Recent advances in the field of transition metal-catalyzed cycloadditions have made them among the most efficient methods to assemble polycyclic carbocycles and heterocycles.1 Cycloadditions employing carbodiimides are surprisingly scarce in the literature.2 An intramolecular Pauson-Khand-type reaction of alkynyl carbodiimides has recently been described,3 while [2 + 2 + 2] cycloadditions of diynes and carbodiimides have been documented to provide mostly inseparable mixtures.4,5 To the best of our knowledge, there have been no reports of successful enantioselective [m + n + o] type cycloadditions involving carbodiimides as a 2π component.
 |
(1) |
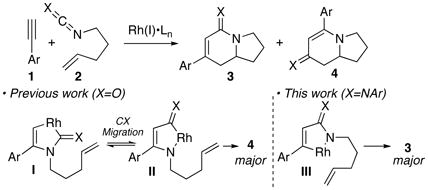
We previously described an unusual cycloaddition between phenyl acetylene 1a and isocyanate 2 (X=O, eq 1), where the major pathway proceeds via metalacycles I and II involving a CO migration process.6 The resulting cycloaddition affords product 4 (X=O) in good efficiency while cycloadduct 3 can only be formed as the minor component (3:4 = 1:7, Ar=Ph). In an effort to selectively access products of type 3, we envisioned that a cycloaddition employing carbodiimides (2, X=NAr) should favor the formation of metalacycle III by placing the bulky imido moiety further away from the rhodium center (I vs. III). Herein, we report the successful application of this strategy, providing a complementary selectivity to the cycloaddition previously described using isocyanates.7 Further, this reaction offers a novel entry into the asymmetric synthesis of bicyclic amidines (3, X=NAr).8
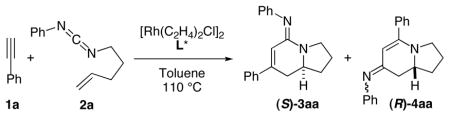
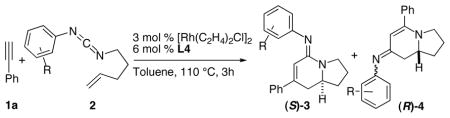
Our investigations began with the cycloaddition of phenyl acetylene 1a and the phenyl-substituted pentenyl carbodiimide 2a employing our previously developed reaction conditions (entries 1—2, Table 1).6b These conditions afford the desired bicyclic amidine 3 in moderate yields with the ligand L2 providing the highest enantioselectivity. The p-tol-TADDOL derived ligand L3 provides a very efficient reaction with half the catalyst loading and shorter reaction times (entry 3). Further optimization led to the identification of m-xylyl-TADDOL derivative L4 as the best ligand, affording the bicyclic amidine with good chemical yield and excellent enantioselectivity (entry 4). The amino group on the ligand proves to have a significant effect on the reaction; replacing the pyrrolidinyl moiety with the piperidine can further improve the product selectivity for 3, although the enantioselectivity decreases (entry 5). It is significant to note that the type 4 product resulting from a rare isocyanide (CNR) migration9 can be observed in the reaction mixture as the minor component.
Table 1.
Ligand Screen
 | ||||||
|---|---|---|---|---|---|---|
| entry | [Rh(C2H4)2Cl2] | L* (mol %) | time (h) | 3: 4b | yield (%) of 3aac | ee(%) of 3aad |
| 1 | 5% | L1 (10) | 12 | 2.4: 1 | 40 | 84 |
| 2 | 5% | L2 (10) | 12 | 2.8: 1 | 57 | 94 |
| 3 | 3% | L3 (6) | 3 | 3.4: 1 | 64 | 95 |
| 4 | 3% | L4 (6) | 3 | 3.4: 1 | 70 | 97 |
| 5 | 3% | L5 (6) | 3 | 4.8: 1 | 78 | 89 |
Conditions: 1 (2 equiv), 2 (0.16 mmol), Rh catalyst, L in PhMe at 110 °C.
Product selectivity (3: 4) is determined by 1H NMR of the unpurified reaction mixture.
Isolated yield.
Determined by HPLC analysis using a chiral stationary phase.
Table 2 summarizes the scope of the enantioselective [2 + 2 + 2] cycloaddition of phenyl acetylene 1a and a variety of aryl-substituted carbodiimides. The reaction tolerates both electron-rich and electron-poor substituents at various positions to afford bicyclic amidines 3 in good yields and excellent enantioselectivity (entries 1—5). Aryl carbodiimides with strong electron-withdrawing groups provide much greater product selectivity (entries 4—5). For larger scale reactions, the catalyst loading may be reduced to 1 mol% [Rh(C2H4)2Cl]2 with virtually identical yield and enantioselectivity.
Table 2.
Carbodiimide Scope
 | ||||
|---|---|---|---|---|
| entry | R | 3:4b | yield (%) of 3c | ee (%) of 3d |
| 1 | p-OMe, 2b | 3.6: 1 | 70 | 94 |
| 2 | o-OMe, 2c | 3.8: 1 | 68 (62)e | 98 (96)e |
| 3 | m-Cl, 2d | 3.4: 1 | 67 | 97 |
| 4 | o-CF3, 2e | 9.4: 1 | 82 | 97 |
| 5f | p-CN, 2f | 9.5: 1 | 55 | 92 |
Conditions: 1 (2 equiv), 2 (0.16 mmol), Rh catalyst, L in PhMe at 110 °C.
Product selectivity (3: 4) is determined by 1H NMR of the unpurified reaction mixture.
Isolated yield.
Determined by HPLC analysis using a chiral stationary phase.
0.8 mmol scale of 2c, 1 mol% Rh catalyst and 2 mol% L4.
5 mol % Rh catalyst and 10 mol % L4.
For further substrate development, we chose o-anisidine derived carbodiimide as the standard cycloaddition partner. This selection was based on the optimal enantioselectivity obtained and their potential roles as an oxidatively cleavable protecting group of the resulting cycloadducts.10
The asymmetric synthesis of amidine 6a possessing a nitrogen-substituted quaternary center can be achieved in high efficiency from the corresponding disubstituted alkenyl carbodiimide (eq 2).6c Although a much more sluggish reaction, cycloaddition of carbodiimide 8 to construct the desired [4.4.0] bicyclic amidine 9a proceeds in a moderate yield with excellent enantiocontrol (eq 3).
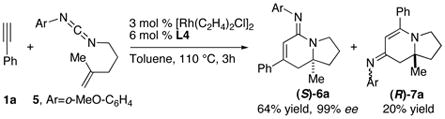 |
(2) |
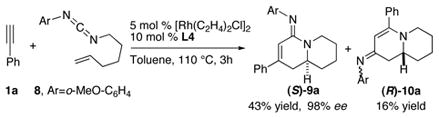 |
(3) |
The cycloadditions of a variety of terminal alkynes and carbodiimides 2c and 5 were examined (Table 3). The electronic and steric effects of alkynyl partners play important roles to the reaction outcomes. Aryl acetylenes substituted with various electron-poor groups participate in the cycloaddition readily to furnish almost exclusively the bicyclic amidine 3 or 6 with good yields and excellent enantioselectivity (entries 1—6, 14—15).11 The reactions of alkyne 1h, which possesses a moderate σ-withdrawing group, proceed with the same efficiency to afford the desired amidines in good product ratio (entries 7, 16). m-Tolyl acetylene 1i, which is slightly more electron-rich than 1a, and ethynyl thiophene 1j, which is sterically smaller than 1a, undergo the cycloaddition to provide the corresponding amidines in high enantiomeric excess with product ratio similar to those with 1a (entries 8, 10). The moderate product ratio can be greatly improved while maintaining the excellent enantiocontrol by using the o-CF3-phenyl carbodiimide 2e (entries 9, 11). On the other hand, the reaction of electron-rich aryl acetylene 1k proceeds with an opposite product selectivity (entries 12—13), a trend that is consistent with our previous study.6b Cycloadditions with alkyl acetylenes require a slightly higher catalyst loading to ensure complete conversion (entries 17—22), but generate bicyclic amidines 3 with high efficiency (88—96% ee).
Table 3.
Terminal Alkyne Scope
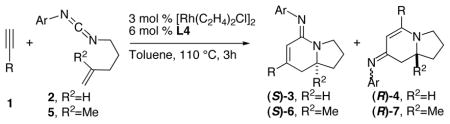 | |||||
|---|---|---|---|---|---|
| entry | R | carbodiimide | 3: 4 or 6: 7b | yield (%) of 3 or 6c | ee (%) of 3 or 6d,e |
| 1 | p-Br-C6H4, 1b | 2c | 16: 1 | 75 | 98 |
| 2 | m-F-C6H4, 1c | 2c | > 19: 1 | 77 | 99 |
| 3 | 3,5-F-C6H3, 1d | 2c | > 19: 1 | 66 | 99 |
| 4 | p-Ac-C6H4, 1e | 2c | > 19: 1 | 78 | 99 |
| 5 | p-CF3-C6H4, 1f | 2c | > 19: 1 | 68 | 96 |
| 6 | m-CN-C6H4, 1g | 2c | > 19: 1 | 62 | 94 |
| 7 | m-OMe-C6H4, 1h | 2c | 6.3: 1 | 69 | 99 |
| 8 | m-Me-C6H4, 1i | 2c | 3.4: 1 | 61 | 98 |
| 9 | “ | 2e | 8.3: 1 | 74 | 98 |
| 10 |
|
2c | 3.2: 1 | 58 | 98 |
| 11 | “ | 2e | 7.2: 1 | 79 | 97 |
| 12 | p-OMe-C6H4, 1k | 2c | 1: 2.8 | 20 (52)g | 99 |
| 13 | “ | 2e | 1: 1 | 37 (36)g | 96 |
| 14 | 3,5-F-C6H3, 1d | 5 | > 19: 1 | 79 | 98 |
| 15 | p-Ac-C6H4, 1e | 5 | 16: 1 | 74 | 99 |
| 16 | m-OMe-C6H4, 1h | 5 | 4.5: 1 | 66 | 96 |
| 17f | n-Hex, 1l | 2c | > 19: 1 | 74 | 91 |
| 18f | (CH2)4CO2Me, 1m | 2c | > 19: 1 | 68 | 92 |
| 19f | CH2CH2OTBS, 1n | 2c | > 19: 1 | 76 | 96 |
| 20f | (CH2)2CH2OTBS, 1o | 2c | > 19: 1 | 73 | 94 |
| 21f | (CH2)3CH2Cl, 1p | 2c | > 19: 1 | 60 | 88 |
| 22f | CH2CH2Ph, 1q | 2c | > 19: 1 | 70 | 92 |
Conditions: 1 (2 equiv), 2 (0.16 mmol), Rh catalyst, L in PhMe at 110 °C.
Product selectivity (3: 4) is determined by 1H NMR of the unpurified reaction mixture.
Isolated yield.
Determined by HPLC analysis using a chiral stationary phase.
Absolute configuration assigned by analogy to (S)-3bc (established by X-ray analysis).
5 mol % Rh catalyst and 10 mol % L4 employed.
Isolated yield of 4 (a 2:1 mixture of imine isomers).
The resulting bicyclic amidines 3 are potentially useful chiral building blocks. Under appropriate conditions, the olefin and the amidine moiety can each be selectively reduced while leaving the other functionality untouched for further transformation (eq 4).
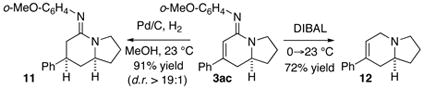 |
(4) |
In summary, we have developed the first enantioselective [2 + 2 + 2] cycloaddition utilizing carbodiimides to give bicyclic amidines with excellent enantiocontrol. Observation of the isocyanide migration process during the cycloaddition is notable. Studies to explore the synthetic utility of bicyclic amidines 3 are ongoing.
Supplementary Material
Acknowledgments
Dedicated with deep respect to the memory of our friend and colleague Professor Albert I. Meyers (1932–2007). We thank NIGMS (GM080442), Eli Lilly, Boehringer Ingelheim and Johnson & Johnson for support. TR is a fellow of the Alfred P. Sloan Foundation and thanks the Monfort Family Foundation for a Monfort Professorship.
Footnotes
Supporting Information Available: Experimental procedures, characterization, 1H and 13C NMR spectra are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews: Aubert C, Buisine O, Malacria M. Chem Rev. 2002;102:813. doi: 10.1021/cr980054f.Murakami M. Angew Chem Int Ed. 2003;42:718. doi: 10.1002/anie.200390200.Nakamura I, Yamamoto Y. Chem Rev. 2004;104:2127. doi: 10.1021/cr020095i.Gandon V, Aubert C, Malacria M. Chem Commun. 2006:2209. doi: 10.1039/b517696b.Chopade PR, Louie J. Adv Synth Catal. 2006;348:2307.
- 2.Intermolecular cyclotrimerization of alkynes and carbodiimides: Hong P, Yamazaki H. Tetrahedron Lett. 1977:1333.Hoberg H, Burkhart G. Synthesis. 1979:525.Diversi P, Ingrosso G, Lucherini A, Malquori S. J Mol Catal. 1987;40:267.Takahashi T, Tsai F, Li Y, Wang H, Kondo Y, Yamanaka M, Nakajima K, Kotora M. J Am Chem Soc. 2002;124:5059. doi: 10.1021/ja017507+.
- 3.(a) Mukai C, Yoshida T, Sorimachi M, Odani A. Org Lett. 2006;8:83. doi: 10.1021/ol052562z. [DOI] [PubMed] [Google Scholar]; (b) Saito T, Sugizaki K, Otani T, Suyama T. Org Lett. 2007;9:1239. doi: 10.1021/ol063123i. [DOI] [PubMed] [Google Scholar]; (c) Aburano D, Yoshida T, Miyakoshi N, Mukai C. J Org Chem. 2007;72:6878. doi: 10.1021/jo071137b. [DOI] [PubMed] [Google Scholar]
- 4.See text: Yamamoto Y, Kinpara K, Saigoku T, Takagishi H, Okuda S, Nishiyama H, Itoh K. J Am Chem Soc. 2005;127:605. doi: 10.1021/ja045694g.Bonaga LVR, Zhang HC, Moretto AF, Ye H, Gauthier DA, Li J, Leo GC, Maryanoff BE. J Am Chem Soc. 2005;127:3473. doi: 10.1021/ja045001w.
- 5.A successful cycloaddition of solid supported diynes and carbodiimides was reported: Young DD, Deiters A. Angew Chem Int Ed. 2007;46:5187. doi: 10.1002/anie.200700802.
- 6.(a) Yu RT, Rovis T. J Am Chem Soc. 2006;128:2782. doi: 10.1021/ja057803c. [DOI] [PubMed] [Google Scholar]; (b) Yu RT, Rovis T. J Am Chem Soc. 2006;128:12370. doi: 10.1021/ja064868m. [DOI] [PubMed] [Google Scholar]; (c) Lee EE, Rovis T. Org Lett. doi: 10.1021/ol800086s. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For other metal catalyzed [2 + 2 + 2] cycloadditions involving isocyanates. Co: Earl RA, Vollhardt KPC. J Org Chem. 1984;49:4786.(b) ref. 2a. (c) ref. 4b. Ru: Yamamoto Y, Takagishi H, Itoh K. Org Lett. 2001;3:2117. doi: 10.1021/ol016082t.(e) ref. 4a. Ni: Hoberg H, Oster BW. Synthesis. 1982:324.Duong HA, Cross MJ, Louie J. J Am Chem Soc. 2004;126:11438. doi: 10.1021/ja046477i.Rh: Tanaka K, Wada A, Noguchi K. Org Lett. 2005;7:4737. doi: 10.1021/ol052041b.
- 8.Synthesis of chiral amidines: Meyers AI, Miller DB, White FH. J Am Chem Soc. 1988;110:4778.Convery MA, Davis AP, Dunne CJ, MacKinnon JW. Chem Commun. 1994:2557.Papandreou G, Tong MK, Ganem B. J Am Chem Soc. 1993;115:11682.Ostendorf M, Dijkink J, Rutjes FPJT, Hiemstra H. Eur J Org Chem. 2000:115.Heck M, Vincent SP, Murray BW, Bellamy F, Wong C, Mioskowski C. J Am Chem Soc. 2004;126:1971. doi: 10.1021/ja037822r.Kumagai N, Matsunaga S, Shibasaki M. Angew Chem Int Ed. 2004;43:478. doi: 10.1002/anie.200352750.Chang S, Lee M, Jung DY, Yoo EJ, Cho SH, Han SK. J Am Chem Soc. 2006;128:12366. doi: 10.1021/ja064788i.
- 9.(a) Barnhart RW, Bosnich B. Organometallics. 1995;14:4343. [Google Scholar]; (b) Tanaka K, Fu GC. Chem Commun. 2002:684. doi: 10.1039/b200208f. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kobayashi S, Ishitani H, Ueno M. J Am Chem Soc. 1998;120:431. [Google Scholar]; (b) Saito S, Hatanaka K, Yamamoto H. Org Lett. 2000;2:1891. doi: 10.1021/ol000099e. [DOI] [PubMed] [Google Scholar]
- 11.ortho-Substituted aryl alkynes are not tolerated under current conditions. Cycloaddition with 1-chloro-2-ethynyl benzene gives product 3 in only 35% yield and 31% ee while 2-ethynyltoluene provides no cycloadduct.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


