Abstract
CD36 is a multifunctional membrane receptor present on mononuclear phagocytes, platelets, and other cells that serves as a scavenger receptor for oxidized phospholipids, apoptotic cells and certain microbial pathogens. On macrophages, CD36 interaction with oxidized LDL (oxLDL) triggers a signaling response that is pro-inflammatory and pro-atherogenic. The signaling pathway involves activation of src-family kinases, MAP kinases, and Vav family guanine nucleotide exchange factors and results in ligand internalization, foam cell formation and inhibition of migration. On platelets, CD36 interaction with oxLDL and cell-derived microparticles transduces intracellular signals that render them more reactive to low concentrations of classical agonists. In vitro studies and in vivo experiments in CD36 null mice have revealed an important mechanistic role for CD36 in atherosclerosis and thrombosis. Identification of the precise CD36 signaling pathways in specific cells elicited in response to specific ligands may yield novel targets for drug development in athero-thrombotic disorders.
CD36 is an 88,0000MW glycoprotein that contributes to the genesis of several important pathological processes highly relevant to atherosclerosis and thrombosis. Its gene is a member of an evolutionarily conserved family that in vertebrates also includes SRB1 (Scavenger Receptor B1), a cellular receptor for high density lipoprotein, and LIMP2, a lysosomal integral membrane protein of unclear function (1). CD36 has an unusual structural organization, with two transmembrane domains, two very short intracytoplasmic domains, and a large heavily glycosylated extracellular domain. Both of the intracellular domains contain pairs of cysteines that are fatty acid acylated, and thus presumably closely apposed to the inner leaflet of the cell membrane (2).
CD36 is expressed on the surface of platelets, adipocytes, skeletal and cardiac myocytes, capillary endothelial cells, most professional phagocytes, and epithelial cells in gut, kidney and breast (3). This diverse range of expression reflects its multi-functionality. CD36 independently binds three major classes of extracellular ligands and effects different cellular functions in a tissue and cell-specific context. It was first identified as a receptor for the matrix glycoprotein thrombospondin-1 (4), and later shown to bind to several other proteins that contain the so-called thrombospondin type-1 structural homology region (TSR), including thrombospondin-2 and brain angiogenesis inhibtitor-1 (5). On capillary endothelial cells the interaction of CD36 with TSR-containing proteins mediates a potent anti-angiogenic, pro-apoptotic response (6).
CD36 is also known as FAT (fatty acid translocase) because it binds long chain free fatty acids and facilitates their transport into cells (7). On myocytes this serves to supply the cells with an energy source for beta-oxidation, while on adipocytes it results in lipid storage. In the gut, CD36 participates in fat and fat-soluble vitamin absorption. Recent studies of CD36 orthologs in insects showed that CD36 family members are expressed on sensory cells on antennae where they participate in mediating a signaling response to fatty acid-derived pheromones, thus mediating social and sexual behavior (8). In rodents, a fatty acid sensing function for CD36 has been shown in taste buds and proximal gut. The former mediates preferential feeding behavior for fatty food and the latter serves to prepare the more distal gut for an incoming fatty meal.
The remainder of this manuscript will focus on the role of CD36 in macrophage and platelet biology and in this context, on its third major function—as a pattern recognition or “scavenger” receptor. Studies of insects and mice (9, 10) revealed that CD36 is part of the innate immune system, recognizing molecules present on the surface of certain microbial pathogens, including staphylococcus, myocbacteria and fungi, mediating their clearance by phagocytic cells. As with many innate immune receptors, CD36 also recognizes and scavenges endogenously derived molecules, including oxidized low density lipoproteins (oxLDL) (11), advanced glycated proteins, fibrillar Aβ amyloid peptides, and components on the surface of apoptotic cells, cell-derived microparticles and shed photoreceptor outer segments (12, 13).
Our lab has been particularly interested in oxLDL because of its pathogenic role in atherosclerosis. An early step in the atherogenic process is transmigration of blood monocytes across an injured or dysfunctional area of endothelium into the sub-endothelial space (intima) where they differentiate into macrophages (14). LDL particles also cross the endothelium and become trapped in the intima connective tissue. Under the influence of pro-inflammatory cytokines, the macrophages produce reactive oxygen and nitrogen species which oxidize the unsaturated phospholipids present in LDL. Once oxidized, the LDL particles lose their affinity for the LDL receptor but gain affinity for scavenger receptors, including CD36, and are thus internalized by intima macrophages. A unique feature of CD36 is that expression of its gene is significantly up-regulated by the ligand. When internalized, specific oxidized lipids present in oxLDL can serve as ligands or precursors of ligands for the nuclear hormone receptor PPARγ (15). This receptor, once engaged and activated, functions as a transcription factor that drives expression of many metabolic genes, including CD36. The net effect of these interactions is that in the setting of abundant generation of oxLDL, CD36 expression on macrophages is increased and the uptake of oxLDL “loads” the cells with cholesterol to the point where natural efflux pathways are overwhelmed, creating what are called “foam cells”, the earliest visible characteristic of the developing atheromatous lesion. We have hypothesized that a modern “western” diet and lifestyle with its attendant hyperlipidemia and oxidant stress converts a biological process originally evolved to be homeostatic (i.e. clearance of modified lipoproteins from the vessel wall) to one that is pathologic—generation of foam cells.
The development of a genetically targeted CD36 null mouse line has played a critical role in defining the cellular functions of CD36 and in demonstrating in vivo relevance to athero-thrombotic disorders (16). Macrophages from CD36 null mice bind and internalize substantially less oxLDL than do those from wild type mice and consequently do not effectively form foam cells (17). The decrement is somewhat dependent on the manner in which the LDL is oxidized, with the largest effects seen with LDL oxidized by a highly relevant myeloperoxidase-based system or by an in vivo method. Breeding CD36 null mice into several different atherosclerosis-prone mouse strains, including apoE null and LDL receptor null, substantially reduced the amount of plaque formed in these animals. The degree of athero-protection differs in different models, but all showed either smaller lesions or less complex lesions (17–20). We reported up to a 70% reduction in total plaque area in high fat diet fed apoE null mice and hypothesized that the amount of protection in different models relates to the level of systemic inflammation and oxidant stress.
In considering the mechanisms by which CD36 promotes athero-thrombotic activity in vascular cells we will review four important sets of experimental results that led to the following conclusions:
CD36 recognizes and binds specific oxidized phospholipid moieties in OxLDL
CD36 initiates an intracellular signaling cascade in macrophages that provides a so-called “eat me” signal leading to oxLDL uptake and foam cell formation
CD36 signaling pathways modulate cytoskeletal dynamics leading to trapping of macrophages in plaque
CD36 signaling in platelets leads to a “hyper-active” state and promotes thrombosis.
CD36 recognizes specific oxidized phospholipid species
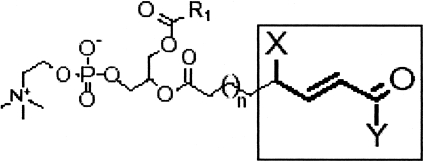
De-lipidated oxLDL does not bind CD36, suggesting that the ligand(s) for CD36, unlike for SRA1, are most likely oxidized lipids, perhaps oxidized phospholipids or oxysterols. Podrez and colleagues from the lab of Stanley Hazen (21) used high performance liquid chromatography to separate the component lipids of oxLDL and an in vitro binding assay with CD36 cDNA transfected cells to identify those fractions within the oxLDL particles that were recognized specifically by CD36. They then utilized mass spectrometry to define the precise molecular nature of the lipids in the CD36-binding fractions and synthetic chemistry to verify that indeed the specific components bound to CD36 with high affinity when incorporated into unilammelar phospholipid liposomes (21). These oxidized phospholipids all share a common structural motif; the unsaturated fatty acid in the sn-2 position of the phosphatidyl choline is truncated at the site of the most proximal double bond and contains either a terminal carboxylic acid or aldehyde with a double bond in the beta position and an alcohol or ketone in the gamma position (see Figure 1). Importantly these oxidized PC molecules (now known as oxPCCD36) can be detected in reasonably abundant levels within atherosclerotic plaque from experimental animals or human subjects, but are absent or present in much lower levels in normal arterial segments. They can also be detected in the circulation of hyperlipidemic, pro-therogenic mice and human hyperlipemic patients with known atherosclerotic disease (22). More recent structural analysis of oxLDL suggests that the truncated sn-2 oxidized lipid, because of its hydrophilic nature, protrudes from the surface of particle into the aqueous phase (much like a “whisker”) where it is thus accessible to bind CD36 (23). The Hazen lab has recently found similar “signature” oxidized sn-2 phospholipids in the phosphatidyl serine molecules expressed on the surface of apoptotic cells or shed photoreceptor outer segments, thereby showing that CD36 scavenging of cells and cell fragments shares a common structural basis with scavenging oxLDL (24). How this relates to recognition of components of microbial pathogens, such as staphylococcal lipoteichoic acid, remains to be determined. An important feature of oxPCCD36 is that they do not bind to scavenger receptors unrelated to CD36, including SRA1, thus they represent an ideal experimental reagent to determine those downstream consequences of oxLDL binding to cells that are mediated by CD36.
Fig. 1.
Structural features of oxPCCD36 - the signature CD36 ligands found in oxLDL. Truncated oxidized unsaturated fatty acids in the sn-2 position of glycerol-phospholipids containing a terminal aldehyde or carboxylic acid, a double bond in the beta position, and a ketone or alcohol in the gamma position are ligands for CD36. X is OH or = O; Y is H or OH.
Macrophage CD36 signaling cascades mediate oxLDL uptake and foam cell formation
Studies from our lab and others had shown that despite an intracellular structure lacking known signaling domains (e.g. kinase, phosphatase, g-protein binding, or scaffolding domains), CD36 is indeed capable of transducing cell signals. On microvascular endothelial cells, the anti-angiogenic activity of CD36 was shown to involve activation of a specific src-family non-receptor tyrosine kinase, fyn, and a specific mitogen-activated serine/threonine (MAP) kinase family member, p38 (25). CD36 had been shown by several groups to co-immunoprecipitate with src-family members in platelets and endothelial cells. So we hypothesized that the cytoplasmic domain of CD36 may function to assemble a signaling complex in macrophages and other cells. Since the N-terminal cytoplasmic domain is extremely short (5–7 amino acid residues) and probably closely associated with the internal leaflet of the plasma membrane, we focused our initial studies on the carboxy-terminal domain. This region, while also quite short (13 amino acids), contains a CXCX5K motif homologous to a region in the intracellular domain of CD4 and CD8 that is known to interact with signaling molecules.
To address this hypothesis, we created a recombinant peptide containing these 13 amino acids linked to a glutathione-S-transferase (GST) tag, and used it to affinity-purify cytoplasmic proteins from human monocytic cells (26). Several of these proteins were identified by mass spectrometry and immunoblot, including the src family member, Lyn, and the upstream MAP kinase kinase, MEKK2. We then compared macrophages from CD36 null mice to those of wild type mice to define the role of these signaling molecules in responses to oxLDL and/or oxPCCD36. The key findings were that incubation of macrophages with oxLDL led to a dose- and time- dependent activation of the MAP kinases JNK1 and JNK2. This was not seen in CD36 null cells and was blocked if src kinases were inhibited with pharmacologic agents. Importantly, the specific src family members, lyn and fyn were co-precipitated with anti-CD36 antibodies from macrophages, with a significant oxLDL-dependent enrichment of the activated form of lyn in the CD36 signaling complex. The physiologic and patholologic relevance of these findings were demonstrated by showing that aortic tissue from atherosclerotic mice had significantly increased levels of activated JNK compared to tissue from non-atherosclerotic aortae. Also specific inhibition of JNK activation or JNK activity blocked CD36-mediated uptake of oxLDL and markedly inhibited foam cell formation in in vitro assays. Recent studies showing that mice genetically deficient in JNK were protected from experimental atherosclerosis further highlighted the importance of this pathway (27).
The CD36 signaling pathway also mediates other macrophage scavenger functions, for example uptake and clearance of microbial pathogens and of apoptotic cells. Stuart and colleagues showed that site-directed mutation of specific amino acids (tyrosine463 or cysteine464) in the carboxy-terminal cytoplasmic tail of CD36 abrogated microbial uptake (28), suggesting that these sites are necessary for forming a signaling complex. For some microbial ligands, including staph glycolipids, CD36 acts as a co-receptor with toll-like receptor (TLR)-2 to effect uptake (29); and for CD36-mediated uptake of apoptotic cells, beta 3 or beta 5 integrins function as necessary co-receptors (30, 31). CD36-mediated uptake of oxLDL or oxPCCD36 liposomes, however, does not require either TLRs or integrins.
The most recent studies from our lab have attempted to define the mechanisms by which Lyn and JNK contribute to CD36-mediated foam cell formation (i.e. what are the downstream effectors). Members of the Vav family of guanine nucleotide exchange factors (GEFs) have been identified as potential key signaling intermediates (32). These molecules are activated by src-family kinases, and in addition to their function in activating Rac and Rho family small molecular weight G proteins, also function as scaffolds for a variety of relevant signaling molecules including phospholipase C and the large MW GTPase, dynamin. In vitro, oxLDL induced rapid activation of macrophage Vav in a CD36 and Src kinase-dependent manner, and in vivo, aortae removed from western diet-fed hyperlipidemic mice showed significantly more activated Vav protein than aortae from CD36 null mice fed the same diet (33). Importantly, CD36-dependent uptake of oxLDL in vitro and foam cell formation in vivo were significantly reduced in Vav null macrophages concomitant with impaired calcium signaling and PLC-γ1 activation. Inhibition of dynamin, a Vav-interacting protein that is involved in endocytic vesicle fission, blocked oxLDL uptake and inhibited foam cell formation. Immunofluorescence microscopy studies showed that mobilization of dynamin-2 by oxLDL was impaired in Vav null cells, and that disruption of the CD36/Vav pathway caused a defect in maturation of oxLDL-containing endocytic vesicles.
Macrophage CD36 signaling pathways modulate cytoskeletal dynamics leading to trapping of macrophages in plaque
In addition to playing a key role in macrophage foam cell formation, CD36 modulates other important pro-atherogenic and pro-inflammatory macrophage phenotypes. For example, exposure of human monocyte-derived macrophages to oxLDL activates the pro-inflammatory transcription factor NFκb in a CD36-dependent manner (34). We have focused recent studies on migration, because trapping of lipid-laden macrophages in the arterial intima is a critical but reversible step in the atherogenic process, and work from other labs many years ago showed that oxLDL could inhibit migration and thus promote macrophage retention in lesions (35). We found using a murine in vivo assay for macrophage migration out of inflamed peritoneum that oxLDL but not native LDL completely inhibited emigration of wild type but not CD36-null cells (36). In vitro, oxLDL also inhibited migration in a modified Boyden Chamber assay and also induced rapid spreading, actin polymerization, and loss of cell polarity in CD36-expressing, but not CD36-negative macrophages. Mechanistically, these phenomena were triggered by a CD36 signaling pathway that resulted in activation of focal adhesion kinase (FAK) by src-family kinases (probably Lyn) coupled with inactivation of src homology 2-containing phosphotyrosine phosphatase (SHP-2). The latter was due to NADPH oxidase-mediated generation of reactive oxygen species (ROS) with resulting oxidative inactivation of critical cysteine residues in the active site of SHP-2. The net effect of these molecular events is that oxLDL induces a sustained activation of FAK and this loss of ability to dynamically regulate actin assembly and disassembly. Importantly, macrophage migration in vitro in the presence of oxLDL could be restored by treating the cells with antioxidants or NADPH oxidase inhibitors which also restored dynamic activation of FAK. Recent unpublished studies suggest that Vav family members may also be involved in this pathway by contributing to Rac GTPase activation and thereby influencing cell polarity. These studies identify CD36 and its downstream signaling partners as potential targets for novel therapeutics aimed at reversing atherogenesis.
CD36 signaling in platelets leads to a “hyper-active” state and promotes thrombosis
CD36 was first identified as a platelet surface glycoprotein and named glycoprotein IV or IIIb based on its migration on SDS-PAGE gels (37, 38). Although initially thought to function as a collagen receptor, this was not borne out as better reagents became available, and for many years its function on platelets remained undefined. We hypothesized that because of its capacity to recognize ligands such as oxLDL, advanced glycated proteins, apoptotic cells and cell-derived microparticles that are generated during many common chronic diseases, including athersclerosis, diabetes, cancer and systemic inflammatory disorders, platelet CD36 could act as a circulating “sensor” for these disease states. Furthermore, because of its potential to generate intracellular signals, CD36 could perhaps contribute to the platelet hyper-reactivity and pro-thrombotic state associated with these conditions.
To test this hypothesis we first demonstrated that, indeed, oxLDL and oxPCCD36 bind specifically and saturably to the surface of resting platelets and that this binding can be blocked with monoclonal antibodies to CD36 or by using platelets from CD36 null mice or CD36-deficient human subjects (22). When platelets were incubated with oxLDL at concentrations likely to be present in patients with atherosclerosis, we found that they became more sensitive to activation by low doses of classic agonists, such as ADP. This could be detected as an increase in platelet aggregation responses and by increased expression of platelet activation markers, such as P-selectin and the activated form of the fibrinogen receptor, integrin αIIbβ3. These effects were not seen if CD36 null platelets were used in the assays.
ApoE null mice fed a high fat “western” diet are a commonly used model of hyperlipidemia, oxidant stress and atherosclerosis. Eitzman and colleagues showed that these mice are also a good model for hyper-coagulability and the prothrombotic state associated with these conditions (39). They form occlusive thrombi faster than wild type mice when challenged with vascular injury, for example tail transection or ferric chloride application to the carotid artery. We showed that genetic deletion of CD36 in the apoE null strain “rescued” this prothrombotic phenotype (i.e. the tail vein bleeding times and carotid occlusion times were indistinguishable from those of wild type mice on normal chow diets). Importantly, when platelets from wild type mice, but not CD36 null mice, were washed and re-suspended in plasma from the high fat diet-fed apoE null mice, they became more sensitive to activation by low doses of ADP (22), suggesting that there is a platelet “sensitizing” factor in hyperlipidemic plasma - probably oxLDL. These studies thus mechanistically link CD36, hyperlipidemia and oxidant stress to a pro-thrombotic phenotype.
The signaling pathway in platelets activated by CD36 is similar to that seen in macrophages (40). OxLDL induces recruitment of Fyn and Lyn to CD36 with preferential accumulation of the activated, phosphorylated form of Fyn. This then leads to rapid activation of Vav and JNK family members. Pharmacologic inhibition of these signaling molecules, or use of platelets from mice genetically deficient in them, abrogates the hyper-reactivity of platelets induced by oxLDL in vitro. In vivo studies in mice showed that hyperlipidemic animals had increased “basal” levels of activated JNK in their circulating platelets and increased evidence of JNK activation in experimentally induced thrombi. Genetic deletion of Vav family members, like that of CD36, rescued the high-fat diet induced prothrombotic phenotype in mice and pharmacologic inhibition of JNK in wild type, but not CD36 null mice, and prolonged thrombosis times, suggesting that the CD36 pathway could be a therapeutic target for specific pro-thrombotic conditions.
Cell-derived microparticles are endogenous CD36 ligands that participate in hemostasis and thrombosis
Microparticles (MP) are small (200-1000 nM) membrane bound vesicles that bud off of normal cells as a consequence of apoptosis and/or cell activation. They can be derived from most circulating blood cells, vascular endothelial cells and tumor cells, and can be detected in the circulation of most normal human subjects (41). Their levels in the blood can increase dramatically during certain diseases, and several groups are exploring them as biomarkers for diagnostic and prognostic purposes. For example, levels of leukocyte-derived MP may be increased in patients with acute and chronic inflammatory disorders, endothelial-derived MP in patients with sickle cell disease and other vasculopathies, platelet-derived MP in patients with athero-inflammatory disorders, and tumor-derived MP in patients with cancer (42). There is considerable interest in the role of MP in thrombosis. Since MP express surface proteins reflective of their cell of origin, most attention in this regard has been on MP that express tissue factor, the initiating agent in thrombin generation. Leukocyte-derived and tumor-derived MP are particularly rich sources of circulating tissue factor. The former may play an important role in normal hemostasis, while the latter may contribute to the hyper-coagulable state associated with cancer (43).
A characteristic feature of MP is that like apoptotic cells, they lose their membrane asymmetry and thus express anionic phospholipids, such as PS on their surface. Since oxPS can serve as a CD36 ligand, we hypothesized that MP (includng tissue factor-negative MP) could bind specifically to CD36 on resting platelets and thereby contribute to platelet activation under both normal and pathological conditions. To test this hypothesis we developed fluoresecence-based microscopy and flow cytometry assays to detect platelet-MP interactions (44). Using MP derived ex vivo from cultured endothelial cells or monocytes or isolated from human subjects we found that MP bind specifically to the surface of resting platelets. Binding could be blocked by antibodies to CD36 or competitive CD36 ligands (oxLDL) and binding was not detected in platelets from CD36 null donors. Binding could also be blocked by pre-incubating the MP with annexin V to mask exposed PS. These studies thus showed that MP binding to resting platelets is mediated by a physical interaction between the PS exposed on the MP surface with CD36 on the platelet surface.
The functional consequence of platelet-MP binding is similar to that of oxLDL binding; the platelets become more sensitive to aggregation and activation by low doses of agonist, such as ADP. As expected, CD36 null platelets were not sensitized by MP. The in vivo relevance of these observations remains to be fully explored, but intriguing data point to an important role in both normal hemostasis and pathological thrombosis. Although CD36 null mice do not have a bleeding diathesis and form thrombi at rates indistinguishable from wild type mice in response to vascular injury induced by high doses of ferric chloride, we found that the CD36 null mice had modestly prolonged times to form occlusive thrombi in large and medium sized vessels (carotid arteries and mesenteric venules and arterioles) in response to more modest injury induced by low doses of ferric chloride (44). These data suggest that endogenous CD36 ligands are generated during vascular injury (see model in Figure 2) and contribute to platelet activation. Indeed we found immunological evidence for endothelial-derived MP in experimentally induced thrombi in mice and showed that the thrombi from CD36 null mice contained less endothelial MP immuno-reactivity than those from wild type animals.
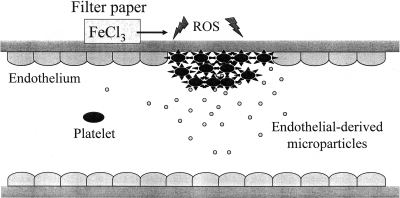
Fig. 2.
Model of microparticle MP-platelet interactions during thrombus formation. Vascular injury is induced experimentally by overlaying a piece of filter paper saturated with ferric chloride (FeCl3) on the adventitial side of a blood vessel. This induces oxidant stress, formation of reactive oxygen species (ROS) and endothelial injury with MP release. The MP then interact with platelets in a CD36-dependent manner, enhancing platelet reactivity and becoming incorporated into the developing thrombus.
SUMMARY
CD36 plays a significant role in the pathogenesis of atherosclerosis by serving as a highly specific receptor for oxidized phospholipids prevalent in oxLDL. The interaction of oxLDL with CD36 triggers a signaling cascade that is necessary for oxLDL uptake and foam cell formation and that alters cytoskeletal dynamics and inhibits migration, thus contributing to trapping of foam cells within the atherosclerotic plaque. CD36 on platelets also interacts with specific oxidized lipids in oxLDL and on the surface of MP and triggers an analogous signaling cascade that renders platelets more sensitive to activation by classic agonists and thereby promotes hemostasis and pathological thrombosis. CD36-mediated signaling pathways are cell context dependent, but as shown in the model in Figure 3, are defined by certain common themes, such as recruitment of src-family kinases and activation Vav family guanine nucleotide exchange factors and specific MAP kinases. Careful dissection of CD36 signaling pathways in the context of specific cells and ligands may yield novel insights for drug development of a variety of disorders. Because CD36 ligands are generated in vivo by oxidant stress, hyperlipidemia, inflammation and cancer, CD36 might mechanistically link these processes to vascular pathology. Thus, targeting CD36 and/or its signaling pathway may provide a novel approach for development of anti-thrombosis and anti-atherosclerosis therapies relevant to specific high risk states. Importantly, CD36 deficiency in humans is not rare and is not associated with any significant pathologic phenotype, including excess bleeding. Similarly, CD36 null mice are phenotypically normal under most conditions. These observations strongly suggest that targeting CD36 pharmacologically is feasible.
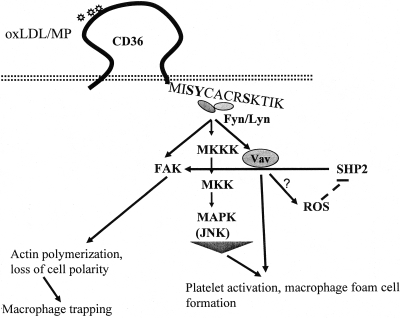
Fig. 3.
Model of CD36 signaling in platelets and macrophages. CD36 binds to oxLDL or MP via a specific domain in the extracellular domain, triggering an interaction of active fyn and lyn src family kinases with its intracytoplasmic C-terminal domain. Downstream events include activation of focal adhesion kinase (FAK), upstream MAP kinase kinases (MKKK and MKK), the effector MAP kinase JNK, and the guanine nucleotide exchange factor Vav; generation of reactive oxygen species (ROS); and oxidative inactivation of the phosphatase SHP2. Together these molecular actions in macrophages lead to ligand internalization and foam cell formation as well as unregulated actin polymerization and loss of cell polarity causing a migration defect and trapping of the cells in athero-inflammatory lesions. In platelets these signaling events lead to enhanced platelet reactivity and promote thrombosis.
ACKNOWLEDGEMENTS
This work was supported by NIH P50 HL81011 and P01 HL087018 (R.L.S.) and the Cleveland Clinic/Case Western Reserve University NIH-sponsored Clinical and Translational Science Award (CTSA).
Footnotes
Potential Conflicts of Interest: None disclosed.
REFERENCES
- 1.Calvo D, Dopazo J, Vega MA. The CD36, CLA-1 (CD36L1), and LIMPII (CD36L2) gene family: cellular distribution, chromosomal location, and genetic evolution. Genomics. 1995;25:100–6. doi: 10.1016/0888-7543(95)80114-2. [DOI] [PubMed] [Google Scholar]
- 2.Tao N, Wagner SJ, Lublin DM. CD36 is palmitoylated on both N- and C-terminal cytoplasmic tails. J Biol Chem. 1996;271:22315–20. doi: 10.1074/jbc.271.37.22315. [DOI] [PubMed] [Google Scholar]
- 3.Febbraio M, Hajjar DP, Silverstein RL. CD36: A Class B Scavenger Receptor involved in angiogenesis, atherosclerosis, inflammation and lipid metabolism. J Clin Inv. 2001;108:785–91. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asch AS, Barnwell J, Silverstein RL, et al. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987;79:1054–61. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur B, Sandberg EM, Devi NS, et al. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cane Res. 2009;69:1212–20. doi: 10.1158/0008-5472.CAN-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson DW, Pearce SFA, Zhong R, et al. CD36 Mediates the Inhibitory Effects of Thrombospondin-1 on Endothelial Cells. J Cell Biol. 1997;138:707–17. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abumrad NA, el-Maghrabi MA, Amri EZ, et al. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–8. [PubMed] [Google Scholar]
- 8.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science Signaling. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoebe K, Georgel P, Rutschmann S, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–7. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 10.Franc NC, Dimarcq JL, Lagueux M, et al. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–43. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- 11.Endemann G, Stanton LW, Madden KS, et al. CD36 is a receptor for oxidised Low Density Lipoprotein. J Biol Chem. 1993;268:11811–6. [PubMed] [Google Scholar]
- 12.Savill J, Hogg N. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–22. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryeom S, Sparrow J, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments on retinal pigment epithelium. J Cell Sci. 1996;109:387–95. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- 14.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 15.Tontonoz P, Nagy L, Alvarez JG, et al. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–52. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 16.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–62. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 17.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the Class B scavenger receptor, CD36, protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–56. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy DJ, Kuchibhotla SD, Guy E, et al. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Arterioscler Thromb Vase Biol. 2009;29:1481–7. doi: 10.1161/ATVBAHA.109.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore KJ, Kunjathoor W, Koehn SL, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning-Tobin JJ, Moore KJ, Seimon TA, et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vase Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podrez EA, Batyreva E, Shen Z, et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–23. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 22.Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a pro-thrombotic phenotype. Nat Med. 2007;13:1086–95. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg ME, Li XM, Gugiu BG, et al. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–96. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 24.Sun M, Finnemann SC, Febbraio M, et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36- mediated phagocytosis by retinal pigment epithelium: A potential mechanism for modulating outer segment phagocytosis under oxidant stress condition. J Biol Chem. 2006;281:4222–30. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez B, Volpert OV, Crawford SE, et al. Signals leading to apoptosis- dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 26.Rahaman SO, Lennon DJ, Febbraio M, et al. CD36-dependent activation of c- Jun N-terminal kinase by oxidized LDL is required for macrophage foam cell formation. Cell Metabol. 2006;4:211–21. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricci R, Sumara G, Sumara I, et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell. Science. 2004;306:1558–61. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 28.Stuart LM, Deng J, Silver JM, et al. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–85. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Triantafilou M, Gamper FG, Haston RM, et al. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–11. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 30.Albert ML, Pearce SFA, Francisco L, et al. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to CTLs. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finnemann SC, Silverstein RL. Differential roles of CD36 and αvβ5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J Exp Med. 2001;194:1289–98. doi: 10.1084/jem.194.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson B, Koenigsknecht-Talboo J, Grammes C, et al. Fibrillar beta-amyloid- stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–50. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 33.Rahaman SO, Zhou G, Swat W, et al. Vav proteins mediate a CD36-dependent proatherogenic macrophage phenotype. (submitted) [Google Scholar]
- 34.Janabi M, Yamashita S, Hirano K, et al. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterio Thromb Vase Biol. 2000;20:1953–60. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- 35.Quinn MT, Parthasarathy S, Fong LG, et al. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;84:2995–8. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and contributes to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–45. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clemetson KJ, Pfueller ST, Luscher EF, et al. Isolation of the membrane glycoproteins of human blood platelets by lection affinity chromatography. Biochim Biophys Acta. 1977;464:493–508. doi: 10.1016/0005-2736(77)90025-6. [DOI] [PubMed] [Google Scholar]
- 38.Knowles DM, Tolidijian B, Marboe C, et al. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984;132:2170–3. [PubMed] [Google Scholar]
- 39.Eitzman DT, Westrick RJ, Xu Z, et al. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein E-deficient mice. Arterioscler Thromb Vase Biol. 2000;20:1831–4. doi: 10.1161/01.atv.20.7.1831. [DOI] [PubMed] [Google Scholar]
- 40.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized LDL. Circ Res. 2008;102:1512–9. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Combes V, Simon AC, Grau GE, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 43.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh A, Li W, Febbraio M, et al. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in vivo. J Clin Invest. 2008;118:1934–43. doi: 10.1172/JCI34904. [DOI] [PMC free article] [PubMed] [Google Scholar]