Abstract
Recent studies suggest that the heart possesses an intrinsic system that is intended to delimit tissue injury, as well as orchestrate homoeostatic responses within the heart. The extant literature suggests that this intrinsic stress response is mediated, at least in part, by a family of pattern recognition receptors that belong to the innate immune system, including CD14, the soluble pattern recognition receptor for lipopolysaccharide, and Toll like receptors-2, 3, 4, and 6. Although this intrinsic stress response system provides a short-term adaptive response to tissue injury, the beneficial effects of this phylogenetically ancient system may be lost if myocardial expression of these molecules either becomes sustained and/or excessive, in which case the salutary effects of activation of these pathways may be contravened by the known deleterious effects of inflammatory signaling. Herein we present new information with regard to activation of innate immune gene expression in the failing human heart. Taken together, these new observations provide provisional evidence that the innate immune system is activated in human heart failure, raising the interesting possibility that this pathway may represent a target for the development of novel heart failure therapeutics.
OVERVIEW OF INNATE IMMUNITY
The adult mammalian myocardium responds to tissue injury by synthesizing an ensemble of proteins that allow the heart to delimit cell injury through upregulation of cytoprotective factors and/or activate mechanisms that facilitate tissue repair. Although the exact mechanisms that are responsible for orchestrating these different stress responses within the heart are not known, there is a growing body of literature which suggests that the innate immune system plays an important role in terms of initiating, integrating and maintaining the myocardial response to environmental stress. Traditionally, the immune system has been divided into innate and adaptive components, each of which has a different role in helping the host to differentiate self from non-self. The innate immune system is activated by a family of “pattern recognition receptors” that reside in a variety of cell types, including cardiac myocytes. These pattern recognition receptors recognize invariant patterns (so-called pathogen associated molecular patterns) shared by groups of microorganisms but not by host tissues. Typical examples of pathogen-associated molecular patterns include the lipopolysaccharides (LPS) of Gram-negative organisms, the teichoic acids of Gram positive organisms, the glycolipids of mycobacterium, the mannans of yeast and the double-stranded RNAs of viruses. These pathogen-associated molecular patterns are unique to these pathogens and in some cases are required for their virulence. Thus, one of the quintessential features of the innate immune system is that it serves as an “early warning system” that enables the host to accurately and rapidly discriminate self from non-self. Recent studies have shown that the heart possesses a functionally intact innate immune system, and that the cardiac innate immune system is activated nonspecifically in response to all forms of acute myocardial injury, especially during ischemia/reperfusion injury (reviewed in (1, 2)). However, as will be discussed below, this adaptive system can become maladaptive when it is activated in a sustained manner.
While the concept of an immune system that is designed to discriminate between “self” and “non-self” works well for classical immunological disorders involving T cells and B cells, it has been difficult, heretofore, to apply these concepts to the expression of inflammatory mediators in the heart, wherein tissue injury is encountered more frequently than invading pathogens. Recently, Matzinger (3) has suggested an alternative view of the role of the immune system that lends itself more readily to understanding the role of inflammatory mediators in the heart. The so-called “Danger Model” of immunity suggested by Matzinger proposes that cell damage, rather than “foreignness,” is what initiates an immune response. The Danger Model further proposes that what really matters from an evolutionary perspective is whether a given entity causes damage or not. Matzinger's model suggests that injured and/or stressed tissues release intracellular or extracellular “alarm”/“danger” signals that are capable of activating the immune system. According to the Danger Model of immunity, the pattern recognition receptors of the innate immune system recognize specific epitopes or ligands that indicate that cells are injured or dying. And indeed, molecules released by stressed cells (heat shock protein 60 (4)) and/or injured tissue (fibronectin (5)) are sufficient to activate the innate immune response (6). Once these danger signals are recognized by pattern recognition receptors, they activate the components of the innate immune response, including NF-κB, proinflammatory cytokines and nitric oxide (1), that in turn activate the immune system. Alternatively, these danger signals may activate pro-apoptotic pathways that lead to cell death without activating the immune system. In addition to the classical exogenous danger signals discussed above, recent evidence suggests that reactive oxygen intermediates (ROI) serve as danger signals in metabolically stressed cells (7). Given that the heart is a metabolically active tissue, it is perhaps not surprising that ROI serve as danger signals in cardiac myocytes as well (8). Moreover, the extant literature suggests that ROI are sufficient to trigger signaling through innate immune receptors (8).
INNATE IMMUNITY AND THE ADULT MAMMALIAN HEART
As noted above, the heart posses a germ-line encoded “innate” stress response that is activated in response to diverse forms of tissue injury. For example, mediators and effectors of the innate immune response, such as proinflammatory cytokines, nitric oxide and chemokines, are expressed by cardiac myocytes in response to tissue injury and/or challenge with classical pathogen-associated molecular patterns (e.g lipopolysaccharide [LPS] and viral particles) (9–11). Moreover, the heart expresses classical pattern recognition receptors for pathogen-associated molecular patterns. Indeed, cardiac myocytes express at least five classical receptors that belong to the innate immune system (so-called pattern recognition receptors), including CD14, the soluble pattern recognition receptor for lipopolysaccharide (12), and Toll like receptors-2, 3, 4, and 6 (TLR2, TLR3, TLR4 and TLR6, respectively) (8). Taken together, these observations provide evidence for a functionally intact innate immune system in the heart.
INNATE IMMUNE SIGNALING PATHWAYS
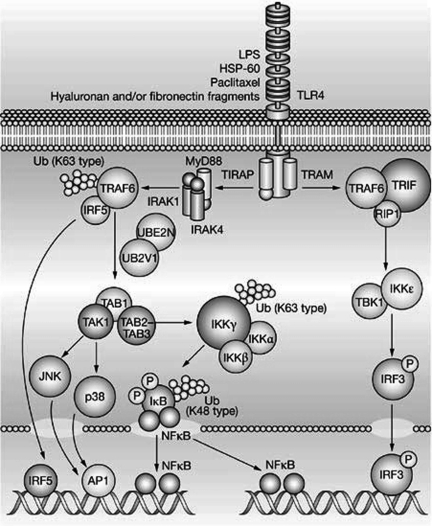
The signaling pathway that is used by the TLR family of receptors is highly homologous to that of the IL-1 receptor (IL-1R) family. All TLRs (except for TLR3) interact with an adaptor protein termed myeloid differentiation factor 88 (MyD88) via their Toll Interleukin Receptor (TIR) domains. When stimulated, MyD88 recruits IL-1 receptor associated kinase (IRAK) to the receptor. IRAK is then activated by phosphorylation and associates with tumor necrosis receptor-associated factor 6 (TRAF6), leading to NF-κB activation through the classical IKK-IκBα dependent pathway depicted in Figure 1 (13). Although the adaptor molecule TIR domain-containing adapter protein (TIRAP) was initially thought to contribute to MyD88-independent signaling, studies have shown that TIRAP is required for TLR2 and TLR4 mediated activation of NF-κB.
Fig. 1.
The Toll-like receptor signaling pathway. (Key: AP1, activator protein 1; HSP-60, heat shock protein 60; IκB, inhibitor of nuclear factor κB; IKKα, inhibitor of nuclear factor κ-B kinase α; IKKβ, inhibitor of nuclear factor κB kinase-β; IKKε, inhibitor of nuclear factor κ-B kinase ε; IKKγ, inhibitor of nuclear factor κ-B kinase γ; IRAK1, interleukin 1 receptor-associated kinase 1; IRAK4, interleukin 1 receptor-associated kinase 4; IRF3, interferon regulatory factor 3; IRF5, interferon regulatory factor 5; JNK, c-jun N-terminal kinase; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response protein; NF-κB, nuclear factor κB; RIP1, receptor-interacting protein 1; TAB1, TAK1 - binding protein 1; TAB2-TAB3, TAK1-binding proteins 2 and 3; TAK1 (M3K7), transforming growth factor-β-activated kinase 1; TBK1, serine-threonine-protein kinase; TIRAP, TIR domain-containing adaptor protein; TLR4, Toll-like receptor 4; TRAF6, tumor necrosis factor receptor-associated factor 6; TRAM, TRIF-related adaptor molecule; TRIF, TIR-domain-containing adaptor inducing interferon β; Ub, ubiquitin; UB2V1, ubiquitin-conjugating enzyme E2 variant 1; UBE2N, ubiquitin-conjugating enzyme E2N (Reproduced with permission from Frantz, S., Ertl, G., & Bauersachs, J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 4, 444–454, 2007).
ADAPTIVE EFFECTS OF INNATE IMMUNE RESPONSES IN THE HEART
There is increasing evidence which supports the point of view that short-term expression of proinflammatory cytokines is beneficial in the heart. It is noteworthy that the family of proinflammatory molecules that comprise this innate stress response system are phylogenetically ancient, and thus likely to have evolved in organisms with relatively short life spans (weeks to months). Thus, activation of the “innate” stress response system was never intended to provide long-term adaptive responses to the host organism. As will be discussed towards the end of this review, sustained and/or dysregulated expression of proinflammatory cytokines is sufficient to produce tissue injury and provoke overt cardiac decompensation.
With respect to the cytoprotective aspects of cytokine signaling, several lines of investigation suggest that the activation of systemic TLR4 by LPS in vivo or ex vivo protects the myocardium following myocardial ischemia/reperfusion (I/R) injury (14). For example, hearts isolated from rats that were pretreated with a low dose of LPS (0.5 mg/kg) 24 hours prior to terminal sacrifice had preserved LV function after I/R injury compared with the saline treated control hearts (15). The cytoprotective effects of LPS occured between 12 and 24 hours after the administration of LPS, were sensitive to inhibition with cycloheximide, and were shown to be mediated via TLR4 (16). Analogous to ischemic pre-conditioning, the cytoprotective effects of LPS were mediated by nitric oxide synthase 2 NOS2 and the serine/threonine protein kinase Akt (protein kinase B) (14). Moreover, recent studies from our laboratory have shown that ischemia-reperfusion injury is mediated via a TLR2-TIRAP dependent signaling pathway that involves protein kinase C (17). These latter findings are consistent with the point of view that TLR2-TIRAP dependent signaling comprises part of an evolutionarily conserved “early warning system” in the heart that is activated in response to tissue injury
MALADAPTIVE EFFECTS OF INNATE IMMUNE RESPONSES IN THE HEART
Although the short-term, self-limited expression of stress-activated cytokines may provide the heart with an adaptive response to environmental injury, this protective response may occur at the cost of unwanted deleterious effects that occur when cytokines are elaborated either for sustained periods of time, or when cytokines are expressed at supraphysiological, pathological levels. As shown in Table 1, sustained expression of cytokines may produce frank maladaptive effects in the heart. There is also a growing appreciation that TLRs may play an important role in modulating tissue injury in the heart. Indeed, recent studies from this and other laboratories have shown that TLR-4 is critical for upregulating the expression of TNF, IL-1β, IL-6 and NOS2 in the heart following stimulation with lipopolysaccharide (LPS) (11, 18), and that CD-14 and TLR4 are essential for LPS-mediated LV dysfunction (19, 20). Moreover, mice with a missense mutation of TLR4 or targeted disruption of TLR4 (21–23), TLR2, (24) or MyD88 (25) have reduced infarct sizes when compared to wild-type controls. Similarly, mice pre-treated with a TLR4 antagonist (Eritoran) (26) had smaller infarct sizes when compared to vehicle treated animals. Mortality and LV remodeling are reduced in mice with targeted disruption of TLR4 or TLR2 (27, 28). Although the mechanism(s) for the deleterious effects of TLR signaling following I/R injury and/or myocardial infarction has not been established, a recent study using reconstituted bone marrow cells from wild-type and TLR4 deficient mice suggests that TLR4 receptors on bone marrow-derived hematopoietic cells may contribute to cardiac dysfunction by modulating the recruitment of neutrophils to the myocardium (29).
TABLE 1.
Maladaptive Cardiovascular Effects on Innate Immunity and Proinflammatory Cytokine Signaling
| Produces left ventricular dysfunction |
| Produces pulmonary edema in humans |
| Produces cardiomyopathy in humans |
| Promotes left ventricular remodeling experimentally |
| Produces abnormalities in myocardial metabolism experimentally |
| Produces β-receptor uncoupling from adenylate cyclase experimentally |
| Produces abnormalities of mitochondrial energetics |
| Activation of the fetal gene program experimentally |
In addition to mediating LV dysfunction during sepsis and following I/R injury, there are several provisional experimental reports which suggest that the innate immune system may play an important role in the development and progression of heart failure. For example, studies have shown that expression of TLR4 is increased in the hearts of patients with advanced heart failure (18, 30). Interestingly, in normal mammalian myocardium, TLR4 expression is diffuse and is predominantly confined to cardiac myocytes, whereas TLR staining is increased and is more localized in human heart failure (31). However, the significance of these findings is unclear.
Given the paucity of data on the role of innate immunity in the failing human heart, we sought to examine the role of the innate immune system in the failing human heart. Accordingly, we examined the expression profiles of genes that are involved in innate immune signaling (Table 2). For this analysis we used a publicly available gene array data set (Cardiogenomics Consortium [http://www.cardiogenomics.med.harvard.edu]) that was obtained from the explanted hearts of 79 subjects, including 32 patients with ischemic cardiomyopathy (ICM), 26 patients with idiopathic dilated cardiomyopathy (DCM), and 7 patients with viral cardiomyopathy (VCM), as well as 14 non-failing (NF) hearts (see data Supplement for methodology). Expression data for innate immune signaling genes were first analyzed using Principal Component Analysis (PCA), which is a mathematical modeling procedure that transforms a number of possibly correlated variables into a smaller number of uncorrelated variables that are termed principal components (32). The first principal component accounts for as much of the variability in the data as possible, and each succeeding component accounts for as much of the remaining variability of the data as possible. PCA allows one to visualize complex data sets in simple 3-dimensional display format, wherein the x-axis displays the values for the first principal component, the y-axis displays the values for the second principal component, and the z-axis displays the values for the third principal component.
TABLE 2.
Innate Immune Signaling Pathway Genes
| Gene | Probe # | Gene | Probe # | Gene | Probe # | Gene | Probe # |
|---|---|---|---|---|---|---|---|
| TLR1 | 1 | TRIF | 1 | IRF3 | 1 | MKK6 | 2 |
| TLR2 | 1 | MYD88 | 1 | IRF7 | 1 | MKK7 | 4 |
| TLR3 | 1 | MD-2 | 1 | RIP1 | 2 | MEKK1 | 2 |
| TLR4 | 4 | CD14 | 1 | RIP3 | 1 | p38 | 4 |
| TLR5 | 1 | TIRAP | 4 | A20 | 2 | TAK1 | 3 |
| TLR6 | 1 | TRAF3 | 2 | PI3K | 4 | JNK | 5 |
| TLR7 | 2 | TRAF6 | 1 | IKBα | 1 | FADD | 1 |
| TLR8 | 2 | TOLLIP | 3 | IKKβ | 3 | CASP8 | 3 |
| TLR9 | 1 | MAVS | 4 | IKKγ | 2 | TAB1 | 2 |
| TLR10 | 2 | ECSIT | 1 | IKKα | 1 | TAB2 | 2 |
| IRAK-1 | 2 | UEV1A | 2 | IKKP) (A) | 2 | ST2L | 1 |
| IRAK-2 | 2 | UBC13 | 3 | NFKB1 | 1 | SIGIRR | 2 |
| IRAK-M | 3 | SOCS1 | 3 | RelA | 2 | TWIST1 | 1 |
| IRAK-4 | 1 | SOCS3 | 4 | MKK3 | 3 | NOD2 | 1 |
| TRAM | 2 | TBK1 | 2 | MKK4 | 2 |
This table represents the 59 innate immune genes (120 probe sets) present on the Affymetrix HG-U133 plus 2.0 oligonucleotide microarray (54,675 probe sets) platform.
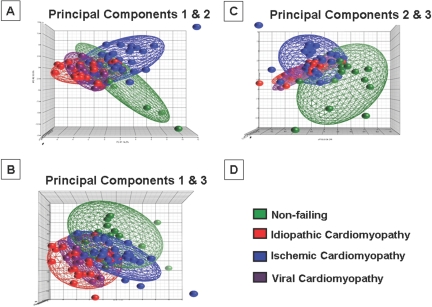
The 3-dimensional (3-D) PCA plot illustrated in Figure 2 shows two important findings with respect to innate immune gene expression in human heart failure. The first is that the numerical values for the principal component analysis for failing and non-failing hearts were clearly distinct in each of the 3-D projections depicted in Figure 2, suggesting that the innate immune system is differentially expressed/activated in heart failure in response to tissue injury. As shown, the numerical values for the PCA plots for NF hearts were clustered differently from the numerical values for the PCA plots for the ICM, DCM, VCM hearts, which tended to cluster together (best seen in Figure 2A). The second important finding is that within the broad grouping of gene expression profiles in heart failure patients, the PCA profiles were different in ICM patients when compared to DCM patients, raising the interesting possibility that the innate immune system is activated in response to the nature of the pathological tissue injury pattern. In this regard it is interesting to note that the PCA plots for VCM patients were similar to that observed in DCM. This latter observation is of interest, insofar as occult and/or persistent viral myocarditis has been suggested as a potential etiology for idiopathic dilated cardiomyopathy (33). Whether the differences in the PCA plots in ICM and DCM can be explained by an occult and/or lingering viral infection in the DCM patients cannot be addressed from the present provisional study.
Fig. 2.
Principal component analysis of changes in innate immune gene expression in failing and non-failing human hearts. Innate immune genes (Table 2) were subjected to a principal component analysis (PCA), and the first, second and third principal components were displayed in a 3-D graphic format (see Data Supplement for details). The 3-dimensional PCA analyses depicted in this figure accounted for 36.6% of the total variation of the entire data set.
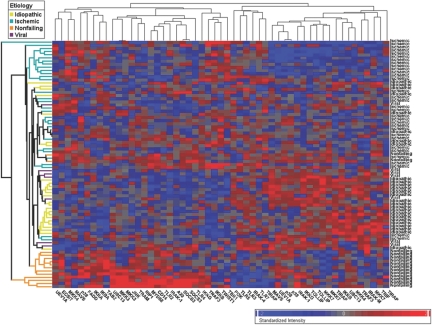
To extend the observations with respect to the PCA analysis, we performed an unsupervised hierarchical clustering analysis of DCM, ICM, VCM and non-failing human hearts based on expression profile of the differentially expressed innate immune genes. As shown in Figure 3 the ICM, DCM, and NF changes in gene expression were clustered in the upper, middle, and lower portions of the heat map, respectively, suggesting that [1] there were distinct gene expression profiles for innate immune genes in failing and non-failing hearts, and [2] that there were distinct gene expression profiles for innate immune genes in ICM and DCM hearts. Of interest, 6 out of 7 VCM patients were clustered within the DCM block, as shown by the dendogram on the left hand side of the heat map, implying that innate immune gene expression profiles were overlapping in DCM and VCM hearts.
Fig. 3.
Hierarchical cluster analysis of innate immune genes. An unsupervised hierarchical cluster analysis was performed on the family of innate immune genes depicted in Table 2. Increased gene expression is depicted in red, and decreased gene expression is depicted in blue.
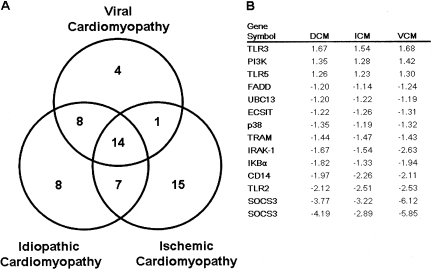
To determine whether there were significant differences in innate immune gene expression in DCM, ICM and VCM hearts, we analyzed expression levels for each of the innate immune genes shown in Table 2, relative to the non-failing human hearts. Group comparison with ANOVA test revealed that there were 37, 37, and 27 transcripts, respectively, whose expression levels were significantly different in DCM, ICM, and VCM when compared to NF hearts. The Venn diagram illustrated in Figure 4 reveals that of the 91 transcripts in Table 2 that were analyzed, there were only 14 transcripts whose changes were similar in all forms of cardiomyopathy. As shown in Panel 4B, there were 3 genes whose expression levels increased and 11 innate immune genes whose expression decreased in all forms of cardiomyopathy. Consistent with the PCA and hierarchical clustering analyses depicted in Figures 2 and 3, the VCM hearts had more transcripts in common with DCM than with ICM (22 vs. 15).
Fig. 4.
Venn diagram of changes in gene expression that are common and unique to ischemic (ICM), idiopathic dilated (DCM) and viral cardiomyopathy (VCM). ANOVA testing was performed on the change in gene expression in DCM, ICM, and VCM hearts, relative to NF hearts, for the ensemble of innate immune genes displayed in Table 2. There were 37, 37, and 27 transcripts, respectively, in DCM, ICM, and VCM hearts. The Venn diagram depicts the number of innate immune genes whose changes in gene expression that are common to all forms of cardiomyopathy (n = 14), those that are common to DCM and ICM (n = 21 [14 + 7]), those that are common to ICM and VCM (n = 22 [14 + 8), those that are common to VCM and ICM (n = 15 [14 + 1]), and those that are unique to DCM alone (n = 8), those that are unique to ICM (n = 15), and those that are unique to VCM (n = 4).
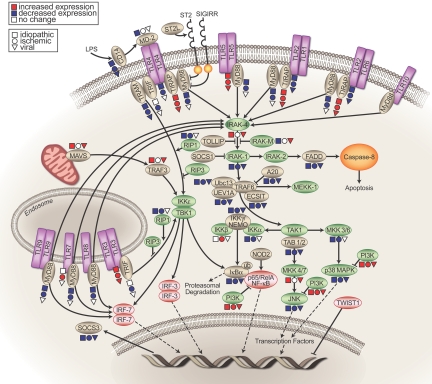
To determine the functional significance of the changes in innate immune gene expression in cardiomyopathies of different etiologies, we displayed the changes in the pattern of innate immune gene expression (i.e., increased, decreased or unchanged) relative to NF hearts on a map of known TLR signaling pathways. Figure 5 discloses several interesting findings with respect to the expression of genes in the TLR signaling pathway in human heart failure. The expression levels for several of the membrane bound TLRs that are expressed in cardiac myocytes, namely TLR2 and TLR4, were decreased in all forms of cardiomyopathy. However, TLR3, which is primarily located within endosomes, was increased in all forms of cardiomyopathy. Second, the expression level of TIRAP (see Figure 1), a scaffolding protein essential for MyD88 signaling through TLR2 and TLR4, was increased. Moreover, there was increased expression of IRAK4, a kinase that is immediately downstream from TIRAP-dependent signaling, in idiopathic and viral cardiomyopathy. Additionally, ECSIT, an inhibitory protein that decreases IRAK-dependent signaling, A20, an inhibitory protein that decreases TLR activation of NF-kB though deubiquitination of TRAF6, and IκBα an inhibitory protein that dampens NF-kB-dependent signaling (downstream IRAK signaling), were each decreased in all forms of cardiomyopathy. Although our gene array analysis suggests deceased expression of TLR2 and TLR4 mRNA, it is notable that prior studies have demonstrated increased TLR protein levels in human heart failure (18). When this observation is coupled with the finding that there is increased gene expression of signaling molecules that are downstream from TLR2 and TLR4 signaling (TIRAP and IRAK 4), and decreased expression of molecules that inhibit TLR2 and TLR4 signaling (ECSIT, A20 and IκBα) raises the interesting possibility that enhanced innate immune signaling may contribute to the overall pathogenesis of human heart failure through sustained expression of proinflammatory mediators that are sufficient to contribute to the heart failure phenotype (see Table 1).
Fig. 5.
Functional significance of changes in innate immune gene expression in human heart failure. Changes in innate immune gene expression in ischemic, idiopathic dilated and viral cardiomyopathies were compared to non-failing hearts for the transcripts shown in Table 2 and were projected onto a map of Toll-like receptor (TLR) signaling. Increased gene expression is depicted in red, decreased gene expression is depicted in blue, and no change in gene expression is depicted as an open symbol. Changes in ischemic cardiomyopathy are depicted as an open symbols, changes in idiopathic cardiomyopathy is depicted as an square symbols, and changes in viral cardiomyopathy are depicted as triangles.
CONCLUSION
Recent studies suggest that the heart possesses an intrinsic or “innate stress response” system that is intended to delimit tissue injury, as well as orchestrate homoeostatic responses within the heart. With that said, the short-term beneficial effects of inflammatory mediator signaling may be lost if myocardial expression of these molecules becomes sustained and/or excessive, in which case the salutary effects of these proteins may be contravened by their known deleterious effects. Indeed, studies presented herein, as well as from other laboratories raise the interesting possibility that dysregulation of Toll-like receptor signaling may contribute to disease progression in heart failure. Based on the foregoing arguments, the important question that remains to be addressed is whether or not it will be possible to modulate the inappropriate/maladaptive consequences of innate immune activation and proinflammatory cytokine expression in the mammalian heart, while still preserving the important advantages that this phylogenetically conserved immune system provides to the host. Future studies will be necessary in order to determine whether modulation of the innate immune system will represent a novel cardiovascular target, particularly in conditions that are accompanied by tissue injury.
ACKNOWLEDGEMENTS
This research was supported by research funds from the N.I.H. (RO1 HL58081, HL-73017-0, HL089543-01 and T32HL007081). The authors acknowledge the following public source for the microarray data: Genomics of Cardiovascular Development, Adaptation, and Remodeling, NHLBI Program for Genomic Applications, Harvard Medical School (URL: http://www.cardiogenomics.org [accessed November 2009]).
Footnotes
Potential Conflicts of Interest: None disclosed.
DATA SUPPLEMENT
Methods
Microarray Data Source.
Microarray data were obtained from Cardiogenomics Consortium (http://www.cardiogenomics.med.harvard.edu), a NHLBI sponsored program for Genomic Applications. The Affymetrix HG-U133 plus 2.0 oligonucleotide microarray (54,675 probe sets) platform was used for gene array. At the time of data accession, gene expression profile was available for 79 subjects, including 32 patients with ischemic cardiomyopathy (ICM), 26 patients with idiopathic dilated cardiomyopathy (DCM), and 7 patients with viral cardiomyopathy (VCM). The characteristics of the patient cohort are presented in Supplemental Table 1. The remaining 14 myocardial samples were obtained from explanted non-failing (NF) hearts that could not be transplanted for technical reasons.
Supplemental TABLE 1.
Clinical Characteristics of Patient Cohorts
| Idiopathic | Ischemic | Viral | Non-Failing | p-value | |
|---|---|---|---|---|---|
| Age (years) | 46.9 ± 19.0 | 52.2 ± 10.2 | 38.3 ± 16.3 | 52.4 ± 13.4 | 0.144 |
| Gender (F) | 38.5% | 15.6% | 0.0% | 50.0% | 0.003 |
| Hypertension | 25.0% | 73.1% | 20.0% | 54.5% | 0.004 |
| Diabetes | 24.0% | 39.3% | 0.0% | 9.1% | 0.093 |
| BMI (kg/m5) | 24.6 ± 3.5 | 25.6 ± 4.0 | 23.9 ± 4.2 | 24.4 ± 4.5 | 0.672 |
| LVEF (%) | 12.8 ± 6.3 | 21.9 ± 12.0 | 16.8 ± 2.7 | 61.3 ± 8.5 | <0.001 |
| LVEDD (cm) | 7.8 ± 1.4 | 7.1 ± 1.1 | 7.7 ± 1.3 | 4.8 ± 1.5 | 0.009 |
| LVESD (cm) | 6.9 ± 1.3 | 5.9 ± 1.8 | 7.0 ± 1.2 | 2.6 ± 1.3 | <0.001 |
| LVPW (cm) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.3 | 0.095 |
| PA systolic | 49.1 ± 14.3 | 39.0 ± 13.9 | 48.5 ± 10.5 | 24.0 ± 0.0 | 0.016 |
| PCWP | 23.4 ± 8.1 | 17.6 ± 7.3 | 32.3 ± 2.7 | 11.0 ± 1.4 | <0.001 |
Data Processing and Normalization.
Gene expression data for each patient obtained from analysis output files (*.txt), were generated by analysis of image files using Affymetrix MAS 4.0 software. The gene expression profiles were then subjected to global scaling in order to correct for intensity related biases. Probe sets that had more than 90% absent calls across all subjects were filtered. A total of 28,511 probe sets from 79 patients that were confidently detected at a detection p value < 0.05. These data were imported into Partek Genomics Suite v6.4 software (Partek, St Louis, MO) for further processing. The expression levels of the probe sets were log-transformed to normalize the intensity distribution. Insofar as the date of chip processing had a significant effect on the gene expression profile (data not shown), the Partek Batch Remover™ with ANOVA function was applied in order account for this effect. The filtered, log-transformed, and normalized data set was then screened for a total of 120 probe sets mapping to 59 genes (Table 2) that were known to play an important role in innate immune signaling. Out of 120 probe sets, 91 were confidently detected and used in further analyses.
Principal Component Analysis and Hierarchical Clustering.
Principal Component Analysis (PCA) was used to analyze the genes depicted in Table 2. The Principal Component (PC) Analysis module of Partek GS was used to generate a 3-dimensional projection map (X, Y, and Z axes representing PC#1, PC#2, and PC#3, respectively), as well as calculate projection values. A PCA scatterplot graph was then generated for the four patient groups (idiopathic cardiomyopathy, ischemic cardiomyopathy, viral cardiomyopathy, and non-failing controls) using a matrix of 79 patients with expression values for 91 innate immune probe sets that are present on the Affymetrix microarray platform.
The expression matrix was also subjected to an unsupervised hierarchical clustering based on significantly regulated innate immune genes in any pair wise comparisons in order to provide a visual assessment of the ability of the innate immune transcript profile to classify subjects based on their etiology of heart failure. Agglomerative hierarchical clustering (combination of two rows/columns or clusters at each step) was performed using Partek GS. The Euclidian distance was used to measure dissimilarity (the distance between two rows or columns) and average linkage (the average distance between 11 pairs of objects in two different clusters) was used as the measure of distance between two clusters.
Statistical Analysis
Individual genes that were differentially expressed between groups (Idiopathic vs Non-Failing, Ischemic vs. Non-Failing, Viral vs. Non-Failing, and Ischemic vs. Idiopathic) were identified using 1-way ANOVA with linear contrasts at a false discovery rate (FDR) of 0.05 using Partek GS. Demographic and phenotypic variables were analyzed using SPSS v17.0 software (SPSS, Chicago, IL). Continuous data were compared using 1-way ANOVA with Tukey's post-hoc analysis whereas chi-square test was used for categorical variables.
DISCUSSION
May, Gainesville: I am really struck by the role of protein kinase C. This is not my area so I am not sure about signaling here, but there is an inability in the preconditioning phenomenon here for protein kinase C epsilon to be attracted to the membrane. Usually that occurs through diacylglycerol. So what happens to phospholipid and phosphatidylinositol regulation in the toll … (inaudible) … is it lost and you could get preconditioning? You wouldn't get cardiomegaly?
Mann, St. Louis: Yes. Well again, preconditioning would determine the infarct size. The infarct size would determine the ability to go on and develop heart failure. PI 3-kinase is not involved in this when we've looked at it as a potential mechanism. We did a lot of other studies I didn't have time for. What we think the Tolls are doing is allowing for PKC to basically act as scaffolding proteins that allow PKC to come in and be phosphorated and then translocate to the membrane. The problem with proving that is that the antibodies are really terrible, and it's hard to do the immunoprecipitation studies and then do Western blotting. So we have not been able to prove it. There is one paper out in the literature that actually showed a physical association of PKC with the Tolls and that's why we think it is all upstream. All of this happens very early on in the receptor, and the downstream part that leads to inflammation and tissue damage really is irrelevant to this cytoprotective part. So again, we haven't worked it out. The antibodies aren't there. We have tried doing GC-Mass speck. That was a disaster. We are still working on it.
May, Gainesville: Is phospholipase C gamma at all involved, because that's what really converts the forms of the diacylglycerol?
Mann, St. Louis: Don't know.
Schreiner, San Francisco: Doug, as you know, there is a growing emphasis on the role of mitochondrial dropout and dysfunction both in the models of ischemia/reperfusion and heart failure. I think an emphasis that has gained strength through the cyclosporine data last year that was published on limiting infarcts in humans presenting with myocardial infarctions. Would you explain how some of the downstream consequences of this process could conceivably impact mitochondria on either a beneficial or an injurious fashion?
Mann, St. Louis: We haven't had time to really look at whether this affects the mitochondrial permeability transition pore and whether these molecules might, in some way, be dampening that. There is a very strong story now that a lot of the preconditioning has to do with stabilizing mitochondrial function. We just haven't had time to delve into it. It is a really good area. We looked at the traditional things like PI 3-kinase and couldn't find any hints there, but the mitochondria are really good downstream targets.
Mudge, Boston: This was very interesting. Just to bring this back into the clinical arena, the heart transplant model is an interesting model, because in the long-term follow up, they rarely dilate and they primarily have diastolic dysfunction. I am just wondering if you could speculate on why that might be.
Mann, St. Louis: So, there's a whole other part of this, the so-called RAGE receptors, which I didn't talk, about that are incredibly profibrotic, that lead to crosslinking and whatnot, and I suspect that one of the things that happens in the transplanted heart that gets incredibly fibrotic doesn't dilate because its so fibrosed in. It basically gets encased in cement and can't. Studies from our group and others have shown that even in the transplanted heart with all of the immunosuppressants you give, there are low levels of low-grade inflammation, and I suspect that one of the things that is driving the fibrosis is that these receptors are being tickled in some way and are sending out profibrotic signals. But the RAGE receptor we have done work on is incredibly interesting, because it is responsible for crosslinking. It senses these crosslink collagen fibers, the AGEs, and maybe it is reactivating inflammation that way. So, I suspect that low-grade inflammation may be giving you the profibrotic phenotype here.
Martin, Chevy Chase: It is a fascinating presentation, and in terms of the preconditioning model of repeated ischemic events, which is a tough way to think about this clinically, would other preconditioning processes, like intense exercise, ever trigger a similar Toll receptor pathway that might reduce the risk of heart failure in susceptible people?
Mann, St. Louis: So obviously, doing repetitive bouts of ischemia is not what you would want to do in the clinic. There are two things. One is to develop mimetics that can tickle the system, and as I said, the Tolls, at least with Toll 2, we tickle it with lipopeptide. We can pharmacologically get preconditioning. There are a number of other ways to get pharmacologic preconditioning as well. There was a very interesting paper in The Lancet, probably about six to nine months ago, where they inflated a blood pressure cuff, made the arm ischemic, and then released it. I think they did that several times, and then they went and looked at CK release after an angioplasty, and they were able to get a diminution of CK release. I think that I recall the paper correctly. So there are novel ways of potentially stimulating these systems that don't involve causing a mini heart attack. This is exactly what happens in the brain too, with very similar paradigm in the brain with stroke and repetitive bouts of ischemia. They will protect the brain from a larger stroke. These paradigms I think will hold up in all tissues.
Lange, San Antonio: You've mentioned in the IRAK knockout mice, it wasn't necessary for the preconditioning response. Talk about TRAM and TRIF. Is that necessary in those?
Mann, St. Louis: So TRAM and TRIF are mainly with Toll 4. Toll 4 appears to be dispensable, at least in our model. TRAM and TRIF are also involved with TLR3 signaling. We are just getting the knockouts in now, but I think for the model that we've shown that pathway is dispensable. It appears to be completely TRIF-dependent, and it appears to be early out in the process, and the downstream part that really causes inflammation and tissue damage we think is completely dispensable.
REFERENCES
- 1.Mann DL. Tumor Necrosis Factor and Viral Myocarditis: The Fine Line Between Innate and Inappropriate Immune Responses in the Heart. Circulation. 2001;103(5):626–9. doi: 10.1161/01.cir.103.5.626. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 4.Vabulas RM, Ahmad-Nejad P, da Costa C, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276(33):31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 5.Okamura Y, Watari M, Jerud ES, et al. The EDA domain of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276:10229–33. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 6.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13(1):114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 7.Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med. 1999;26(12):232–8. doi: 10.1016/s0891-5849(98)00194-4. [DOI] [PubMed] [Google Scholar]
- 8.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor-kappa B by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197–203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 9.Kapadia S, Lee JR, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest. 1995;96:1042–52. doi: 10.1172/JCI118090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh M, Tamura G, Segawa I, Tashiro A, Hiramori K, Satodate R. Expression of cytokine genes and presence of enteroviral genomic RNA in endomyocardial biopsy tissues of myocarditis and dilated cardiomyopathy. Virchows Archiv. 1996;427:503–9. doi: 10.1007/BF00199511. [DOI] [PubMed] [Google Scholar]
- 11.Baumgarten G, Knuefermann P, Nozaki N, Sivasubramanian N, Mann DL, Vallejo JG. In vivo expression of proinflammatory mediators in the adult heart after endotoxin administration: the role of toll-like receptor-4. J Infect Dis. 2001;183(11):1617–24. doi: 10.1086/320712. [DOI] [PubMed] [Google Scholar]
- 12.Cowan DB, Poutias DN, del Nido PJ, McGowan FX., Jr CD14-independent activation of cardiomyocyte signal transduction by bacterial endotoxin. Am J Physiol Heart Circ Physiol. 2000;279(2):H619–29. doi: 10.1152/ajpheart.2000.279.2.H619. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Carpio DF, Zheng Y, et al. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166(12):7128–35. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 14.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296(1):H1–12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JM, Grosso MA, Terada LS, et al. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci U S A. 1989;86:2516–20. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Zhao H, Graveline AR, et al. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;291(4):H1900–9. doi: 10.1152/ajpheart.00112.2006. [DOI] [PubMed] [Google Scholar]
- 17.Dong JW, Vallejo JG, Taffet G, Baker JS, Mann DL. Toll-Like Receptors Modulate Ischemic Preconditioning Through a Toll-IL-1 Receptor Domain-Containing Adaptor Protein Dependent Pathway. Circulation. 2006;11418:II-313. [Google Scholar]
- 18.Frantz S, Kobzik L, Kim YD, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104(3):271–80. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemoto S, Vallejo JG, Knuefermann P, et al. Escheria coli lipolysaccharide-induced left ventricular dysfunction: the role of toll-like receptor-4 in the adult mammalian heart. Am J Physiol. 2002;282(6):H2316–23. doi: 10.1152/ajpheart.00763.2001. [DOI] [PubMed] [Google Scholar]
- 20.Knuefermann P, Nemoto S, Misra A, et al. CD14-deficient mice are protected against lipopolysaccharide-induced cardiac inflammation and left ventricular dysfunction. Circulation. 2002;106(20):2608–15. doi: 10.1161/01.cir.0000038110.69369.4c. [DOI] [PubMed] [Google Scholar]
- 21.Chong AJ, Shimamoto A, Hampton CR, et al. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004;128(2):170–9. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 22.Kim SC, Ghanem A, Stapel H, et al. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol. 2007;7:5. doi: 10.1186/1472-6793-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyama J, Blais C, Jr, Liu X, et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109(6):784–9. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 24.Favre J, Musette P, Douin-Echinard V, et al. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27(5):1064–71. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Zhao H, Xu X, et al. Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. Am J Physiol Heart Circ Physiol. 2008;295(3):H1311–8. doi: 10.1152/ajpheart.00119.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamoto A, Chong AJ, Yada M, et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114(1 Suppl):I270–4. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 27.Shishido T, Nozaki N, Yamaguchi S, et al. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108(23):2905–10. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 28.Riad A, Jager S, Sobirey M, et al. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180(10):6954–61. doi: 10.4049/jimmunol.180.10.6954. [DOI] [PubMed] [Google Scholar]
- 29.Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95(7):700–7. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- 30.Birks EJ, Felkin LE, Banner NR, Khaghani A, Barton PJ, Yacoub MH. Increased toll-like receptor 4 in the myocardium of patients requiring left ventricular assist devices. J Heart Lung Transplant. 2004;23(2):228–35. doi: 10.1016/S1053-2498(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 31.Hilenski LL, Terracio L, Sawyer R, Borg TK. Effects of extracellular matrix on cytoskeletal and myofibrillar organization in vitro. Scan Microsc. 1989;3:535–48. [PubMed] [Google Scholar]
- 32.Ringner M. What is principal component analysis? Nat Biotechnol. 2008;26(3):303–4. doi: 10.1038/nbt0308-303. [DOI] [PubMed] [Google Scholar]
- 33.Parrillo JE, Cunnion RE, Epstein SE, et al. A prospective randomized controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–8. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]