and the involvement of multiple transcription factors (e.g., NF-kB, Egr-1, c-jun, c-fos, KLF-2) has been documented in vitro and in vivo (20–24).
HEMODYNAMICS AS A “LOCAL RISK FACTOR” FOR ATHEROGENESIS
Given the well-established observation that uniform laminar shear stresses are characteristically associated with atherosclerotic lesion-protected geometries in vivo, we initially turned to high-through-put molecular biological strategies (e.g., differential display of expressed transcripts using RT-PCR) to compare the patterns of endothelial genes that are either upregulated or downregulated in cultured human endothelial cells in response to physiological levels of steady laminar shear stress, a comparable level of turbulent (non-laminar) shear stress, and a soluble, proinflammatory cytokine stimulus (Interleukin-1 beta) (25). This approach revealed distinctive patterns of endothelial gene expression not previously appreciated, including a set of genes that appears to be upregulated in a sustained fashion by steady laminar shear stress, but not by turbulent shear stress. Certain of these differentially regulated transcripts encode endothelial genes with known relevance to atherogenesis, such as eNOS (the endothelial isoform of nitric oxide synthase), COX-2 (the inducible isoform of cyclooxygenase), and Mn-SOD (manganese-dependent superoxide dismutase). These endothelial enzymes exert potent anti-thrombotic, anti-adhesive, anti-inflammatory and anti-oxidant effects, both within the endothelial lining and in interacting cells, such as platelets, leukocytes, and vascular smooth muscle. The biological consequences of these steady laminar shear upregulated endothelial genes, thus, would be predicted to be vasoprotective or anti-atherogenic (26).
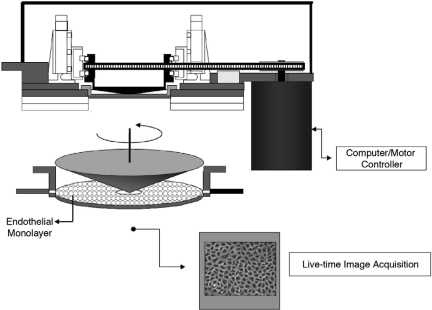
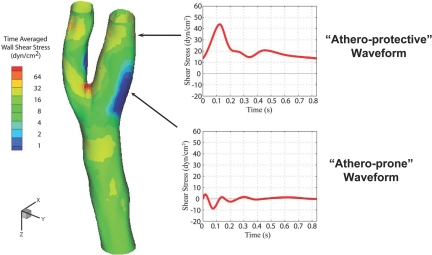
To further explore the implications of this biomechanical, “athero-protective gene” hypothesis, we modified our experimental strategy in two important ways. First, we developed a high-through-put cDNA microarray platform (and associated database management and statistical analytic tools) to perform an unbiased, global assessment of endothelial gene regulation (27–29); and second, we further refined the basic cone and plate viscometer apparatus to create a “Dynamic Flow System” which was capable of simulating actual human arterial waveforms on cultured human endothelial monolayers (Figure 2) (30). We then performed finite element analysis of the distinct flow patterns within various regions of the human carotid bifurcation to accurately define the near-wall shear stresses present in the carotid sinus, an “atherosclerosis-prone” area, and the distal internal carotid artery, an “atherosclerosis-protected” area (Figure 3) (31). The corresponding “athero-prone” and “athero-protective” flows were recreated utilizing the Dynamic Flow Device, and the resultant patterns of endothelial gene expression analyzed. The results of these experiments revealed a dramatic pattern of differential gene regulation by these two pathophysiological relevant biomechanical stimuli (31). Interestingly, whereas the absolute number of affected genes was surprisingly small (less than 200 of the more than several thousand represented on the DNA-microarrays), the scope of their pathophysiologic implications was very broad, encompassing genes involved in signal transduction, transcriptional regulation, inflammation, angiogenesis, growth regulation, coagulation and lipid metabolism. Some interesting patterns emerged: a) Certain chemokines and chemokine receptors, including IL-8, CXCR4 and TTX3 were transcriptionally upregulated by the athero-prone waveform stimulation, suggesting the acquisition of a proinflammatory endothelial phenotype; 2) Chronic exposure to the athero-prone waveform resulted in a sustained secretion of the proatherogenic cytokine IL-8, whereas athero-protective stimulation suppressed endothelial IL-8 production; 3) Preconditioning endothelial monolayers with 24 hours of exposure to the athero-protective waveform prevented the subsequent induction, by cytokine stimulation, of the atherosclerosis-related adhesion molecule VCAM-1, while athero-prone preconditioning failed to silence VCAM-1 expression; 4) Stimulation with the athero-prone waveform, but not the athero-protective waveform, resulted in activation and nuclear-translocation of the NF-kB, a transcription factor closely linked to the atherogenic process in vivo (31). Taken together, these observations strongly suggest that an organized program of genetic regulation is differentially induced in vascular endothelium in response to biomechanical stimulation, and this may contribute to regional variations in atherosclerosis-susceptibility in vivo.
Fig. 2.
Schematic Diagram of Dynamic Flow System. This cone-and-plate fluid mechanical apparatus incorporates an optically transparent cone rotating over a cultured monolayer of endothelial cells, thus allowing direct microscopic visualization during the application of precisely controlled, computer-simulated arterial waveforms (see Blackman et al., 2002) (30).
Fig. 3.
Wall Shear Stresses in Regions of the Human Carotid Artery Bifurcation Relatively Resistant or Susceptible to Atherosclerosis. This color-coded model of the human common carotid artery bifurcation displays the pattern of time-averaged wall shear stress magnitudes, calculated from noninvasive MRI and ultrasound flow measurements in normal subjects (see Dai et al., 2004 (31) for details of fluid mechanical calculations). Prototypic “athero-protective” and “athero-prone” waveforms representative of those present in the distal internal carotid artery (a lesion-resistant region) and the carotid sinus (a lesion-susceptible region), respectively, were then derived for use as input stimuli in the Dynamic Flow System (see Figure 2).
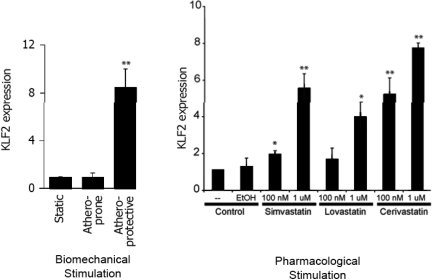
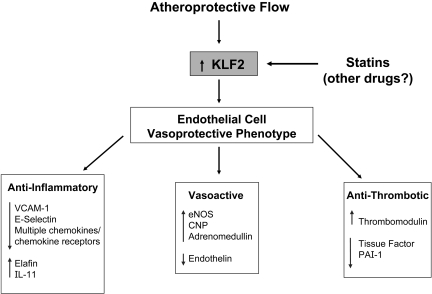
We undertook further analysis of the large body of transcriptional profiling data generated from the above and subsequent studies in an effort to identify the key transcription factors involved in orchestrating the biomechanical responsiveness of human endothelial cells. One of the transcription factors most robustly upregulated selectively by athero-protective waveform stimulation was the Kruppel-like factor 2 (KLF2). Originally, one of a small number of genes reported to be upregulated in cultured endothelial cells by prolonged (7 day) exposure to steady laminar shear stress (32), the in vivo pattern of KLF2 expression in blood vessels is correlated with atherosclerois-protected versus atherosclerosis-susceptible geometries, thus, suggesting a relationship to atherogenesis (33). In our in vitro gene profiling experiments, KLF2 consistently showed a differential regulation by athero-protective waveform stimulation in cultured human endothelial cells (Figure 4) (34). Interestingly, its developmental expression in the zebra fish is dependent on a beating heart and corresponds to the onset of blood flow (35). Further, in vitro studies revealed involvement of the MEK5/ERK5/MEF pathway in linking biomechanical stimulation to KLF2 expression in human endothelial cells. Using a “systems biology” approach, KLF2 has been implicated as a central integrator of various programs of endothelial gene expression governing the “athero-protective phenotype” (Figure 5) (35, 36). Indeed, silencing KLF2 in cultured human endothelial cells results in a loss of their athero-protective flow-induced anti-inflammatory properties and resistance to oxidative stress. A major insight into the potential role of KLF2 as a “master regulator” of endothelial vasoprotection came from the demonstration that its expression in human endothelial cells is upregulated in a dose-dependent fashion by Statins, the most commonly prescribed class of lipid-lowering drugs. This appears to be a class-generalized, endothelial-directed action of the statins that is evident at pharmacologically relevant doses in vitro (Figure 4) (34, 37), and is independent of their intended effects on lowering plasma lipids in vivo. The dependence of many statin-dependent transcription effects in endothelium on KLF2 implicates this transcription factor in the well-recognized, but poorly understood “pleiotropic” beneficial cardiovascular effects of this class of drugs, thus adding a further dimension to our understanding of the “atheroprotective phenotype” of vascular endothelium.
Fig. 4.
Dual Regulation of Transcription Factor Kruppel-like Factor 2 (KLF2) in Cultured Endothelium. The expression of the transcription factor KLF2 in cultured human endothelial cells is selectively regulated by athero-protective biomechanical stimulation and also by treatment with pharmacologically relevant concentrations of various Statins, in a dose-dependent fashion (original data adapted from Parmar et al., 2005) (34).
Fig. 5.
Coordinated Regulation of Endothelial Atheroprotective Phenotype. This diagram depicts the proposed central role of the transcription factor KLF2 in the coordinated regulation of multiple endothelial-genetic programs that contribute to a “vasoprotective phenotype” (see Parmar et al., 2006) (35). In the physiological setting, the biomechanical stimuli present in atherosclerosis-resistant vascular geometries would serve to maintain local endothelial KLF2 expression; alternatively treatment with Statins could act to upregulate KLF2 in lesion-prone areas or augment its physiologic regulation in the face of atherosclerotic risk factors.
CONCLUSIONS AND FUTURE DIRECTIONS
The vascular endothelial lining of the circulatory system comprises a vital interface whose functional properties are dynamically modulated by humoral and biomechanical stimuli. The localization of atherosclerotic lesions to arterial geometries associated with disturbed flow patterns suggests an important role for local hemodynamic forces as “risk factors” in atherogenesis. Mechanistic insights linking biomechanical stimulation to patterns of gene regulation in vascular endothelium have begun to reveal intrinsic, homeostatic programs that support “vasoprotection”. The identification of “critical regulatory nodes” in these networks may point the way to novel therapeutic interventions in cardiovascular health and disease.
ACKNOWLEDGEMENTS
The author wishes to acknowledge the close collaboration of his colleague, Dr. Guillermo Garcia-Cardena, in the studies described here. I also thank the past and present members of the Gimbrone and Garcia-Cardena Laboratories, in the Center for Excellence in Vascular Biology at the Brigham and Women's Hospital, for their contributions, especially Jamie Topper, Guohao Dai, Brett Blackman, Kush Palmar, Jason Comander, Yvonne Ou, Yuzhi Zhang, Eric Wang, Jeanne-Marie Keily, Kay Case, Keith Anderson and George Stavrakis. In addition, the longstanding interdisciplinary collaboration of Prof. C.F. Dewey and Roger Kamm, and their colleagues in the Fluid Mechanics Laboratory at Massachusetts Institute of Technology, is much appreciated. The original research described here was supported primarily by grants from the National Heart, Lung and Blood Institute.
Footnotes
Potential Conflicts of Interest: None disclosed
DISCUSSION
Alexander, Atlanta: Michael, thank you for a spectacular lecture, and I must say, it is scintillating to see the progress that has been made over the years, starting from basic concepts. I can remember early conversations with you to the effect that an objective of cardiovascular therapy would be to make every endothelial cell “think” that it is in a protected laminar shear area, and now it seems as if you are doing just that.
Quesenberry, Providence: Just a general question about an area in which we have been working that involves the endothelium more and more, and that is ectosomal microvesicle transfer of phenotype. This process potentially adds a whole layer of complexity to vascular biology. I would be interested if you have any comments.
Gimbrone, Boston: Cells certainly do communicate in wondrous ways—including the transfer of surface properties. We see that in the context of platelet-endothelial interactions—the age old premise of whether endothelial cells are “nurtured” by platelets (Gimbrone et al., Nature, 1969) and, indeed, how that might be accomplished— perhaps through the secretion of a humoral factor, or, alternatively, the transfer of small pieces of their membranes, via microvesicles, as they make their way through the myriad vessels of the body. Whether this process occurs to different degrees in the context of different biomechanical environments remains an open question.
REFERENCES
- 1.Virchow R. Der ateromatose Prozess der Arterien. Wien Med Wochenshr. 1856;6:825–41. [Google Scholar]
- 2.Altschul R. New York: The Macmillan Co.; 1954. Endothelium: Its Development, Morphology, Function and Pathology. [Google Scholar]
- 3.Pubmed. National Institute of Health; 2009. U.S. National Library of Medicine. [Google Scholar]
- 4.Gimbrone MA J. Vascular Endothelium in Health and Disease. In: Haber E., editor. Molecular Cardiovascular Medicine. New York: Scientific American Medicine; 1995. pp. 49–61. [Google Scholar]
- 5.Gimbrone MA, Topper JN. Biology of the Vessel Wall: Endothelium. In: Chien K, et al., editors. Molecular Basis of Cardiovascular Disease Part V Vascular Biology & Atherogenesis. Philadelphia: W.B. Saunders; 1998. pp. 331–48. [Google Scholar]
- 6.Ross R. Atherosclerosis-an inflammatory disease. N Eng J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Gimbrone MA, Jr, Nagel T, Topper JN. Biomechanical activation: an emerging paradigm in endothelial adhesion biology. J Clin Invest. 1997;99:1809–13. doi: 10.1172/JCI119346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 9.Natural history of aortic and coronary atherosclerotic lesions in youth. Findings from the PDAY Study. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb. 1993;13:1291–8. doi: 10.1161/01.atv.13.9.1291. [DOI] [PubMed] [Google Scholar]
- 10.Karino T. Microscopic structure of disturbed flows in the arterial and venous systems, and its implication in the localization of vascular diseases. Int Angiol. 1986;5:297–313. [PubMed] [Google Scholar]
- 11.Giddens DP, Zarins CK, Glagov S. The role of fluid mechanics in the localization and detection of atherosclerosis. J Biomech Eng. 1993;115:588–94. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- 12.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–91. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 13.Nerem RM, Levesque MJ, Cornhill JF. Vascular endothelial morphology as an indicator of the pattern of blood flow. Journal of Biomech Eng. 1981;103:172–6. doi: 10.1115/1.3138275. [DOI] [PubMed] [Google Scholar]
- 14.Bussolari SR, Dewey CF, Jr, Gimbrone MA., Jr Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instr. 1982;53:1851–4. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- 15.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. Journal of biomechanical engineering. 1981;103(3):177–85. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 16.Remuzzi A, Dewey CF, Jr, Davies PF, Gimbrone MA., Jr Orientation of endothelial cells in shear fields in vitro. Biorheology. 1984;21:617–30. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- 17.Davies PF. Flow-mediated endothelial mechanotransduction. Phys Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr, Gimbrone MA., Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94:885–91. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Jr, Gimbron MA., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993;90:4591–5. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh HJ, Li NQ, Frangos JA. Pulsatile and steady flow induces c-fos expression in human endothelial cells. J Cell Phys. 1993;154:143–51. doi: 10.1002/jcp.1041540118. [DOI] [PubMed] [Google Scholar]
- 21.Khachigian LM, Anderson KR, Halnon NJ, Gimbrone MA, Jr, Resnick N, Collins T. Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A-chain promoter. Arterioscler Thromb Vasc Biol. 1997;17:2280–6. doi: 10.1161/01.atv.17.10.2280. [DOI] [PubMed] [Google Scholar]
- 22.Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–75. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korenaga R, Ando J, Kosaki K, Isshiki M, Takada Y, Kamiya A. Negative transcriptional regulation of the VCAM-1 gene by fluid shear stress in murine endothelial cells. Am J Physiol. 1997;273:C1506–15. doi: 10.1152/ajpcell.1997.273.5.c1506. [DOI] [PubMed] [Google Scholar]
- 24.Malek AM, Izumo S. Molecular aspects of signal transduction of shear stress in the endothelial cell. J Hypertension. 1994;12:989–99. [PubMed] [Google Scholar]
- 25.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93(19):10417–22. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topper JN, Gimbrone MA., Jr Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Molec Medicine Today. 1999;5:40–6. doi: 10.1016/s1357-4310(98)01372-0. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98(8):4478–85. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comander J, Weber GM, Gimbrone MA, Jr, Garcia-Cardena G. Argus-a new database system for Web-based analysis of multiple microarray data sets. Genome Res. 2001;11:1603–10. doi: 10.1101/gr.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comander J, Natarajan S, Gimbrone MA, Jr, Garcia-Cardena G. Improving the statistical detection of regulated genes from microarray data using intensity-based variance estimation. BMC Genomics. 2004;5:17. doi: 10.1186/1471-2164-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackman BR, Garcia-Cardena G, Gimbrone MA., Jr A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J Biomech Eng. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 31.Dai G, Kaazempur-Mofrad MR, Natarajan S, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–6. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dekker RJ, van Soest S, Fontijn RD, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–98. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 33.Dekker RJ, van Thienen JV, Rohlena J, et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–18. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–9. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 35.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen Banerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–15. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen-Banerjee S, Mir S, Lin Z, et al. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–6. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]