Abstract
Despite decades of investigation, the roles of reactive oxygen species (ROS) in atherosclerosis and aging have yet to be defined. We have developed mouse models that allow this question to be addressed experimentally. Given the increase in risk factors for atherosclerosis— particularly related to marked increases in obesity and diabetes in industrialized countries—a better understanding of the molecular mechanisms that define both atherosclerosis and aging is needed.
The past decade has witnessed rapid progress in defining the molecular mechanisms responsible for vascular diseases and, in particular, the etiology of atherosclerosis and in therapeutic approaches to individuals with symptomatic atherosclerosis. Despite these advances, however, myocardial infarction, stroke and heart failure—all most commonly resulting from atherosclerosis—remain the leading causes of death in the United States and in all industrialized countries.
Many risk factors for atherosclerotic vascular diseases can be treated medically or by life-style changes. Medical therapies for hypercholesterolemia, hypertension and diabetes mellitus are effective and reduce the risk of myocardial infarction, stroke and cardiovascular death. Although difficult, smoking cessation also results in a dramatic reduction in cardiovascular risk. Another often-cited risk factor for cardiovascular diseases is family history. In most instances, an increased incidence of myocardial infarction or stroke in families is largely due to hypercholesterolemia or hypertension and is, thus, treatable.
However, it is becoming more and more clear that aging is a risk factor for myocardial infarction and stroke. The very success of preventative therapies results in the progressive increase in the average age of Americans. Over the past five years, we have completed studies designed to better understand the contribution of age to atherosclerosis. Accumulating evidence suggests that oxidative stress increases with age, and that therapeutic and lifestyle approaches that reduce oxidative stress likely slow the development of atherosclerotic cardiovascular disease.
OXIDATIVE STRESS AND AGE
The term oxidative stress is most commonly defined as an imbalance between the production and scavenging of reactive oxygen species (ROS) with measurable increases in ROS in the cells and in the extracellular milieu. Increased cellular ROS (“oxidative stress”) is an important contributor to the pathophysiology of vascular diseases, including atherosclerosis, restenosis, myocardial infarction and stroke (1–6). Increased concentrations of ROS (particularly of superoxide and hydrogen peroxide) lead to enhanced oxidation of low-density lipoprotein (LDL), inactivation of endothelium-derived nitric oxide and vascular dysfunction. Additionally, ROS act as intracellular messengers, and ROS accumulation activates proinflammatory signaling pathways with an increased propensity for the formation of atherosclerotic lesions within the vessel wall.
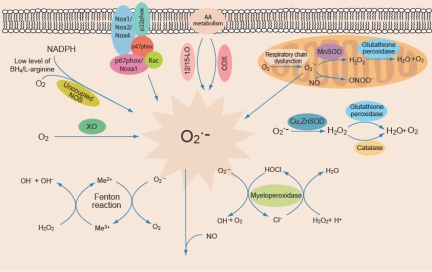
Numerous cellular systems can modulate oxidative stress. These include NADPH oxidases, xanthine oxidase, cyclooxygenases, lipoxygenases—all of which result in increased generation of ROS (Figure 1) (7). Superoxide dismutases and catalase are important in scavenging increased ROS. It is likely that increased ROS levels are important at several definable points in the development of dysfunctional arteries—from endothelial dysfunction (in the absence of identifiable atherosclerotic lesions) to the progressive growth of atherosclerotic lesions and plaque rupture (Figure 2) (8).
Fig. 1.
Sources of ROS in vascular cells. NADPH oxidase, XO (xanthine oxidase), uncoupled NOS (nitric oxide synthase), 12/15-LO (lipoxygenase) and COX (cyclooxygenase) generate superoxide (O2․−). Dysfunctional mitochondrial respiratory chain is another source of O2․− generation. SOD isoforms (MnSOD, and CuZnSOD) dismutate O2․− to produce H2O2 (hydrogen peroxide), which in turn is converted to H2O by glutathione peroxidase (GPx) or catalase. Myeloperoxidase generates HOCl (hypochlorous acid) from H2O2 in the presence of Cl−. H2O2 reacts with transition metals (Me) to produce hydroxyl radicals (OH). Nitric oxide (NO) reacts with O2․− to produce peroxynitrite (ONOO․−) (7).
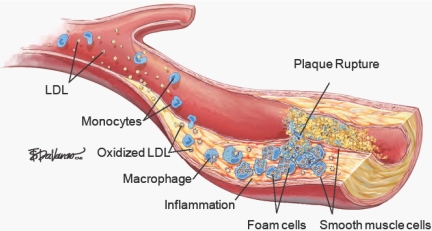
Fig. 2.
Enhanced ROS generation contributes to the development of atherosclerosis. Oxidative stress results in endothelial dysfunction and migration of monocytes into the subendothelial space. ROS produced by endothelial cells, vascular smooth muscle cells, and macrophages oxidize LDL in the subendothelial space initiating events that culminate in the formation of a fibrous plaque. Rupture of fibrous plaque leads to thrombus formation and occlusion of the vessel (8), with permission.
Oxidative stress and oxidative signaling most likely represent a common link between many of the risk factors for atherosclerosis. Although many enzyme systems participate in the generation of ROS, NADPH oxidases and mitochondria are the most important ROS-generating systems in vascular cells under pathophysiological conditions.
Increased oxidative stress is also a hallmark of aging (9–11). Broadly, beneficial lifestyle changes—including exercise and caloric restriction—reduce imbalances caused by excess ROS and slow the development of most markers of aging. Indeed, numerous studies have demonstrated that these same interventions result in decreased markers of oxidative stress in animal models and in humans (12–17).
MOUSE MODELS FOR OXIDATIVE STRESS AND AGING
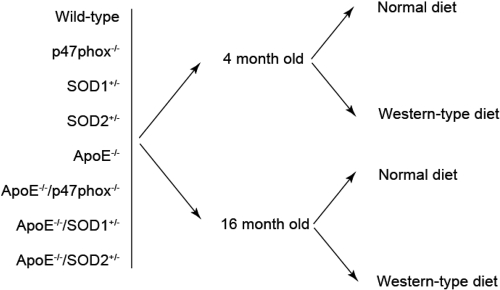
The studies we have undertaken are focused on altering ROS production or scavenging using genetically engineered mouse models. ApoE−/− mice have markedly increased plasma cholesterol levels and develop aortic atherosclerosis in a graded manner. p47phox−/− mice lack a critical subunit of NADPH oxidases. SOD1+/− and SOD2+/− mice are deficient in cytoplasmic and mitochondrial superoxide dismutases, respectively. We examined both histologic and physiologic markers of atherosclerosis in mice at 4 months or 16 months to assess the effect of age and also analyzed the impact of a Western-type diet on atherosclerosis at both ages (Figure 3).
Fig. 3.
Mouse models to study the interaction of oxidative stress, aging and diet on the development of atherosclerosis. We have used altered ROS production (p47phox−/−) or scavenging (SOD1+/− and SOD2+/−) in ApoE−/− mice and investigated the effect of age (4 months vs 16 months) and diet (normal vs Western-type diet) on atherosclerosis development.
The sophistication with which it is possible to measure vascular disease in experimental mouse models has developed rapidly in the past ten years. Two important and interrelated physiologic measures that we have used are: quantification of aortic atherosclerosis (Figure 4) and measurement of arterial compliance (Figure 5) (18). Vascular compliance is inversely related to the peak velocity of blood flow in the aorta as measured by doppler ultrasonography. Finally, ROS production can be quantified by measuring the production of either superoxide or hydrogen peroxide using DHE (dihydroethidum) or DCFDA (2′,7′-dichlorofluorescin diacetate) fluorescence, respectively (Figure 6).
Fig. 4.
Oil red O-positive atherosclerotic lesions in the whole aortas of mice on Western-type diet. The atherosclerotic lesion area can be quantified using ImageJ 1.32j software (National Institute of Health).
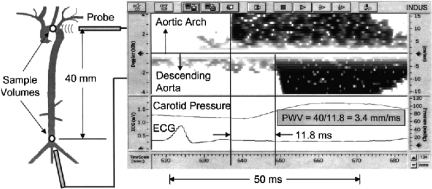
Fig. 5.
Arterial compliance is evaluated by measuring the arrival of the pulse wave from two sites at a known distance apart. When two probes are used simultaneously, one is displayed up and the other down on the spectral display. Alternatively, pulses from two sites measured sequentially using a single probe can be timed with respect to the electrocardiogram (ECG). A pressure wave from a catheter in the carotid artery used in a validation study is also shown (18), with permission.
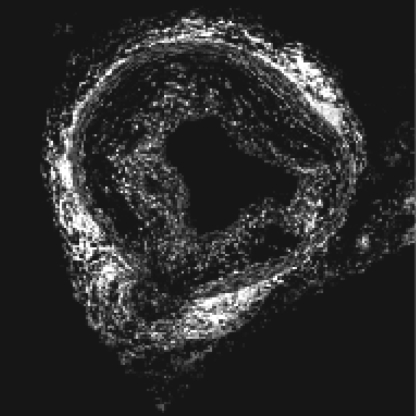
Fig. 6.
Aortic ROS levels are measured by staining cross sections of fresh-frozen aortas with DHE for 10 minutes. A representative ApoE−/− aortic cross section stained with DHE is shown. DHE fluorescence can be quantified by using ImageJ 1.32j software.
IMPLICATIONS FOR UNDERSTANDING HUMAN ARTERIAL DISEASE
Our preliminary data suggest that careful studies of mouse models are relevant to understanding atherosclerosis, aging and human vascular diseases. One of our early findings was that increased oxidative stress results in reduced aortic compliance and increased diastolic blood pressure. There is concomitant increased inflammation at young ages as well as in atherogenesis. While we have not yet determined if mid-life interventions such as dietary changes, exercise or cholesterol reduction, or targeted reductions of intracellular oxidative stress will stop or even reverse atherosclerosis, the model systems we have developed will enable us to ask these questions directly.
SUMMARY
Atherosclerosis and its principal complications, myocardial infarction, stroke and heart failure, are major public health concerns for the citizens of United States and all the industrialized countries. We have developed sophisticated mouse models and measures that will advance a molecular description of the physiologic changes that occur in atherosclerosis and aging and, hopefully, lead to targeted therapies.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants HL-57352 and AG-024282.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Luke, Cincinnati: I was very interested in the diastolic hypertension, which with aging, usually means some renal effects. I presume the renal vasculature is affected by this too. Isolated systolic hypertension in aging is more common than diastolic hypertension. I'd like to hear your comments on that.
Runge, Chapel Hill: Well, we've puzzled over that too because we didn't see much systolic hypertension. We only saw diastolic hypertension in these mice. Certainly the vessels in every organ are involved, including those in the kidney although I did not show you those data. If you modulate the NADPH enzyme complex in any of a number of different ways you get the same exact results. So, I think the diastolic hypertension is an effect of modulating NADPH oxidase activity. It's not, as you correctly state, it's not a true recapitulation of what's going on in terms of elderly vessels though, but it's a model.
Mann, St. Louis: Marschall, wonderful talk. The other flavor of heart failure that we deal with today is this so called heart failure with preserved EF. Although the mechanisms are still being argued out, one mechanism, which I think is incredibly important, particularly for aging and diabetes, is this interaction of a stiff ventricle and stiff vessels. So basically, any salt load gets pushed back into the lungs, because it has no place to go. So far we've had modest success with reversing cardiac hypertrophy. What is really known about reversing vessel stiffness, particularly in aging where there is probably so much of a problem with the cross linking and, as a corollary of diabetes, where you get a lot of vascular stiffness too. Are these ROS generating systems involved? Is there a component of reversibility that's achievable that we might look at as a new therapeutic target in diastolic heart failure?
Runge, Chapel Hill: I think that's a great question, and what I can tell you is that the pathways that end up being activated when you have increased superoxide by any of the mechanisms that I talked about, end up causing increased collagen deposition and all the things that you see in aging arteries. There are mouse models that we have not studied, using conditional transgenic approaches, which allow reversal of a given phenotype later in life. In particular, with NADPH oxidase “over-activity”, reversal back to baseline results in partial resolution of aortic thickening. I don't know if this sort of intervention is the sort of approach that would result in a targeted drug approach or not. There is a small company that is targeting the Nox's (components of NADPH oxidase) and has some very interesting preliminary data. They will soon be starting clinical trials using Nox inhibitors for a variety of indications, one of which has to do with vascular function.
Mann, St. Louis: And is there a Rubicon, if you will, for the vasculature? Is there a point after which that they can't reverse this, or is this something that is potentially reversible, even in the octogenarians?
Runge, Chapel Hill: Great question. The only data that I can tell you about, and you and Wayne and others frankly know more about this than I do, is that there are studies looking at vascular reactivity before and after exercise, even in elderly people over the age of 70, that shows improved vascular function with exercise and potentially with statins also.
Mann, St. Louis: Thank you.
Abboud, Iowa City: Thanks very much. Very interesting combination of models that can give us some insight. I noticed that the mitochondrial reactive oxygen seems to be more damaging in vascular stiffness than cytoplasmic reactive oxygen. Any insight into mechanisms there, or is it just the magnitude of generation of reactive oxygen is greater from the mitochondria?
Runge, Chapel Hill: That is also an excellent question. I don't know the answer, but I think that just as in the heart, as was mentioned earlier, the role of the mitochondria in vascular function is going to be very important.
Cohen, Chapel Hill: Thank you for your talk. NADPH oxidase and superoxidase work originated from the granulocyte field and the phagocyte field. In those slides you show constitutive formation of superoxide in blood vessels—what's the kind of belief system of why should a blood vessel want to have any superoxide generated?
Runge, Chapel Hill: Another great question. I didn't state this but production of reactive oxygen species is absolutely necessary for normal cell function. If you completely block superoxide generation, vascular cells become apoptotic or non-viable. In virtually all biological systems, some level of generation of reactive oxygen species and downstream signaling is part of normal physiology. The studies I reported on involve overproduction of ROS.
Boxer, Ann Arbor: Another very important component is nitric oxide, and as we're learning in sickle cell, the lack of nitric oxide predisposes to pulmonary hypertension. In your model system, have you looked at scavenging of nitric oxide by superoxide and formation of nitrites that would affect reactivity?
Runge, Chapel Hill: We haven't directly looked at that. We tried to quantify peroxynitrite formation unsuccessfully, to date. I agree with you that nitrogen oxide is very important.
Weir, Baltimore: I want to go back to the question that Robin Luke brought up about the diastolic blood pressure, because I would think that as you lose aortic compliance with an increased pulse wave reflection and pulse wave amplification, you'd actually get a higher systolic and a lower diastolic and a wider pulse pressure, because this is what tends to correlate with left ventricular hypertrophy and other comorbidities. Maybe I missed that in terms of what you are saying?
Runge, Chapel Hill: No. No, you are saying exactly what we saw, and I don't have a good explanation for it. The only explanation that I have, which I'll throw out again, is that part of the diastolic hypertension is resulting from the diastolic dysfunction of the heart. That too doesn't carry a lot of water. I really just do not have a good explanation for what we have observed.
Oates, Nashville: Well, I think your point about the mitochondrial contribution reminds me of the work that sort of ties together yours and Wayne's talk. Jack Roberts showed that a marker of lipid peroxidation, the F2-isoprostanes, which are elevated in obese individuals, that within three days of starting a 25% reduction in calories, that the isoprostane levels fall to normal before any weight loss, obviously, can have occurred. So that suggests that providing calories to your mitochondria very acutely generates free radicals and lipid peroxidation.
Runge, Chapel Hill: Very interesting point. Thank you.
REFERENCES
- 1.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brümmer J, Gutzki FM, Berger J, Frölich JC, Böger RH. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109:843–8. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 2.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 3.Givertz MM, Colucci WS. New targets for heart-failure therapy: endothelin, inflammatory cytokines, and oxidative stress. Lancet. 1998;352(suppl 1):SI34–8. doi: 10.1016/s0140-6736(98)90017-4. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrova M, Bochev P, Markova V, Bechev B, Popova M, Danovska M, Simeonova V. Dynamics of free radical processes in acute ischemic stroke: influence on neurological status and outcome. J Clin Neurosci. 2004;11:501–6. doi: 10.1016/j.jocn.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med. 2005;39:841–52. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Cipollone F, Fazia M, Iezzi A, Pini B, Costantini F, De Cesare D, Paloscia L, Materazzo G, D'Annunzio E, Bucciarelli T, Vecchiet J, Chiarelli F, Cuccurullo F, Mezzetti A. High preprocedural non-HDL cholesterol is associated with enhanced oxidative stress and monocyte activation after coronary angioplasty: possible implications in restenosis. Heart. 2003;89:773–9. doi: 10.1136/heart.89.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madamanchi NR, Vendrov A, Runge MS. Reactive oxygen species signals leading to vascular dysfunction and atherosclerosis. In: Willard H, Ginsburg G, editors. Genomic and Personalized Medicine. New York, NY: Academic Press; 2008. [Google Scholar]
- 8.Vavalle JP, Runge MS. In: Identifying Patients at Highest Risk for Acute Coronary Syndromes: Plaque Rupture and “Immediate Risk”. In: Runge MS, Stouffer GA, Patterson WC, editors. Netter's Cardiology. 2nd ed. Philadelphia, PA: Elsevier Inc.; (in press) [Google Scholar]
- 9.Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J Appl Physiol. 2008;105:1695–705. doi: 10.1152/japplphysiol.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dr|$$|Adoge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci. 2005;360:2355–72. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pansarasa O, Bertorelli L, Vecchiet J, Felzani G, Marzatico F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic Biol Med. 1999;27:617–22. doi: 10.1016/s0891-5849(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 12.Rad|$$|Aaak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18:749–50. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- 13.Bloomer RJ. Effect of exercise on oxidative stress biomarkers. Adv Clin Chem. 2008;46:1–50. doi: 10.1016/s0065-2423(08)00401-0. [DOI] [PubMed] [Google Scholar]
- 14.Werner C, Fürster T, Widmann T, Pöss J, Roggia C, Hanhoun M, Scharhag J, Büchner N, Meyer T, Kindermann W, Haendeler J, Böhm M, Laufs U. Physical Exercise Prevents Cellular Senescence in Circulating Leukocytes and in the Vessel Wall. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.861005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–5. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 16.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–94. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- 17.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–3. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 18.Hartley CJ, Taffet GE, Reddy AK, Entman ML, Michael LH. Noninvasive cardiovascular phenotyping in mice. ILAR J. 2002;43:147–58. doi: 10.1093/ilar.43.3.147. [DOI] [PubMed] [Google Scholar]