Abstract
Purpose
Late gastrointestinal and genitourinary morbidity from external beam irradiation used to treat adenocarcinoma of the prostate continue to be a concern of physicians, and patients alike. Additionally for high risk/locally advanced patients the appropriate use of hormonal manipulation in addition to radiation therapy (RT) may increase toxicity. We analyzed three large RTOG studies 85-31, 86-10, and 92-02 to try to address the aforementioned issues.
Methods
2,922 patients were accrued with a median follow up of 10.3 years for surviving patients. The RTOG scoring scheme was used to assess GI, GU, and other toxicities. Toxicity reported was grade 3 or higher late toxicity. Patient toxicity level was assessed by study and by treatment type combining RT only vs. RT + short course hormone therapy (STH) vs. RT + long term hormone therapy (LTH).
Results
Multivariate analysis reveals that age > 70 was statistically significantly associated with a decrease in late any grade 3+ toxicity (HR= 0.78, p=0.0476) adjusted for treatment type. Comparing treatment type, patients treated with RT+STH had a statistically significant lower probability of grade 3+ GI, GU, and other toxicity compared to RT alone (p = .00006; p=0.0037; p=0.0127, respectively). Patients treated with RT+LTH had a statistically significant lower probability of grade 3+ GU toxicity compared to RT alone (p=0.023).
Conclusion
These data show that external beam radiation therapy remains a safe option for locally advanced/high risk prostate cancer, and the use of hormonal manipulation does appear to be protective for GU and GI toxicity depending upon length of treatment.
Keywords: Prostate cancer, toxicity, hormone therapy
Introduction
Significant long term sequelae from definitive treatment of adenocarcinoma of the prostate via external beam radiation therapy (RT) continues to be a significant concern of radiation oncologists, urologists, and patients. The RTOG has developed a morbidity scoring scheme in an effort to standardize the reporting of sequelae following management of adenocarcinoma of the prostate and other tumors via radiation therapy.(1)
Previously the RTOG reported on long term significant sequelae from two large randomized trials 75-06 and 77-06 involving 1,020 patients.(2) These patients were treated during the late 70’s and early 80’s, and the risk of major sequelae (grade 3 or higher toxicity) was found to be acceptably low. Subsequently, a number of trials were executed in the late 80’s and early 90’s, RTOG 85-31, 86-10, and 92-02 the late sequelae of which are available for analysis. In addition to radiation therapy these trials employ different forms of hormonal manipulation (LHRH agonist + antiandrogen vs. LHRH agonist alone).(3, 4, 5,6) It was suggested in the results of RTOG 92-02 that long term hormone suppression increase the incidence of GI toxicity.(5,6) Other authors have also suggested a relationship between RT, GI toxicity and androgen deprivation.(7,8)
The purpose of this study is to evaluate the incidence of long term treatment sequelae grade 3 or higher (grade 3+) in patients treated on RTOG studies 85-31, 86-10 and 92-02, looking to evaluate any changes in late toxicity incidence with specific evaluation of the potential effect of hormone therapy on late GI, GU, and other sequelae in patients receiving definitive radiation therapy for adenocarcinoma of the prostate.
Methods and Materials
2,922 eligible patients were accrued into RTOG protocols 85-31 (n=945), 86-10 (n = 456), and 92-02 (n = 1,521). Among those patients, 2906 patients (85-31, n=944; 86-10, n = 454; and 92-02, n = 1,508) with late toxicity information were used for this analysis. Specific parameters for accrual can be found in prior publications (3, 4, 5) yet each trial accrued patients with locally advanced non-metastatic (no distant mets, M0) prostate cancer. All patients were treated with whole pelvis radiation therapy 44-46 Gy at 1.8 – 2.0 Gy/fraction, and doses up to 50 Gy were acceptable. The prostate ± seminal vesicles following the whole pelvis RT received a boost such that the total dose was 65 – 70 Gy. (3,4,5) The hormone therapy ranged from none in one arm of RTOG 85-31 and 86-10 to short term hormone therapy (STH) consisting of two months of neoadjuvant total androgen suppression (TAS) with flutamide 250mg 3 times a day and a LHRH agonist (Goserelin) plus 2 months concurrent TAS with RT (in 1 arm of 86-10 and both arms of 92-02). Lastly, adjuvant hormone therapy (LHRH only) was used in 1 arm of RTOG 85-31 indefinitely and 1 arm of RTOG 92-02 for 2 years duration.
Toxicity is reported according to the RTOG scoring scheme including GI, GU, and other toxicity.(1) Late toxicity is reported as occurring ≥ 90 days after the start of RT. The data was analyzed by study treatment arms and then all six treatment arms (2 from each study) were collapsed into 3 categories: RT only, RT+STH, and RT + LTH:
| RT only | RT + hormones at relapse arm of 85-31 |
| RT only arm of 86-10 | |
| RT+STH | RT + hormones arm of 86-10 |
| RT + Short-Term Hormones arm of 92-02 | |
| RT + LTH | RT + immediate hormones arm of 85-31 |
| RT + Long-Term Hormones arm of 92-02 |
Statistics
To analyze whether age or treatment type was associated with the time to late toxicity, multivariate Cox proportional hazard regression models(9) were utilized for the combined treatment groups. Age (≤ 70 years (reference level; R.L.) vs. > 70 years) and treatment type (RT Alone (R.L.) vs. RT+STH vs. RT+LTH) Adjusted hazard ratios (HRs) were calculated for these covariates using the Cox proportional hazards model with associated 95% confidence intervals (CI) and P-values for probability estimates. All statistical comparisons were two-tailed and a P-value of <0.05 was considered statistically significant. Statistical Analysis System (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Table 1 shows the number of patients, median follow up for all prostate and surviving patients, median PSA, race, KPS and Gleason Score of patients on RTOG 85-31, 86-10, and 92-02 by treatment arm. Given that these were phase III randomized trials, there is no statistically significant difference in any of these parameters except PSA in 86-10 since this parameter was not part of the routine practice at the outset of the study, and therefore, not a requirement for study entry. The median follow up for 85-31 was 8.1 years and 11.1 for living patients. Median follow up for 86-10 was 6.9 years and 12.2 for living patients. Median follow up for 92-02 was 8.1 years with 9.9 years for living patients. Median age was 70 across all three studies. The median PSA value was approximately 20 for all 3 studies, and a minimum of 42% of patients had a GS of ≥ 7. Both PSA and GS revealed the high risk and/or locally advanced nature of the tumors in these studies.
Table 1.
Patient Characteristics
| 85-31 | 86-10 | 92-02 | ||||
|---|---|---|---|---|---|---|
| RT Alone | RT+LTH | RT | RT+STH | RT+STH | RT+LTH | |
| n= | 467 | 477 | 231 | 223 | 755 | 753 |
| Median f/u | 8.1 yrs | 6.9 yrs | 8.1 yrs | |||
| Median f/u living patients |
11.1 yrs | 12.2 yrs | 9.9 yrs | |||
| Median age | 70 | 70 | 71 | 70 | 70 | 70 |
| Median PSA | 21.8 | 22 | 33.8 | 22.5 | 20.9 | 19.9 |
| Race: White Black |
90% 8% |
90% 9% |
89% 9% |
87% 10% |
84% 12% |
84% 14% |
| KPS 90–100 | 94% | 93% | 96% | 92% | 91% | 93% |
| GS 2–6 7–10 missing |
37% 42% 21% |
34% 44% 21% |
29% 44% 27% |
35% 43% 23% |
18% 55% 27% |
16% 59% 24% |
Maximum late GI, GU, and other toxicity as well as maximum toxicity per patient is shown in Table 2 by study. Regarding GI toxicity, there were 3 deaths (grade 5 toxicity), all of which occurred in study 92-02. One was in the short term hormone arm, and two were in the long term hormone arm. The first was a sigmoid perforation with sepsis and death, another a small bowel obstruction with surgical resection and ultimate peritonitis and death, and the last was a patient with rectal bleeding and known radiation proctitis who refused laser treatment as well as transfusion, and ultimately died. Grade 4 GI toxicity occurred in < 1 – 1% of patients in all 3 studies. The most common types of grade 4 GI toxicity were proctitis and rectal bleeding.
Table 2.
Toxicity Data by Study
| 85-31 | 86-10 | 92-02 | ||||
|---|---|---|---|---|---|---|
| RT Alone | RT+LTH | RT | RT+STH | RT+STH | RT+LTH | |
| n | 467 | 477 | 231 | 223 | 755 | 753 |
|
Late Max GI - Toxicity |
||||||
| Gr 3 | 4% (n=17) | 2% (n=11) | 3% (n=7) | 2% (n=4) | 1% (n=6) | 2% (n=16) |
| Gr 4 | 1 %( n=3 ) | 1 % (n=6) | 1 %( n=3 ) | <1% (n=1) | <1 %(n=2 ) | <1% (n=3) |
| Gr 5 | 0% | 0% | 0% | 0% | <1% (n=1) | <1% (n=2 |
| Gr≥3 | 4% (n=20) | 4% (n=17) | 4% (n=10) | 2% (n=5) | 1% (n=9) | 3% (n=21) |
|
Late Max GU -Toxicity |
||||||
| Gr3 | 8% ( n=36) | 6% (n=31) | 6% (n=15) | 7% (n=15) | 4% (n=32) | 3% (n=26) |
| Gr4 | 1% (n=5) | 1% (n=4) | 2% (n=4) | 1% (n=2) | <1% (n=2) | 2% (n=13) |
| Gr5 | 0% | 0% | 0% | 0% | 0% | 0% |
| Gr≥3 | 9% (n=41) | 7% (n=35) | 8% (n=19) | 8% (n=17) | 5% ( n=34) | 5% (n=39) |
|
Late Other - Toxicity |
||||||
| Gr 3 | 1% (n=7) | 2%(n=8) | 2% (n=3) | <1% (n=1) | 1% (n=5) | 1% (n=8) |
| Gr 4 | <1% (n=2) | 0% | 1% (n=2) | 0% | 0% | <1% (n=1) |
| Gr 5 | 0% | 0% | 0% | 0% | 0% | 0% |
| Gr≥3 | 2% (n=9) | 2% (n=8) | 2% (n=5) | <1% (n=1) | 1% (n=5) | 1% (n=9) |
| Max Total Toxicity | ||||||
| Gr3 | 11% (n=53) | 10% (n=50) | 8% (n=19) | 9% (n=19) | 6% (n=42) | 6% (n=46) |
| Gr4 | 2% (n=10) | 2% (n=9) | 3% (n=6) | 1% (n=3) | 1% (n=4) | 2% (n=15) |
| Gr 5 | 0% | 0% | 0% | 0% | < 1% (n=1) | < 1% (n=2) |
| Gr≥3 | 13% (n=63) | 12% (n=59) | 11% (n=25) | 10% (n=22) | 6% (n=47) | 8% (n=63) |
Regarding maximum GU toxicity, there were no deaths, and < 1 – 2% of patients experienced grade 4 toxicity, the most common types being cystitis and hematuria.
Late other toxicity showed no deaths, and < 1–2% of patients had grade 4 complications. These consisted of osteoporosis, malignant ascites, rectovesicle fistula, pain, and rectourethral fistula. There were no statistically significant differences in maximum toxicity per patient for any of the three studies when analyzed by treatment arm.
Table 3 shows the late grade 3+ toxicity by treatment grouping of RT only, RT+STH, and RT + LTH. Maximum grade 3+ late GI toxicity was 4% for RT only, 1% for RT+STH, and 3% for RT + LTH. Maximum grade 3+ late GU toxicity was 9%, 5%, and 6% for RT only, RT+STH, and RT + LTH, respectively. Maximum grade 3+ late other toxicity was 2%, 1%, and 1% respectively for RT alone, RT+STH, and RT + LTH.
Table 3.
Toxicity Data by RT vs RT+STH vs RT+LTH
| RT | STH+RT | RT+LTH | |
|---|---|---|---|
| n= | 698 | 978 | 1230 |
| GI | |||
| Gr ≥ 3 | 4% (n=30) | 1% (n=14) | 3% (n=38) |
| Gr 5 | 0% | <1% (n=1) | <1% (n=2) |
| GU | |||
| Gr ≥ 3 | 9%(n=60) | 5% (n=51) | 6% (n=74) |
| Gr 5 | 0% | 0% | 0% |
| Other | |||
| Gr ≥ 3 | 2%(n=14) | 1% (n=6) | 1% (n=17) |
| Gr 5 | 0% | 0% | 0% |
|
Max Total Toxicity |
|||
| Gr ≥ 3 | 13% | 7% | 10% |
| Gr 5 | 0% | 0% | 0% |
Table 4 reports the results of the multivariate analysis with two covariates, age (≤ 70 vs. > 70) and treatment type (RT alone, RT+STH, and RT + LTH). It shows that age > 70 was statistically significantly associated with a decrease in late total grade 3+ toxicity (HR= 0.78, p=0.0476) after adjusting for treatment type. Patients treated with RT+STH had a statistically significant lower probability of grade 3+ GI, GU, and other toxicity compared to RT alone when adjusting for age (HR=0.33, p = .00006; HR=0.57, p=0.0037; HR=0.30, p=0.0127, respectively). RT+STH had a statistically significant decrease in grade 3+ late total toxicity over RT alone (HR=0.54, p = 0.0001). Patients treated with RT+LTH had a statistically significant lower probability of grade 3+ GU toxicity compared to RT alone (HR=0.67, p=0.023). Also, RT + LTH showed a trend towards decrease in late total grade 3+ toxicity (HR= 0.77, p = 0.0566). No other statistically significant differences were seen.
Table 4.
Cox Proportional Hazard Models for Time to Late Grade 3+ Toxicity
| Covariates | HR* | 95% C.I. * | p-value† | ||
|---|---|---|---|---|---|
| Late Grade 3+ | Age | ||||
| Total Toxicity | |||||
| ≤ 70 | RL | ||||
| > 70 | 0.78 | (0.62, 1.00) | 0.0476+ | ||
| Treatment | |||||
| RT only | RL | ||||
| Short-term HR | 0.54 | (0.39, 0.74) | 0.0001+ | ||
| Long-term HR | 0.77 | (0.62, 1.01) | 0.0566 | ||
| Late Grade 3+ GI | Age | ||||
| Toxicity | |||||
| ≤ 70 | RL | ||||
| > 70 | 0.71 | (0.45, 1.11) | 0.1289 | ||
| Treatment | |||||
| RT only | RL | ||||
| Short-term HR | 0.33 | (0.17, 0.62) | 0.0006+ | ||
| Long-term HR | 0.70 | (0.43, 1.13) | 0.1423 | ||
| Late Grade 3+ | Age | ||||
| GU Toxicity | |||||
| ≤ 70 | RL | ||||
| > 70 | 0.90 | (0.67, 1.20) | 0.4636 | ||
| Treatment | |||||
| RT only | RL | ||||
| Short-term HR | 0.57 | (0.40, 0.84) | 0.0037+ | ||
| Long-term HR | 0.67 | (0.48, 0.95) | 0.0230+ | ||
| Late Grade 3+ Other | Age | ||||
| Toxicity | |||||
| ≤ 70 | RL | ||||
| > 70 | 0.66 | (0.33, 1.29) | 0.2214 | ||
| Treatment | |||||
| RT only | RL | ||||
| Short-term HR | 0.30 | (0.11, 0.77) | 0.0127+ | ||
| Long-term HR | 0.74 | (0.33, 1.29) | 0.4109 | ||
HR=Hazard ratio, RL=Reference level, C.I. is the confidence interval.
p-value is from Chi-square test using the Cox proportional hazards model.
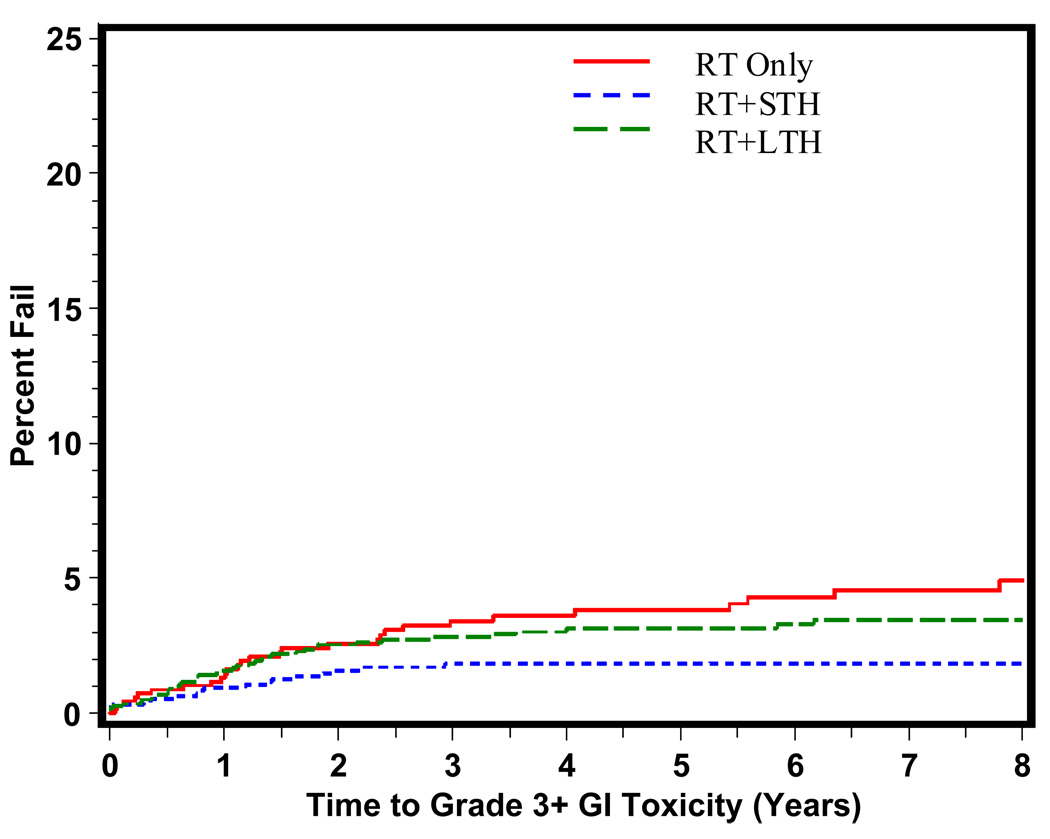
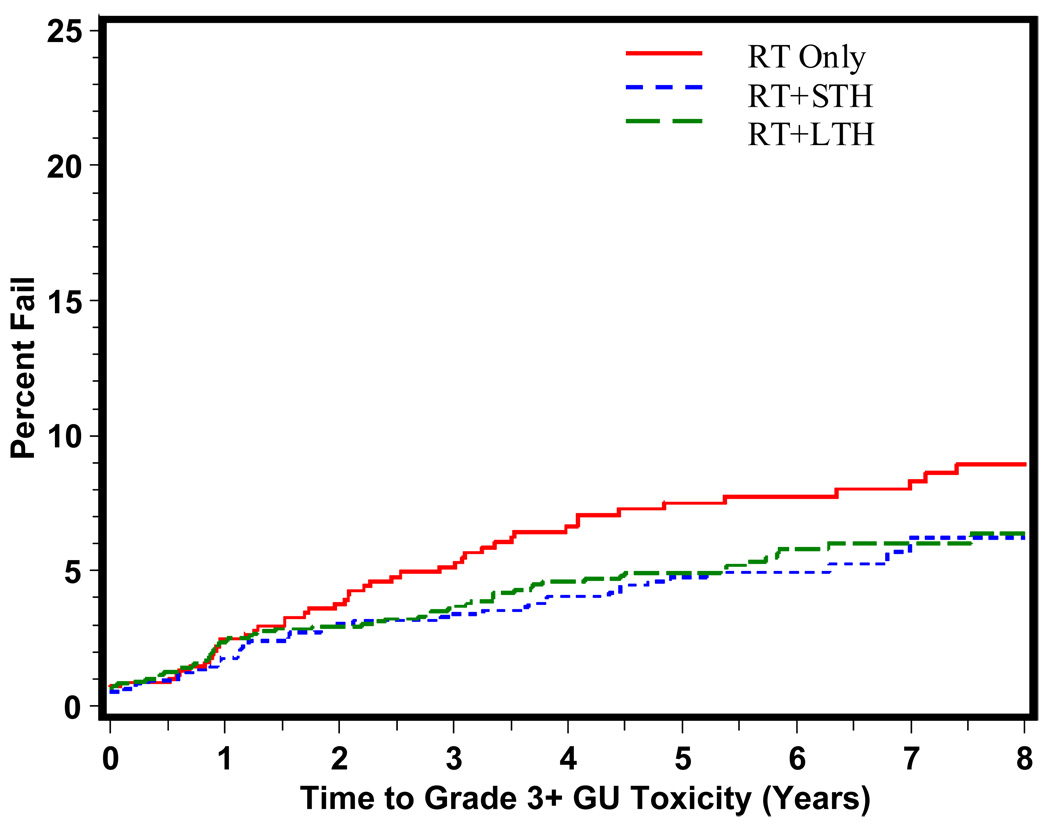
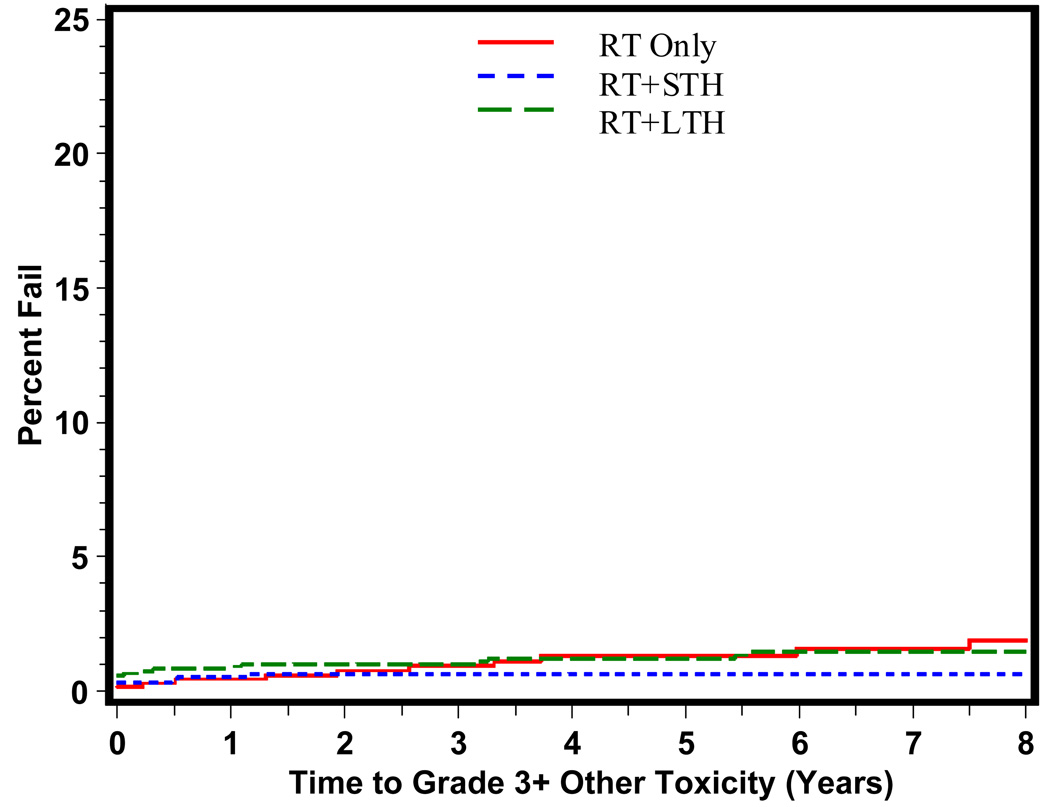
The time to grade 3+ late GI, GU, and other toxicity is shown in Figures 1, 2, and 3 respectively. As predicted, the majority of grade 3+ late GI toxicity occurs in the first 1–3 years, whereas the GU toxicity occurs over a longer period of time, 2–5 years. RT+STH was statistically significantly associated with a decreased time to grade 3+ late GI toxicity (p = 0.006). Time to grade 3+ late GU toxicity shown in Figure 2 reveals a statistically significant difference in time to grade 3+ late GU toxicity for RT+STH and LTH + RT compared to RT alone (p = 0.0037 and p = 0.0230, respectively).
Figure 1.
Time to Late Grade 3+ GI Toxicity By Treatment Type
Figure 2.
Time to Late Grade 3+ GU Toxicity By Treatment Type
Figure 3.
Time to Late Grade 3+ Other Toxicity By Treatment Type
Conclusions
The trials analyzed here (RTOG 85-31, 86-10, and 92-02) have helped to establish that patients with high risk tumors (locally advanced and/or high grade) do benefit in terms of overall and disease specific survival by adding both neoadjuvant and adjuvant hormone therapy to RT. Yet what is the cost of this addition in terms of toxicity especially late toxicity? The data presented here including almost 3,000 patients show that late grade 3+ GI, GU, and other toxicity is certainly present for some patients with RT ± hormone therapy, but clearly is not increased with the use of hormone therapy over RT alone. In fact this data firmly refutes the notion that hormone therapy is detrimental with regards to late grade 3+ toxicity. RT+STH actually results in a statistically significant decrease in late grade 3+ toxicity compared to RT alone (p = 0.0001, Table 4) This is true for each of the toxicities analyzed i.e., GI, GU, and other toxicity. RT + LTH results in a statistically significant decrease in late grade 3+ GU toxicity (p = 0.0230) compared with RT alone and is not statistically different from RT alone with regards to late grade 3+ GI and other toxicity. Although prior data suggested that long term adjuvant (LHRH) therapy was associated with an increase in GI toxicity (5, 6, 7) this was not found in this very large cohort of similarly treated patients. Thus the question has been answered. Yet is there some price to pay for aggressive treatment of locally advanced/high risk prostate cancer treated with neoadjuvant hormones + RT and concurrent hormone + adjuvant hormones? The answer is yes, but certainly no worse than aggressive RT alone as seen in this data. 8% of patients treated with RT + LTH and 7% of patients treated with RT+STH exhibited grade 3+ toxicity. The majority of this toxicity was grade 3 with < 1% – 2% at the grade 4 level across GI, GU, and other toxicity. The risk of any grade 3+ toxicity for RT alone was 15%, 9% of which was GU toxicity.
Certainly there is some level of late grade 3+ toxicity that is unavoidable. Yet one must remember that the data presented here reflects treatment during the late 80’s and early 90’s before IMRT to treat pelvic lymph nodes and the primary tumor was utilized. We will await trials such RTOG 05–21 to try to understand whether more conformal technology represented by IMRT ultimately translates into a decrease in late complications as we hope it will. Until that time we have shown with this data that the current benchmark of neoadjuvant hormone therapy + radiation therapy and concurrent hormone + adjuvant LHRH is not only effective treatment for patients with locally advanced/high risk prostate cancer, but safe treatment as well. This data is especially important in light of the concerns for hormonal manipulation related to other toxicities such as cardiac and bone. This data proves that hormonal manipulation is not only helpful regarding disease control as shown in multiple studies,(3,4,5) but in the tolerance of the radiation therapy that these patients need. Further studies need to be done to elucidate the mechanism of the protective effect of the hormonal manipulation.
Acknowledgments
Supported by the Division of Cancer Treatment and Diagnosis, National Cancer Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the RTOG and EORTC. Int J Rad Onc Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 2.Lawton CA, Won M, Pilepich M, et al. Long-term treatment sequelae following external beam radiation for adenocarcinoma of the prostate: Analysis of RTOG studies 75-06 and 77-06. Int J Rad Onc Biol & Phys. 1991;21:935–939. doi: 10.1016/0360-3016(91)90732-j. [DOI] [PubMed] [Google Scholar]
- 3.Pilepich M, Winter K, Lawton C, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma – long term results of phase III RTOG 85-31. Int J Rad Onc Biol & Phys. 2005;61(5):1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Pilepich M, Winter K, John M, et al. Phase III RTOG trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Rad Onc Biol & Phys. 2001;50(5):1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 5.Hanks G, Pajak T, Porter A, et al. Phase III trial of long term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: RTOG protocol 92-02. J Clin Onc. 2003;21(21):3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Hanks GF, Bae K, Porter A, et al. Ten year follow-up of RTOG 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Proceedings of the 48th Annual ASTRO Meeting; 2006. p. S13. Abs #22. [Google Scholar]
- 7.Sanguineti G, Agostinelli S, Foppiano F, et al. Adjuvant androgen deprivation impacts late rectal toxicity after conformal radiotherapy of prostate carcinoma. British Journal of Cancer. 2002;86:1843–1847. doi: 10.1038/sj.bjc.6600266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M, Pickles T, Agranovich A, et al. Impact of neoadjuvant androgen ablation and other factors on late toxicity after external beam prostate radiotherapy. Int J Rad Onc Biol & Phys. 2004;58(1):59–67. doi: 10.1016/s0360-3016(03)00777-6. [DOI] [PubMed] [Google Scholar]
- 9.Cox DR. Regression models and life tables. J R Stat Soc Series B. 1972;34:187–229. [Google Scholar]