Abstract
Tnk1/Kos1 is a non-receptor protein tyrosine kinase implicated in negative regulation of cell growth by a mechanism involving inhibition of Ras activation and requiring Tnk1/Kos1's intrinsic catalytic activity. Tnk1/Kos1 null mice were created by homologous recombination by deleting the catalytic domain. Upon aging, both Tnk1+/− and Tnk1−/− mice develop spontaneous tumors, including lymphomas and carcinomas at high rates (i.e. 27%, and 43%, respectively), indicating that Tnk1/Kos1 is a tumor suppressor. Tissues from Tnk1/Kos1-null mice exhibit proportionally higher levels of basal and growth factor-stimulated Ras activation. Mechanistically, Tnk1/Kos1 requires either or both Y277 and Y287 sites to be intact for enzymatic activity and phosphorylation of its substrate, growth factor receptor binding protein 2 (Grb2). Data indicate that following tyrosine phosphorylation of Grb2 by Tnk1/Kos1, the Grb2-Sos1 guanine exchange factor (GEF) complex that mediates growth factor stimulated Ras activation becomes disrupted, resulting in the reversal of Ras activation. Conversely, the loss of Tnk1/Kos1 activity results in constitutive activation of Ras due to prolonged stabilization/activation of the Grb2-Sos1 GEF activity. Tnk1/Kos1 is the first tyrosine kinase discovered to have tumor suppressor activity, and the mechanism of spontaneous tumor formation involves constitutive, indirect activation of Ras. Thus, Ras may display “oncogenic activity” without undergoing “oncogenic” mutation. We now find that a cohort of patients with diffuse large B-cell lymphoma (DLBCL) display downregulation of Tnk1/Kos1 that may account for tumorigenesis in humans.
ROLE OF PROTEIN TYROSINE KINASES ON GROWTH
Receptor as well as non-receptor protein tyrosine kinases (RPTKs, NRPTKs) are major upstream regulators of growth and differentiation (1, 2). PTKs function by phosphorylating protein substrates on tyrosine residues, a dynamic event that is opposed by the action of protein tyrosine phosphatases (3, 4). Activation of either RPTKs or NRPTKs can trigger the activation or inactivation of immediate downstream signaling components involved in regulating Ras, a critical protooncogene that plays a central role in controlling diverse cellular processes, including cell growth, differentiation and cytoskeletal organization (5, 6). Ras (H-Ras, N-Ras and K-Ras) belong to a family of small GTPases (p21) that bind GTP and GDP and possess intrinsic GTPase activity to facilitate hydrolysis of GTP to GDP (7, 8). Further, the hydrolysis of Ras-GTP to Ras-GDP is stimulated by the interaction of Ras with GTPase activating proteins or GAPs, for example NF1 (7, 8). Therefore, p21Ras activity cycles between the active GTP-bound and the inactive GDP-bound forms (7, 8). The activation of Ras is dependent on the recruitment of guanine nucleotide exchange factors (GEFs), like Sos1/2) to ligand activated receptors by Grb2 (Growth factor receptor binding protein 2), an adaptor protein (9, 10). The activated Grb2-Sos1 GEF complex mediates the exchange of GTP with GDP-bound Ras, and disruption or uncoupling of this complex results in decreased Ras activity (11–13). The activation of Ras generates a network of signals by initiating activation of a cascade of effectors directly or indirectly (8). For example, the Ras-MAP3K pathway is activated upon association of activated Ras with the serine/threoine kinase Raf (MAPKKK), which leads to the sequential phosphorylation and activation of MAPKKs (MEK 1 & 2). MEKs phosphorylate MAP kinases (MAPKs i.e., ERKs (14, 15) and activated ERKs then directly phosphorylate cytosolic downstream substrates and can be translocated to the nucleus to activate nuclear transcription factors (16, 17). Importantly, activation of Ras can trigger other pathways as well, including PI3 kinase, NF-κB and can activate other members of the Ras superfamily, including Cdc42, Rac and Rho (8, 18). Regulation of Ras is a dynamic process which is affected by mutations in Ras (at positions 12, 13 and 61) that reduce its intrinsic GTPase activity and lead to “oncogenic” activation of Ras resulting in cellular transformation and tumorigenesis (7, 8). Interestingly, the malignant phenotype induced by oncogenic Ras can be inhibited by Rap1A (a small GTPase of the Ras super-family) (19, 20), indicating that even oncogenic Ras can be potentially inhibited by cellular regulatory mechanisms. Also, Ras may become aberrantly activated in the absence of any oncogenic mutation as result of the loss of a negative Ras regulator. For example, the loss of NF1 (21) tumor suppressor leads to hyperactivation of Ras and is associated with Juvenile Myelomonocytic Leukemia (JMML) (22). Furthermore, non-mutational but constitutive activation of Ras may be facilitated by failure to quench Ras GEF activity, but it is not clear how this potential mechanism is regulated. For example, increased Ras expression/activation is reported in lymphomas, where Ras is found to be rarely mutated (7, 8, 23). However, the mechanism is not clear, and a role for a PTK has not yet been demonstrated.
Human Tnk1 (Thirty eight negative kinase) is a 72 kDa NRPTK that was identified and cloned from CD34+ve/Lin-ve/CD38-ve hematopoietic stem/progenitor cells (24). We initially identified the alternate spliced variant of Tnk1/Kos1, a 47 kDa protein, from differentiating murine embryonic stem (ES) cells and named it Kos1 (Kinase of embryonic stem cells) (25). Tnk1/Kos1 is located on human chromosome band 17p13.1 (equivalent to mouse chromosome 11) and belongs to the Ack family of NRPTKs (24, 25). However, unlike Ack1 and Ack2 (26, 27), Tnk1/Kos1 lacks a CRIB motif that can bind the GTP-bound form of activated Cdc42, a member of the Ras superfamily of GTPase, indicating that Tnk1/Kos1 may function in a different manner from other members of the Ack family. Interestingly, while Tnk1 was found to be expressed in fetal blood cells/tissues, Kos1 is ubiquitously expressed, including in undifferentiated murine ES cells, mouse embryos and all adult tissues (25, 28). We recently reported that expression of Tnk1/Kos1 may be regulated at the promoter level (29–31). Our initial characterization studies with Tnk1/Kos1 and later with 72 kDa Tnk1 revealed that both isoforms can negatively regulate cell growth (25, 30). Collectively, these findings point to a potential role for Tnk1/Kos1 in both embryogenesis and adult tissue homeostasis. Interestingly, expression of either Tnk1 or Kos1 in cells can suppress cell growth by indirectly inhibiting both endogenous Ras and oncogenic H-RasG12V that leads to down-regulation of the Raf1-MAPK pathway (25,31). Also in support of a role for Tnk1/Kos1 in negative cell growth, Azoitei et. al. recently reported that Tnk1 can inhibit NF-κB survival signaling (30). Mechanistically, tyrosine auto-phosphorylation of Tnk1/Kos1 was found to be required for both its enzymatic activity and growth inhibitory function (25, 30, 31). The kinase-dead Tnk1/Kos1 mutants both fail to undergo autotyrosine phosphorylation and are unable to inhibit cell growth when expressed, but rather function as a “dominant stimulator” of cell growth in association with aberrantly hyper-activated endogenous Ras. Furthermore, the “dominant stimulator” Tnk1/Kos1 mutant is transforming when expressed in murine 3T3 cells (25).
THE NOVEL NONRECEPTOR PROTEIN TYROSINE KINASE TNK1/KOS1 IS A TUMOR SUPPRESSOR
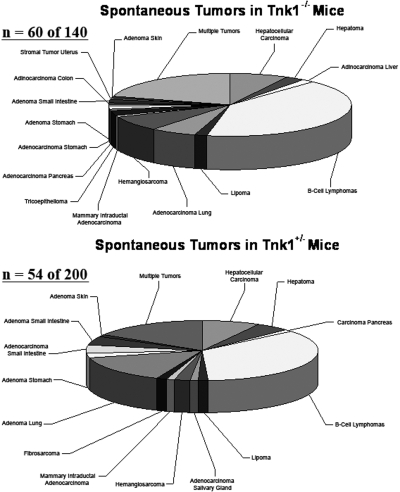
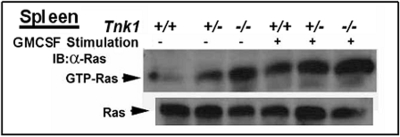
Since Tnk1/Kos1 requires its intrinsic PTK activity to suppress cell growth by indirectly inhibiting Ras activity, this suggests potential tumor suppressor activity. Therefore, we generated Tnk1/Kos1 knockout mice by homologous recombination to delete its catalytic domain (31). As we reported both Tnk1/Kos1 homozygous and heterozygous null mice develop spontaneous tumors upon aging (31). Initially we analyzed a total of 102 mice, 28 Tnk1−/−, 52 Tnk1+/− and 22 Tnk1+/+ littermates, and found that the incidence of tumor formation was high for the knockout animals, while no tumors were found in the 22 Tnk1+/+ wild type littermates. We have now extended this analysis with a total of 382 mice and find that 60 of 140 (43%) Tnk1−/− mice and 54 of 200 (27%) Tnk1+/− mice develop spontaneous neoplasms in various tissues with an average latency of 17 months (Figure 1). Furthermore, only one of 42 Tnk1+/+ mice develop a lymphoma. Interestingly, the majority of the tumors that develop in either the Tnk1−/− (47 of 60; 78 %) or Tnk1+/− (31 of 54; 57%) null mice are B-cell lymphomas of the DLBCL type (Figure 1). Of 47 such malignant B-lymphomas that developed in Tnk1−/− mice, 28 could be classified as DLBCL (58%), MALT (29%), follicular (11%) and plasmacytoma (4%) or SLL (4%). Likewise for the Tnk1+/− mice, 14 of 31 are DLBCL (57%), MALT (29%) and follicular lymphoma (14%). Significantly, 40% of Tnk1−/− mice (24 of 60) and 26% of Tnk1+/− mice (14 of 54) had more than one tumor type develop in the same animal. The occurrence of spontaneous neoplasms in various tissues is likely due to the ubiquitous expression of Tnk1/Kos1 (25). For example, hepatocellular carcinomas were observed as the second most frequent neoplasm in Tnk1−/− mice (15%; 9 of 60) and Tnk1+/− mice (13%; 7 of 54). These results clearly indicate that loss of Tnk1 predisposes mice to spontaneous development of tumors. We can conclude that Tnk1/Kos1 is a tumor suppressor gene and that tumor formation is closely associated with aberrant activation of Ras (31). Importantly, the elevated (i.e., hyperactivated) Ras activity associated with activation of MAPK and GEF activities in 4 week old Tnk1/Kos1 null mice point to Ras as a/the primary effector for Tnk1/Kos1 activity in mice (Figure 2).
Fig. 1.
Spectrum of spontaneous tumors that develop in Tnk1/Kos1 knockout mice. Sixty of 140 (43%) Tnk1−/− homozygous mice and 54 of 200 (27%) Tnk1+/− heterozygous mice developed tumors with aging. The majority of the tumors are classified as diffuse large B-cell lymphomas.
Fig. 2.
High Endogenous Ras activity in Tnk1/Kos1 null mice prior to tumor development. Endogenous Ras activity in the spleen of six week old mice was determined (25,31). A dose dependent elevation of Ras activity (hyper active Ras) in spleen associated with allelic loss of Tnk1 gene in heterozygous and homozygous null mice both in the absence and presence of 100 ng/ml of Granulocyte Macrophage-Colony Stimulating Factor (GMCSF).
LOSS OF TNK1/KOS1 EXPRESSION OCCURS IN MALIGNANT HUMAN AND MURINE B-CELL LYMPHOMAS
Tumor suppressors may undergo allelic loss which leads to reduced or absent expression (32, 33). An initial meta-analysis of Tnk1 mRNA in 69 human diffuse large B-cell lymphoma (DLBCL) patients revealed a significant loss of Tnk1 expression compared to 25 normal B-lymphocyte specimens. In addition, new preliminary data from 63 newly diagnosed DLBCL patient samples analyzed using high density (HD) single polynucleotide polymorphism (SNP) array and gene expression profiling reveal that a cohort (i.e., 14 of 63; 22%) of these patients demonstrate an allelic loss and/or downregulation of Tnk1 expression (Hoare, Shipp and May unpublished data), indicating the clinical relevance of Tnk1/Kos1 for human lymphomagenesis (and potentially other tumors) is high. Importantly, the allelic loss of the Tnk1 gene may result from promoter methylation as observed in Tnk1+/− heterozygous mice (31). The Tnk1/Kos1 knockout model provides the scientific evidence how tumors can arise as a result of loss of heterozygosity, a well reported mechanism for loss of tumor suppressor gene expression (32, 33). But how Tnk1/Kos1 promoter methylation is regulated in these cells is not clear and will require additional studies.
TNK1/KOS1 FUNCTIONS TO UNCOUPLE THE GRB2-SOS1 GEF COMPLEX WHICH INVOLVES THE LOSS OF RAS ACTIVATION
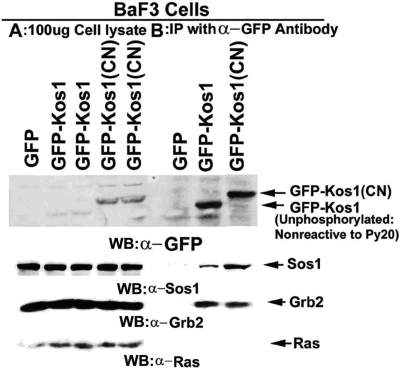
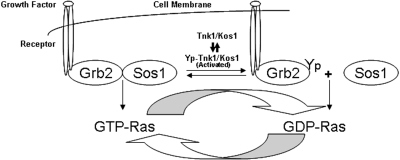
Expression of either exogenous human or murine Tnk1/Kos1 in cells inhibits Ras activity and results in suppression of the Ras-Raf1-MAPK pathway (31). To determine the mechanism by which Tnk1/Kos1 can suppress Ras activation, we initially expressed GFP-Kos1 or a kinase-dead Kos1 (CN) in BaF3 cells. Results revealed that Tnk1/Kos1 associates with the Grb2-Sos1 complex but not with Ras (Figure 3). From these data, we concluded that Tnk1/Kos1 can suppress Ras activity but in an indirect manner, potentially by inhibiting the Grb2-Sos1 GEF complex. Subsequently, in vitro PTK assays using rGrb2 or the Grb2-Sos1 complex as substrates have revealed that Tnk1/Kos1 can directly tyrosine phosphorylate Grb2 but not Sos1. This indicates a role for Tnk1/Kos1 activity in negatively regulating (i.e., inhibiting) the GEF complex. Furthermore, the “kinase-dead” Tnk1/Kos1 mutant was able to stimulate Ras activity but failed to phosphorylate Grb2 (Figures 4, 6). Therefore, we proposed that prolonged stabilization of the Grb2-Sos1 complex that occurs in the absence of Tnk1/Kos1 activity leads to aberrant activation of Ras (Figure 2) (31). We found that Tnk1/Kos1 mediated tyrosine-phosphorylation of Grb2 leads to the uncoupling of Grb2-Sos1 GEF complex (25,31). These data indicate a mechanism by which Tnk1/Kos1 can indirectly inhibit Ras activity. Conversely, in the absence Tnk1/Kos1 expression/activity, the abundance of reduced or decreased tyrosine phosphorylated Grb2 enables stabilization/activation of the Grb2-Sos1 GEF complex and as a result, aberrant activation Ras occurs in tissues or tumors from the Tnk1/Kos1 knockout mice (Figure 2) (31). Therefore, we propose that the dynamic reversal of Grb2-Sos1 GEF complex can be regulated by Tnk1/Kos1 as indicated in the model (Figure 5). However, the mechanism(s) by which Tnk1/Kos1 is activated to quench the Ras (Grb2-Sos1) GEF activity and suppress Ras activation is not yet known but becomes the focus of future studies. We propose that understanding the mechanism by which Tnk1/Kos1 is activated may point the way for developing a targeted therapeutic approach to quench hyperactivated Ras in cancers.
Fig. 3.
Tnk1/Kos1 directly associates with Sos1 and Grb2 but not with Ras. GFP, GFP-Kos1 and GFP-Kos1(CN) were immuno-precipiated from BaF3 cells cell lysates (1mg) using a anti-GFP antibody (24). Western analysis of 100 μg of cell lysates was compared with the anti GFP antibody pull-downs.
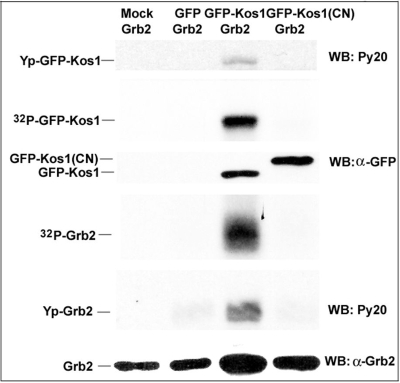
Fig. 4.
Grb2 is the substrate of Tnk1/Kos1. Cos7 cells were transfected with GFP, GFP-Kos1 and kinase-dead GFP-Kos1(CN). GFP and GFP fusion proteins were immuno-precipitated using an anti GFP antibody and an in vitro PTK assay was performed using 0.25 μg of recombinant Grb2 and [γ-32P]ATP. Western analysis was performed using anti GFP, Py20, and anti Grb2 antibodies. Grb2 is tyrosine phosphorylated by GFP-Kos1, which undergo auto- tyrosine phosphorylation. GFP-only or GFP-Kos1(CN) fail to tyrosine phosphorylate Grb2 (31).
Fig. 6.
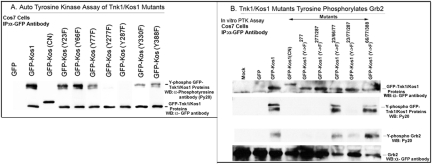
PTK activity of Tnk1/Kos1 tyrosine (Y) to phenyl alanine (F) mutants. Panel A: The Tnk1/Kos1 point mutants (Y→F) were created by site-directed mutagenesis. Cos7 cells were transfected with GFP, GFP-Kos1 or GFP-Kos1 mutants and were immuno precipitated using an anti GFP antibody. Protein bands were resolved on a 10% SDS-PAGE and transferred on to nitro-cellulose paper. Auto tyrosine kinase activity of GFP, GFP-Kos1 or GFP-Kos1-mutants was determined using Py20. Anti-GFP antibody was used to determine the expression of GFP and the GFP fusion proteins (24). Panel B: The immunoprecipitates containing GFP-Kos1 or Kos1 (Y→F) mutants were subjected to invitro PTK assay using 0.25 μg Grb2 and cold ATP (2). The reaction mix was subjected to SDS-PAGE as above for Western analysis using the phospho-tryosine antibody Py20, anti-GFP and anti-Grb2 antibodies.
Fig. 5.
Tnk1/Kos1 uncouples Grb2-Sos1 GEF complex. Receptor mediated Ras activation is regulated by guanine nucleotide exchange factors, which catalyze the exchange of GDP for GTP. The model depicts the mechanism by which activated Tnk1/Kos1 inhibits the Ras GEF activity by uncoupling the activated Grb2-Sos1 GEF complex to indirectly suppress Ras and abort downstream Ras signaling.
ACTIVATION OF TNK1/KOS1 REQUIRES PHOSPHORYLATION OF TYROSINE SITES 277 AND 287
Since autotyrosine phosphorylation of Tnk1/Kos1 was initially found to be required for its function, we performed site directed mutagenesis studies to identify the critical tyrosine site(s) required for its enzymatic activity. All 9 of the potential tyrosine residues (Y) were individually mutated to phenylalanine (F) which cannot be phosphorylated. In addition, we constructed compound mutants in which two or three sites were simultaneously replaced. The mutants were initially evaluated for autokinase activity and then for their ability to tyrosine phosphorylate Grb2 (Figure 6 A and B). Results indicate that two sites, Y277 and Y287, are necessary for Tnk1/Kos1 activity. We found that these two Y→F mutants fail to undergo autophosphorylation and are unable to directly phosphorylate Grb2. Thus, when expressed in cells, the Tnk1/Kos1 Y277F or Y287F mutant will mimic the effects of kinase-dead GFP-Kos1 (CN) to stimulate anchorage independent cell growth by inducing aberrant activation of Ras. Interestingly, several SNP-generated missense mutations have been reported to occur within the kinase domain of Tnk1/Kos1 contained in primary human tumors (34–36). We now speculate that these point mutants may have “oncogenic” potential by mimicking the function of the kinase-dead Tnk1/Kos1 in transforming cells.
CONCLUSION
The loss of Tnk1/Kos1 activity that occurs in null mice results in aberrantly high Ras activity, which is associated with spontaneous development of tumors in mice. Malignant B-cell lymphomas are the most common form of cancer that develops in the Tnk1 null mice. While PTKs are known to be “oncogenic” when mutated (e.g. EGFR, PDGFR, Src, Bcr-Abl), Tnk1/Kos1 is the first such tyrosine kinase discovered to have tumor suppressor activity. Mounting evidence from human B-cell lymphoma specimens from patients now points to a causative role of loss of Tnk1/Kos1 activity in human tumorigenesis. Thus, while Ras is reported to be infrequently mutated to oncogenic Ras in human B-cell lymphomas (7, 8, 22), the loss of Tnk1/Kos1 activity may account for the non-mutational “activation” of Ras found in these tumors. Furthermore, the mechanism for the activation of Ras as a result of the loss of Tnk1/Kos1 expression or activity is due to prolonged stabilization/activation of the Grb2-Sos1 GEF complex. Conversely, the uncoupling of the Grb2-Sos1 GEF complex involves tyrosine phosphorylation of Grb2 by Tnk1/Kos1 and serves as a mechanism to suppress Ras activity indirectly. Further characterization of this novel mechanism of Ras regulation by Tnk1/Kos1 is expected to help point the way to the development of more effective anti-Ras strategies in cancers where Ras is found to be aberrantly activated but not oncogenically mutated.
ACKNOWLEDGEMENT
This work was supported by the NIH /NCI grants CA109150 and CA44649.
The abbreviations used are:
- Tnk1
Thirty-eight negative kinase
- Kos1
Kinase of stem cells
- Grb2
Growth factor receptor binding protein 2
- Sos1/2
Sons of seven 1/2
- GEF
Guanine exchange factor
- GAP
GTPase activating protein
- NRPTK
Nonreceptor protein tyrosine kinase
- RPTK
Receptor protein tyrosine kinase
- IP
Immunoprecipitation
- WB
Western blotting
- SDS
Sodium dodecyl phosphate
- PAGE
Polyacrylamide gel electrophoresesis.
DISCUSSION
Quesenberry, Providence: Beautiful work, Strat. I am just interested in the potential role of this in normal stem cell biology and maybe taking a step further, if you believe that cancer stem cells exist, other than CML, which I think is an open-ended question. What about its role in cancer stem cells as far as cancers in general?
May, Gainesville: Yes, well as far as these mice go in terms of a role for TNK1/KOS1 in hematopoiesis, these mice are live born, about normal Mendelian ratios; they live a full 24-month life, and, they can reproduce. The homozygous null mice, about 10% or so die right around birth. We don't really know what the cause is, but the hemogram looks pretty normal. We have not followed hematopoiesis out completely. I don't know whether there is some degree of anemia or white cells or platelets affected, but the hemogram does look pretty normal. So, I don't have any evidence that it's required. It certainly isn't required for normal hematopoiesis. However, we have not stressed these animals. So, I don't know how well they will tolerate stress. They may or may not tolerate stress. So, I cannot tell about that. But in terms of its role in other tumors, we have examined two different polymorphisms. Both have come from (human) lung tumors that were published. Both of them seem to have lost tyrosine kinase activity, and these polymorphisms are in an open reading frame. One of them happens to be in a kinase insert region and the other is outside of that region. So, I think that it may have a potential role in other tumors as well and certainly could because of its ubiquitous expression.
Alexander, Atlanta: We see a varitey of tumors in knockout mice, predominantly lymphomas Many gene abnormalities in phosphatases have been reported to promote malignancy of one description or another involving protein phosphatases or phosphoinositide phosphatases, and those are well-described. This does, indeed, appear to be a novel mechanism by which a tyrosine kinase is inhibitory to growth. Are there other examples not yet described?
May, Gainesville: No. Not that I am aware of. There are a number of non-receptor protein tyrosine kinases that are believed to be tumor suppressors as well. Shk is one of the best examples of that. The other non-receptor kinase is the LYNs and the FYNs and the SARCs, and all of those are oncogenic if they get mutated or cooperate with other oncogenic changes to induce malignancy So these mutations are examples of positive or activating mutations where the effect by Tnk is inhibitory, at least to the tyrosine kinase activity. So, I would expect there would be more. It's just that this happens to serendipitously pop out as the first one documented. It's not the first one that's been thought to be a tumor suppressor but the first documented example of a tyrosine kinase as a tumor suppressor
Berger, Cleveland: What strain of mice are you using, and are you recapitulating the tumor spectrum of that strain, or do you think you have a unique tumor strain?
May, Gainesville: Maybe one of all of this strain, this is the 129 strain that's crossed to the Black 6 mices, none of the wild-type mice developed tumors in the time period that we were looking at. These tumors develop a little bit late. They develop between 12 and 17 months. So, it's not something like loss of P53, where you start to get tumors within, you know, 12 weeks or so. So, that gives us an opportunity to look and see if we can compress that latent period by crossing with other cooperating mutations. But in the mice that we study we don't see, routinely, a lot of lymphomas. There are certain strains of mice that do get spontaneous lymphomas at a certain rate, 3, 4, 5%. This is not one of them.
REFERENCES
- 1.Neet K, Hunter T. Vertebrate non-receptor protein-tyrosine kinase families. Genes Cells. 1996;1:147–69. doi: 10.1046/j.1365-2443.1996.d01-234.x. [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 3.Fischer EH, Charbonneau H, Tonks NK. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991;253:401–6. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- 4.Avraham H, Avraham S, Taniguchi Y. Receptor protein tyrosine phosphatases in hematopoietic cells. J Hematother Stem Cell Res. 2000;9:425–32. doi: 10.1089/152581600419080. [DOI] [PubMed] [Google Scholar]
- 5.Erpel T, Courtneidge SA. Src family protein tyrosine kinases and cellular signal transduction pathways. Curr Opin Cell Biol. 1995;7:176–82. doi: 10.1016/0955-0674(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 6.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–8. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 7.Bos JL. Ras oncogene in human cancer. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 8.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–31. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;292:154–96. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 10.Aronheim A, Engelberg D, Li N, al-Alawi N, Schlessinger J, Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994;78:949–61. doi: 10.1016/0092-8674(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 11.Corbalan-Garcia S, Yang SS, Degenhardt KR, Bar-Sagi D. Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol Cell Biol. 1996;16:5674–82. doi: 10.1128/mcb.16.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherniack AD, Klarlund JK, Conway BR, Czech MP. Disassembly of Son-of-sevenless proteins from Grb2 during p21ras desensitization by insulin. J Biol Chem. 1995;4:1485–8. [PubMed] [Google Scholar]
- 13.Li S, Couvillon AD, Brasher BB, Van Etten RA. Tyrosine phosphorylation of Grb2 by Bcr/Ab1 and epidermal growth factor receptor: a novel regulatory mechanism for tyrosine kinase signaling. EMBO J. 2001;23:6793–804. doi: 10.1093/emboj/20.23.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita T, Yokoto T, Arai K, Atsushi M. Suppression of apoptotic death in hematopoietic cells by signalling through the IL-3/GM-CSF receptors. EMBO J. 1995;14:266–75. doi: 10.1002/j.1460-2075.1995.tb07000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig EA, Stevens MV, Vaillancourt RR, Camenisch TD. MAP3Ks as central regulators of cell fate during development. Dev Dyn. 2008;237:3102–14. doi: 10.1002/dvdy.21750. [DOI] [PubMed] [Google Scholar]
- 16.Deng X., Gao F., Flagg T., May W.S. Mono- and Multi-Site Phosphorylation Enhance Bcl2's Anti-Apoptotic and Inhibition of Cell Cycle Entry Functions. Proc Natl Acad Sci U S A. 2004;101:153–8. doi: 10.1073/pnas.2533920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng X., Gao F., Flagg T., Anderson J., May W.S. Bcl2's Flexible Loop Domain Regulates p53 Binding and Survival. Molecular Cell Biology. 2006;26:4421–34. doi: 10.1128/MCB.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 19.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 20.Bardeesy N, Sharpless NE. RAS unplugged: negative feedback and oncogene-induced senescence. Cancer Cell. 2006;6:459–72. doi: 10.1016/j.ccr.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 21.De Clue JE, Papageorge AG, Fletcher JA, et al. Abnormal regulation of mammalian p21Ras contributes to malignant tumor growth in von Recklinghausen (type1) neurofibromatosis. Cell. 1992;69:265–73. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- 22.Lauchle JO, Braun BS, Loh ML, Shannon K. Inherited predispositions and hyperactive Ras in myeloid leukemogenesis. Pediatr Blood Cancer. 2006;46:579–85. doi: 10.1002/pbc.20644. [DOI] [PubMed] [Google Scholar]
- 23.Mangues R, Corral T, Lu S, Symmans WF, Liu L, Pellicer A. NF1 inacivation cooperates with N-Ras in vivo lymphogenesis activating Erk by a mechanism independent of its Ras-GTPase interacting activity. Oncogene. 1998;17:1705–16. doi: 10.1038/sj.onc.1202097. [DOI] [PubMed] [Google Scholar]
- 24.Hoehn GT, Stokland T, Amin S, Ramirez M, et al. Tnk1, a novel intracellular tyrosine kinase gene isolated from human umbilical cord blood CD34+/Lin-/CD38− stem/progenitor cells. Oncogene. 1996;12:903–13. 1996. [PubMed] [Google Scholar]
- 25.Hoare K, Hoare S, Smith OS, Kalmaz G, Small D, May S. Kos1, a nonreceptor tyrosine kinase that suppresses Ras signaling. Oncogene. 2003;22:3562–77. doi: 10.1038/sj.onc.1206480. [DOI] [PubMed] [Google Scholar]
- 26.Manser E, Leung T, Salihuddin H, Tan L, Lim L. A nonreceptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature. 1993;363:364–67. doi: 10.1038/363364a0. [DOI] [PubMed] [Google Scholar]
- 27.Yang W, Cerione RA. Cloning and characterization of a novel Cdc42-associated tyrosine kinase Ack2, from bovine braining. J. Biol. Chem. 1997;272:24819–24. doi: 10.1074/jbc.272.40.24819. [DOI] [PubMed] [Google Scholar]
- 28.Felscow DM, Civin CI, Hoehn GT. Characterization of the tyrosine kinase Tnk1 and its binding with phospholipaseCγ1. BBRC. 2000;273:294–301. doi: 10.1006/bbrc.2000.2887. [DOI] [PubMed] [Google Scholar]
- 29.Hoare S, Hoare K, Reinhard MK, Flagg TA, May WS. Functional characterization of murine Tnk1 promoter. Genes. 2009;444:1–9. doi: 10.1016/j.gene.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azoitei N, Brey A, Busch T, Fulda S, Adler G, Seufferlein T. Thirty-eight negative kinase1 (Tnk1) facilitates TNFα-induced apoptosis by blocking NF-κB activation. Oncogene. 2007;26:6536–45. doi: 10.1038/sj.onc.1210476. [DOI] [PubMed] [Google Scholar]
- 31.Hoare S, Hoare K, Reinhard M, Lee YJ, Oh P, May S. Tnk1/Kos1 knockout mice develop spontaneous tumors. Cancer Research. 2008;68:8723–32. doi: 10.1158/0008-5472.CAN-08-1467. [DOI] [PubMed] [Google Scholar]
- 32.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–96. [PubMed] [Google Scholar]
- 33.Tate G, Suzuki T, Nemoto H, Kishimoto K, Hibi K, Mitsuya T. Allelic loss of the PTEN gene and mutation of the TP53 gene in choriocarcinoma arising from gastric adenocarcinoma: analysis of loss of heterozygosity in two male patients with extragonadal choriocarcinoma. Cancer Genet Cytogenet. 2009;193:104–8. doi: 10.1016/j.cancergencyto.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Davis H., Hunter C, Smith R, et al. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res. 2005;65:7591–5. doi: 10.1158/0008-5472.CAN-05-1855. 2005. [DOI] [PubMed] [Google Scholar]
- 35.Ruhe JE, Streit S, Hart S, Wong C-H, Specht K, et al. Genetic alterations in the tyrosine kinase transcriptome of human cancer cell lines. Cancer Res. 2007;67:11368–76. doi: 10.1158/0008-5472.CAN-07-2703. [DOI] [PubMed] [Google Scholar]
- 36.Lierman E, Van Miegroet H, Beullens E, Cools J. Identification of protein tyrosine kinases with oncogenic potential using a retroviral insertion mutagenesis screen. Haematologica. 2009;94:1440–4. doi: 10.3324/haematol.2009.007328. [DOI] [PMC free article] [PubMed] [Google Scholar]