Abstract
Methods developed in the early 1970s to highly purify factor VIII (FVIII) from the plasma of large numbers of blood donors led, for the first time, to concentrates of FVIII that enabled hemophiliac to self-treat, providing independence and opening the way to safe surgery and other treatments. But, with the introduction of blood-borne viruses such as HIV-1 and hepatitis C viruses into the blood supply, these concentrates also transmitted HIV and hepatitis to a high percentage of hemophiliacs. Nevertheless, from the depths of the AIDS epidemic in hemophilia came extraordinary scientific advances that led to recombinant FVIII, the identification of HIV as the agent causing AIDS, the eventual development of effective treatments for AIDS, gene transfer approaches using lentiviruses, and treatments for hepatitis C. All of these have improved the lives of current and future hemophiliacs and have brought us to the threshold of a cure.
“Things don't get better or worse: they evolve and transform themselves”
—L. Berio
When I came into the field in the early 1970s, hemophilia care was in a state of transition. Treatment for patients was traditionally with fresh frozen plasma (FFP), which contained factor VIII and factor IX, and with infusion of FFP, one could achieve plasma levels in the range of 30% before volume overload was encountered. Cryoprecipitate, a cold precipitate of fresh frozen plasma which could be re-dissolved in FFP, contained concentrated amounts of factor VIII and became the preferred treatment in the mid-to-late 1960s. In the late 1960s, glycine-precipitated plasma fractions containing even higher concentrations of factor VIII were developed and were being used in the early 1970s. At that time, the average lifespan of a hemophiliac was about 42 years. Hepatitis B and a form of blood-borne hepatitis that was neither hepatitis A nor hepatitis B, called non-A, non-B hepatitis and now known as hepatitis C, was appearing in the hemophilia population. AIDS was 7 or so years away from the first report in humans. There was essentially no treatment for the 25–30% of hemophiliacs who developed inhibitory antibodies to infused clotting factor, rendering them unresponsive to subsequent treatment.
What has happened in the 35 years since then is a remarkable story. It is remarkable because of the changes that have occurred that now make the lives of hemophiliacs better than ever before. But it is also remarkable because of the tragedy that occurred along the way with the development of blood-borne infections. Ultimately, the story that I wish to tell is about the incredible power of medical research to take a negative and turn it into a positive.
AIDS IN HEMOPHILIA—THE DARK DAYS
With the development of cryoprecipitate and even more so with the development of glycine-precipitated factor VIII concentrates (1), hemophiliacs could receive treatment at home and learn self-treatment. This opened up a whole new world in which hemophiliacs could treat themselves more rapidly, limiting the blood in their joints; treat themselves at home, decreasing their dependence on the emergency room; treat themselves prophylactically prior to activities that might cause bleeding; and travel. In addition, the ability to achieve normal factor VIII levels with glycine-precipitated concentrates facilitated surgery and enabled hemophiliacs to undergo joint replacement procedures that had not been possible previously. It seemed that hemophilia treatment had never been better. All of this changed when AIDS made its way into the blood supply. AIDS was first described in December 1981 in a paper by Gottlieb and colleagues in the New England Journal of Medicine (2). The first description of AIDS in hemophilia appeared about six months later in the Center for Disease Control's Medical and Morbidity Weekly Report (3). The CDC report provided strong evidence that AIDS was spread by blood. Altogether, almost 5000 hemophiliacs were to become infected with HIV before concentrates were rendered safe, and more than 4000 of the estimated 10,000 hemophiliacs in the US would eventually die of AIDS (Fig. 1). At my center, there were more than 135 deaths due to AIDS before effective treatments were developed. These were dark times for individuals in the hemophilia community. The freedom patients had gained disappeared as they stopped treating all but the most serious bleeds. Treaters were confused about what to recommend. Patients that the other members of the hemophilia team and I had followed for years began to die of complications related to AIDS. Because the treatment of hemophilia is from birth to death, the staff of a hemophilia center becomes unusually attached to their patients and the patients to the staff. We knew their goals and aspirations; we knew their shortcomings and their failures. We had seen how their goals had to be modified by their hemophilia, and now we saw that their goals might never be achieved. There was a larger concern in the hemophilia community that AIDS would wipe out the disorder. It was a devastating time and one that has been chronicled by others (4).
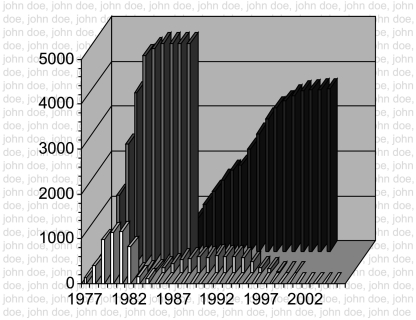
Fig. 1.
AIDS and Hemophilia 1977–2002. The graphs show national numbers for yearly and cumulative infection with HIV-1 and death in hemophilia. Front row bars, number of individuals infected by year; second row bars, number of deaths due to AIDS by year; third row bars, cumulative infections by year; last row bars, cumulative deaths by year. There were 3915 deaths in hemophilia nationwide at the time of this analysis; 135 hemophiliacs died of AIDS at the UNC Center. The names in the background were the 135 patients who died at the UNC Center, but have been changed to John Doe to comply with HIPAA regulations. (copyright G. C. White, II).
From these depths came a number of scientific discoveries that have now changed the treatment of hemophilia. These discoveries were fueled by the tragedy that was happening in the hemophilia community and driven by a robust collaboration between academic medical centers, the fledgling biotechnology industry and pharmaceutical companies. In retrospect, these discoveries came relatively rapidly, but they did not come fast enough to save scores of infected individuals.
THE CLONING OF FACTOR VIII AND THE DEVELOPMENT OF RECOMBINANT PRODUCTS
The first scientific discovery I want to focus on is the cloning of the F8 gene and the development of safe concentrates. The initial response by the manufacturers of concentrates to the discovery that AIDS was transmitted by their products was to heat-treat their concentrates (5, 6). This was really an attempt to inactivate non-A, non-B hepatitis in the concentrates, which it did not do (7). But it did inactivate HIV-1. Later, solvent-detergent extraction was developed and found to be effective in removing lipid enveloped viruses, such as HIV-1, from the glycine-precipitated concentrates (8, 9). As a consequence of these two steps, heat treatment and solvent detergent extraction, there were essentially no new cases of HIV-1 after 1985. Still later, methods to purify clotting factors by immunoaffinity chromatography were developed, and this added to the viral reduction and therefore the safety of the products (10, 11). In addition, with the availability of ultrapure FVIII, it was possible to obtain for the first time partial amino acid sequence of FVIII. This, in turn, enabled two groups, one at Genetics Institute in Boston and one at Genentech in South San Francisco, to clone the F8 gene (12–15). At the time it was cloned, F8 was by far the largest gene so cloned. Once cloned, the race was on to develop recombinant forms of factor VIII. Just three years later, in March of 1987, the first infusion of recombinant factor VIII was given at our Hemophilia Center (16) (Fig. 2). The individual selected to receive the product, G.M., was a highly articulate 43-year old man, who had been one of the first recipients of glycine-precipitated factor VIII when it had been developed. During his informed consent, I explained to him that the product had been made in Chinese Hamster Ovary (CHO) cells and that one of the concerns was that, being produced in a non-human cell, it might not fold properly and antibodies might develop. I explained that by all methods of comparison, the CHO-produced protein was identical to the protein isolated from human plasma, but antibody formation was still a potential risk. When the day for the initial infusion came, there was a news crew in our Clinical Research Unit to film the occasion. The lights were bright and I was hot and nervous. As I was infusing the material, I noticed that G.M. was sitting with his eyes closed and his chin was resting on his chest. I asked if he was ok, but he did not answer. I asked again, but no answer. Louder, I said, “speak to me”. He looked up at me and started making hamster noises, intending to suggest that the infusion of a product manufactured in hamster cells had turned him into a hamster. After the product was licensed, the patient was invited to Genetics Institute Headquarters in Cambridge, MA for a celebration. During the celebration, Gabe Schmergel, the CEO of Genetics Institute, invited the patient to make some comments. After slowly and painfully climbing to a balcony half way up the stairs, he delivered a powerful story about what it was like to grow up with hemophilia without adequate treatment, how as a child he had lost a beloved older brother from a bleed, and how important the development of safe recombinant factors was to him and all people with hemophilia; his comments had the entire company in tears.
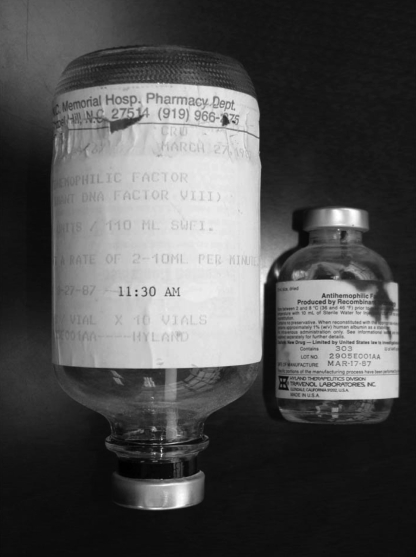
Fig. 2.
Initial Vials of rFVIII infused to a Hemophiliac. The infusion set for the first administration of rFVIII to a human on March 27, 1987 is shown on the left. The vial containing the first clinical lot (#2905E001AA1) of rFVIII manufactured on March 17, 1987 is shown on the right (copyright G. C. White, II).
Today, more than 88% of the factor VIII administered in the US is recombinant. Follow-on products formulated in buffers that contain no human proteins have been subsequently developed, and so-called third-generation products are available now that are completely devoid of human proteins. As a result, current products are considered exceedingly safe, certainly safer than the risk of driving to the clinic to see the physician.
GENERIC FACTORS, GENETIC ENGINEERING AND THE SUPER-MOLECULES OF THE FUTURE
The cost of recombinant FVIII is approximately $0.90 per unit and the average adult hemophiliac may use 50,000 units a year in a typical on-demand therapy regimen. For a prophylaxis regimen, the cost can be two or even three times the cost of on-demand therapy. One of the great promises of recombinant DNA technology has been that it might be used to reduce the cost of treatment through improvements in the efficiency of FVIII production or by engineering functionally enhanced molecules, especially molecules with a longer half-life in the circulation. Although efforts in this direction were held up for a number of years by patents on FVIII production and purification and by the orphan drug law, the orphan exclusivity originally granted to the first generation FVIII products in February 1993 expired in February 2000, and some of the patents on FVIII have expired in the ensuring years. As a result, there is now a flurry of activity by a number of companies to develop new products.
The first targets have been the development of biosimilar materials at a reduced cost. Despite early explorations in duckweed and tobacco, the post-translational modifications in FVIII and FIX preclude production in plants. For reasons that remain incompletely understood, FVIII is inefficiently synthesized. Sequences in the B-domain, a portion of the molecule that is excised when FVIII is activated, are believed to be responsible for the inefficient synthesis (17), and retention of FVIII in the endoplasmic reticulum leads to the generation of an unfolded protein response (18) that is toxic to the cell. Better understanding of the mechanisms involved will eventually enable the engineering of a molecule that can be produced more cheaply. For now, increased competition between products synthesized in mammalian systems similar to the currently available products will have some effect on cost.
At the same time that biosimilar products are being developed, there is considerable interest by pharmaceutical companies in developing genetically engineered molecules that have improved properties (Table I). Once again, the cloning of factor VIII was critically important to this concept of developing genetically improved molecules. Cloning enabled the production of large quantities of protein, which permitted the crystallization of portions of the molecule, which provided structure. Crystals of the C-domain repeats have been achieved (19), and low-resolution structure of the B-domain deleted human FVIII has been published recently (20). Cloning and expression of FVIII also permitted mutagenesis studies which provided information about function. Altogether, these techniques have resulted in an unprecedented understanding of structure and function of FVIII, which forms the basis for the current interest in genetic engineering.
TABLE 1.
Next Generation Products
| Prolong clotting factor circulation |
| Polyethylene glycol |
| Immunoglobulin fusion molecules |
| Target degradation pathway |
| Decrease inactivation of clotting factor |
| Multiple sequences that mediate inactivation |
| Inhibit subunit dissociation |
| Increase clotting factor activity |
| Increase rate of inactivation |
| Increase catalytic activity |
| Increase synthesis of clotting factor |
A significant limitation of the current products is their rate of clearance. The half-life of FVIII is approximately 12 hours. Recent studies suggest that one mechanism for the removal of FVIII is through binding to the low-density lipoprotein receptor related protein (LRP) (21, 22). Binding involves sequences in the A3 domain of FVIII and the second cluster of LDL receptor class A repeats of LRP (23, 24). In LRP-deficient mice, the FVIII half-life is increased by nearly 30%, showing that LRP is involved in clearance but that other mechanisms are also involved. While there is interest in mutant forms of FVIII that are unable to bind LRP, the first real attempts to develop a form of FVIII with a longer half-life will be to pegylate the molecule. Pegylation has been demonstrated to prolong the half-life of other proteins and is an approach that has been approved by regulatory agencies. First-in-human trials with pegylated FVIII are currently underway.
All of these advances in knowledge about the structure, function, production and catabolism of FVIII are the direct outcome of the cloning of FVIII. They all came about because of the need of the hemophilia community for safer concentrates, free of blood-borne viruses. They have all occurred because of the tragedy of AIDS.
THE IDENTIFICATION AND CHARACTERIZATION OF HIV-1 AND HCV
Another important scientific discovery was the identification of HIV-1 (known then as LAV or HTLV-III) as the agent that causes AIDS (25–29). Hemophilia played an important role in the identification of HIV-1 as the causative agent of AIDS in both the Gallo and Montagnier labs. In the Gallo lab, it was plasma samples from a patient at our Hemophilia Center at the University of North Carolina that contained very high levels of antibody that was key to the identification of HIV, as published in Science in 1984 (Tables 2 and 3). A patient, E.T. in the Tables, presented with enlarged lymph nodes, and lymphoma was in the differential. Both lymph node and plasma samples were sent to Gallo as part of a collaboration to examine AIDS in the hemophilia population. The identification and subsequent characterization of the HIV-1 virus was important for diagnosis but also, critically, for the development of treatments. The first anti-retroviral agent, Zidovudine, came out in 1985 (30, 31). Our center participated in those initial clinical trials, but patients continued to die. Later, the role of the HIV protease in viral replication was identified (32) and inhibitors of the HIV protease were developed, providing for the first time highly active anti-retroviral therapy (HAART), which has revolutionized the treatment of AIDS (33, 34). In 1992, a 15-year old hemophiliac named Ricky Ray, who challenged attempts in Florida to bar him from attending school, died setting in motion events that eventually led to the Ricky Ray Bill for victims of AIDS.
TABLE 2.
Response of cloned T-cell populations to infection with HTLV-III
| Characteristics after infection | Clones |
|||||||
|---|---|---|---|---|---|---|---|---|
| H3 | H4 | H6 | H17 | H31 | H31 | H35 | H38 | |
| Total cell number (×106) | ||||||||
| At 6 days | 1 | 1.5 | 1.5 | 0.3 | 0.4 | 0.3 | 0.5 | 1.8 |
| At 14 days | 2.2 | 7.3 | 7.5 | 10 | 4.7 | 5.0 | 4.5 | 3.2 |
| Multinucleated cells (%) | ||||||||
| At 6 days | 24 | 42 | 32 | 7 | 13 | 14 | 30 | 45 |
| At 14 days | 45 | 48 | 45 | 30 | 22 | 45 | 60 | 60 |
| Innunofluorescence positive cells (%) | ||||||||
| At 6 days | ||||||||
| Rabbit antiserum to HTLV-III | 55 | 56 | 32 | 32 | 39 | 21 | 10 | 87 |
| Patient serum (E.T.) | 56 | 29 | 21 | ND | ND | ND | ND | 73 |
| At 14 days | ||||||||
| Rabbit antiserum to HTLV-III | 50 | 74 | 60 | 97 | 71 | 40 | 20 | 80 |
| Patient serum | 45 | 47 | 56 | 78 | 61 | 43 | 22 | 89 |
| Reverse transcriptase activity (×104 cpm/ml) | ||||||||
| At 6 days | 2.4 | 1.8 | 2.1 | 4.1 | 2.6 | 1.4 | 1.7 | 2.5 |
| At 14 days | 16.2 | 18.1 | 16.1 | 20.2 | 17.1 | 13.4 | 15.1 | 18.2 |
Culture fluids from the infected parental cell line were positive for particulate RT activity, and about 20 percent of the infected cell population was positive in an indirect immune fluorescence assay in which we used serum from a hemophilia patient with pre-AIDS (patient E.T.). Serum from E.T. also contained antibodies to proteins of disrupted HTLV-III but did not react with cells infected with HTLV-I or HTLV-II. (from Popovic, et al. [25] with permission)
TABLE 3.
Isolation of HTLV-III from patients with AIDS and pre-AIDS
| Patient | Diagnosis | Origin | Virus expression |
|||
|---|---|---|---|---|---|---|
| RT Activity (×104 cpm) | Percent positive cells in immune fluorescence assay |
Electron microscopy | ||||
| Rabbit anti-serum | Serum from E.T. | |||||
| R.F. | AIDS (heterosexual) | Haiti | 0.25 | 80 | 33 | ND |
| S.N. | Hemophiliac (lymphadenopathy) | United States | 6.3 | 10 | ND | + |
| B.K. | AIDS (homosexual) | United States | 0.24 | 44 | 5 | + |
| L.S. | AIDS (homosexual) | United States | 0.13 | 64 | 19 | + |
| W.T. | Hemophiliac (lymphadenopathy) | United States | 3.2 | 69 | ND | ND |
The percentage of T cells positive for viral antigen ranged from 10 to 80 percent, as determined by immune fluorescence assays with serum from patient E.T. and with antiserum from rabbits infected repeatedly with disrupted HTLV-III. (from Popovic, et al. [25] with permission)-S.N. and W.T. were from the UNC Center.
Research also proceeded on hepatitis C. The virus was eventually cloned and identified as a member of the flaviviridae family (35). As with HIV, increased understanding of hepatitis C virus (HCV) resulted in the development of new approaches to treatment. Hemophiliacs and our center played an important role in the development of interferon α-2b plus ribavirin as an effective form of treatment (36). Nevertheless, hepatitis C, especially hepatitis due to the type 1 genotype, remains difficult to treat, and sustained virologic responses occur in less than half of patients with this genotype on pegylated interferon and ribavirin. Following the observations that protease inhibitors were an effective therapy in HIV, efforts proceeded to characterize the virally encoded chymotrypsin-like serine proteinase located in the N-terminal domain of non-structural protein 3 (NS3) in HCV. Activity of this critical enzyme required NS4A, a 54-residue polyprotein cleavage product, to form a stable complex with the NS3 domain (37, 38). Recent work shows that inhibitors of the NS3/4A proteinase in combination with interferon and ribavirin significantly improved sustained virologic response rates in genotype 1 HCV compared with interferon and ribavirin alone (39).
VIRUSES AND GENE TRANSFER IN HEMOPHILIA
At the same time that the treatment of AIDS progressed, the genome of HIV and the function of the various gene products were being characterized, and retroviruses, first Moloney murine leukemia virus and later lentiviruses including HIV, were being developed as gene transfer vectors (40–42). Characteristics that made them strong gene transfer agent candidates were their efficient entry into cells, their ability to integrate into the chromosome, albeit in a random manner, the ability to pseudotype the virus to target it to specific cells, the ability to render the virus replication-defective through employment of a packaging system, and the size of the virus genome. Thus, the virus that had caused so much suffering and death in the hemophilia population had developed some potential to give back to the community. After two ex vivo gene transfer trials, one in China and one in the US (43), the first in vivo gene transfer trial was initiated (44). This was a trial using a Moloney-based retrovirus containing the cDNA for factor VIII. The first subject that I enrolled in the study was a 51-year old hemophiliac, who had severe joint disease involving multiple joints and was blind in one eye from an accident complicated by bleeding. He participated in the trial because he had two grandsons, and he did not want them to go through what he had gone through. He did well and had levels of factor VIII up to 1.7% and a reduction in his frequency of bleeding, but overall the trial did not show enough of a dose-response effect to move forward as a therapeutic. Later studies using adeno-associated virus as a vector achieved factor levels up to 11.8% but with significant hepatotoxicity (45). Although there are no current ongoing clinical trials, hemophilia continues to be an important target for gene transfer. It is a good target because the gene is known; there are good assays for factor VIII and IX; levels of clotting factor do not have to be tightly regulated as, for example, insulin; there are good large and small animal models that facilitate pre-clinical studies; and satisfactory treatment is available so gene transfer can proceed at a safe pace.
CONCLUSIONS
At the outset, I indicated that this is ultimately a story of the power of medical research and a remarkable response by scientists to a tragedy. It's a story that needs to be told; it needs to be told to those who think that our research isn't translational enough; to those who fund research because it illustrates what research can accomplish; and to students so they can see the value of research. After the advances of the past 35 years, the average life span of a hemophiliac today is over 60 years, nearly that of a non-hemophiliac male. And the quality of life is considerably improved. There have been no new cases of product-transmitted hepatitis C or HIV in well over a decade, and many of the patients who survived to see the development of protease inhibitors remain alive today. The availability of safe replacement therapy has re-kindled efforts to implement primary prophylaxis treatment for children and adolescents and, in some countries, adults.
But, this is not the end of this story; it remains a story in evolution. Hopefully, the treatment of hemophilia in 35 years will look quite different from today, whether it is longer-lasting concentrates that mean treatment is needed but once a month, or concentrates that are cheap enough that patients can be treated daily for their entire life, or even oral forms of factor. Hopefully, gene transfer will be a reality in 35 years, and we can talk realistically about a cure. The remarkable scientific advances of the past 35 years hold out the promise that all of these things might be a reality in the next 35. I am grateful to have been a part of the story and that I have been able to see these amazing changes. I can not and will not forget those who were involved.
Footnotes
Potential Conflicts of Interest: Dr. White has received honoraria from the Baxter, Wyeth and Bayer companies, been a consultant for Archemix and Inspiration, and is currently a member of a Baxter DSMB.
DISCUSSION
Schiffman, Providence: Gil that was a beautiful presentation. Can you talk a little bit about von Willebrand antigen and its possible use in preventing the degradation of recombinant Factor VIII? Has that been studied? We know that natively, von Willebrand factor is a protector of Factor VIII degradation. Has this been a manipulation that you have considered?
White, Milwaukee: Yes, I think it's a story in evolution. Clearly there is some relationship between von Willebrand and Factor VIII. Von Willebrand has to dissociate from Factor VIII for Factor VIII to be activated and yet Factor VIII has to be carried around by von Willebrand factor. So, it's a necessary relationship. One of the major questions now is whether the immunogenicity of von Willebrand factor containing Factor VIII is less than the immunogenicity of von Willebrand factor deleted Factor VIII and I think it's a story in evolution. It's a story I wasn't really convinced of until the last year or two, but I think there is some evidence now that there may be a difference, and I think studies to look at this more carefully are going to be very important, but I don't think the answer is known at this point in time.
High, Philadelphia: So, Gil that was really a terrific overview of how hemophilia care has changed in the last three to five decades, and it really was a great talk. I wanted to ask your perspective on two questions. One is that what you told us about today is really about improvement in life expectancy for hemophilia patients in the western world, and I wonder, because you've been so active on the international scene, if you can give us your perspective on whether these advances with, for example, the generation of generic recombinant factors, when will these benefits move into the rest of the world's hemophilia population, which, you know, still suffers from that low life expectancy?
White, Milwaukee: That's a great question. I think clearly these advances can be applied everywhere. It's just a monetary issue. They have to be applied everywhere. We, as a world, need to do it. I do think generic Factor VIII and generic Factor IX are going to be a reality. I think their production is going to be cheaper, but I don't think we have to wait until we have those generic products to do the things that we need to do in underdeveloped parts of the world. Manufacturers do make products available at reduced rates in underdeveloped parts of the world. Another part of the question you raise is providers. You don't have treaters in underdeveloped parts of the world that understand the products. So, I think it has got to be not only the products; its got to be us as physicians that go out and try and help. A good example is the HIV field. I think Mike Cohen, for example, and his group from UNC used to go over to Africa and provide care over in Africa, and I think we as a hemophilia group probably ought to be doing the same sort of thing. It takes both. It is going to take product, and it is going to take people, but it is something we have to do.
High, Philadelphia: My other question for you is just from your perspective. Now that we have a generation of young people who are emerging into young adulthood with fairly intact joints, really an absence of joint disease, do you think that those people are going to require the same intensity of prophylaxis through adulthood, or do you think that we are going to be able to get away with something less, because the cost of maintaining that kind of prophylaxis for an adult is really prohibitive I'm afraid.
White, Milwaukee: Well just to amplify, it's prohibitive because we get bigger, and we weigh more so it takes more Factor VIII or Factor IX. So the cost, once you're above about 17 or 18-years-old, really does become enormous. So the answer, I think, is probably a two-part answer. One, I do think it is going to take fairly aggressive prophylaxis to keep them in good health; but will it take the same level of prophylaxis? Maybe not, because I think we get smarter as we get older, and I think we don't try to do things that we shouldn't do; and so we start driving a little less fast in cars, and we stop jumping off of heights that we shouldn't jump off of. So I think a little less prophylaxis will be okay, but I think it will still be prophylaxis.
Boxer, Ann Arbor: Gil, could you comment on other novel ways to deliver Factor VIII? Say through platelets and megakaryoctes.
White, Milwaukee: I'd be glad to. So Factor VIII is needed where a developing clot is occurring, and one way the people have envisioned targeting that is to deliver Factor VIII in platelets. Platelets naturally go to where a clot is needed, and if you could target Factor VIII to that area through platelet release it would be a good thing. I thought your question was going to be something a little bit different, Larry. There are some studies that are going on now with a fusion molecule between Factor IX and an Fc receptor. That Fc receptor is a receptor that can be taken up in nasal epithelia and respiratory epithelia through the neonatal Fc receptor, and so there is some interest now in delivering Factor VIII through nasal and respiratory passages, which I think is also a positive thing.
Runge, Chapel Hill: Is there an individual range in terms of the levels of Factor VIII replacement that you have to have to have adequate prophylaxis from person-to-person, or is it generally the same level from one person to another, albeit the dose might be different?
White, Milwaukee: I think what we try and do is just give a level, but I think the real answer is that we're all individuals, and it will take an individual approach. Generally, if you can keep levels above about 3 or 4%, it works fine. But, like every other drug, Factor VIII is individually and pharmacogenomically metabolized, and I think in the future that probably is going to be something that is done. You determine what a person's individual fall-off of Factor VIII is and then tailor a prophylaxis regimen to that.
REFERENCES
- 1.Brinkhous KM, Shanbrom E, Roberts HR, et al. A new high-potency glycine-precipitated antihemophilic factor (AHF) concentrate. Treatment of classical hemophilia and hemophilia with inhibitors. Jama. 1968;205:613–7. [PubMed] [Google Scholar]
- 2.Gottlieb MS, Schroff R, Schanker HM, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305:1425–31. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 3.Pneumocystis carinii pneumonia among persons with hemophilia A. MMWR Morb Mortal Wkly Rep. 1982;31:365–7. [PubMed] [Google Scholar]
- 4.Gray L, Chamberlain C. The Gift of Experience. Conversations about Hemophilia. Brunswick, ME: Camden Writers; 2007. [Google Scholar]
- 5.Petricciani JC, McDougal JS, Evatt BL. Case for concluding that heat-treated, licensed anti-haemophilic factor is free from HTLV-III. Lancet. 1985;2:890–1. doi: 10.1016/s0140-6736(85)90155-2. [DOI] [PubMed] [Google Scholar]
- 6.Rouzioux C, Chamaret S, Montagnier L, et al. Absence of antibodies to AIDS virus in haemophiliacs treated with heat-treated Factor VIII concentrate. Lancet. 1985;1:271–2. doi: 10.1016/s0140-6736(85)91043-8. [DOI] [PubMed] [Google Scholar]
- 7.Colombo M, Mannucci PM, Carnelli V, et al. Transmission of non-A, non-B hepatitis by heat-treated factor VIII concentrate. Lancet. 1985;2:1–4. doi: 10.1016/s0140-6736(85)90055-8. [DOI] [PubMed] [Google Scholar]
- 8.Horowitz B, Wiebe ME, Lippin A, et al. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion. 1985;25:516–22. doi: 10.1046/j.1537-2995.1985.25686071422.x. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz MS, Rooks C, Horowitz B, et al. Virus safety of solvent/detergent-treated antihaemophilic factor concentrate. Lancet. 1988;2:186–9. doi: 10.1016/s0140-6736(88)92288-x. [DOI] [PubMed] [Google Scholar]
- 10.Lusher JM. Viral safety and inhibitor development associated with monoclonal antibody-purified F VIII:C. Ann Hematol. 1991;63:138–41. doi: 10.1007/BF01703244. [DOI] [PubMed] [Google Scholar]
- 11.Addiego JE, Jr, Gomperts E, Liu SL, et al. Treatment of hemophilia A with a highly purified factor VIII concentrate prepared by anti-FVIIIc immunoaffinity chromatography. Thromb Haemost. 1992;67:19–27. [PubMed] [Google Scholar]
- 12.Toole JJ, Knopf JL, Wozney JM, et al. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984;312:342–7. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- 13.Gitschier J, Wood WI, Goralka TM, et al. Characterization of the human factor VIII gene. Nature. 1984;312:326–30. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
- 14.Wood WI, Capon DJ, Simonsen CC, et al. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984;312:330–7. doi: 10.1038/312330a0. [DOI] [PubMed] [Google Scholar]
- 15.Vehar GA, Keyt B, Eaton D, et al. Structure of human factor VIII. Nature. 1984;312:337–42. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- 16.White GC, 2nd, McMillan CW, Kingdon HS, et al. Use of recombinant antihemophilic factor in the treatment of two patients with classic hemophilia. N Engl J Med. 1989;320:166–70. doi: 10.1056/NEJM198901193200307. [DOI] [PubMed] [Google Scholar]
- 17.Miao HZ, Sirachainan N, Palmer L, et al. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103:3412–9. doi: 10.1182/blood-2003-10-3591. [DOI] [PubMed] [Google Scholar]
- 18.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 19.Liu ML, Shen BW, Nakaya S, et al. Hemophilic factor VIII C1- and C2-domain missense mutations and their modeling to the 1.5-angstrom human C2-domain crystal structure. Blood. 2000;96:979–87. [PubMed] [Google Scholar]
- 20.Ngo JC, Huang M, Roth DA, et al. Crystal structure of human factor VIII: implications for the formation of the factor IXa-factor VIIIa complex. Structure. 2008;16:597–606. doi: 10.1016/j.str.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Lenting PJ, Neels JG, van den Berg BM, et al. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. J Biol Chem. 1999;274:23734–9. doi: 10.1074/jbc.274.34.23734. [DOI] [PubMed] [Google Scholar]
- 22.Saenko EL, Yakhyaev AV, Mikhailenko I, et al. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. J Biol Chem. 1999;274:37685–92. doi: 10.1074/jbc.274.53.37685. [DOI] [PubMed] [Google Scholar]
- 23.Bovenschen N, Boertjes RC, van Stempvoort G, et al. Low density lipoprotein receptor-related protein and factor IXa share structural requirements for binding to the A3 domain of coagulation factor VIII. J Biol Chem. 2003;278:9370–7. doi: 10.1074/jbc.M212053200. [DOI] [PubMed] [Google Scholar]
- 24.Sarafanov AG, Makogonenko EM, Pechik IV, et al. Identification of coagulation factor VIII A2 domain residues forming the binding epitope for low-density lipoprotein receptor-related protein. Biochemistry. 2006;45:1829–40. doi: 10.1021/bi0520380. [DOI] [PubMed] [Google Scholar]
- 25.Popovic M, Sarngadharan MG, Read E, et al. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 26.Gallo RC, Salahuddin SZ, Popovic M, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–3. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 27.Schupbach J, Popovic M, Gilden RV, et al. Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science. 1984;224:503–5. doi: 10.1126/science.6200937. [DOI] [PubMed] [Google Scholar]
- 28.Sarngadharan MG, Popovic M, Bruch L, et al. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984;224:506–8. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- 29.Montagnier L, Gruest J, Chamaret S, et al. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984;225:63–6. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- 30.Mitsuya H, Weinhold KJ, Furman PA, et al. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985;82:7096–100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarchoan R, Klecker RW, Weinhold KJ, et al. Administration of 3′-azido-3′-deoxythymidine, an inhibitor of HTLV-III/LAV replication, to patients with AIDS or AIDS-related complex. Lancet. 1986;1:575–80. doi: 10.1016/s0140-6736(86)92808-4. [DOI] [PubMed] [Google Scholar]
- 32.Kramer RA, Schaber MD, Skalka AM, et al. HTLV-III gag protein is processed in yeast cells by the virus pol-protease. Science. 1986;231:1580–4. doi: 10.1126/science.2420008. [DOI] [PubMed] [Google Scholar]
- 33.Collier AC, Coombs RW, Schoenfeld DA, et al. Combination therapy with zidovudine, didanosine and saquinavir. Antiviral Res. 1996;29:99. doi: 10.1016/0166-3542(95)00928-0. [DOI] [PubMed] [Google Scholar]
- 34.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–9. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 35.Choo QL, Kuo G, Weiner AJ, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–62. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 36.Fried MW, Peter J, Hoots K, et al. Hepatitis C in adults and adolescents with hemophilia: a randomized, controlled trial of interferon alfa-2b and ribavirin. Hepatology. 2002;36:967–72. doi: 10.1053/jhep.2002.35529. [DOI] [PubMed] [Google Scholar]
- 37.Kwong AD, Kim JL, Rao G, et al. Hepatitis C virus NS3/4A protease. Antiviral Res. 1998;40:1–18. doi: 10.1016/s0166-3542(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 38.Bartenschlager R. The NS3/4A proteinase of the hepatitis C virus: unravelling structure and function of an unusual enzyme and a prime target for antiviral therapy. J Viral Hepat. 1999;6:165–81. doi: 10.1046/j.1365-2893.1999.00152.x. [DOI] [PubMed] [Google Scholar]
- 39.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–38. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 40.Eglitis MA, Kantoff P, Gilboa E, et al. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985;230:1395–8. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- 41.Yu SF, von Ruden T, Kantoff PW, et al. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986;83:3194–8. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilboa E. Retroviral gene transfer: applications to human therapy. Prog Clin Biol Res. 1990;352:301–11. [PubMed] [Google Scholar]
- 43.Roth DA, Tawa NE, Jr, O'Brien JM, et al. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. 2001;344:1735–42. doi: 10.1056/NEJM200106073442301. [DOI] [PubMed] [Google Scholar]
- 44.Powell JS, Ragni MV, White GC, 2nd, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102:2038–45. doi: 10.1182/blood-2003-01-0167. [DOI] [PubMed] [Google Scholar]
- 45.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–7. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]