Abstract
PURPOSE/OBJECTIVE
This trial was designed to test the hypothesis that TAS and WP radiotherapy (RT) followed by a prostate boost improves progression free survival (PFS) by at least 10% compared to TAS and PO RT. This trial was also designed to test the hypothesis that neoadjuvant hormonal therapy (NHT) followed by concurrent TAS and RT improves PFS compared to RT followed by adjuvant TAS (AHT) by at least 10%.
METHODS/MATERIALS
Patients eligible for the study included those with clinically localized adenocarcinoma of the prostate and elevated prostate specific antigen (PSA) < 100 ng/ml. Patients were stratified by T stage, PSA, and Gleason score (GS) and required to have an estimated risk of lymph node (LN) involvement > 15%.
RESULTS
The results of neoadjuvant vs. adjuvant hormone therapy and WP vs PO show no statistically significant difference in PFS or OS. Results show a statistically significant difference in PFS (p=0.035) in favor of the WP+NHT arm over PORT+NHT and WPRT + AHT. Overall survival for the four arms shows a statistically significant difference (p=0.027).
CONCLUSIONS
This trial has shown that there is an interaction between the timing of hormone therapy and radiation field size for this patient population. Based on this analysis and randomized trials to date which show a benefit to radiation therapy and hormone therapy in locally advanced prostate cancer patients, it is clear that NHT + WPRT remains the standard of care.
Combining hormonal manipulation with radiation therapy for patients with locally advanced and/or high risk prostate cancer has become a standard based on multiple prospective randomized trials.(1,2,3,4) In each of these trials the scope of the radiation included the pelvic lymph nodes.
However, some authors believe that it is not necessary to treat the pelvic lymph nodes either because they feel that lymph node disease is tantamount to distant metastasis or potentially that the hormone manipulation will address microscopically involved lymph nodes as it appears to help prevent/treat potential microscopic distant metastasis. (1,2,3,4)
In addition each of the trials referenced above the androgen suppression was done somewhat differently. RTOG protocol 85-31 (1) and the EORTC trial (3) gave adjuvant LHRH yet RTOG 86-10(2) and 92-02(4) gave neoadjuvant androgen suppression. So in addition to the scope of the radiation required, the question of timing of androgen suppression remained. Is neoadjuvant better/worse than adjuvant or are they equivalent?
RTOG study 94-13 was designed to answer both questions. This trial was a multicenter prospective randomized trial designed to answer the questions concerning the value of prophylactic WP RT in patients who had an assessed risk of lymph node involvement of > 15% (Roach formula) .(5) It was also designed to answer the question of timing of hormonal therapy (neoadjuvant vs. adjuvant) on progression free survival (PFS). This analysis is an update of the results of 94-13 with specific focus on the unexpected interaction between field size and timing of hormone therapy.
Methods/Materials
Eligibility
Patients eligible for this trial included histologically confirmed clinically localized adenocarcinoma of the prostate with an elevated prostate specific androgen (PSA) ≤ 100 ng/ml. Patients were stratified by T stage (T1c, T2a vs. T1b, T2b vs. T2c to T4), PSA (< 30 vs. ≥ 30 ng/ml), and Gleason score (GS < 7 vs. 7–10). PSA stratification was based on the median PSA observed in an earlier high risk patient study.(6,7) Additionally eligible patients were required to have an estimated risk of lymph node (LN) involvement of > 15%, based on the equation + LN = (2/3) PSA + [(GS − 6) × 10].(5,8) Patients with T2c to T4 tumors were also eligible if they had a GS ≥ 6 even if their calculated risk of LN involvement did not reach 15%. Patients who were surgically staged or who had metastatic disease were ineligible. Other eligibility criteria included Karnofsky Performance Status (KPS) ≥ 70%; no prior hormone therapy, radiation, or chemotherapy; and liver function tests ≤ 1.2 × the upper limit of normal. In addition all patients signed a consent form prior to randomization.
All patients in the trial received TAS which consisted of goserelin acetate 3.6 mg/month subcutaneously or leuprolide acetate 7.5 mg/month intramuscularly, and flutamide 250 mg t.i.d. orally for four months. Patients receiving NHT began hormone therapy two months before RT and continued to receive it during RT. Those patients receiving adjuvant hormone therapy (AHT) began their drugs immediately following the completion of RT.
RT was given at 1.8 Gy/fraction to a total dose of 70.2 Gy calculated at isocenter. WP RT consisted of conventional four-field “box” technique with a minimum unblocked field size of 16 × 16 cm to a maximum central axis dose of 50.4 Gy. Patients receiving WP RT were treated with an additional 19.8 Gy to the prostate using a cone down boost technique. Prostate-only (PO) RT was limited to the prostate and seminal vesicles, with a maximum unblocked field size of 11 × 11 cm to a total central axis dose of 70.2 Gy. A urethrogram was required as part of the simulation, and the inferior edge of the field was to be placed at least 1cm below the point where the contrast narrowed (apex of the penile urethra) to ensure coverage of the prostate.(9)
Statistical Analysis
This trial used a 2×2 factorial design to test whether WP RT and TAS improved PFS compared to PO RT and TAS and whether NHT and RT improved PFS compared to AHT and RT. Secondary objectives included comparing treatments with regard to local failure (LF), time to distant failure, and overall survival. (OS) The design assumed no significant statistical interaction between the treatments, and was designed to detect a 10% difference in the 5 year PFS rates with a significant level of 0.025 and a statistical power of 80%. It was assumed that the PFS estimate was exponentially distributed. A 2-sided log-rank test was used. The initial sample size was increased by 10% because of the possibility that patients may be found retrospectively ineligible or lost to follow up. Thus, the target sample size for the study was 1,200 patients.
PFS and OS were estimated with the Kaplan-Meier method and the unstratified log-rank statistical analysis was used to test for differences. (10) Biochemical failure, local failure, regional nodal failure, and distant metastasis were estimated using the cumulative incidence method,(11) and Gray’s test was used to test for differences.(12) These methods account for competing risks, such as death without experiencing an event. It is important to note that the analysis of local, regional nodal, distant, and biochemical failure do not take into account competing events, with the exception of death. Thus, the number of failures for the individual events do not sum to the total number of disease progression for a given treatment arm. However, the analysis of time to first occurrence of an individual event (eg, LF) will account for other competing events. Thus the sum of these individual failures will be equal to the number of disease progressions for each treatment arm.
The RTOG toxicity scoring scale was used to assess treatment related toxicities.(13)
End Point Definition
The primary endpoint for this study was PFS. Secondary endpoints included OS, LF, distant metastasis, (DM) and PSA failure. A failure event for PFS was defined as the first occurrence of local progression, regional/nodal failure, distant failure, or biochemical (PSA) failure or death due to any cause. The failure event for OS was defined as death due to any cause. LF was defined as tumor recurrence (positive rebiopsy at least 2 years after treatment), tumor regrowth by 50% or tumor that never cleared.
The definition of biochemical (PSA) failure used in this trial was developed in 1994 as part of a study designed by the American Society for Therapeutic Radiation and Oncology (ASTRO) consensus conference.(14) Biochemical (PSA) failure was defined as two consecutive and significant PSA rises separated by at least one month. For a PSA level that was ≤ 1.5 ng/ml, an increase ≥ 0.3 ng/ml was considered a significant rise. For a PSA level that was more than 1.5 ng/ml, a significant rise was defined as an increase of 20% or more. For example, if the current PSA level was 2.0 ng/ml an increase would be considered significant if the next recorded value (separated by at least one month) was ≥ 2.4 ng/ml. Patients with slowly rising PSA’s (< 20%/year) were considered free from PSA failure as long as their PSA remained < 4.0 ng/ml. The decision to use this relatively high threshold value to define failure was based on the desire to use a definition with high specificity for clinically meaningful outcome.(15) In the decision to define failure as two rises reflected the desire to maintain high sensitivity. Given the recent interest and enthusiasm for new definition of biochemical failure of nadir + 2 ng/ml, the data was also analyzed using that definition.
Data Monitoring
This study was approved by the National Cancer Institute and by the Committee on Human Subject Research and the institutional review board at each of the participating RTOG institutions. A formal RTOG Data Monitoring Committee was in place to oversee the study’s progress. The interim analysis results were presented to the Data Monitoring Committee in April of 2001. Following this review the study was deemed mature enough to warrant disclosure of the findings relative to the primary endpoint as stated in the study design. The O’Brien-Flemming(16,17) alpha-spending function boundary was 2.397, with a nominal significance level of 0.0165.
Results
This study was activated April 1, 1995 and closed June 1, 1999 with a total of 1,323 patients accrued. Ninety-eight percent of the patients (1,292 of the 1,323 patients) were considered eligible and properly entered onto the study. The pre-treatment characteristics of the patients are summarized in Table 1. The median age was 70 years and nearly 23% were of African American descent. The median PSA was 22.6 ng/ml and 2/3 of the patients had clinical disease stages T2c–T4 with 73% of patients having a Gleason Score of ≥ 7. Patients were balanced between all four arms for Gleason score, PSA, stage, race, and estimated risk of lymph node involvement. The median follow up since completion of therapy for all patients was 6.6 years. Median follow up for patients alive at the time of the analysis is 7.0 years (Range 2–10.4 years)
Table 1.
Pretreatment Characteristics
| Hormones + RT Whole Pelvis + Boost (n=320) |
Hormones + RT Prostate Alone (n=319) |
RT Whole Pelvis + Boost + Hormones (n=319) |
RT Prostate Alone + Hormones (n=321) |
|||||
|---|---|---|---|---|---|---|---|---|
| Age (year) | ||||||||
| Median | 70 | 70 | 70 | 70 | ||||
| Range | 46–83 | 44–87 | 50–84 | 45–85 | ||||
| PSA (ng/mL) | ||||||||
| Median | 23.4 | 23.3 | 22.1 | 23.5 | ||||
| Range | 2.9–97.6 | 2.1–95.1 | 3.0–98.0 | 3.9–98.2 | ||||
| T-Stage | n | % | n | % | n | % | n | % |
| T1c, T2a | 74 | 23 | 75 | 24 | 69 | 22 | 68 | 21 |
| T1b, T2b | 32 | 10 | 34 | 11 | 22 | 7 | 41 | 13 |
| T2c-T4 | 214 | 67 | 210 | 66 | 228 | 71 | 212 | 66 |
| PSA | ||||||||
| <30 | 215 | 67 | 215 | 67 | 213 | 67 | 218 | 68 |
| ≥30 | 105 | 33 | 104 | 33 | 106 | 33 | 103 | 32 |
| Gleason (Institution) |
||||||||
| < 7 | 85 | 27 | 83 | 26 | 86 | 27 | 86 | 27 |
| 7–10 | 235 | 73 | 236 | 74 | 233 | 73 | 235 | 73 |
| Karnofsky status | ||||||||
| 70 | 6 | 2 | 5 | 2 | 5 | 2 | 6 | 2 |
| 80 | 25 | 8 | 17 | 5 | 22 | 7 | 19 | 6 |
| 90 | 138 | 43 | 139 | 44 | 132 | 41 | 136 | 42 |
| 100 | 151 | 47 | 158 | 50 | 160 | 50 | 160 | 50 |
| Race | ||||||||
| White | 230 | 72 | 218 | 68 | 222 | 70 | 218 | 68 |
| African American | 71 | 22 | 88 | 28 | 77 | 24 | 88 | 27 |
| Other | 19 | 6 | 13 | 4 | 20 | 6 | 15 | 5 |
| Estimated Risk of Lymph Node Involvement |
||||||||
| > 15–35% | 239 | 75 | 244 | 76 | 237 | 74 | 244 | 76 |
| > 35% | 81 | 25 | 75 | 24 | 82 | 26 | 77 | 24 |
Outcome of Primary Objectives: Radiation Volume and Hormone Therapy Sequence
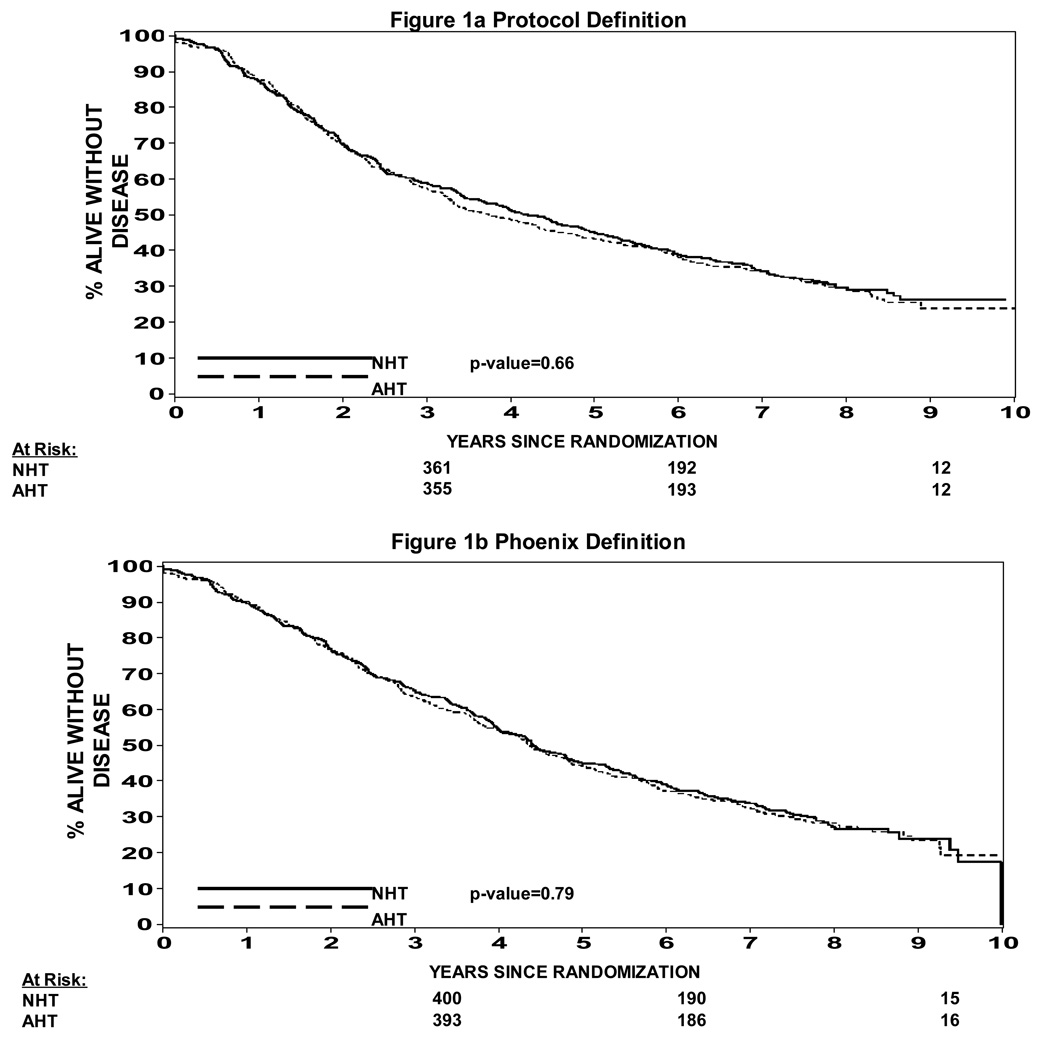
The effect of hormone therapy sequence on progression free survival is summarized in Figure 1a and 1b. There was no statistical difference in progression free survival looking at the neoadjuvant hormone therapy arms (Arms 1 & 2) vs. the adjuvant hormone therapy arms (Arms 3 & 4). (Figure 1a protocol definition of biochemical failure) Figure 1b looks at biochemical failure as a nadir + 2 ng/ml definition and again shows no difference in progression free survival based on hormone therapy sequence. (p=0.79)
Figure 1.
Figure 1a: Progression Free Survival NHT vs AHT (protocol definition of biochemical failure)
Figure 1b: Progression Free Survival NHT vs AHT (nadir + 2 definition of biochemical failure)
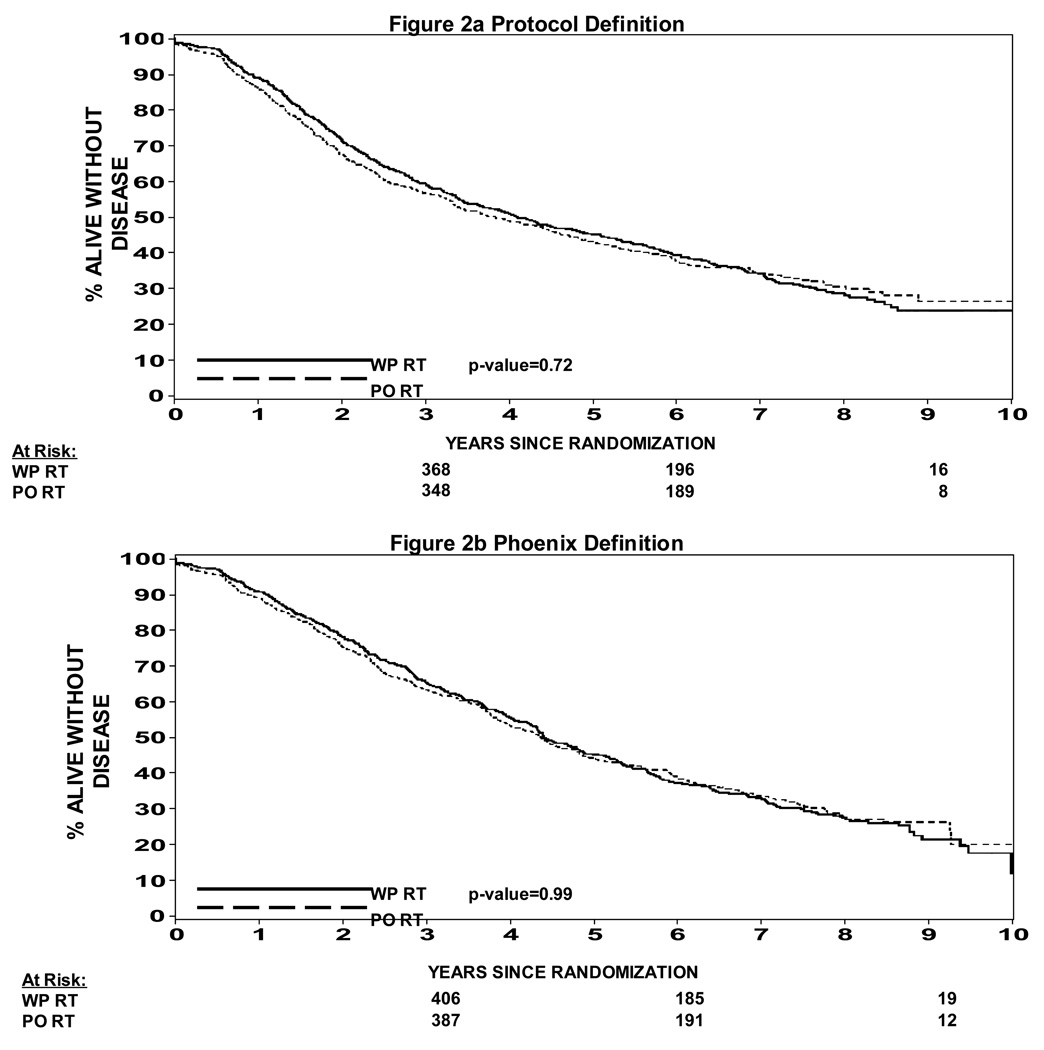
The effective treatment volume on progression free survival is shown in Figures 2a and 2b. There was no statistically significant difference in progression free survival based on the protocol definition of biochemical failure Figure 2a (p = 0.72) or with the nadir + 2 ng/ml definition as shown in Figure 2b (p=0.99).
Figure 2.
Figure 2a: Progression Free Survival WPRT vs PORT (protocol definition of biochemical failure)
Figure 2b: Progression Free Survival WPRT vs PORT (nadir + 2 definition of biochemical failure)
Analysis by Treatment Arm and Evidence of Hormone Timing plus RT Field Size Interactions
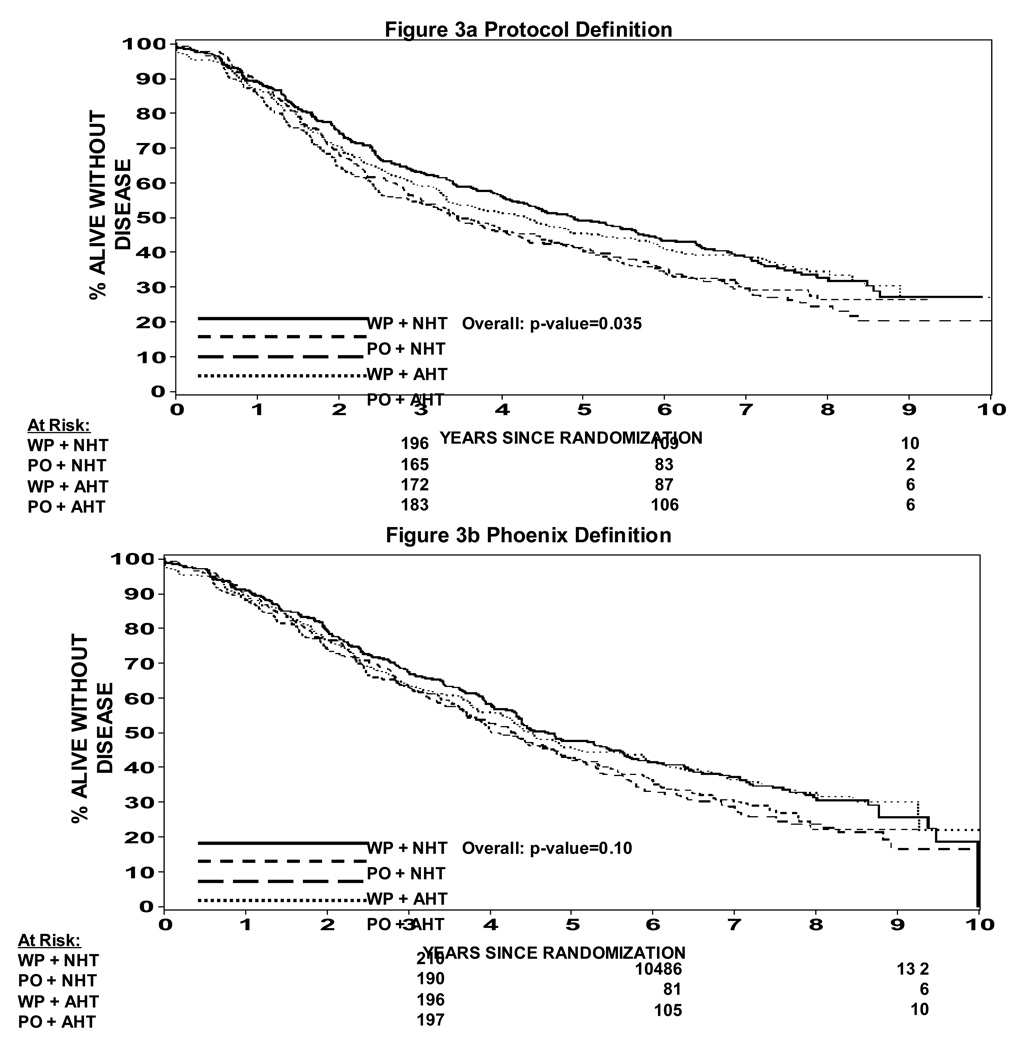
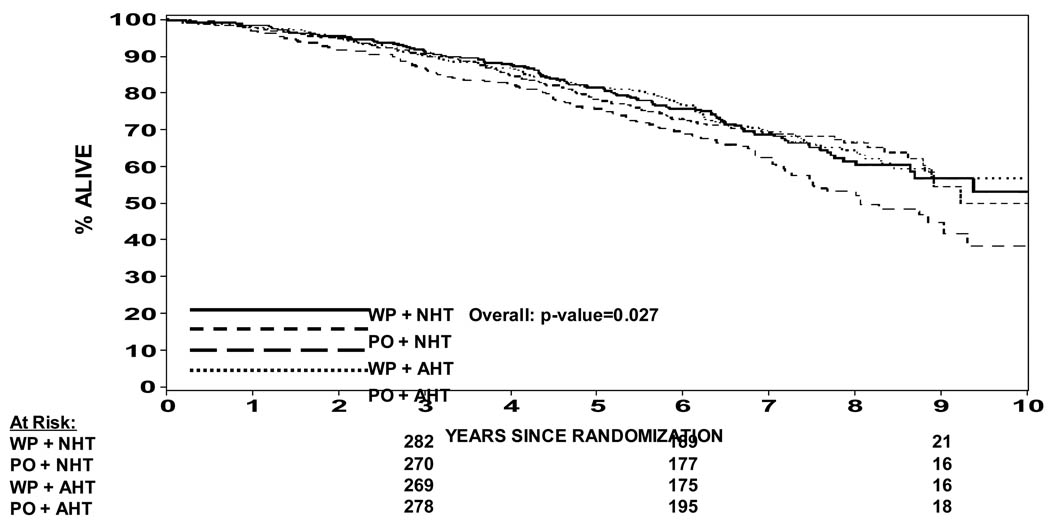
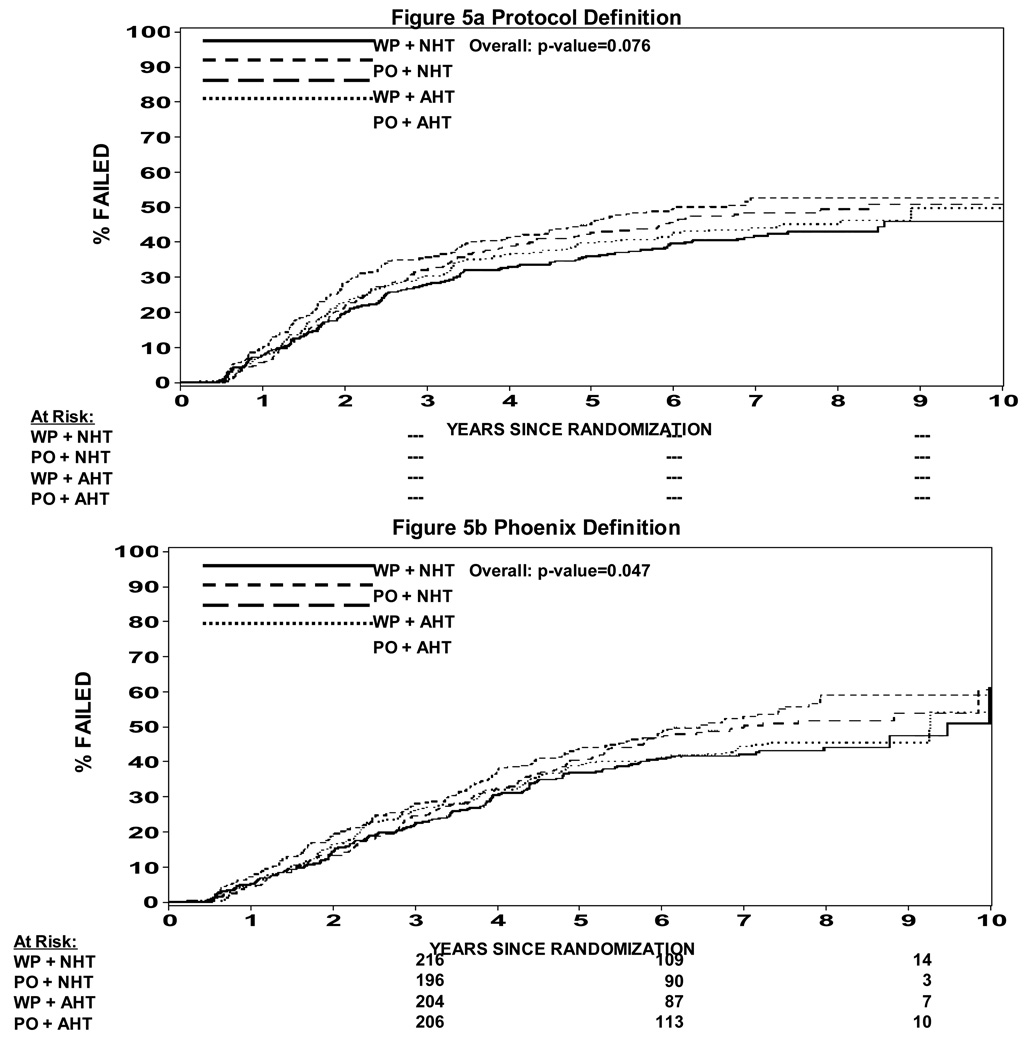
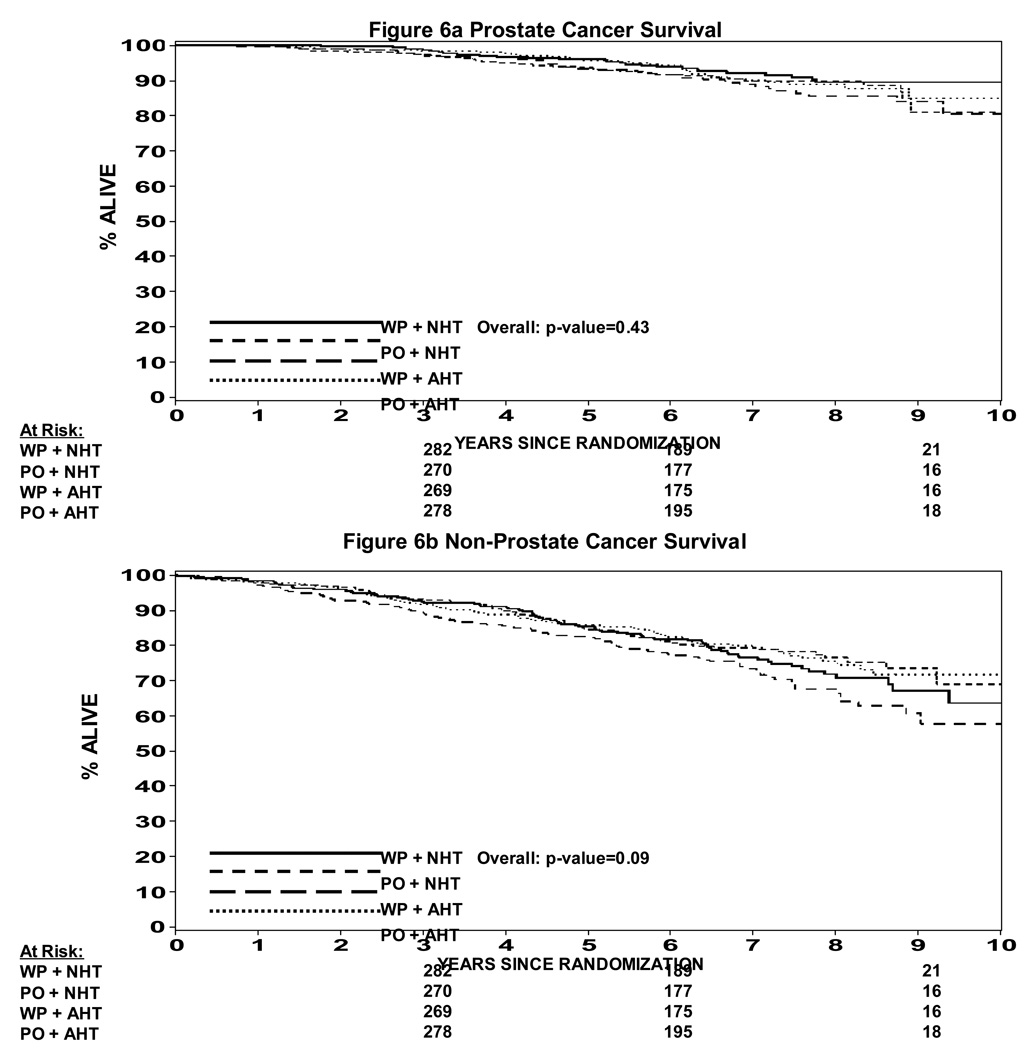
In an attempt to understand potential interactions between radiation field size and hormone timing which was not expected in the development of this study, a Cox proportion hazard model was used to determine whether there was evidence of an interaction. Table 2 shows a statistically significant difference in progression free survival when analyzed by treatment arm (p=0.035). A pairwise comparison is also shown in Table 2 showing that WP RT + NHT trends toward a difference over PORT + NHT (p = 0.023) and over WPRT + AHT (p = 0.014), but not different than PO RT + AHT (p = 0.63). This data can also be seen in Figure 3a and 3b. Figure 3a shows the progression free survival by treatment arm using the protocol definition of biochemical failure vs. Figure 3b which shows the progression free survival using a nadir + 2 ng/ml definition of biochemical failure. In the nadir + 2 ng/ml definition the p value is not statistically significant (p = 0.1). The p values shown in Table 2 are not adjusted for multiple comparisons such that if they were the level of statistical significance would be a p < .008. Therefore, none of them would be statistically significantly different. We thought it was important to present the data nevertheless because this study was not powered to show a difference between the four arms. Overall survival is shown in Figure 4. Overall survival was statistically significantly different amongst the four arms (p = 0.027). Pair wise comparison of the four arms in the study can be seen in Table 3. Again the p values are not adjusted for multiple comparisons, but of note is the fact that WP RT + AHT trended towards being worse than every other arm of this study. Local and distant failure by treatment arm shows that there was no statistically significant difference amongst the four arms in regards to local failure or distant failure (p = 0.67 and p = 0.40 respectively). Biochemical failure by treatment arm is shown in Figure 5a and 5b respectively. Based on the protocol definition of biochemical failure there was no statistically significant difference between the four arms with (p = 0.076). With a nadir + 2 ng/ml definition of biochemical failure there was a statistically significant difference in biochemical failure (p = 0.047). Pairwise comparison shows a trend towards statistical significance between WPRT + NHT and PORT + NHT in favor of the WPRT (p=.009). No other trends or statistically significant relationships were seen. Cause specific survival by treatment arm is shown in Figure 6a and 6b. Figure 6a shows prostate cancer survival by treatment arm and Figure 6b nonprostate cancer survival by treatment arm. There was no statistically significant difference between prostate cancer survival or nonprostate cancer survival among the four arms (p = 0.43 and 0.09 respectively).
Table 2.
Progressive Free Survival by Treatment Arm
| Progressive Free Survival | ||
|---|---|---|
| WP RT + NHT | 200/320 | *p=0.035 |
| PO RT + NHT | 214/316 | |
| WP RT + AHT | 223/319 | |
| PO RT + AHT | 200/320 | |
| Pairwise Comparison | ||
|---|---|---|
| Comparison | p value* | |
| WP RT + NHT vs | PO RT + NHT | 0.023 |
| WP RT + AHT | 0.014 | |
| PO RT + AHT | 0.63 | |
| PO RT + NHT vs. | WP RT + AHT | 0.97 |
| PO RT + AHT | 0.09 | |
| WP RT + AHT vs. | PO RT + AHT | 0.07 |
Log rank for comparing the progresion free survival curves
p value is from the log rank for comparing progression free survival
Figure 3.
Figure 3a: Progression Free Survival by Treatment Arm (protocol definition of biochemical failure)
Figure 3b: Progression Free Survival by Treatment Arm (nadir + 2 definition of biochemical failure)
Figure 4.
Overall Survival by Treatment Arm
Table 3.
Overall Survival by Treatment Arm
| Overall Survival | ||
|---|---|---|
| WP RT + NHT | 104/320 | *p=0.027 |
| PO RT + NHT | 99/316 | |
| WP RT + AHT | 130/319 | |
| PO RT + AHT | 101/320 | |
| Pairwise Comparison | ||
|---|---|---|
| Comparison | p value* | |
| WP RT + NHT vs | PO RT + NHT | 0.9629 |
| WP RT + AHT | 0.019 | |
| PO RT + AHT | 0.80 | |
| PO RT + NHT vs. | WP RT + AHT | 0.019 |
| PO RT + AHT | 0.86 | |
| WP RT + AHT vs. | PO RT + AHT | 0.01 |
Log rank test for comparing the overall survival curves
p value is from the log rank for comparing overall survival curves
Figure 5.
Figure 5a: Biochemical Failure by Treatment Arm (protocol definition)
Figure 5b: Biochemical Failure by Treatment Arm (nadir + 2 definition)
Figure 6.
Figre 6a: Cause Specific Survival (prostate cancer survival)
Figure 6b: Non Prostate Cancer Survival
Based on progression free survival as shown in Figures 3a and 3b as well as Table 2 and overall survival as shown in Figure 4 as well as Table 3, what appears to be clear is that amongst the four arms WP RT + AHT is the least effective form of treatment resulting in the greatest number of failures and ultimately deaths. Table 2 and Table 3 also suggest that within WP RT there is an effect of hormone therapy timing in that both end points of progression free survival as well as overall survival trend toward statistical significance in favor of WPRT + NHT over WPRT + AHT (p=0.014 and p=0.019 respectively). Since the study was designed assuming there was no interaction between field size and timing of hormone therapy the study was not powered to show a statistical difference between these two.
Complications by Treatment Type
Acute RT toxicity grades ≥ 3 was not statistically different amongst the four arms of the study. Acute hormone toxicity grades ≥ 3 were statistically worse p = .003 in the NHT arms vs. the AHT arms; WP RT + NHT (8%), PORT + NHT (5%), WPRT + AHT (3%), and PORT + AHT (3%). The vast majority of this toxicity was lymphopenia.
Late toxicity in the form of genitourinary toxicity of ≥ 3 was not statistically significant amongst the four arms (p=0.16). Late GI toxicity of ≥ 3 was statistically significantly different (p=0.002) with 5% of patients in the WPRT + NHT arm experience Grade 3 or higher late toxicity vs. 1% in the PORT + NHT, 2% in the WPRT + AHT, and 2% in the PORT +AHT arms.
Discussion
Questions of timing of hormone therapy as well as radiation field size in patients with adenocarcinoma of the prostate with a risk of lymph node involvement of > 15% were the primary goals of this study. Progression Free Survival as can be seen in Figures 1a and 1b shows that there was no difference in progression free survival by timing of hormones. Figures 2a and 2b show that there was no difference in progression free survival by radiation field size. Unfortunately the lack of benefit of whole pelvis vs. prostate only radiation or adjuvant vs. neoadjuvant hormonal manipulation in this group of patients was not likely to be found because in the design of the study it was assumed that there would be no interaction between field size and timing of hormone therapy. Clearly this exists, and therefore, the analysis of this study becomes more complex.
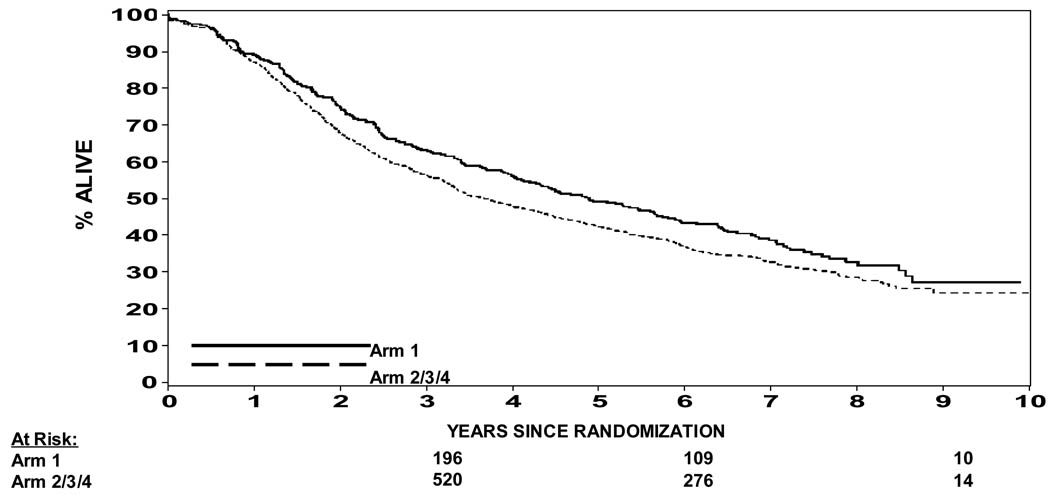
What is clear is that the study was not powered to compare the four treatment arms one against the other. Yet when one looks at the WPRT + NHT arms compared to the PORT + NHT arm there is a trend towards statistical significance in the endpoints of PFS (p=0.023), biochemical failure per protocol definition (p=0.013) and biochemical failure nadir + 2 ng/ml definition (p=0.009). This suggests that if one selects to use NHT for this population of prostate cancer patients there appears to be a benefit to WPRT over PORT. To expand on the concept of NHT based on the data presented here, Figure 7 shows the PFS curves for WPRT + NHT vs. the other 3 arms of this trial. This analysis is not statistically valid based on the design of the trial, but seemed reasonable to evaluate as one tries to understand the interactions between timing of hormone therapy and field size. From Figure 7 one certainly could make an additional argument in favor of WPRT + NHTthat would correspond to the results of RTOG 92-02 and 86-10. This result is further supported by a subset analysis of arms 1 & 2 of 94-13 by Roach et al.(18) This analysis shows a clear benefit in both biochemical control and PFS in favor of the WPRT.(18) Given the results of RTOG protocol 92-02 as well as 86-10(2,4) it seems reasonable that for this population one would select to use neoadjuvant hormonal manipulation.
Figure 7.
Progression Free Survival (protocol definition of biochemical failure) Arm 1 vs. Arm 2–4
Another clear observation is that WPRT + AHT is the least desirable option with regards to overall survival. Table 3 reveals that WPRT and AHT trends towards worse survival compared to the other three arms, p=0.019 vs. WPRT + NHT, p=0.019 vs. PORT+NHT p=0.01 vs. PORT+AHT. This can be graphically seen in Figure 4. The observation that WPRT+AHT would offer a worse outcome is somewhat surprising given the results of WPRT + NHT. Yet when one evaluates the published randomized data suggesting the use of hormone therapy in this group of patients, none of the data to date has looked at short course adjuvant hormone therapy.(1,2,3,4) So the result although unexpected was not incongruous with prior data because the prior data for adjuvant therapy utilized only long term adjuvant therapy, (i.e., 2–3 years).
So why would WPRT appear to be beneficial when hormone therapy is given neoadjuvantly and concurrently as in Arm 1, yet detrimental to survival when hormones are given adjuvantly? And why don’t we see that interaction in the PORT arms (Arms 2 & 4)?
One possible explanation for the benefit of WPRT + NHT over WPRT + AHT may lie in the immune modulation of anti-androgen therapy. Mercader et al(19) have shown that anti-androgen ablation therapy results in T-cell infiltration of the prostate which increases apoptosis. This peaks at about 3–4 weeks into treatment with hormone therapy. It may be possible that T-cell infiltration occurs within the involved lymph nodes, such that there is an increase in apoptosis preradiation therapy and during radiation therapy which may make the radiation therapy more effective at the doses used to treat the lymph nodes. Since we do not see a statistical difference in PORT + NHT vs. PORT + AHT the above phenomena may be dose dependent, such that the doses of 70 Gy are high enough that we cannot see the effects. Other authors have noted a beneficial effect of intratumoral T-cells in other malignancies such as ovarian carcinoma.(20)
Given that there is no question that there is an interaction between timing of hormone therapy and radiation therapy field size in this patient population, one is left with the question of how best to treat these patients. Based on this analysis and the randomized trials to date(1,2,3,4) which show a benefit to radiation therapy and hormone therapy in this patient population, it is clear that NHT+WPRT remains the standard of care. Further follow up will help elucidate this stance with regards to cause specific and overall survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pilepich M, Winter K, Lawton C, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma long term results of phase III RTOG 85-31. Int J Rad Onc Biol Phys. 2005;61(5):1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Pilepich MV, Winter K, Fu KKM, et al. Phase III Radiation Therapy Oncology Group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Rad Onc Bio Phys. 2001;50(5):1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Collette L, Blank L, et al. Long term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study, a phase III randomized trial) Lancet. 2002;360:103–108. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 4.Hanks GF, Pajak T, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: RTOG Protocol 92-02. Journ Clin Oncol. 2003;21(21):3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Roach M. Re: The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150:1923–1924. doi: 10.1016/s0022-5347(17)35937-2. [DOI] [PubMed] [Google Scholar]
- 6.Roach M, Lu J, Pilepich MV, et al. Predicting long term survival, and the need for hormonal therapy: A meta-analysis of RTOG prostate cancer trials. Int J Rad Onc Biol & Phys. 2000;47:617–627. doi: 10.1016/s0360-3016(00)00577-0. [DOI] [PubMed] [Google Scholar]
- 7.Pilepich MV, Winter K, Roach M, et al. Phase III RTOG trial 86-10 of androgen deprivation before and during radiotherapy in locally advanced carcinoma of the prostate. Presented at Am Soc of Clin Onc; May 16–19, 1998; Los Angeles, CA. [Google Scholar]
- 8.Roach M, Marquez C, Yuo H, et al. Predicting the risk of lymph node involvement using the pre-treatment specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Rad Onc Biol & Phys. 1993;28:33–37. doi: 10.1016/0360-3016(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 9.Roach M, Pickett B, Holland J, et al. The role of the urethragram during simulation for localized prostate cancer. Int J Rad Onc Biol & Phy. 1993;25:299–307. doi: 10.1016/0360-3016(93)90352-v. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Non-parameteric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific and conditional failure probabilities. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 12.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 13.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the RTOG and EORTC. Int J Rad Onc Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 14.Cox JD, Grignon DJ, Kaplan RS, et al. Consensus statement: Guidelines for PSA following radiation therapy. Int J Rad Onc Biol & Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 15.Kestin LL, Vicini FA, Martinex AA. Practical application of biochemical failure definitions: What to do and when to do it. Int J Rad Onc Biol & Phys. 2002;53:304–315. doi: 10.1016/s0360-3016(02)02707-4. [DOI] [PubMed] [Google Scholar]
- 16.Fleming TR, Green SJ, Harrington DP. Considerations for monitoring and evaluating treatment effects in clinical trials. Control Clin Trials. 1984;5:55–66. doi: 10.1016/0197-2456(84)90150-8. [DOI] [PubMed] [Google Scholar]
- 17.Fleming TR, Harrington DP, O’Brien PC. Deisgns for group sequential tests. Control Clin Trials. 1984;5:348–361. doi: 10.1016/s0197-2456(84)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Roach M, DeSilvio M, Valicenti R, et al. Whole-pelvis, “mini-pelvis,” or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal theapy in patients treated in the RTOG 94-13 trial. Int J Rad Onc Biol & Phys. 2006 doi: 10.1016/j.ijrobp.2006.05.074. (In Press) [DOI] [PubMed] [Google Scholar]
- 19.Mercader M, Bodner B, Moser M, et al. T-cell infiltration of the prostate induced by androgen withdrawel in patients with prostate cancer. Proceedings of the National Acadamy of Science (PNAS) 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Conejo-Garcia J, Katsaros D, et al. Intratumoral T-cells, recurrence, and survival in epithelial ovarian cancer. New Eng Jou of Med. 2004;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]