Abstract
Appreciation for the role of aldosterone and mineralocorticoid receptors in cardiovascular disease is accelerating rapidly. Recent experimental work has unveiled a strong relationship between brain mineralocorticoid receptors and sympathetic drive, an important determinant of outcome in heart failure and hypertension. Two putative mechanisms are explored in this manuscript. First, brain mineralocorticoid receptors may influence sympathetic discharge by regulating the release of pro-inflammatory cytokines into the circulation. Blood-borne pro-inflammatory cytokines act upon receptors in the microvasculature of the brain to induce cyclooxygenase-2 activity and the production of prostaglandin E2, which penetrates the blood-brain barrier to activate the sympathetic nervous system. Second, brain mineralocorticoid receptors may influence sympathetic drive by upregulating the activity of the brain renin-angiotensin system, resulting in NAD(P)H oxidase dependent superoxide production. A potential role for superoxide dependent mitogen-activated protein kinase signaling pathways in the regulation of sympathetic nerve activity is also considered. Other potential downstream signaling mechanisms contributing to mineralocorticoid receptor mediated sympathetic excitation are under investigation.
Introduction
The traditional view of aldosterone as a hormone acting primarily upon receptors in the kidneys and the colon to conserve sodium has undergone substantial modification in the past two decades (Connell & Davies, 2005), as evidence has emerged for its involvement in cardiac and vascular fibrosis (Weber et al., 1995), central nervous system mechanisms regulating sodium appetite (De Nicola et al., 1992) and sympathetic nerve activity (Francis et al., 2001a), and experimental models of hypertension (Gomez-Sanchez et al., 1990) and heart failure (HF) (Francis et al., 2001a; Lal et al., 2004). Interest in the role of aldosterone in cardiovascular diseases was heightened by the Random Aldactone Evaluation Study (RALES), a large clinical trial demonstrating that the addition of a small oral dose of the mineralocorticoid receptor (MR) antagonist spironolactone (SL) to the regimen of otherwise optimally managed patients with established heart failure dramatically reduced morbidity and mortality (Pitt et al., 1999). The mechanism(s) accounting for these beneficial effects were unknown (Rousseau et al., 2002).
Activation of MR in the brain has long been associated with increased salt appetite (De Nicola et al., 1992) and sympathetically mediated hypertension (Gomez-Sanchez, 1997). It is therefore reasonable to hypothesize that blocking the activation of brain MR might account, at least in part, for the salutary influences of SL in HF. That hypothesis was tested in a rat model of systolic HF induced by coronary artery ligation, to mimic coronary artery disease, a leading cause of systolic HF in humans (Writing Group et al., 2009).
The rat model of systolic HF
Ischemia-induced HF in rats resembles end-stage human systolic HF (Kjaer & Hesse, 2001) in many respects (Francis et al., 2001b). The left ventricular (LV) ejection fraction is reduced immediately following coronary artery ligation, to about 35% with >80% being normal for rats. Over the ensuing 4–6 weeks, LV ejection fraction does not change significantly, but LV end-diastolic pressure rises and LV volume increases to a greater extent than LV mass, increasing the stress on the LV wall and presaging end-stage HF (Francis et al., 2001b). The sympathetic and renin-angiotensin systems are activated in response to the low cardiac output of the injured LV. Circulating angiotensin II (Leenen et al., 1999), aldosterone (Yu et al., 2008), atrial natriuretic factor (Francis et al., 2001b), and pro-inflammatory cytokines (Francis et al., 2004a; Kang et al., 2006) are all increased. These HF rats have an increased appetite for sodium, offered as a 1.8% sodium chloride solution, and their renal excretion of sodium and water is reduced (Francis et al., 2001a; Francis et al., 2001b; Francis et al., 2004b). There is no measurable increase in body weight, but wet lung to body weight and right ventricular to body weight ratios increase (Kang et al., 2006; Kang et al., 2008), indicating volume accumulation and increased pulmonary artery pressures and stress on the right heart. Occasionally these animals are found to have ascites or pleural effusions 4–6 weeks after coronary artery ligation.
Brain MR and manifestations of HF in the rat
SL or vehicle was infused continuously into the 3rd cerebral ventricle of rats with ischemia-induced HF for 4 weeks, beginning 24 hours after coronary artery ligation. HF rats treated with ICV SL had less renal sympathetic nerve activity (RSNA), improved baroreflex control of RSNA and heart rate, and normalization of the sodium consumption and renal handling of sodium and water than HF rats treated with ICV vehicle (Francis et al., 2001a). The reduction in salt appetite is a behavioral response that would be predicted by the well-known effects of MR agonists to stimulate sodium ingestion (De Nicola et al., 1992). The improvements in kidney function, with increased urine volume and sodium excretion, are less easily explained. However, there is evidence that inhibition of brain MR affects kidney function via the renal nerves (Rahmouni et al., 1999). Thus, it seems likely that the beneficial effect of ICV SL on renal function in this study resulted from the reduction in RSNA. Of note, a systemic infusion of the same dose of SL had no effect on renal function over the first two weeks of treatment. These findings suggest that activation of brain MR contributes to the dysregulation of sympathetic drive and to sympathetically-mediated renal dysfunction in HF.
How do brain MR regulate sympathetic drive?
The mechanisms by which activation of brain MR stimulates the sympathetic nervous system in HF remain obscure. Activation of MR can elicit both genomic and non-genomic actions (Grossmann & Gekle, 2008). Our data suggest that stimulation of brain MR upregulates the activity of other central excitatory systems, thereby augmenting the excitatory neurochemical milieu in cardiovascular regions of the brain.
Brain MR and the pro-inflammatory cytokines
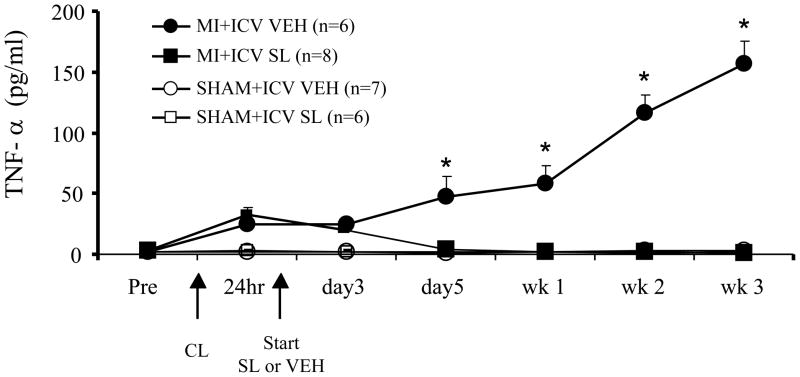
One excitatory mediator that responds to manipulations of brain MR is tumor necrosis factor – alpha (TNF-α), a pro-inflammatory cytokine. Plasma levels of TNF-α are high in patients with severe HF (Dibbs et al., 1999) and correlate with adverse outcome (Deswal et al., 2001). Rats with ischemia-induced HF also have high circulating levels of TNF-α. However, a continuous ICV infusion of SL, initiated within 24 hours of coronary artery ligation, prevents the expected rise in plasma TNF-α levels (Figure 1) (Francis et al., 2003b). Conversely, in normal rats, treatment with the MR agonist deoxycorticosterone acetate in a dose sufficient to induce sodium appetite increases plasma TNF-α levels; and this effect is prevented by ICV administration of SL (Francis et al., 2003a). These two studies demonstrated the surprising finding that plasma levels of pro-inflammatory cytokines are regulated, at least in part, by brain MR.
Figure 1.
Effect of central mineralocorticoid receptor blockade on plasma TNF-α levels in rats following myocardial infarction (MI). Rats underwent implantation of a cannula for chronic ICV administration of the mineralocorticoid receptor antagonist spironolactone (SL) or vehicle (VEH). Two weeks later they underwent coronary artery ligation (CL) to induce MI or a sham operation (SHAM). MI and SHAM rats received a continuous ICV infusion of SL or VEH via osmotic minipump for three weeks, beginning approximately 24 hours after coronary artery ligation and echocardiographic confirmation of left ventricular function. Jugular venous samples were collected for measurement (by ELISA) of plasma TNF-α level at the intervals indicated. * P<0.05, SL vs VEH in MI rats. Adapted from (Francis et al., 2003b)
How might the influence of brain MR on circulating cytokines affect sympathetic drive? A brief review of the literature regarding the effect of pro-inflammatory cytokines on the hypothalamic-pituitary-adrenal (HPA) axis may provide context. Blood-borne pro-inflammatory cytokines activate the HPA axis to increase circulating glucocorticoid, catecholamines and sympathetic drive (Turnbull & Rivier, 1999; Chrousos, 2000; Dunn, 2000; Rivest et al., 2000). However, since the pro-inflammatory cytokines are too large to readily cross the blood-brain barrier, it is not obvious how they accomplish this. Several mechanisms have been proposed (Turnbull & Rivier, 1999; Chrousos, 2000). A leading theory is that they act upon receptors in the vasculature to induce cyclooxygenase-2 (COX-2), resulting in the production of prostaglandins that are able to cross the blood-brain barrier to activate E-prostanoid receptors on central neurons (Rivest et al., 2000). In a series of experiments exploring this hypothesis in detail, Sawchenko and colleagues demonstrated that acutely injected interleukin-1 beta (IL-1β) induces COX-2 activity in perivascular macrophages lining the cerebral vasculature (Schiltz & Sawchenko, 2002, 2003), that microinjection of prostaglandin E2 (PGE2) into the rostral ventrolateral medulla (RVLM) activates corticotropin releasing hormone (CRH) neurons in the paraventricular nucleus of the hypothalamus (PVN)(Ericsson et al., 1997), and that IL-1β-induced activation of CRH neurons is prevented by interrupting noradrenergic pathways ascending from the RVLM (Ericsson et al., 1994). These studies strongly suggest that PGE2 mediates the acute affects of systemically administered pro-inflammatory cytokines on the HPA axis.
We first examined this potential link between blood-borne cytokines, COX-2 and sympathetic drive further in normal rats. An acute intracarotid injection of TNF-α, directed toward the brain, elicited increases in heart rate, RSNA, arterial pressure, and neuronal excitation in both PVN and RVLM (Zhang et al., 2003). All these responses were prevented in rats pretreated with ICV administration of the cyclooxygenase inhibitor ketorolac. These findings were consistent with the known sympatho-excitatory effect of ICV PGE2 (Hoffman & Schmid, 1979; Feuerstein et al., 1982), and confirmed the role of PGE2 as the central mediator of sympathetic responses to an acutely administered cytokine challenge.
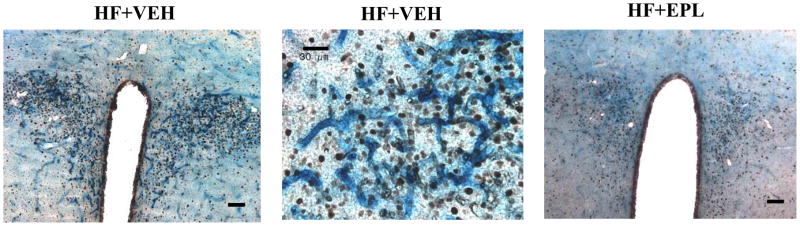
We then hypothesized that COX-2 activity and PGE2 production by the cerebral vasculature might contribute to sympathetic excitation in HF, a setting characterized by chronic elevations of circulating cytokines. In HF rats, we found intense COX-2 staining in the microvasculature of the PVN (Kang et al., 2006), subsequently localized to perivascular macrophages in the PVN (Yu et al., 2007). This intense COX-2 staining was closely associated with marked activation of PVN neurons, as indicated by Fra-like activity (Kang et al., 2006). Notably, HF rats treated orally with the MR antagonist eplerenone for 6 weeks had lower plasma levels of IL-1β, less COX-2 staining in the vessels penetrating the PVN and less PVN neuronal excitation (Figure 2) (Kang et al., 2006). Studies using the cytokine synthesis inhibitor (pentoxifylline) and the recombinant human soluble tumor necrosis factor-α receptor complex (etanercept) yielded very similar results (Kang et al., 2006), suggesting that the central effects of eplerenone were secondary to the reduction in blood-borne cytokines. Combined treatment with eplerenone and etanercept had no additional effect (Kang et al., 2006). In addition, treatment with eplerenone, pentoxifylline, etanercept and eplerenone plus etanercept had similar effects on CSF PGE2, a biological index of COX-2 activity and the presumed mediator of cytokine-induced sympathetic drive, as well as on plasma norepinephrine, a surrogate measure of sympathetic drive (Kang et al., 2006).
Figure 2.
Effect of chronic oral administration of the mineralocorticoid receptor antagonist eplerenone (EPL) on cyclooxygenase-2 (COX-2) expression and neuronal excitation in the paraventricular nucleus of hypothalamus (PVN) in rats with ischemia-induced heart failure (HF). Representative immunohistochemical images showing COX-2 (blue stain) and Fra-like activity (black dots) in sections taken from the PVN of rats with HF six weeks following coronary artery ligation. Left panel: coronal section showing the full expanse of the PVN in a rat with HF. Bar = 100 μm. Middle panel: a higher power view illustrating the localization of COX-2 staining to the extensive microvasculature penetrating the PVN and the proximity of COX-2 staining to chronically excited (Fra-like positive) neurons in a rat with HF. Bar = 30 μm. Right panel: coronal section showing the full expanse of the PVN in a HF rat treated orally with EPL for six weeks. Bar = 100 μm. Adapted from (Kang et al., 2006)
Taken together, these studies suggest that one mechanism for the MR-mediated increase in sympathetic nerve activity in HF is a centrally mediated increase in circulating cytokines. How and where in the central nervous system MR agonists act to effect an increase in circulating cytokines remains to be determined.
Brain MR and the brain renin-angiotensin system
Aldosterone levels increases in brain as well as in the plasma in rats with HF (Yu et al., 2008). Aldosterone of adrenal origin can cross the blood-brain barrier, though brain levels are tightly regulated (Connell & Davies, 2005), and aldosterone levels in the brain have been shown to fluctuate in response to changes in plasma levels (Gomez-Sanchez et al., 2005; Yu et al., 2008). In adrenalectomized rats, brain aldosterone levels are present but very low (Gomez-Sanchez et al., 2005; Yu et al., 2008) and increase in proportion to peripherally infused aldosterone (Yu et al., 2008). However, the brain itself is also capable of producing aldosterone (Connell & Davies, 2005), so the origin of the aldosterone in the brain of HF rats remains uncertain (Yu et al., 2008; Huang et al., 2009).
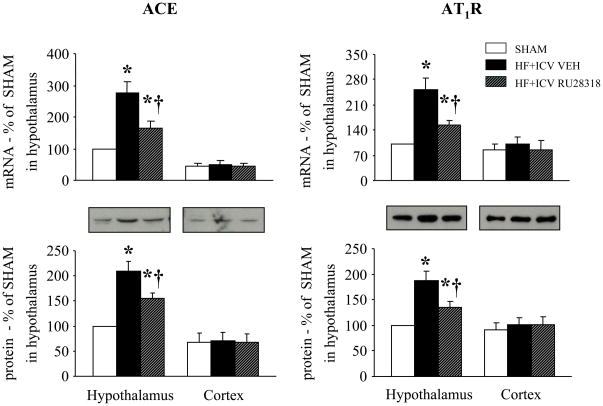
Aldosterone has actions in the brain similar to those of angiotensin II, promoting sodium and water consumption and increasing sympathetic drive (De Nicola et al., 1992; Gomez-Sanchez et al., 1996; McKinley et al., 1996). We hypothesized that activation of brain MR might upregulate the activity of the brain renin-angiotensin system. We tested this hypothesis in HF rats treated for 4 weeks with a continuous ICV infusion of the MR antagonist RU28318 (Yu et al., 2008). Compared with sham-operated control rats, untreated HF rats had increased hypothalamic mRNA and protein for angiotensin converting enzyme and angiotensin II type 1 receptors (AT1R), two key components of the brain renin-angiotensin system (Figure 3), consistent with previous findings from our laboratory and others (Tan et al., 2004; Guggilam et al., 2008; Kang et al., 2008; Wei et al., 2008). They also had increased hypothalamic NAD(P)H oxidase activity, as demonstrated by increased mRNA for the NAD(P)H oxidase subunits p47phox and gp91phox, increased NAD(P)H oxidase dependent superoxide production, and increased intracellular superoxide in the PVN, as reported previously (Guggilam et al., 2007; Kang et al., 2008). Activation of the renin-angiotensin system, with downstream NAD(P)H oxidase dependent superoxide production, is closely associated with increased sympathetic drive (Zimmerman et al., 2002; Gao et al., 2004). These untreated HF rats had increased Fra-like activity in the PVN, signifying chronic neuronal excitation, and increased plasma norepinephrine, consistent with increased sympathetic nerve activity. All of these findings were ameliorated in the HF rats treated with ICV RU28318 (Yu et al., 2008). Notably, these same manifestations were elicited in normal rats subjected to a week-long continuous ICV infusion of aldosterone, and were blocked by concomitant ICV administration of RU28318 (Zhang et al., 2008). These studies support the hypothesis that activation of brain MR augments sympathetic drive, at least in part, by upregulating the activity of the brain renin-angiotensin system. An alternative explanation for the activation of NAD(P)H oxidase might be a non-genomic direct effect of aldosterone that has been demonstrated in peripheral tissues (Callera et al., 2005).
Figure 3.
Effect of central mineralocorticoid receptor blockade on components of the brain renin-angiotensin system. mRNA and protein expression of angiotensin converting enzyme (ACE, left panels) and angiotensin II type 1 receptors (AT1R, right panels) in hypothalamus and cortex of rats with heart failure (HF) treated for 4 weeks with a continuous ICV infusion via osmotic minipump of VEH or the mineralocorticoid receptor antagonist RU28318 and of sham operated control rats (SHAM) * P<0.05 vs. SHAM; † P<0.05, HF+ICV RU28318 vs. HF+ ICV VEH. Adapted from (Yu et al., 2008).
Downstream signaling mechanisms - substrates for excitatory interactions
The neurochemical milieu in cardiovascular regions of the heart failure brain is complex. The brain renin-angiotensin system is upregulated (Tan et al., 2004; Liu et al., 2006), the pro-inflammatory cytokines are increased (Francis et al., 2004a; Kang et al., 2008), and aldosterone is present in higher than normal levels (Yu et al., 2008). It is likely that these excitatory mediators utilize common effector mechanisms. Thus, angiotensin II (Gao et al., 2004), the pro-inflammatory cytokines (Guggilam et al., 2007)and aldosterone (Yu et al., 2008) all activate NAD(P)H oxidase in the brain, and all three are known to stimulate NAD(P)H oxidase dependent mitogen-activated protein kinase (MAPK) pathways – i.e, p44/42 MAPK, p38 MAPK and c-Jun N-terminal kinase (Torres & Forman, 2003). Gene products downstream from these three major MAPK pathways include AT1R, the pro-inflammatory cytokines TNF-α, IL-1β, and COX-2. It is easy to imagine that AT1R generated by this mechanism might bind with ambient angiotensin II, and that PGE2 produced by COX-2 might bind with ambient E-prostanoid receptors, both ultimately resulting in increased sympathetic nerve activity. Similarly, one might anticipate a feed-forward mechanism, by which the gene products of the MAPK pathways tend to reactivate and perpetuate it, perhaps contributing to the sustained sympathetic drive characteristic of the HF syndrome.
Previously published work has demonstrated that acute inhibition of brain p44/42 MAPK activity reduces mean arterial pressure, heart rate, and renal sympathetic nerve activity in rats with established HF (Wei et al., 2008), and that systemically administered angiotensin II upregulates the activity of all three MAPK pathways in the PVN and the subfornical organ in normal rats (Wei et al., 2009). Preliminary data suggests that aldosterone may also utilize this pathway – a 4 hour systemic infusion of aldosterone increases expression of phosphorylated p44/42 and p38 MAPK in hypothalamus and specifically in PVN, and ICV infusion of a p44/42 MAPK inhibitor (but not a p38 MAPK inhibitor) prevents increases in heart rate, mean arterial pressure, and RSNA induced by the aldosterone infusion (Zhang et al., 2009).
Summary
The mechanisms by which activation of MR induces an increase in sympathetic discharge remain obscure. The data presented here suggest several possibilities, including indirect effects mediated by pro-inflammatory cytokines and/or upregulation of the brain renin-angiotensin, and possibly even direct effects mediated by NAD(P)H oxidase dependent generation of superoxide and superoxide dependent cell-signaling pathways. Further studies are needed to elucidate the significance of these mechanisms and others that may contribute but are not yet appreciated.
Acknowledgments
This work has been supported by a Department of Veterans Affairs Merit Review, NIH RO1s HL HL063915 and HL073986, an American Heart Association Heartland Grant-In-Aid, and the University of Iowa.
Footnotes
DISCLOSURES
The author has nothing to disclose
References
- Callera GE, Montezano AC, Yogi A, Tostes RC, He Y, Schiffrin EL, Touyz RM. c-Src-dependent nongenomic signaling responses to aldosterone are increased in vascular myocytes from spontaneously hypertensive rats. Hypertension. 2005;46:1032–1038. doi: 10.1161/01.HYP.0000176588.51027.35. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann N Y Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Grillo C, Gonzalez S. Physiological, biochemical and molecular mechanisms of salt appetite control by mineralocorticoid action in brain. Brazilian Journal of Medical & Biological Research. 1992;25:1153–1162. [PubMed] [Google Scholar]
- Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and Cytokine Receptors in Advanced Heart Failure: An Analysis of the Cytokine Database from the Vesnarinone Trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- Dibbs Z, Kurrelmeyer K, Kalra D, Seta Y, Wang F, Bozkurt B, Baumgarten G, Sivasubramanian N, Mann DL. Cytokines in heart failure: pathogenetic mechanisms and potential treatment. Proc Assoc Am Physicians. 1999;111:423–428. doi: 10.1111/paa.1999.111.5.423. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–617. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein G, Adelberg SA, Kopin IJ, Jacobowitz DM. Hypothalamic sites for cardiovascular and sympathetic modulation by prostaglandin E2. Brain Res. 1982;231:335–342. doi: 10.1016/0006-8993(82)90370-5. [DOI] [PubMed] [Google Scholar]
- Francis J, Beltz T, Johnson AK, Felder RB. Mineralocorticoids act centrally to regulate blood-borne tumor necrosis factor-{alpha} in normal rats. Am J Physiol Regul Integr Comp Physiol. 2003a;285:R1402–1409. doi: 10.1152/ajpregu.00027.2003. [DOI] [PubMed] [Google Scholar]
- Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004a;286:H2264–2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- Francis J, Wei S-G, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol. 2004b;287:H2138–2146. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Johnson AK, Felder RB. Central mineralocorticoid receptor blockade decreases plasma TNF-alpha after coronary artery ligation in rats. Am J Physiol Regul Integr Comp Physiol. 2003b;284:R328–335. doi: 10.1152/ajpregu.00376.2002. [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Wei S-G, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol. 2001a;281:H2241–2251. doi: 10.1152/ajpheart.2001.281.5.H2241. [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Wei S-G, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol. 2001b;281:R1734–1745. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP. Central hypertensive effects of aldosterone. Front Neuroendocrinol. 1997;18:440–462. doi: 10.1006/frne.1997.0157. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288:E342–346. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Fort CM, Gomez-Sanchez CE. Intracerebroventricular infusion of RU28318 blocks aldosterone-salt hypertension. Am J Physiol Endocrinol Metab. 1990;258:E482–484. doi: 10.1152/ajpendo.1990.258.3.E482. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Zhou M, Gomez-Sanchez CE. Mineralocorticoids, salt and high blood pressure. Steroids. 1996;61:184–188. doi: 10.1016/0039-128x(96)00010-4. [DOI] [PubMed] [Google Scholar]
- Grossmann C, Gekle M. Nongenotropic aldosterone effects and the EGFR: interaction and biological relevance. Steroids. 2008;73:973–978. doi: 10.1016/j.steroids.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: Cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail. 2008;10:625–634. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WE, Schmid PG. Cardiovascular and antidiuretic effects of central prostaglandin E2. J Physiol. 1979;288:159–169. [PMC free article] [PubMed] [Google Scholar]
- Huang BS, White RA, Ahmad M, Tan J, Jeng AY, Leenen FHH. Central infusion of aldosterone synthase inhibitor attenuates left ventricular dysfunction and remodelling in rats after myocardial infarction. Cardiovasc Res. 2009;81:574–581. doi: 10.1093/cvr/cvn222. [DOI] [PubMed] [Google Scholar]
- Kang Y-M, Zhang Z-H, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- Kang Y-M, Zhang Z-H, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer A, Hesse B. Heart failure and neuroendocrine activation: diagnostic, prognostic and therapeutic perspectives. Clin Physiol. 2001;21:661–672. doi: 10.1046/j.1365-2281.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- Lal A, Veinot JP, Leenen FH. Critical role of CNS effects of aldosterone in cardiac remodeling post-myocardial infarction in rats. Cardiovasc Res. 2004;64:437–447. doi: 10.1016/j.cardiores.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Leenen FH, Skarda V, Yuan B, White R. Changes in cardiac ANG II postmyocardial infarction in rats: effects of nephrectomy and ACE inhibitors. Am J Physiol Heart Circ Physiol. 1999;276:H317–325. doi: 10.1152/ajpheart.1999.276.1.H317. [DOI] [PubMed] [Google Scholar]
- Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Neuronal angiotensin II type 1 receptor upregulation in heart failure: activation of activator protein 1 and Jun N-terminal kinase. Circ Res. 2006;99:1004–1011. doi: 10.1161/01.RES.0000247066.19878.93. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clinical & Experimental Pharmacology & Physiology - Supplement. 1996;3:S99–104. [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators [see comments] N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Barthelmebs M, Grima M, Imbs JL, Wybren De J. Brain mineralocorticoid receptor control of blood pressure and kidney function in normotensive rats. Hypertension. 1999;33:1201–1206. doi: 10.1161/01.hyp.33.5.1201. [DOI] [PubMed] [Google Scholar]
- Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- Rousseau MF, Gurne O, Duprez D, Van Mieghem W, Robert A, Ahn S, Galanti L, Ketelslegers J-M, Belgian RALES Investigators Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: Results from the RALES neurohormonal substudy. J Am Coll Cardiol. 2002;40:1596–1601. doi: 10.1016/s0735-1097(02)02382-3. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci. 2003;8:s1321–1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- Tan J, Wang H, Leenen FH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1665–1671. doi: 10.1152/ajpheart.00858.2003. [DOI] [PubMed] [Google Scholar]
- Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Weber KT, Sun Y, Campbell SE, Slight SH, Ganjam VK, Griffing GT, Swinfard RW, Diaz-Arias AA. Chronic mineralocorticoid excess and cardiovascular remodeling. Steroids. 1995;60:125–132. doi: 10.1016/0039-128x(94)00030-g. [DOI] [PubMed] [Google Scholar]
- Wei S-G, Yu Y, Zhang Z-H, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol. 2009;296:H1425–1433. doi: 10.1152/ajpheart.00942.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S-G, Yu Y, Zhang Z-H, Weiss RM, Felder RB. Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats. Hypertension. 2008;52:342–350. doi: 10.1161/HYPERTENSIONAHA.108.110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group Members; Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y and for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Yu Y, Kang YM, Zhang Z-H, Wei S-G, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor kappaB. Hypertension. 2007;49:511–518. doi: 10.1161/01.HYP.0000257356.20527.c5. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wei S-G, Zhang Z-H, Gomez-Sanchez E, Weiss RM, Felder RB. Does Aldosterone Upregulate the Brain Renin-Angiotensin System in Rats With Heart Failure? Hypertension. 2008;51:727–733. doi: 10.1161/HYPERTENSIONAHA.107.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-H, Yu Y, Kang Y-M, Wei S-G, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol. 2008;294:H1067–1074. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- Zhang Z-H, Wei S-G, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R916–927. doi: 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- Zhang Z-H, Yu Y, Wei S-G, Felder RB. Aldosterone induces sympatho-excitation via brain mitogen-activated protein kinase signaling pathways in rat. FASEB J. 2009;23 Program # 610.3. [Google Scholar]
- Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]