Abstract
The past decade has seen substantial growth in research into how changes in the biomechanical and biophysical properties of cells and subcellular structures influence, and are influenced by, the onset and progression of human diseases. This paper presents an overview of the rapidly expanding, nascent field of research that deals with the biomechanics and biophysics of cancer cells. The review begins with some key observations on the biology of cancer cells and on the role of actin microfilaments, intermediate filaments and microtubule biopolymer cytoskeletal components in influencing cell mechanics, locomotion, differentiation and neoplastic transformation. In order to set the scene for mechanistic discussions of the connections among alterations to subcellular structures, attendant changes in cell deformability, cytoadherence, migration, invasion and tumor metastasis, a survey is presented of the various quantitative mechanical and physical assays to extract the elastic and viscoelastic deformability of cancer cells. Results available in the literature on cell mechanics for different types of cancer are then reviewed. Representative case studies are presented next to illustrate how chemically induced cytoskeletal changes, biomechanical responses and signals from the intracellular regions act in concert with the chemomechanical environment of the extracellular matrix and the molecular tumorigenic signaling pathways to effect malignant transformations. Results are presented to illustrate how changes to cytoskeletal architecture induced by cancer drugs and chemotherapy regimens can significantly influence cell mechanics and disease state. It is reasoned through experimental evidence that greater understanding of the mechanics of cancer cell deformability and its interactions with the extracellular physical, chemical and biological environments offers enormous potential for significant new developments in disease diagnostics, prophylactics, therapeutics and drug efficacy assays.
Keywords: Mechanotransduction, Cytoskeleton, Cytoadherence, Signaling pathways, Mechanobiology
1. Introduction
The links between biomechanics and human diseases have been the subject of considerable scientific research effort for a number of decades. The application of traditional “solid” and “fluid” mechanics concepts from physics and engineering to the study of biological and physiological problems has provided valuable insights into the mechanics of organ function, the mechanical response of tissues, joints and articulating surfaces, and the physical and mechanical processes associated with blood flow through the vasculature (see e.g. Refs. [1–7] and the sources cited therein). Classical “continuum mechanics” approaches have also provided a broad and fundamental framework within which phenomenological responses associated with biological systems could be consistently and quantitatively rationalized. Consequently, such approaches have also been adapted, with appropriate modifications, to model the mechanics of deformation of biological cells, subcellular components such as the cytoskeleton and phospholipid bilayer membrane, and biological molecular networks and attachment systems. Examples of concepts adapted from engineering science to the study of cell mechanobiology include:
the application of rubber-like elasticity models to simulate reversible, large deformation elastic response of the human red blood cell (RBC or erythrocyte) [1,8–14];
continuum simulations of the cell membrane and cytoskeleton as well as models of coarse-grained local microscopic deformation processes to capture the structure–property connections [14–26];
the extension of classical Voigt and Maxwell models traditionally used for rationalizing the viscoelastic response of soft engineering materials and creeping solids to the study of whole cell and cytoskeletal deformation [7,8,27–34];
poroelasticity or poroviscoelasticity models and other biphase continuum models [35,36]; cellular solid mechanics [37]; and “tensegrity” models to treat the cytoskeletal structure of a biological cell in a manner similar to that of a “truss-like” engineering structure whose deformation characteristics are appropriately modified to account for the “active” and “dynamic” nature of living cells [38–40];
the use of classical Hertzian elastic indentation analyses (see e.g. [41,42]) to extract cellular mechanical properties by recourse to “contact” experiments involving such techniques as depth-sensing instrumented indentation, atomic force microscopy and scanning force spectroscopy (see Section 3 for further details); and
the application of engineering concepts to extract quantitative understanding of the mechanics of cell adhesion [43–45] and of the mechanics of biological attachments [46].
Continuum mechanics concepts have thus provided a broad quantitative framework to categorize and rationalize biomechanical processes and mechanisms without specifically and directly addressing the issues of length- and time-scales of particular concern to biochemical mechanistic phenomena at the cellular and molecular levels. In the past decade, however, rapid advances in engineering, technology, physical, life and information sciences and medicine, as well as the growing trend to integrate such advances across multidisciplinary boundaries, have produced spectacular progress in the study of mechanics and physics at the levels of individual cells and biomolecules. These advances have significant implications for cancer research and treatment. Specifically,
Advances in biophysical and biomechanical tools have led to the wide availability of instrumentation to probe biological cells and molecules in physiologically appropriate in vitro environments (e.g. [42,47–51]). These tools have provided unprecedented opportunities for imposing and sensing forces and displacements to a precision of a picoNewton and a nanometer, respectively. They have also provided new capabilities to generate force vs. displacement records of mechanical deformation for cells and molecules, and to probe adhesion between specific molecular species (e.g. ligands and receptors) under different stress states, such as those involving tension, in-plane shear or torsion [52–61]. These advances in nanotechnology and subnanoscale physical probing are accompanied by progress in bioimaging in vivo and at the molecular level.
Improvements in computer hardware and software over the past decade have facilitated the development of biomechanical models that are capable of accounting for a large population of relevant molecules in the cytoskeleton of certain types of cells in the simulation of deformation (e.g. [21–26,62,63]). These computational capabilities and visualization tools also address issues that could not be examined systematically and in a well-controlled manner by recourse to experiments alone. Similarly, advances in computer modeling also allow the dynamics of intermolecular interactions at the subcellular level to be quantified in a manner that could not be achieved directly and solely by experiments [21–26,63–65].
Rapid advances in genetics (study of genes) and genomics (study of the whole genome) have provided unique new opportunities for progress in diagnostics, prophylactics and therapeutics in the context of many major classes of human diseases. Such progress has also generated new means by which cellular and molecular mechanics associated with disease onset and progression could be understood by targeting specific genes (e.g. [66– 68]).
In light of these trends and developments, the study of the influence of cellular, subcellular and molecular mechanics on human disease states, including cancer, has emerged as a topic of rapidly expanding scientific interest in recent years. A particular focus of research in this area is to explore the connections among the cell ultrastructure, cellular and cytoskeletal mechanical properties, biological function and human health/disease by specifically addressing the following issues.
How do in vivo biological processes or biochemical factors, the external environment or pathogens influence, and are influenced by, changes in the molecular structure of the cell membrane and cytoskeleton?
How do these structural changes alter single-cell mechanical responses, including elastic and viscoelastic deformation characteristics?
How could such single-cell deformation characteristics be generalized to populations of large numbers of cells?
How well do in vitro biophysical assays capture cell mechanical property changes with sufficient force and displacement resolution, and fidelity to in vivo micromechanical phenomena?
How do changes in cell deformability and shape, in concert with alterations to cytoadherence, affect cell migration?
What are the consequences of these effects for the onset and progression of diseases?
How could the information on structure–mechanical property–biological function–disease state relationships guide the development of novel tools for disease diagnostics, prophylactics and therapeutics, as well as drug efficacy assays?
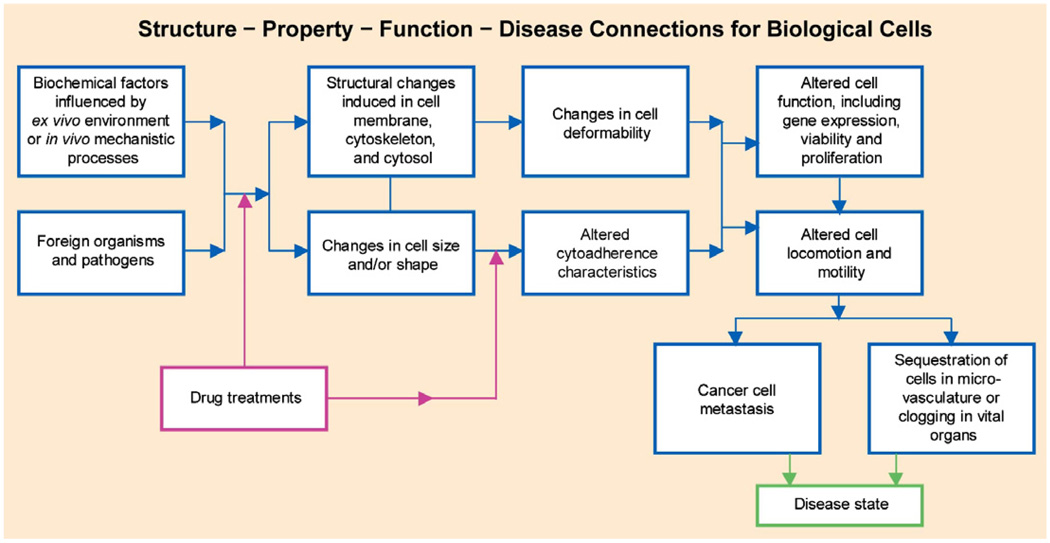
These relationships among cell structure, biomechanics and disease states are schematically illustrated in Fig. 1. This structure–property–function–disease paradigm is of particular interest to the growing community of researchers dealing with the mechanics of cancer cells.
Fig. 1.
Schematic of chemobiomechanical pathways influencing connections among subcellular structure, cell biomechanics, motility and disease state.
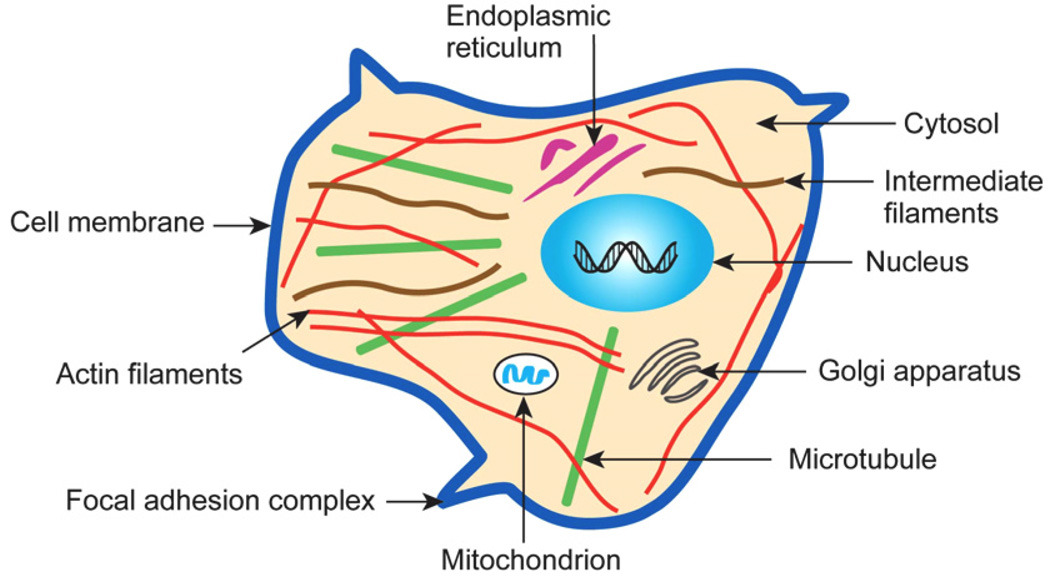
A necessary first step in establishing connections among chemobiomechanical pathways in the context of human diseases inevitably requires systematic studies of cell mechanical properties as a function of disease state under conditions that mirror in vivo phenomena. Given the complexity of the mechanisms underlying this process, it is not surprising that comprehensive studies involving detailed cell mechanics experiments that cover the full range of appropriate disease states are available only for a very limited set of disease classes. This situation is further compounded by the fact that the biological cell is a “dynamic” (far from equilibrium) system whose continuously changing chemical and physical characteristics cannot be characterized by “fixed” mechanical properties [13,69,70]. The cell also has a complex “microstructure” that evolves in response to its chemomechanical environment as well as disease state. A typical eukaryotic cell whose complex microstructure, and hence mechanics, can be significantly altered by cancer, is illustrated schematically in Fig. 2.
Fig. 2.
Schematic illustration of the subcellular structure of a typical eukaryotic cell. Adapted with modifications from Ref. [13]. The structure of key cytoskeletal components is discussed in detail in Section 3.
An example of systematic studies of how disease states influence single-cell and cell-population biomechanics can be found in recent in vitro studies of the effects of the malaria-inducing Plasmodium falciparum parasite on the deformability of human RBCs [68,71–73]. Here the optical tweezers method (described in Section 4) was used to obtain force vs. deformation of Plasmodium-infected RBCs over a force range of 1–200 pN (strains in excess of 100%). These studies have examined the full range of parasite maturation stages during the 48 h time span of the asexual cycle within the RBC at normal physiological and febrile temperatures. Such measurements have also been complemented with microfluidic assays capable of documenting movements of infected RBCs through constricted channels whose inner cross-sections are comparable to those encountered by RBCs circulating in small capillaries [72]. These biomechanical probes of cell deformability have made use of genetic manipulations of specific parasite proteins for altering cell elasticity [67,68]. For this purpose, targeted gene disruption of proteins involved in RBC deformation in Plasmodium has been utilized in conjunction with RBC deformability studies to quantify the contributions of these proteins to cell elasticity.
This paper focuses specifically on the biomechanics and biophysics of cancer cells, and provides an overview of many critical issues pertaining to the deformability of cancer cells with implications for cell signaling, cytoadherence, migration, invasion and metastatic potential. According to a study by the World Health Organization (WHO) [74], 7.6 million people worldwide died of cancer during 2005 alone. WHO estimates that mortality rates due to cancer will continue to rise worldwide, with 9 million cancer deaths by 2015 and 11.4 million by 2030. Major advances have been made in the past several decades in our understanding of the cellular, molecular, genetic and environmental factors influencing the onset and progression of human cancer, in the technology for diagnosing cancer and in the treatment regimes. Despite such progress, overall cancer death rates in the USA have not improved in the past five decades. Each year, approximately 1.4 million people in the USA are diagnosed with cancer, and 600,000 succumb to one of more than 200 different types of cancer [75].
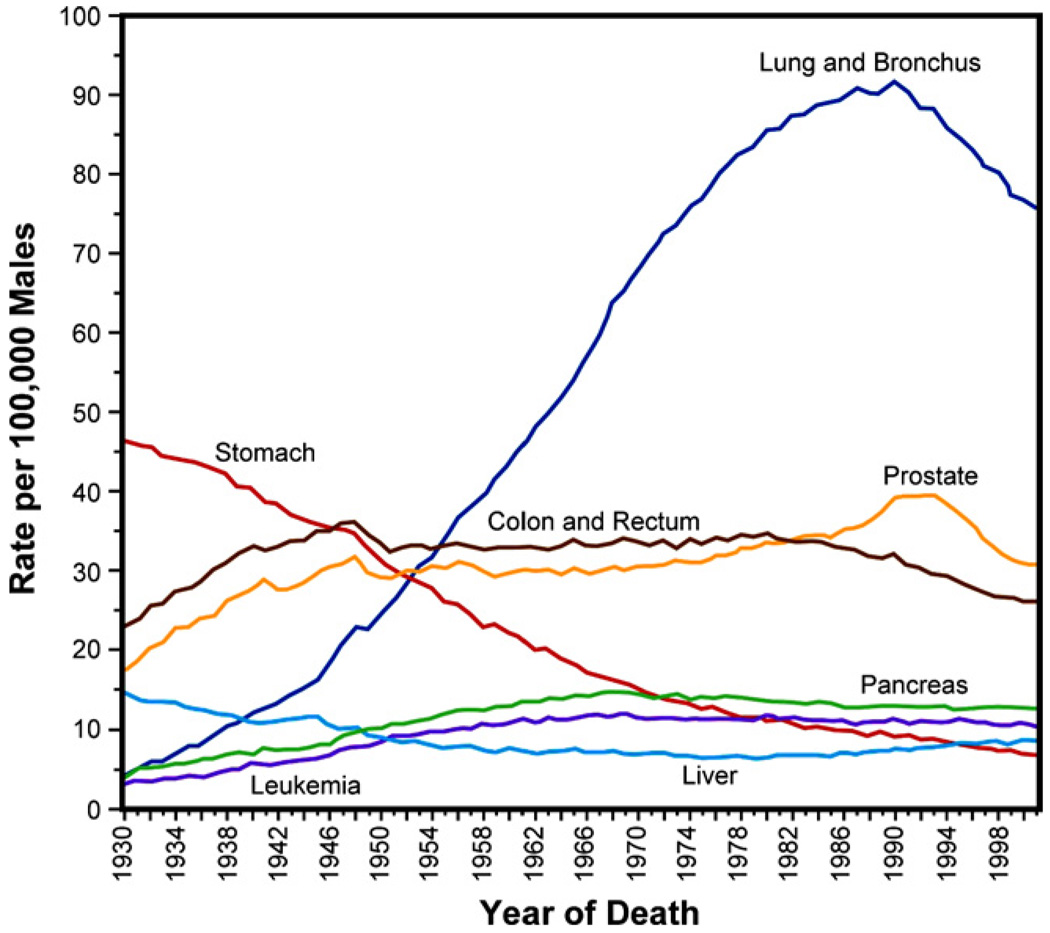
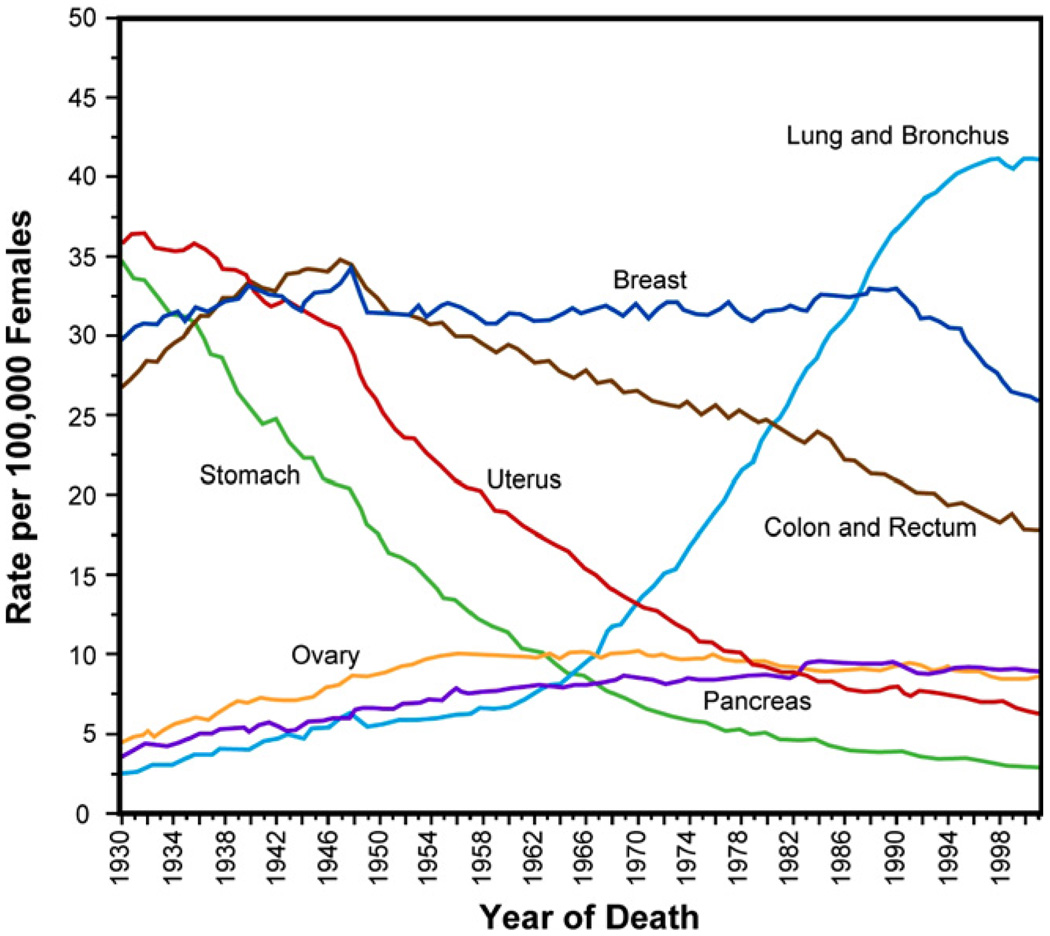
Figs. 3 and 4 show, for males and females in the USA, respectively, the age-adjusted mortality rates due to cancer over seven decades since 1930 [76]. Mortality has declined significantly due to changes in food storage practices and possibly Helicobacter pyroli infection rates for certain types of cancer, such as stomach cancer, and to screening for others, such as cervical and colorectal cancers [77]. However, despite major diagnostic and therapeutic advances and discoveries emanating from cancer research, several types of cancer have remained resistant to treatments particularly when the tumors advance to metastasis, due to a variety of reasons, including lifestyle and environmental factors.
Fig. 3.
Annual cancer death rates among males, age-adjusted to the standard population of the USA in the year 2000, over the period 1930–2001. Adapted from Ref. [76].
Fig. 4.
Annual cancer death rates among females, age-adjusted to the standard population of the USA in the year 2000, over the period 1930–2001. Adapted from Ref. [76].
The paper is arranged in the following sequence. A brief summary of key points from the biology of cancer cells is presented in Section 2 to set the stage for subsequent discussion. Section 3 addresses subcellular cytoskeletal networks and their influence on cell biomechanics. This is followed, in Section 4, by a brief summary of various experimental techniques used to extract quantitative information on the mechanical properties of cancer cells. This summary also includes a broad survey of available information on the biomechanics and biophysics of cancer cells. Section 5 deals with specific case studies that illustrate how different types of subcellular cytoskeletal networks and proteins influence cell biomechanics for human breast cancer, pancreatic cancer and melanomas. An example of experiments and analytical means to determine the energy of adhesion of murine sarcoma cells is described in Section 6. Section 7 reviews possible mechanical signaling pathways between cancer cells and the extracellular matrix through integrins and focal adhesions as cell mechanotransduction acts concurrently with molecular tumorigenic signal pathways to promote malignant transformations. The effects of drugs used to treat cancer and their role in influencing cellular architecture and disease states are the focus of discussion in Section 8. The paper concludes with Section 9, where a summary of key observations and a discussion of possible avenues for further research are provided.
2. Biology of cancer cells
Cancer is a disease that arises from malfunctioning biological cells. The diseased cells proliferate uncontrollably and disrupt the organization of tissue.
Biopolymeric proteins constitute the essential components of the cytoskeleton of the cell. This internal scaffolding, as described in detail in the next section, determines the shape and mechanical rigidity of the cell. Proteins secreted in regions between cells constitute the extracellular matrix (ECM), which clusters and binds the cells together to form tissues. Integrins are cell surface receptors which create clusters known as focal adhesions that serve as binding locations between the cell surface and the ECM. The structures of the cytoskeleton and the ECM are transformed by cancer. Altered protein structures also change the ability of cancer cells to contract or stretch, by influencing their mechanics of deformation. As a result, the motility of cancer cells can be very different from that of normal cells, causing them to migrate through tissue to different sites in the human body and inducing tumor metastasis.
Cancer cells create their own signals for sustained growth and duplication and transmit them between proteins through a process commonly referred to as signal transduction. They reprogram their growth and division through a control circuit assembled out of proteins. The signals of this circuit are transmitted back and forth in a two-way communication that exists between the ECM and the cell interior, including the cytoplasm and nucleus, through the integrins and focal adhesions that bind the cell to the ECM.
The cellular and molecular mechanisms determining the origins of cancer can be traced to the pioneering discovery of Varmus and Bishop [78] of the proto-oncogene, which provided clues about the role of molecules and genes in creating cancer in humans. The proto-oncogene discovery led to the realization that the genomes associated with the cells of normal vertebrates possess a gene with the potential, in some instances, to facilitate the transformation of a normal cell into a cancer cell [77].
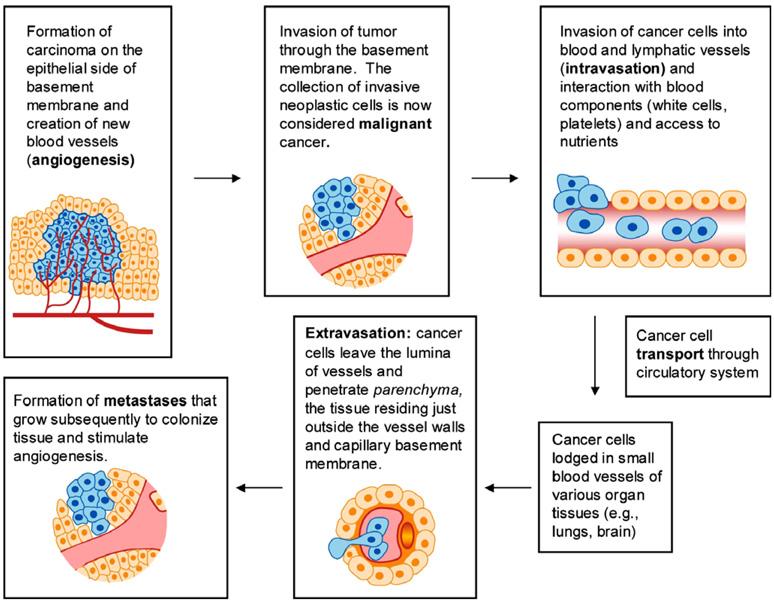
Table 1 lists a brief summary of some key observations on the biology of cancer cells which serve to provide a foundation for subsequent discussion of cancer cell mechanics. More comprehensive discussions of cancer cell biology can be found in the book by Weinberg [77] and the references cited therein. The various steps involved in this invasion–metastasis cascade are shown schematically in Fig. 5.
Table 1.
Some key points from the biology of cancer cells
| Characteristics | Observations and/or definitions |
|---|---|
| Broad classification of tumors |
|
| Major classification of tumors according to their origin |
|
| Cell differentiation | Phenomenon through which a specialized phenotype, such as that representative of cells in a particular tissue site, is acquired by a cell |
| Cell transformation | Transformation refers to the conversion of a normal cell into a cancer cell and/or to the changes to a cell caused by a genetic agent. Normal cells in monolayer culture proliferate until they come into contact with other cells; this results in the cessation of proliferation. Transformed cells, however, do not exhibit such contact inhibition. A cluster of transformed cells from a common progenitor forms a focus, which is a multilayered clump growing in the midst of a monolayer of normal cells in culture. Unlike normal cells, which require attachment to a solid substrate (anchorage dependence) for growth, transformed cells are not anchorage-dependent. Complete cell transformation is often evidenced by tumorigenicity, the ability of cells to create tumors when introduced in animal hosts |
| Cell growth and differentiation | Growth factor (GF) refers to a group of proteins that facilitate cell proliferation and growth by adhering to specific growth factor receptors on the surface of that cell, such as receptor tyrosine kinase (RTK). Epidermal growth factor (EGF) promotes cell proliferation in the epithelial layer of the skin which primarily comprises keratinocytes at different stages of differentiation. EGF receptor works in concert with ErbB proto-oncoprotein, which delivers growth-stimulating signals for cell proliferation. Many varieties of human cancers indicate hyperactive signaling of GF receptors |
| Metastasis | Metastasis refers to the migration of cancer cells from the origin of abnormality to one or more locations in the body. This process also involves the escape of the cancer cells from the tumor mass, migration, invasion of tissue and colonization (see Fig. 5) |
| Oncogene | Oncogene is a gene that can induce cell transformation. When DNA from transformed cells is introduced (transfected) into normal cells, the recipient cells could be induced to undergo transformation. Oncogenes can function across species and tissue boundaries to transform cells. Many human cancers can be triggered by oncogenic mutations in Ras superfamily of G-proteins. (G-proteins, short for guanine nucleotide-binding proteins, are a family of proteins involved in second messenger cascades. They employ a signaling mechanism or molecular switch that exchanges guanosine diphosphate (GDP) for guanosine triphosphate (GTP) as a molecular “switch” to promote or suppress intracellular biochemical reactions.) An oncogenic Ras protein is mutated such that it cannot switch off once activated |
| Possible signaling pathways | A possible pathway for cancer cell proliferation that is responsible for human cancer pathogenesis may involve the protein signaling cascade: GFs → RTKs → Grb2 → Sos → Ras → Raf → MEK → Erk. Anchorage independence and loss of contact inhibition, which are characteristic features of transformed cells, are promoted by signaling pathways that contribute to some cancer cell phenotypes induced by the Ras oncoproteins. This process involves the Ras → Raf → MEK → Erk kinase signaling pathway from the cell surface to the nucleus through the cytoplasm, and regulates translation and transcription factors in the nucleus |
| Cancer cell survival | Normal cells are programmed to undergo apoptosis (programmed cell death) through the activation of caspases. In an attempt to form tumors, cancer cells strive to achieve immortality through a variety of means, including: suppression of caspases, which are enzymes that cause breakdown of the cell during apoptosis by cleaving numerous cellular proteins; enhancements in the levels of anti-apoptotic proteins; inactivation of the p53 protein (which can trigger apoptosis in the event of a cell malfunction) via changes in the p53 gene; or methylation of the promoters of apoptosis-facilitating genes (which suppresses the expression of apoptosis-facilitating genes, or suppresses the production of apoptosis-facilitating proteins) |
| Signaling between ECM and the cell interior via integrins | An important signaling pathway entails the Ras protein to promote a response that suppresses apoptosis Integrins are cell surface receptors physically bound to parts of the extracellular matrix (ECM), and they aggregate to form localized “weld spots” between the cell surface and the ECM at sites known as “focal adhesions”. The two-way communication between the ECM and the cytoplasm via the integrins serves a multitude of functions in cancer cells, including suppression of apoptosis and facilitation of cell motility by modulation of the attachment points to the ECM. A possible signaling pathway here involves ECM → Integrins → MEK → Sos → Ras → Erk |
The information contained in this table is derived from [77].
Fig. 5.
Various steps involved in the cancer cell invasion-metastasis cascade. Adapted with modifications from [77].
3. The cytoskeleton and its effect on cell structure and mechanics
The cytoskeleton, the internal scaffolding comprising a complex network of biopolymeric molecules, primarily determines the cell’s shape and mechanical deformation characteristics [13,69,70]. In concert with accessory proteins, it also plays an important role in such cellular processes as mechanotransduction, mitosis and migration. The concentrations and molecular architecture of different constituent components of the cytoskeleton determine the overall deformability and mechanical response of the cell along with the chemomechanical environment, which determines the interactions of the cell with other neighboring cells and the ECM. Any alterations to the cell function due to biochemical processes occurring within the human body, invasion of foreign organisms or disease development can significantly alter the mechanical properties of the cell. Consequently, measures of the mechanical properties of cells could be used as indicators of its biological state and could offer valuable insights into the pathogenic basis of diseases, including the possible identification of one disease from another (see e.g. [13,69,71–73,79–82]).
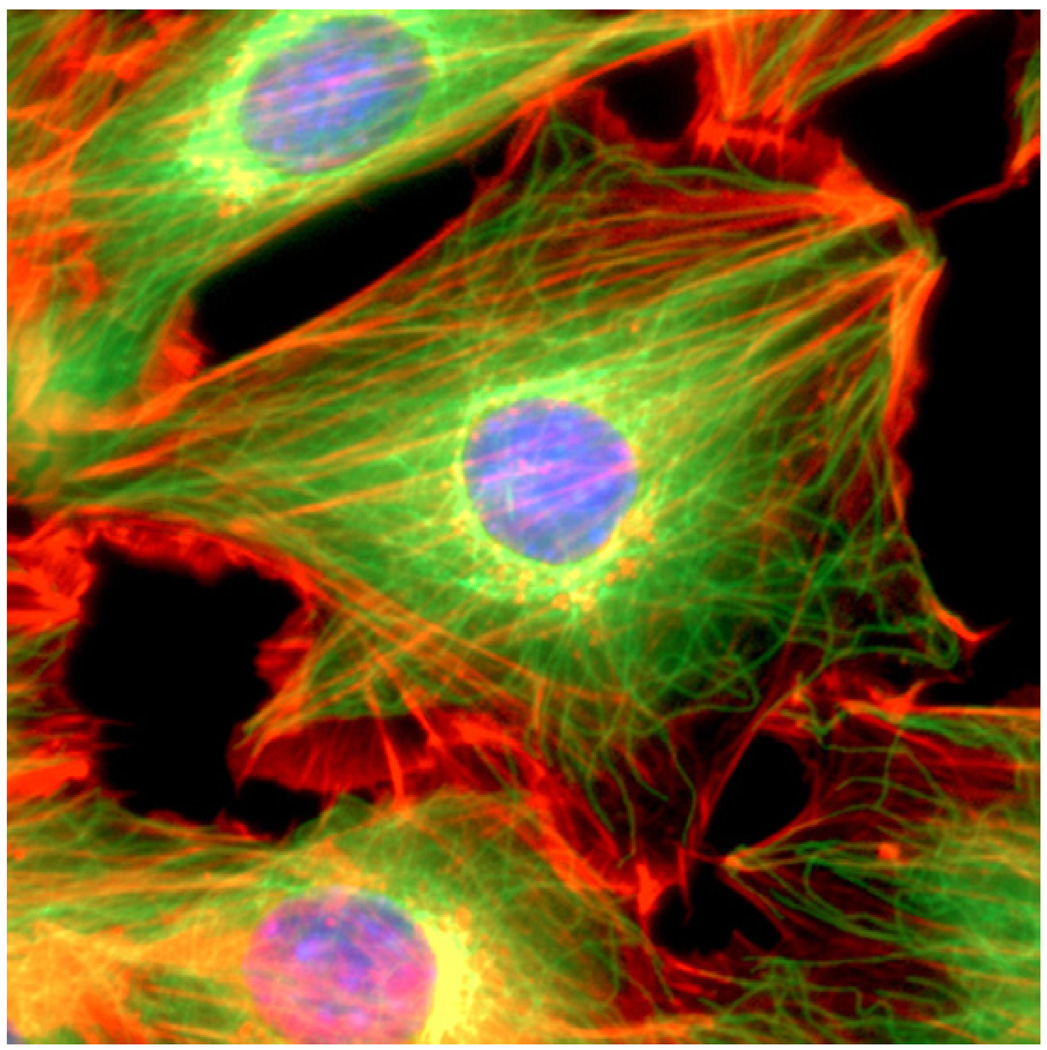
Eukaryotic cells generally contain three distinct types of polymer biomolecules that serve as structural elements in the cytoskeleton of the cell: actin microfilaments, intermediate filaments and microtubules. Fig. 6 is an immunofluorescence image of a 3T3 mouse fibroblast cell showing nuclear DNA, actin microfilaments and alpha-tubulin. An image of the keratin intermediate filament network inside a human pancreas epithelial cancer cell (Panc-1) is shown in a later section.
Fig. 6.
A mouse NIH3T3 fibroblast cell was fixed and stained for DNA (blue) and the major cytoskeletal filaments actin (red) and alpha-tubulin (green). The cell was imaged by fluorescence microscopy on an optical IX70 microscope with a deep-cooled CCD camera. Image courtesy of and with permission from Andrew E. Pelling (London Centre for Nanotechnology and Department of Medicine, University College London).
3.1. Characteristics of three types of cytoskeletal biopolymer filaments
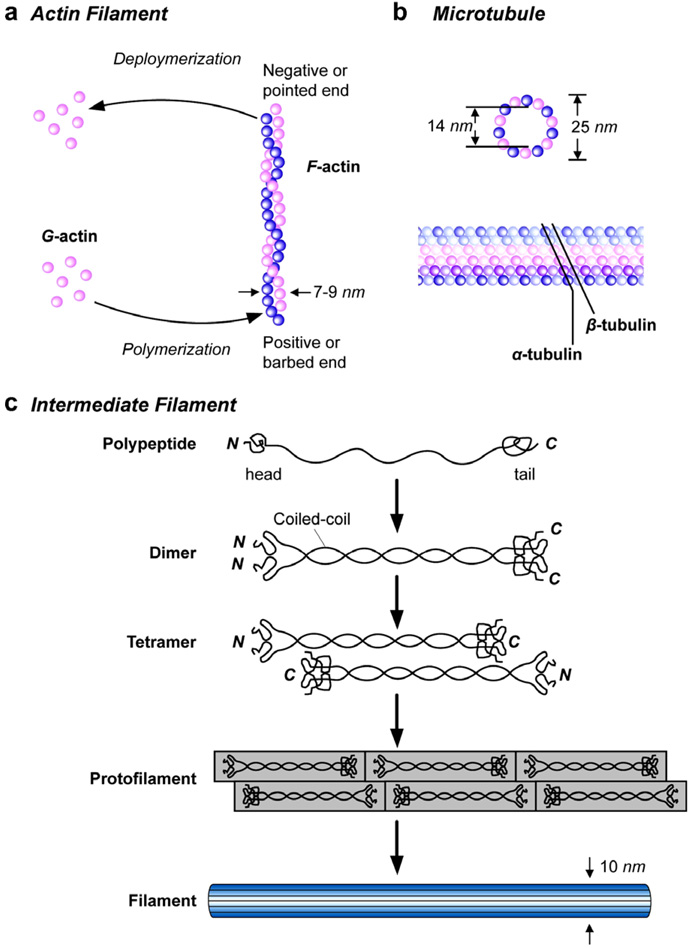
Fig. 7 schematically shows the basic structure of actin microfilaments, intermediate filaments and microtubules [13,36,70]. The basic structural features, elastic properties and key characteristics of these three biopolymer filaments are described in detail in Tables 2–4. The information contained in these tables illustrates the critical role of these cytoskeletal components in influencing not only cell mechanics, but also the interactions of the cell with its external biochemical environment. As noted in these tables, defects in the structure of the cytoskeleton influence a number of diseases, including different types of tumors. Conversely, targeting the cytoskeleton to modify its structural, mechanical and biochemical functions provides an avenue for therapeutics for cancer.
Fig. 7.
Schematic illustrations of the structures of the three basic components of the cytoskeleton of eukaryotic cells. Adapted from [83].
Table 2.
Characteristics of actin microfilaments
| Characteristics | Description |
|---|---|
| Diameter | 6–8 nm |
| Bending stiffness, KB | 7×10−26N m2 |
| Young’s modulus, E | 1.3–2.5 GPa |
| Persistence length, Lp | 15 µm, Lp = KB/(kBT) where kB is the Boltzmann constant and T is the absolute temperature |
| Basic characteristics |
|
Table 4.
Characteristics of microtubules
| Characteristics | Description |
|---|---|
| Diameter | Inner diameter 14 nm, and outer diameter 25 nm |
| Bending stiffness, KB | 2.6 × 10−23 N m2 |
| for a hollow circular rod of outer radius a0 and inner radius ai, where E is the elastic modulus and I is the moment of inertia | |
| Young’s modulus, E | 1.9 GPa |
| Persistence length, Lp | 6 mm in vitro; several orders of magnitude smaller in vivo |
| Basic characteristics |
|
3.2. Viscoelastic properties of individual cytoskeletal biopolymers
Measurements of the individual viscoelastic properties of the three major cytoskeletal fibers, actin microfilaments, vimentin intermediate filaments and tubilin (microtubules) have been performed by Janmey et al. [88] for various concentrations of the biopolymers. They employed a torsional loading apparatus capable of imposing steady and pulsating stresses on the specimens [89]. The stress vs. strain response of the specimens and the storage modulus G′ values as a function of resonance frequency of oscillations were obtained for different concentrations of the biopolymers. The viscoelastic characteristics of the three isolated cytoskeletal polymers were compared with those of gels comprising fibrin protofibrils, which is the basic structural element of blood clots.
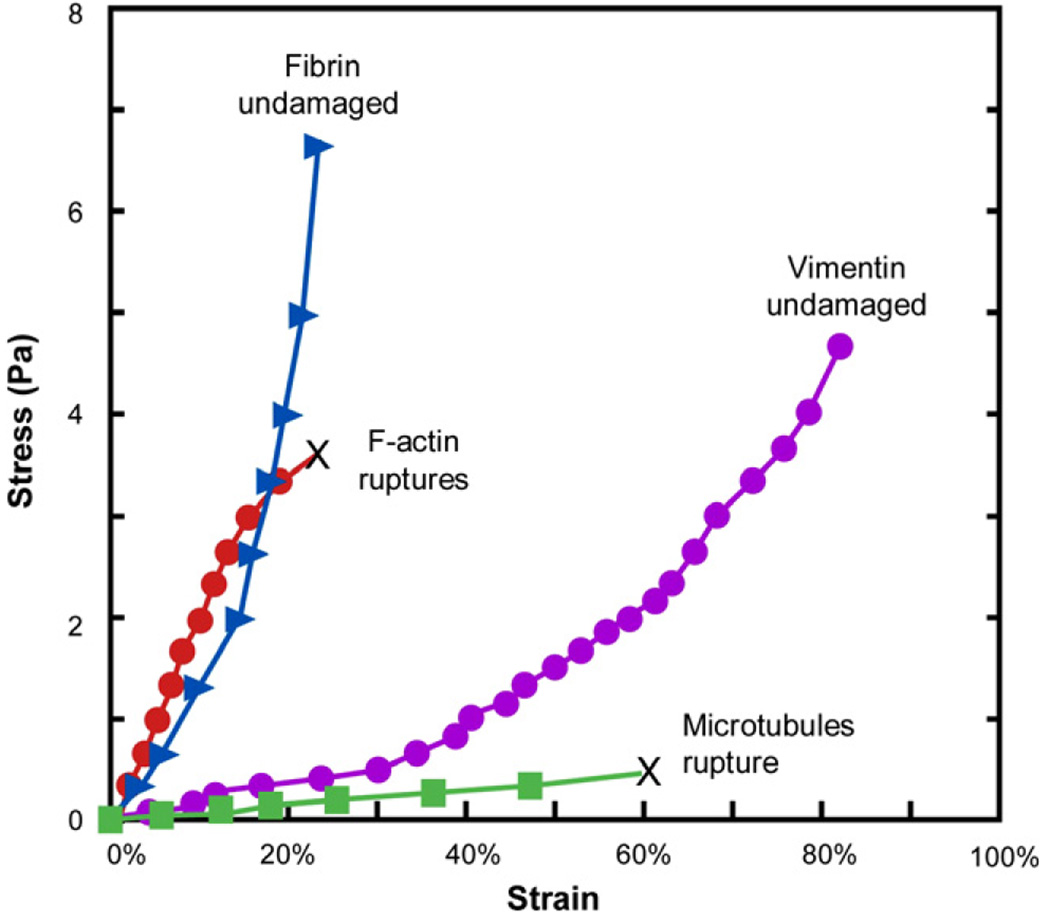
Fig. 8 shows the stress–strain characteristics of actin, vimentin, tubulin and fibrin, all at a fixed protein concentration of 2 mg ml−1. It is clear that the microtubule polymer networks exhibit the largest deformability. The tubulin sample completely loses elasticity beyond a strain of approximately 50%. F-Actin is capable of sustaining much higher stresses and withstands deformation better than both vimentin and microtubules, but begins to rupture and flow at a strain of about 20%. Vimentin and fibrin are more deformable than tubulin and F-actin, but they sustain stresses at much larger strains without flowing freely.
Fig. 8.
Stress–strain curves of actin, vimentin, tubulin and fibrin for which the data points were obtained by monitoring strains at 30 s after the imposition of the shear stress. The concentration of each sample was 2 mg ml−1. F-actin and vimentin networks “rupture” by flowing like a liquid at the value of strain indicated by the symbol “X”. Adapted and replotted from Ref. [88].
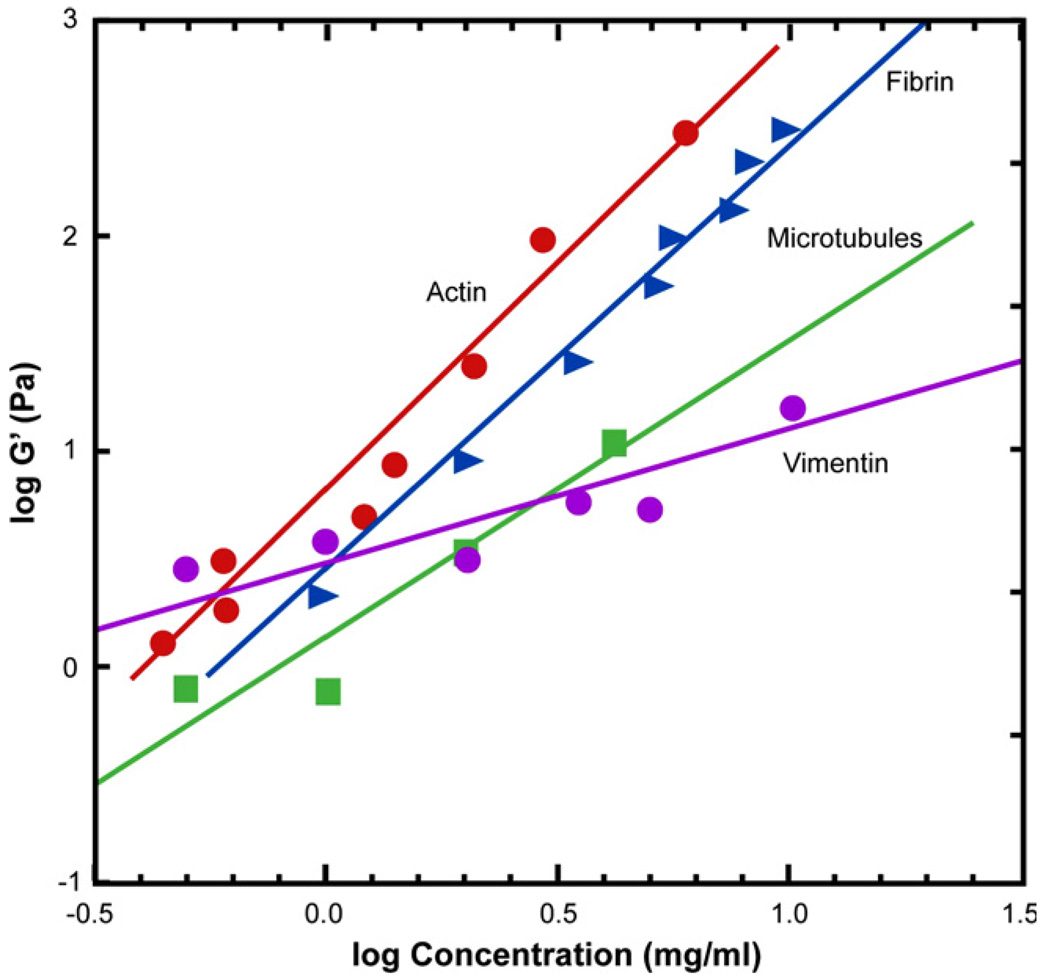
The variation of storage modulus G′ of the four biopolymers as a function of concentration is plotted in Fig. 9 on a log–log scale. Actin and fibrin exhibit larger increases in storage modulus with concentration, with the modulus being proportional to the square of the protein concentration [88].
Fig. 9.
A plot of the storage modulus of the four biopolymers, actin, vimentin, tubulin and fibrin, as a function of their protein concentrations, plotted on a log–log scale. Adapted from Ref. [88].
Although the in vivo biomechanical response of filamental proteins in the cell cytoplasm is likely to be different from that observed in laboratory experiments, the foregoing measurements offer useful insights into the relative roles of different cytoskeletal components in influencing cell biomechanics in both health and disease. The significance of these results can be summarized as follows [88].
Among the different cytoskeletal filaments, F-actin provides the highest resistance to deformation until a certain critical value of local strain. This characteristic offers a rationale for the existence of actin networks at the cell cortex where the networks can easily “fluidize” under high shear stresses to facilitate cell locomotion.
Intermediate filaments are sufficiently compliant to generate moderate deformation, and yet they maintain their resistance to shear deformation at large local strains to provide structural integrity to the cell. They also strain harden to bear the mechanical stress at strain levels where actin networks do not retain their structural integrity.
Microtubules do not have sufficient tensile or shear stiffness to impart significant mechanical integrity to the cytoskeleton. However, they act in concert with the other filamental biopolymers to stabilize the cytoskeleton. Individual intracellular microtubules have been considered resistant to large-scale compression from physiological processes because of lateral reinforcement from the cytoskeleton [103].
The vastly different relative responses of each of these three types of biopolymers also indicate the manner in which the cell is able to engineer its internal composite properties by continuously modulating the components’ relative concentrations and architectures in response to the local biochemical environment and extracellular matrix.
4. Biomechanical assays for cancer cells
A wide variety of experimental biophysical probes have been used to extract the mechanical properties of cancer cells. This section briefly summarizes broad categories of various experimental methods. This is followed by a detailed survey of experimental results on the mechanics of cancer cells from the literature.
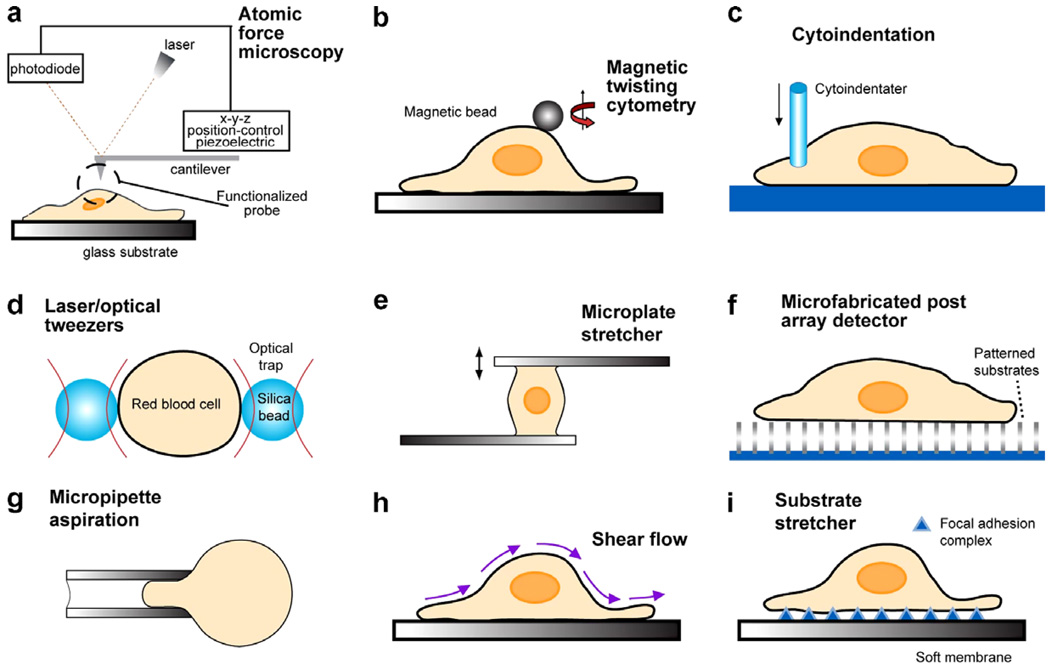
Fig. 10 schematically shows different experimental methods used for biomechanical and biophysical probes of living cells. In this figure, (a)–(c) show three techniques: atomic force microscopy (AFM) [42,47–50], magnetic twisting cytometry (MTC) [34,87,104–106] and instrumented depth-sensing indentation methods [48,51,107, 108]. In these three methods, a portion of the cell surface could be mechanically probed at forces on the order of 10−2−10−6 N and displacements smaller than 1 nm. In AFM, local deformation is induced on a cell surface through physical contact with the sharp tip at the free end of a cantilever. The applied force is then estimated by calibrating the deflection of the cantilever tip, which is detected by a photodiode. MTC entails the attachment of magnetic beads to functionalized surfaces. A segment of the cell surface is deformed by the twisting moment arising from the application of a magnetic field. Elastic and viscoelastic properties of the cell membrane or subcellular components are then extracted from the results through appropriate analysis of deformation.
Fig. 10.
Schematic illustrations of the biomechanical assays used to probe subcellular regions are given in (a)–(c). Biophysical assays commonly used to probe the deformation of single cells are illustrated in (d)–(g). Techniques used to infer cytoadherence, deformation and mobility characteristics of populations of cells are schematically sketched in (h) and (i). This figure is adapted with modifications from Ref. [13].
Fig. 10d–g shows laser/optical tweezers (OT) [14,15,19,71,109–112], mechanical microplate stretcher (MS) [71,113–115], micro-postarray deformation (mPAD) with patterned microarrays that serve as cell substrates [48,116] and micropipette aspiration (MA) [8,11,13,117, 118], respectively. Here forces over the range of 10−12−10−7 N can be induced on the whole cell while submicrometer displacements are monitored optically. With OT, a laser beam is aimed at a high refractive index dielectric bead attached to the cell. The resulting attractive force between the bead and the laser beam pulls the bead towards the focal point of the laser trap. Two beads specifically attached to diametrically opposite ends of a cell could be trapped by two laser beams, thereby inducing relative displacements between them, and hence uniaxially stretching the cell to forces of up to several hundred piconewtons [15]. Another variation of this method involves a single trap, with the diametrically opposite end of the cell specifically attached to a glass plate which is displaced relative to the trapped bead [71]. In the microplate stretcher, force- or displacement-controlled extensional or shear deformation is induced between two functionalized glass plates to the surfaces of which a cell is specifically attached [71,113–115]. In mPAD, a patterned substrate of microfabricated, flexible cantilevers is created and a cell is specifically tethered to the surfaces of these micro-posts. Deflection of these tiny cantilevers due to focal adhesions can then be used to calibrate the force of adhesion [116]. Other patterns, such as discs and spherical islands, can also be created using micro- and nano-fabrication techniques to design different substrate geometries. In MA, a portion of a cell or the whole cell is aspirated through a micropipette by applying suction. Observations of geometry changes along with appropriate analysis then provide the elastic and viscoelastic responses of the cell, usually by neglecting friction between the cell surface and the inside walls of the micropipette [117,118].
Figs. 10g and h illustrate methods with which the substrate deformation (SD) and cytoadherence characteristics of populations of cells could be inferred. Fig. 10h shows a method from which the biomechanical response of populations of cells could be extracted by monitoring the shear resistance of cells to fluid flow [119]. Shear flow experiments involving laminar or turbulent flows are also commonly performed using a cone-and-plate viscometer consisting of a stationary flat plate and a rotating inverted cone. Alternatively, cells could be subjected to forces from laminar flow in a parallel plate flow chamber [13,67,119]. The mechanics of cell spreading, deformation and migration in response to imposed deformation on compliant polymeric substrates to which the cells are attached through focal adhesion complexes is illustrated schematically in Fig. 10i [120,121]. The patterned substrate method shown in Fig. 10f could also be used to probe populations of cells. More detailed descriptions of each of these techniques along with their merits and drawbacks are summarized in Refs. [13,48].
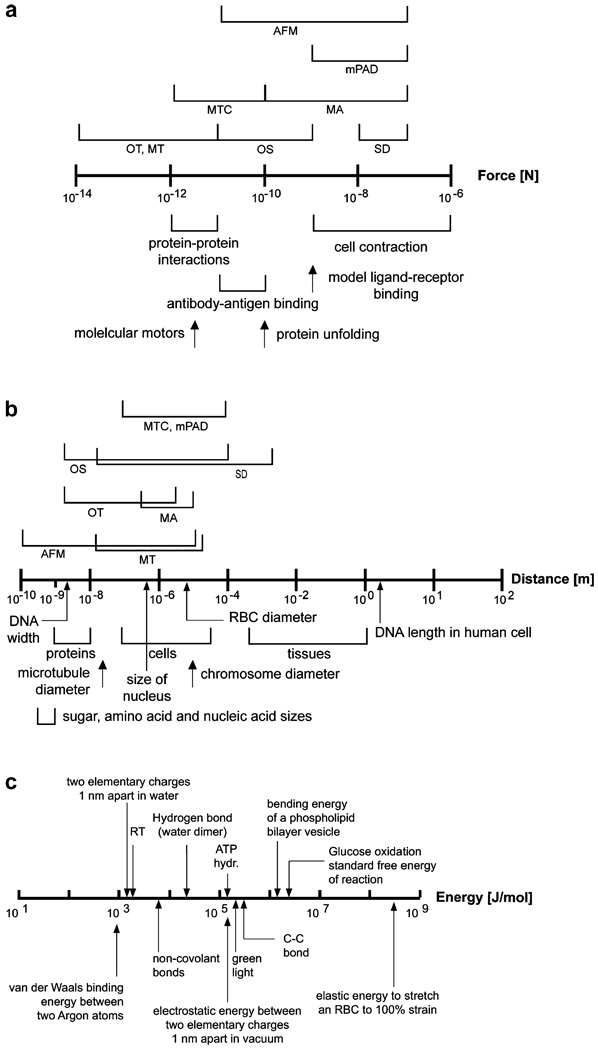
The type of biophysical assay used to probe a particular cell type depends on a variety of factors, including its effective stiffness and time-dependent deformation characteristics, the extent of stretching or contraction it undergoes during mechanical testing, and the force ranges typically achieved during such deformation. Figs. 11a and b summarize the force ranges and length (displacement) scales of relevance, respectively, in select cell and molecular processes of interest in biological systems. Also indicated in these figures are the force and displacement ranges achieved by various biomechanical assays. Fig. 11c illustrates examples of energy scales involved in select biochemical processes of interest in cell and molecular mechanics.
Fig. 11.
Force scales (a) and length scales (b) of relevance to cell and molecular biomechanics. The ranges of forces and displacements probed by different biomechanical assays are also indicated in these two figures. (c) Some energy scales of relevance to biological systems and processes.
In addition to the above methods, a wide variety of simple methods are commonly used to assess mechanical deformability of biological cells without any quantitative measures of forces on the cells or the resulting deformation response. For example, the time of passage of a large population of cells through a micropore filter of a particular pore size is a common method used to simulate cell motility through microvasculature or size-limiting pores in the human body. Such “micropore filtration methods” [122] have also been used to investigate tumor cell deformability as a function of cell size, origin, temperature and pH.
Microfluidic and nanofluidic assays [72,123–125], with relatively rigid or compliant channels fabricated from silicon or poly(dimethylsiloxane) (PDMS), respectively, are also employed to simulate the flow of cells through blood vessels. Such assays could be designed to impose a controlled pressure gradient across the channel, and the resulting flow characteristics of a large population of cells could be recorded in terms of entry time into the channel, cell velocity through the channel, relaxation time for the cell to fully recover its original shape upon egress from a constricted channel, etc. In addition, the inner surfaces of the channels could be lined with an endothelial layer or coated with appropriate receptors to simulate more realistic in vivo biochemical environments. The microfluidic assays could also be used as high throughput cell sorters and employed in conjunction with quantitative cell deformability assays, such as optical tweezers to study the elastic and viscoelastic characteristics of cells. An example of the latter is described in Section 5.2 in the context of deformability of mammary epithelial tumor cells.
Table 5 provides a survey of various observations of the mechanical response of different types of cancer cells, the tools employed to extract the biophysical characteristics and available information on the possible effects of cytoskeleton in modulating cell mechanics.
Table 5.
Summary of select experimental results on cancer cell mechanics, methods used to assess biophysical properties of the cancer cells and available information on underlying mechanisms
| Cell lines | Biomechanical assays used in experiments | Key observations | Reference |
|---|---|---|---|
| Normal human bladder epithelial cells (Hu609 and HCV29) and cancerous ones (Hu456, T24 and BC3726) | Atomic (scanning) force microscopy | Cancer cells are an order of magnitude more deformable (lower in effective stiffness) than normal cells, due to reorganization of the cytoskeleton | Lekka et al. [126,127] |
| Human hepatocytes and hepatocellular carcinoma cells (HCC) | Micropipette aspiration | HCC cells exhibit greater stiffness, but nearly the same viscoelastic properties, when compared with normal hepatocytes | Zhang et al. [128] |
| Non-malignant human mammary epithelial cells (MCF-10), corresponding to human breast cancer (adenocarcinoma) cells (MCF-7), and phorbol ester TPA-treated MCF-7 cells (modMCF-7) with enhanced metastatic potential | Microfluidic optical stretcher | Cancerous MCF-7 cells deformed more than noncancerous MCF-10 cells. Deformability of metastatic modMCF-7 cells was significantly higher than that of MCF-7. Possible mechanisms could include a 30% reduction in F-actin with cytoskeletal reorganization caused by increased metastatic propensity. Possible effects of keratin intermediate filaments at large strains. See Fig. 12 for further details | Guck et al. [129] |
| Human epithelial pancreatic cancer cells (Panc-1) | Mechanical microplate stretcher | Treatment of Panc-1 cells with the bioactive lipid SPC in concentrations comparable to in vivo conditions results in a threefold reduction in elastic stiffness as a result of reorganization of the keratin molecular network in the perinuclear region. See Fig. 13 for further details | Beil et al. [115]; Suresh et al. [71] |
| TRAIL-expressing human leukemic cells (Jurkat) | Micropipette aspiration | Apoptosis-inducing TRAIL cause an increase in elastic stiffness of the cell | Chen et al. [130] |
| Human melanoma and breast carcinoma cells | Micropore filtration | Coexpression of vimentin and keratin intermediate filaments in cancer cells contributes to a more migratory and invasive phenotype | Chu et al. [96] and Hendrix et al. [93], Hendrix et al. [131] |
| Human myeloid (HL60) and lymphoid (Jurkat) leukemia cells | Instrumented indentation with atomic force microscopy | HL60 myeloid cells are up to 18 times stiffer than Jurkat and six times stiffer than human neutrophils. AFM could provide the means to characterize physical properties of leukemia cells so as to elucidate possible origins of vascular complications from acute leukemia | Rosenbluth et al. [132] |
| Human acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) cells incubated with standard induction chemotherapy | Instrumented indentation with atomic force microscopy; microfluidic channel flow assay | Leukemia cell stiffness increased by nearly two orders of magnitude upon exposure to standard induction chemotherapeutic agents, with correspondingly decreased passage through microfluidic channels. Stiffness increase was measured before caspase activation that induces cell death, possibly due to dynamic changes in actin microfilament network | Lam et al. [133] |
| Human colon carcinoma cells (HT-29) (analyzed human cells in rat system) | In vitro flow experiments in a laminar flow chamber and in vivo imaging | Changes in cell elasticity and avidity of cell adhesion molecules after cytoskeletal reorganization influence initial adhesive interactions in vivo | Korb et al. [134] |
| Rat hapatoma cells (AH100B, AH130 and AH66F) and Yoshida sarcoma (YS) cells | Micropipette aspiration | Degree of tumor cell metastasis influenced by cell deformability; larger proportion of recovered cells killed by passage through pulmonary circulation when their resistance to deformation is higher | Sato and Suzuki [135] |
| Nontransformed and transformed rat fibroblast cells | Micropipette aspiration | An increase in cell deformability directly correlates with the progression of the transformed phenotype from a nontumorigenic cell into a tumorigenic, metastatic cell | Ward et al. [136] |
| Lewis lung carcinoma (3LL) mouse cells | Micropore filtration | Nitric oxide, which regulates tumor metastasis via inducible nitric oxide synthase, causes a reduction in deformability of the 3LL cells and influences sequestration of tumor cells in organs | Igawa et al. [137] |
| Three variants of F1 B16 mouse melanoma cells | Micropore filtration | Damage to cytoskeletal components such as microfilaments and microtubules increases the deformability and plays in important role in the metastasis of melanoma cells | Ochalek et al. [138] |
| Mouse erythroleukemia cell line (MEL), as well as MEL transfected with mutant p53 gene (MEL-M) and with p53 wildtype gene (MEL-W) | Micropipette aspiration | MEL-W exhibits less deformability and greater fragility than MEL and MEL-M. Possible causes include structural and compositional changes in the membrane and a reduction in the secondary structures of proteins, such as α-helix and random coils | Yao et al. [139] |
| Mouse fibroblast SV-TV and H-ras-transformed mouse fibroblast cells | Atomic force microscopy | SV-T2 fibroblasts were significantly more deformable than normal fibroblasts BALB 3T3; no appreciable difference between SV-Tw and H-ras-transformed fibroblast | Park et al. [140] |
| BALB/3T3 mouse fibroblast cells and SV-T2 cells transformed from BALB/3T3 with oncogenic DNA virus SV40 | Microfluidic optical stretcher | Deformability of SV-T2 cells significantly increased compared with that of the BALB/3T3 cells, possibly as a result of ~50% reduction in filamentous actin (F-actin) in the former case | Guck et al. [129] |
| Normal or H-ras-transformed NIH 3T3 mouse fibroblast cells on flexible collagen-coated polyacrylamide substrates | Deformation of flexible substrates | Mechanical feedback provided by the substrate regulates cytoadherence, traction forces, cell growth and apoptosis for normal cells and is strongly influenced by substrate stiffness. This loss of feedback in transformed cells, irrespectively of substrate stiffness, could rationalize uncontrolled cell growth | Wang et al. [141] |
| Explanted mouse mammary tumors and healthy mammary glands | Electromechanical indentor to study tissue mechanics | Changes to the stiffness of the ECM activates integrins and growth factor receptors, which further alter the matrix stiffness. The resulting positive feedback loop activates malignant phenotype in breast epithelial cells whereby solid-state mechanotransduction acts in concert with tumorigenic signaling to facilitate malignant transformation | Paschek et al. [142] and Huang and Ingber [143] |
5. Case studies in cancer cell mechanics
This section examines specific examples of cancer cell mechanics with implications for migration, invasion and metastasis.
5.1. Deformability of human mammary epithelial cancer cells
The first example deals with the deformability of non-malignant and malignant human breast cancer cells and of the latter malignant cells chemically modified to increase metastatic efficiency. Guck et al. [129] used a microfluidic optical stretcher, which is a coaxially aligned dual-beam laser tweezers system, to sequentially suspend, trap and deform isolated cells through a contactless microfluidic channel. The net forces from the two beams balance along the beam axis, thereby producing a stable trap. The cells are individually stretched, at forces on the order of 200–500 pN, in fluid suspension at a flow rate of approximately 1 cell min−1.
In this method, the dimensions of major and minor axes, a and b, respectively, of the cell are optically recorded prior to and during stretching in the trap. The difference between the aspect ratio at/bt (i.e. the ratio of the major to the minor axis at time t) at the stretching power and the corresponding aspect ratio at the reference trapping power, ao/bo, are determined to define an approximate measure of normalized deformability, Dt, as
| (1) |
In order to compensate for variations in cell size for different cells in the same population and for different cell conditions or disease states, Eq. (1) is further modified to define a quantitative optical deformability index (ODt):
| (2) |
where Fa and Fb are the integrated stretching forces along the major and minor axes, respectively. The subscripts “ref” and “cell” denote the reference and deformed configurations, respectively. ODt thus provides an approximate measure of the relative cell stiffness or spring constant at any time during cell stretching.
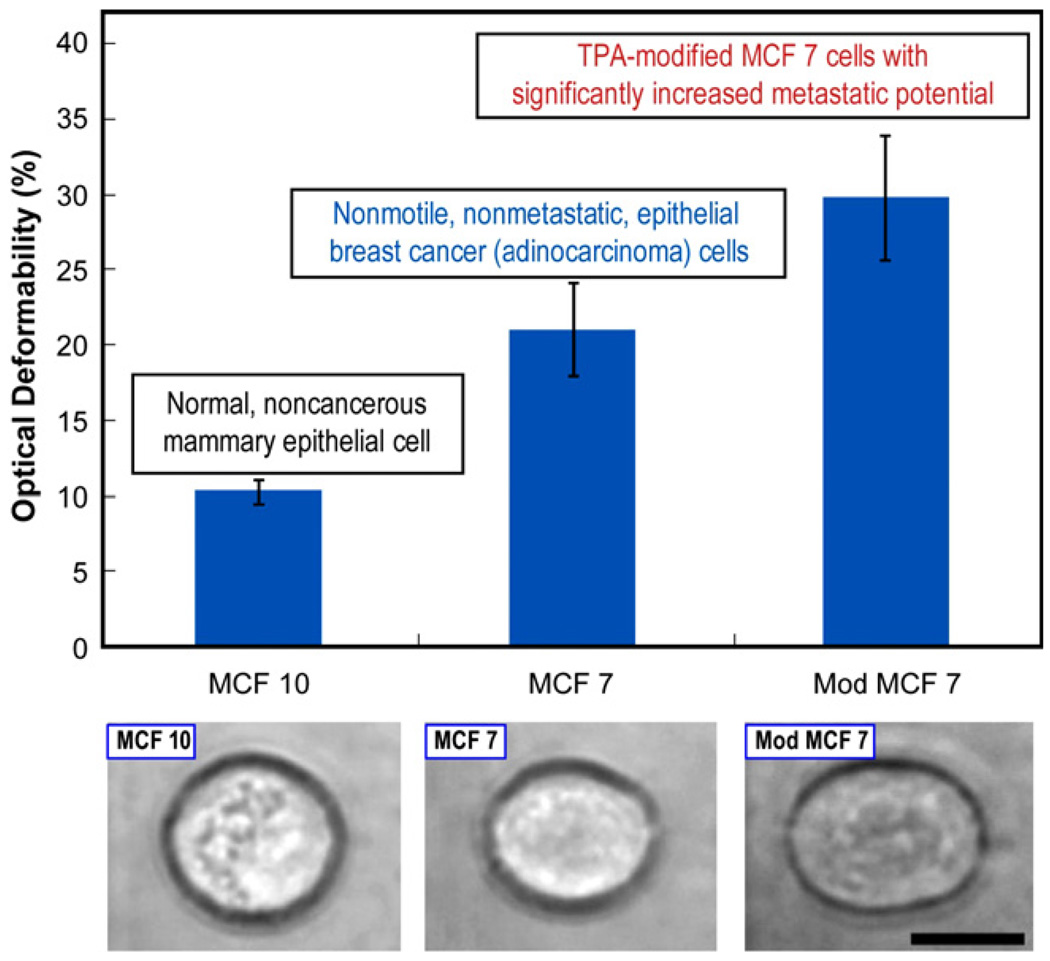
Fig. 12 shows the deformability of nonmetastatic human breast epithelial cells and their malignant counterparts. MCF 10 is a standard model nontumorigenic epithelial cell line derived from benign breast tissue; the cells are immortal, but otherwise normal and noncancerous. MCF 7 is a corresponding human adenocarcinoma cell line in which the cells are nonmotile, nonmetastatic and cancerous. When MCF 7 cells are modified (Mod MCF 7) by treatment with 100 nM 12-O-tetradecanoylphorbol-13-acetate (TPA) for 18 h, an 18-fold increase in the invasiveness and metastatic efficiency is achieved [129]. It is evident from the figure that the cancerous MCF 7 cells are more deformable than the normal MCF 10, and that the meta-static Mod MCF 7 cells are even more deformable than MCF 7 cells. The increased deformability of cancer cells with modified metastatic competence appears to be accompanied by a further reduction in structural strength compared with that of the nonmetastatic cancer cells. These reductions in elastic rigidity appear to arise from a reduction in F-actin concentration of as much as 30% caused during the malignant transformation of the cell [144] at the small strain levels imposed for the results shown in Fig. 12. At larger strain levels, additional contributions to changes in elasticity could also arise from reorganization in the molecular architecture of keratins, which is a major cytoskeletal component of epithelial cells.
Fig. 12.
Relative cell stiffness, as characterized by optical deformability, of healthy (MCF 10) and malignant (MCF 7) human breast cancer epithelial cells (top figure). Also shown in the top figure is a malignant mammary epithelial cell chemically modified to increase its metastatic potential. The optical images of the three cases at the bottom shows an increase in deformability for the cancerous MCF 7 cell compared with the nonmalignant MCF 10, and a further increase in deformability for the Mod MCF 7 cell. These images correspond to a constant stretching laser power of 600 mW. The black scale bar is 10 µm. Adapted from Guck et al. [129]. Reprinted with permission.
The main assessment basis for pathology for breast cancer is the change in morphology of the suspect tissue. However, access to coherent tissue samples inevitably requires biopsy, and is often performed only when a sufficiently noticeable collection of abnormal cells is available. The microfluidic optical stretcher has the advantage of enabling observation and mechanical probing of isolated cells obtained from exfoliative cytology [129]. This method can also be used for microfluidic sorting of the cells on the basis of deformability, thereby obviating the need for genomic and proteomic manipulations of the cells. A drawback of the method is that the forces imposed on the cell by the optical tweezers are not sufficiently large to promote significant deformability, which is necessary to simulate in vivo conditions encountered by migrating tumor cells as they navigate through size-limiting pores. Furthermore, the direct exposure of the cells to the laser beam during stretching is another limitation of the technique.
5.2. Deformability of human pancreatic epithelial cancer cells
Secretion of pancreatic juice into the duodenum in the pancreatic duct and secretion of insulin and glucagons for the regulation of blood glucose levels are two major functions of the human pancreas. Tumor of the pancreas is one of the most lethal forms of cancer in the developed world because of the difficulty in detecting it early and its aggressive propensity for invasion, migration and metastasis. Clinical findings have shown that a bioactive lipid, sphingosylphosphorylcholine (SPC), plays a critical role in the metastatic invasions of gastrointenstinal cancers, including gastric and pancreatic tumors [115]. SPC occurs naturally in human blood components such as blood plasma and high-density lipoprotein particles to promote anti-apoptotic effects.
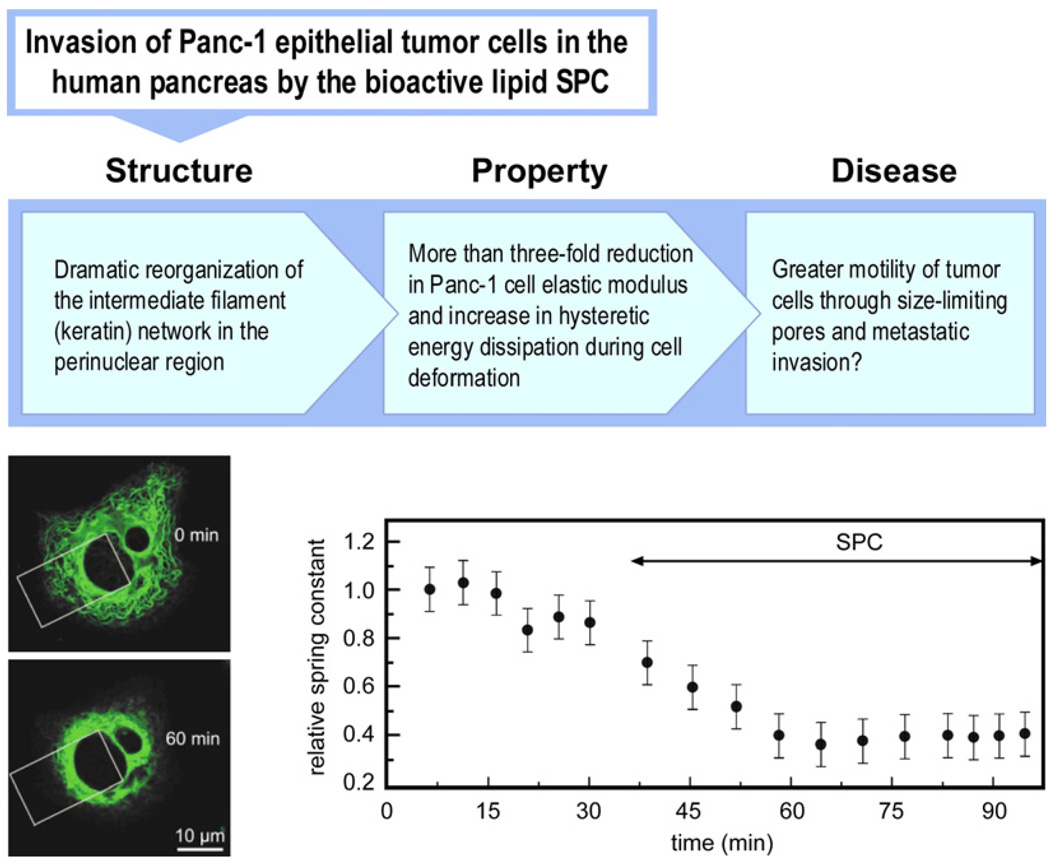
SPC-induced molecular reorganization of the Panc-1 human epithelial pancreatic cancer cells and the consequent changes in mechanical deformability of the cells have been examined as possible pathways that facilitate easier migration and increased metastatic competence of pancreatic tumor cells [71,115]. Human pancreatic epithelial cancer cells express keratins 7, 8, 18 and 19, and the subline of Panc-1 epithelial tumor cells primarily expresses K8 and K18. When human Panc-1 cancer cells are treated with 10 µM SPC to mimic in vivo conditions, phosphorylation leads to a drastic reorganization of keratin into a dense, ring-like molecular structure in the perinuclear region of the cell within 45 min. This is shown in the immunofluorescence images at the lower left of Fig. 13.
Fig. 13.
Possible structure–biomechanics–disease connections in the migration and metastatic efficiency of human Panc-1 epithelial tumor cells. The lower left optical immunofluorescence images were taken using an inverted confocal microscope with a stage pre-heated to 37 °C over a period of 60 min. The top image shows the Panc-1 cancer cell (with two nuclei). When the cell is transfected with 0.5 µg ml−1 of C-HK18-EYFPN1 using Fugene (Roche) and kept in dye-free Dulbecco’s modified Eagle’s medium (20 mM HEPES) in the presence of 10 µM SPC, the keratin network collapses around the nucleus, as shown in the bottom left image. Note the significant reduction, due to SPC treatment, in the spatial distribution of the keratin filaments within the white rectangular area. Microplate mechanical stretch test results of the variation of the effective spring constant of the Panc-1 cell as a function of time before and after SPC treatment. The optical images and biomechanical results are reproduced with permission from Ref. [71].
Biomechanical assays of Panc-1 cell deformability with and without SPC treatment have been performed using the microplate mechanical stretcher method [71,113–115], which is shown schematically in Fig. 10e. For this purpose, a Panc-1 cancer cell is placed between two glass plates functionalized with fibronectin to adhere the cell to the surfaces of the microplates. The cell is then mechanically deformed at physiological temperature by repeated tensile loading over force ranges on the order of several hundred nanonewtons in a microplate cell stretcher to determine its deformability over cell displacements on the order of several micrometers. The resultant spring constant for the cell is calculated as an indicator of its effective elastic rigidity.
Fig. 13 also shows relative changes in the Panc-1 cell effective spring constant as a function of time, first without any SPC treatment and then following SPC treatment. When the reorganization of keratin by SPC is complete, the elastic stiffness of the Panc-1 cell is found to decrease by as much as a factor of 3 compared with its pre-SPC-treatment value. On the basis of these results, it has been postulated [71] that such changes to cell mechanics arising from biochemically induced keratin reorganization could play a critical role in enabling the Panc-1 cancer cell to migrate more easily through size-limiting pores and in facilitating greater metastatic efficiency.
The relatively dominant role of keratin intermediate filaments, over other cytoskeletal filamental biopolymers, in influencing cell deformability was also demonstrated by investigating modifications to cytoskeletal architecture arising from other chemical agents. For example, lysophosphatidic acid (LPA) is known to influence many biological processes, such as platelet activation, mitogenesis, smooth muscle contraction and alteration of the shapes of neurons. These functions are facilitated by the formation of actin stress fibers when the cells are exposed to LPA. If the Panc-1 cancer cell is treated with 10 µM LPA for 15 min, instead of with SPC, microplate mechanical stretch assays do not reveal any significant change in stiffness due to cytoskeletal reorganization [71,115].
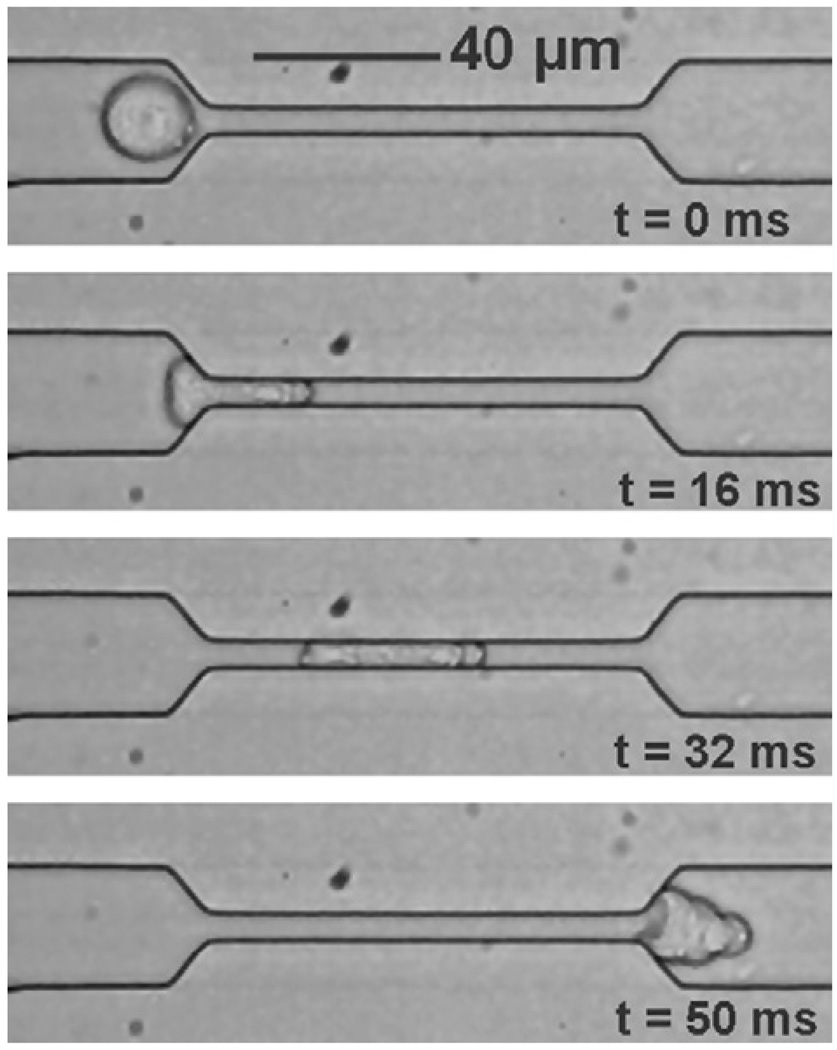
More quantitative connections between changes in cell deformability and motility can be obtained with microfluidic channels. The microfabricated devices offer a means to quantify cell motility characteristics in vitro by providing velocity measurements and shape change and recovery observations as a population of cells flow through channels with well-defined geometrical constrictions and controlled pressure gradients. Fig. 14 shows a microfluidic probe used to study Panc-1 cancer cell migration.
Fig. 14.
A series of sequential optical micrographs showing the movement of a human Panc-1 cancer cell through a PDMS microfabricated fluidic channel, the pressure gradient across which was controlled. The cell approaches the constriction in at a flow rate of ~0.5 µl min−1 (t = 0 ms) and is forced to squeeze into a 9 µm × 18 µm × 150 µm constriction of a PDMS microchannel (t = 16 ms) over a pressure gradient of 4 kPa. After moving through the channel, shape recovery occurs (t = 50 ms). Micrograph obtained by Walter in the laboratory of the author.
5.3. Role of actin and microtubules in influencing cancer cell adhesion
Distant metastatic tumor formation is considered to be influenced strongly by the stable adhesion of cancer cells to the small blood vessel walls. Fluid shear forces and the blood environment surrounding the cells during microcirculation, along with the mechanical response of the cell cytoskeleton, can modulate metastatic potential of circulatory cancer cells in host organs. Korb et al. [134] investigated the role of actin and microtubules in early metastasis in vivo through intravital observation of cytoadherence of colon carcinoma cells within liver microcirculation of rats1 and their invasion into liver parenchyma.
Disruption of tubulin reduced adhesive interactions in vivo, whereas disruption of actin microfilaments increased cell adhesion. During in vitro flow conditions, cytodisruption modulated cell signaling via focal adhesions. These results suggest that changes in cell stiffness and avidity of the cell adhesion molecules (arising from cytoskeletal disruptions) are important for initial adhesive interactions in vivo. However, the broad applicability of these results critically depends on the compatibility of human cells with the immune system of the rat.
5.4. Effects of actin-binding proteins on deformability of melanoma cells
As noted in Table 2, the actin microfilament network stabilizes or gels the cell periphery and the plasma membrane. Actin networks exhibit high elastic moduli and also undergo fluidization at large strains. Processes critical to cell locomotion, such as pseudopod extension, require that the cytoplasm change its viscosity at the cell periphery by transforming from a gelled state to a fluid state.
Solation (destruction) and gelation (reconstruction) of actin microfilaments facilitate the formation of protrusions on the surface of motile cells during their locomotion. Experiments by Cunningham et al. [145] reveal that when dispersed actin filaments are cross-linked and connected to plasma membrane glycoproteins by an actin-binding protein (ABP), which in this case is a homodimer with rod-like 280 kDa subunits with flexible hinges, the cell surface is stabilized. Under these conditions, protrusion activity is organized and locomotion is efficiently achieved. They also show that when cells are ABP-deficient, as in certain human malignant melanoma tumor cell lines, locomotion is impaired and the plasma membrane undergoes circumferential blebbing2 (due to peripheral cytoskeletal instability). If ABP is transfected into such cells, normal locomotion is restored and blebbing is suppressed. ABP transfection also increases the elastic stiffness of the pellets of intact cells by more than a factor of two, from 27 ± 1.1 to 67 ± 4.2 Pa. Since melanoma can be a locally invasive cancer, cell invasion and metastasis and the consequent clinical course of malignancy could be influenced by the migratory efficiency of the cancer cells.
6. Quantification of cytoadherence in murine sarcoma cells
The physics of adhesion between biological cells significantly influences cancer cell motility, invasion and metastasis. However, quantification of cytoadherence is extremely complex, and there are no generally accepted constitutive models or theoretical analysis with which experimental observations of cell adhesion could be systematically rationalized and quantitatively interpreted.
When solid elastic bodies adhere, the underlying mechanics and physics of adhesion could often be analyzed mathematically [146,147]. In general, when two slightly deformable, elastic bodies adhere strongly, the application of a force to separate them causes a finite contact area to develop at the interface prior to the detachment of the surfaces. This behavior is often analyzed by recourse to the Johnson–Kendall–Roberts (JKR) theory [148], which relates the adhesion energy Wa to the force of detachment Fs through the radii of curvature of the contacting materials. If two spherical solids are in adhesive contact, the JKR theory predicts that
| (3) |
where Rm is the harmonic mean of the radii of the adhering spheres. Here, an example of the application JKR theory is presented for the quantification of adhesion energy between murine sarcoma cells.
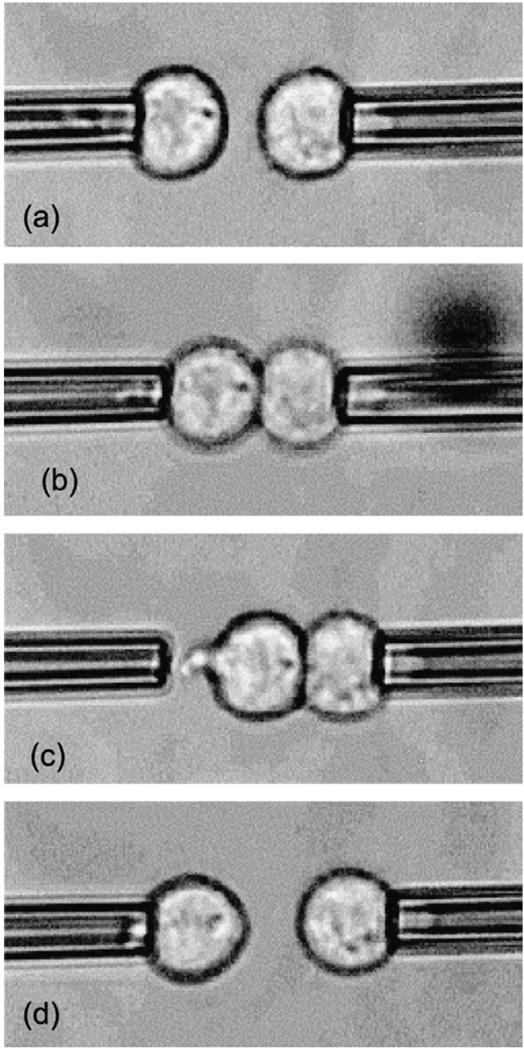
Chu et al. [44] have quantified the energy of adhesion between two murine sarcoma S180 cells through micropipette aspiration experiments by exploiting strong adhesion created by a depletion effect in a highly concentrated dextran solution. These cells do not naturally adhere because of the paucity of superficial adhesion receptors, but strongly attach to each other when forced to touch in the presence of dextran through a pair of micropipettes.
Fig. 15 shows a series of micrographs that illustrate the process of adhesion and separation between the cells. The final separation force between the cells is given by
| (4) |
where ΔP is the average of the aspiration pressures prior to cell separation and R is the inner radius of the micropipette. Since the cells exhibit moderate deformability and finite contact zones during adhesion, it is appropriate to examine the relevance of JKR theory to quantify the energy of adhesion between the cells.
Fig. 15.
The energy of adhesion between two murine sarcoma S180 cells quantified using micropipette aspiration, for different concentrations of the suspending medium dextran. (a) The cells, aspirated through weak pressure with micropipettes, are brought into contact and (b) adhere after 1 s of contact. The aspiration pressure of the right micropipette is then strengthened, and that pipette is moved to the right. As a result, the left cell, tightly adhered to the right cell, leaves the left micropipette, as in (c), or the two cells separate, as in (d). In the case of the behavior shown in (c), the left cell is recaptured by the pipette, aspirated and the right pipette displaced. This process is repeated until the cells separate, and the cell separation force is extracted from the final aspiration pressures. Reproduced with permission from Chu et al. [44].
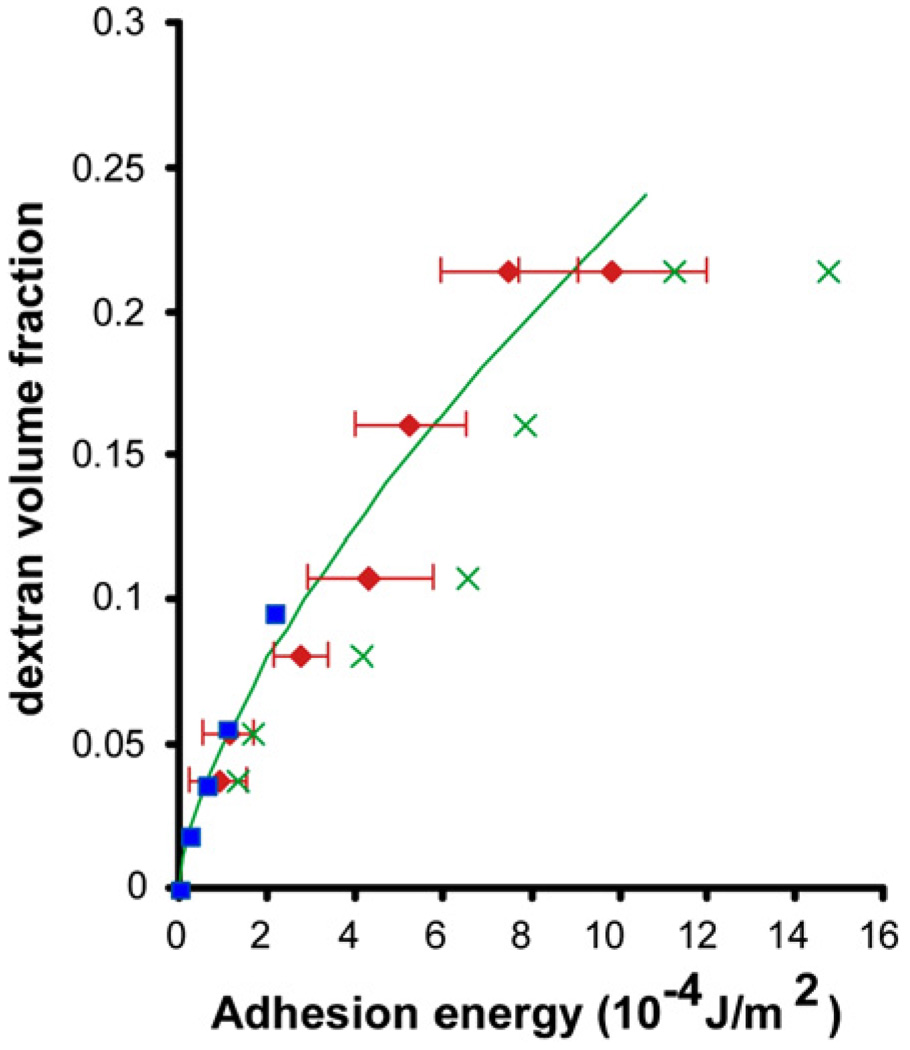
Fig. 16 is a plot of the adhesion energy Wa estimated from Eq. (3) along with experimental measurements for different dextran concentrations. These results can also be compared with experimental measurements of adhesion energies by the depletion of dextran on vesicles [149]. Theoretical analysis of this process [150] shows that the adhesion energy Wa is related to dextran concentration V by the relation
| (5) |
where kBT is the thermal energy (with kB being the Boltzmann constant and T, the absolute temperature) and m is the size of the monomer. Alternatively, the relation between separation force and adhesion energy can also be extracted from the analysis of spherical shells [151] to be
| (6) |
Fig. 16.
Values of adhesion energy between dextran-treated S180 sarcoma cells from micropipette experiments (red points) using Eqs. (3) and (4). The green line shows theoretical predictions of the attractive forces on phospholipids bilayers due to the depletion of dextran, Eq. (5). The predictions of Eq. (6) are shown by the green “×” symbols. Independent experimental results [149] of adhesion energy from contact angle measurements on vesicles are indicated by the blue points. Results adapted from Chu et al. [44].
The results shown in Figs. 15 and 16 point to means by which the adhesion between cells could be quantified, in some special cases, through mechanics analysis.
7. Cancer and the mechanics of extracellular matrix
Cells within different tissues are held together by the ECM. The onset and advance of cancer leads to distinct changes in the stiffness of the surrounding tissue. Consequently, oncologists usually diagnose cancer by sensing the change in elasticity of tissue by palpation [143]. It has long been known that the mechanical response of the ECM itself can play a critical role in tumor formation; a stiffer ECM can result in a stiffer solid tumor [152,153].
There are also clear connections among single-cell mechanics, ECM mechanics, cell mechanotransduction, oncogenic signaling and tumor formation. Select observations illustrating such connections are given below.
Healthy cells attach to a stiffer substrate to spread, stretch and proliferate [154]. However, malignant cells do not display such characteristics [155].
When mammary epithelial cells are cultured on ECM gels, integrins are activated in order to modulate the epidermal growth factor receptor (EGF receptor, or EGFR)3 and to influence cell differentiation and transformation.
Transmembrane EGFRs effect cell mechanotransduction by transferring forces between the compliant, polymeric substrates and the cell through anchoring points known as focal adhesions (e.g. [140,142,154– 156,40,157]). Both the ECM receptors and the focal adhesions link the integrins to the cytoskeleton and align them appropriately for proper cell signaling. However, if the epithelial cells are cultured on flat, rigid substrates instead, these signaling processes are suppressed.
When NIH 3T3 mouse fibroblast cells are cultured on compliant polymeric substrates, they exhibit reduced rates of DNA synthesis, increased rates of apoptosis, as well as reduced cell spreading and traction forces, as compared with the behavior shown on stiffer substrates of comparable chemical composition. However, when the 3T3 cells are H-ras-transformed, substrate stiffness has no effect on cell growth and apoptotic response, and has diminished effects on cell spreading and traction forces [141]. These observations offer valuable insights into the role of ECM mechanical properties in influencing uncontrolled growth of transformed cells.
ECM receptor and focal adhesion sites on the cell exterior also sense the traction forces of the actin microfilaments inside the cell, thereby forming a feedback loop between the cytoskeleton and the ECM through integrins and focal adhesions. Such feedback can also modulate traction by activating the small G protein Rho and its target Rho-associated kinase (ROCK).4 These processes influence cell proliferation [143].
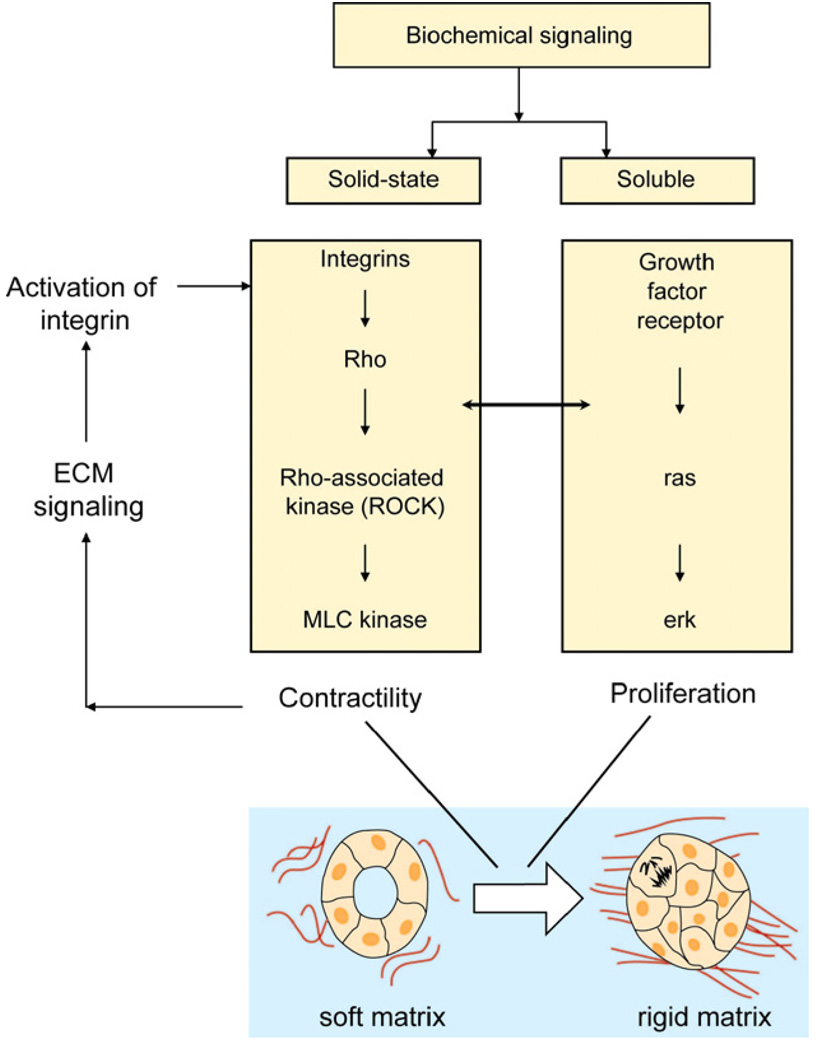
Paszek et al. [142] demonstrate that when the stiffness of ECM is altered, the integrins activated by the rigidity change influence cell contractility by activating Rho. In addition, they modulate cell signaling via the messenger kinase Erk (an enzyme adding phosphate groups from ATP), which is responsible for transmitting signals from the cell surface to the nucleus by the activation of transcription factors so as to influence cell proliferation. The resulting signaling for both contractility and proliferation (see Fig. 17) can further alter the ECM rigidity.
Fig. 17.
Schematic illustration of how mechanical signaling from the ECM acts in concert with biomolecular tumorigenic signaling to activate malignant transformation. Adapted from Ref. [143].
The result is the creation of a closed mechanical signaling loop that acts in concert with the tumorigenic signaling pathways to promote malignant transformation [143].
8. Effects of cancer drugs on cytoskeleton and cell mechanics
Chemotherapeutic agents for cancer are purposely designed to target cell membranes and cytoskeleton so as to induce cytotoxicity and alter cytoadherence. Here, select examples of the effects of chemotherapy on subcellular structures are presented. The effects of chemotherapy on cell mechanics and the attendant consequences for changes in disease states are then described with the specific example of vascular complications arising from chemotherapy.
8.1. Chemotherapy alters subcellular structures
As noted in Tables 2–4, anti-tumor drugs are often purposely designed to target cancer cell membranes and cytoskeleton as they effect cytotoxic action. The following cancer drugs are used as therapeutics for ovarian, breast and lung cancer, malignant melanoma and leukemias [101,158–162].
Doxorubicin tethers to DNA and causes damage to the cell membrane by inducing peroxidation [163].
Etoposide (VP-16), which inhibits DNA topoisomerase II, induces breaks in single- and double-stranded cellular DNA [101].
Taxol enhances tubulin polymerization, suppresses depolymerization of microtubule and inhibits metastasis.
Cancer drugs also modify cytoskeletal architecture by reorganizing F-actin, vimentin and tubulin in K-562 and HL-60 leukemia cell lines during apoptosis. Actin appears to be involved in chromatin remodeling during apoptosis. In addition, translocation of actin from the cytoplasm to the membrane is observed [101].
Chemotherapeutic agents such as vincristine and vinblastine also are designed to prevent the addition of monomers to microtubules, and thereby suppress mitosis entirely [7].
Alterations to cell mechanics induced by chemotherapeutic agents can also result in other clinical complications. The following example describes one such disease which is instigated by the initiation of chemotherapy itself.
8.2. Chemotherapy alters cell mechanics and can induce vascular complications
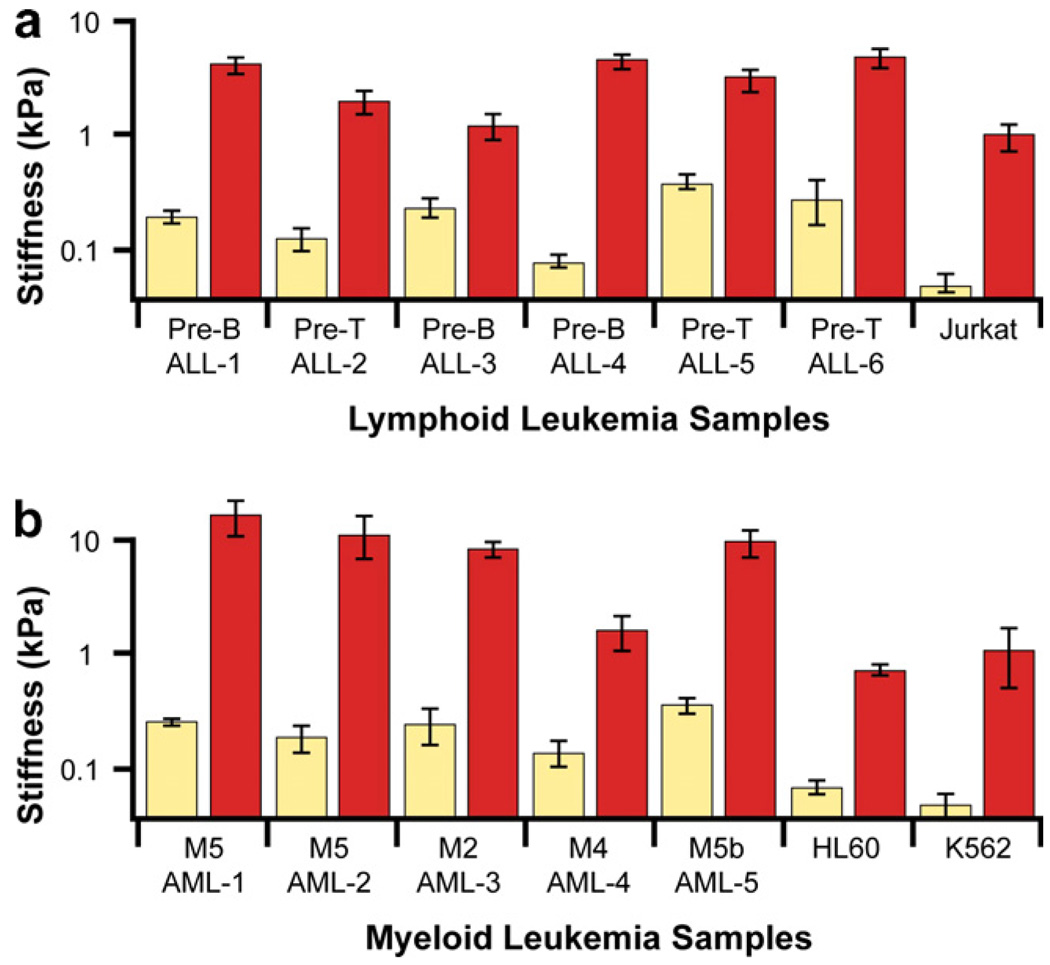
The accumulation of leukemia cells in the blood vessels of vital organs such as the lungs and the brain is known to result in respiratory failure and intracranial hemorrhage [133]. This life-threatening complication of acute leukemia, known as leukostasis, is caused by unknown pathophysiological mechanisms and its diagnosis is usually confirmed only by autopsy. Chemotherapy, which is often essential to treat leukemia, can by itself be a likely risk factor. In some patients, leukostasis develops soon after the initiation of chemotherapy. Advances in chemotherapy treatment regimens for leukemia patients have resulted in a gradual reduction in mortality; however, the mortality rate for the subset of leukemia patients who develop vascular complications has not improved beyond the typical range of 69–74% [164]. Possible causes of vascular complications have been ascribed to mechanical obstruction of blood vessels in vital organs due to an increase in the number of circulating leukemia cells, increased cell stiffness and increased cytoadherence.
Employing the AFM as a quantitative biophysical probe, Lam et al. [133] studied the effects of chemotherapy on changes in elastic properties of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) cells. Samples for the former were obtained from pre-B cell or T-cell precursor cells taken from the peripheral blood of six different, newly diagnosed teenage patients with acute leukemia and with detectable peripheral blasts. Samples for the latter were taken from the peripheral blood or bone marrow of 8- to 78-year-old patients. The AFM chamber was fitted with a PDMS perfusion chamber and a heating stage to facilitate measurements at normal physiological temperature. The ALL and AML cells were exposed to 1 µM dexamethasone and 1 µM daunorubicin, respectively, which are common induction chemotherapeutic agents for the two types of leukemia. Since leukocytes (white blood cells) are nonadherent cells, they were mechanically immobilized in microfabricated wells so as to prevent them from slipping under the AFM cantilever during contact probing. The experiments revealed the following general trends.
In response to treatment with chemotherapeutic agents, the leukemia cells stiffened by up to two orders of magnitude (as revealed by indentation contact stiffness in the AFM based on an elastic contact analysis) during cell death. This is illustrated in Fig. 18 for both ALL and AML cells.
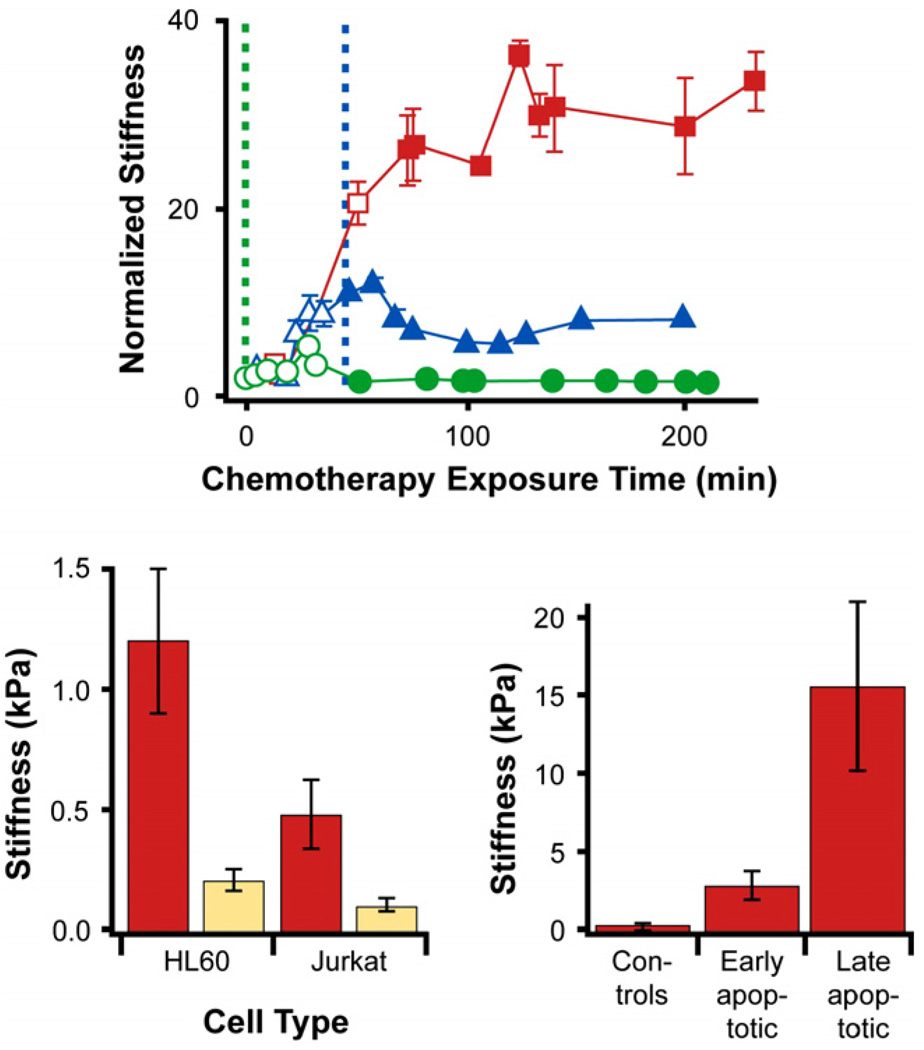
With the progression of chemotherapy-induced cell death, the elastic rigidity of the cells increased significantly as shown in Fig. 19. However, successive measurements of cell stiffness as a function of time for the same duration for a typical control cell, taken from the same patient but not exposed to chemotherapy drugs, did not show any pronounced change in elastic modulus.
Increases in the elastic moduli of cancer cells was observed for different leukemia cell types (primary ALL, primary AML, HL60 and Jurkat), while the rate of stiffness increase was influenced by the type of chemotherapy treatment and cell type (bottom left plot in Fig. 19).
The stiffness increase takes place before caspase activation and reaches a maximum after cell death is completed.
Such cell stiffening appeared to arise from the dynamic reorganization of the actin microfilament network, which underwent polymerization and depolymerization during apoptosis [101,164–169]. When the cancer cells are treated with 2 µM cytochalasin D, an actin polymerization inhibitor, along with the chemotherapeutic agent daunorubicin, cell stiffening is also significantly suppressed as cell death occurs with exposure to the chemotherapy drug (see top plot in Fig. 19).
Fig. 18.
The apparent stiffness of (a) ALL and (b) AML leukemic cells (six different tests for the former and five different samples for the latter) exposed to chemotherapy toxicity. Average apparent stiffness of dead (red) leukemic cells after exposure to chemotherapy is higher compared with untreated (beige) cells. For comparison, leukemic cells Jurkat, HL60 and K562 are also shown. Primary ALL cells and lymphoid leukemic cell lines exposed to 1 µM dexamethasone. Primary AML and myeloid leukemic cell lines exposed to 1 µM daunorubicin. Error bars indicate standard error. Results adapted from Ref. [133].
Fig. 19.
Effects of cell type, chemotherapy exposure time and cell death on the stiffness of leukemia cells. (Top figure) The red line and associated data points show that isolated HL60 cells exposed to 1 µM daunorubicin at time 0 (vertical dashed green line) exhibit increased stiffness (by a factor of more than 30) as a function of time of exposure to this chemotherapy agent. If these cells are now also simultaneously treated with 2 µM cytochalasin D, an actin polymerization inhibitor, at time 0, almost no increase in stiffness is seen (green line and data points) with chemotherapy exposure time and cell death. If the cells are simultaneously treated with 2 µM cytochalasin D, at time t = 45 min (indicated by the vertical dashed blue line) after the 1 µM daunorubicin treatment commences, no further increase (in fact a slight reduction) in stiffness is seen (blue line and data points) with chemotherapy exposure time and cell death. (Lower left figure) The average stiffness of dead cells (beige bars) for both HL60 and Jurkat cell lines exposed to both daunorubicin and cytochalasin D is much lower than that for the same cells exposed only to daunorubicin (red bars). (Bottom right figure) The stiffness of isolated AML cells increases as cell death progresses upon treatment with 1 µM daunorubicin. Results adapted from Ref. [133].
Such correlations between cell mechanics and cell death provide a possible rationale for the clinical observation that some patients develop leukostasis soon after chemotherapy is initiated. The results shown in Figs. 18 and 19 suggest that reduced cell deformability caused by actin network reorganization following chemotherapy initiation might contribute significantly to vascular complications by obstructing microcirculation through the stiffening of leukemia cells during cell death. These results could have some implications for pathophysiology of other diseases where increased stiffness of circulating cells directly affects cell interactions with the endothelium: diabetes, atherosclerosis or autoimmune inflammatory disorders.
9. Concluding remarks
Major advances in cell biology and biophysics have provided extraordinary opportunities to examine the links between the mechanics of cells/subcellular structures and cell functions such as mitosis, signaling, mechanotransduction, ribosomal and vesicle transport, and cell locomotion and motility. A significant outcome of such progress is the availability of techniques to measure the force vs. displacement signature of living cells through a variety of sophisticated biomechanical assays. This mechanical signature, in concert with other chemical, biological and genetic pathways, offers unique new perspectives through which the highly complex mechanistic underpinnings of human health and disease at the molecular and cellular levels could be better understood.
This paper has examined critical issues, results and examples of how changes in cell mechanical responses arising from the biochemical alterations of a stiff and ordered cytoskeleton to an irregular biopolymer network is related to the transformation of a mature, postmitotic, normal cell into an immortal, replicating and motile tumor cell. Our review also points to compelling evidence that clear signaling pathways exist among cell deformability, contractility of the extracellular matrix, cell mechanotransduction, release of integrins and focal adhesions, communication between the cell nucleus and the surface for the activation of transcription factors that influence cell proliferation, and tumorigenic processes. An important step in cancer metastasis involves the penetration of epithelial cells through the endothelial layer, which critically depends on cell elasticity and deformability.
With the rapid growth of the nascent field of cell and molecular mechanics of cancer, a number of challenges remain in translating scientific discoveries from in vitro assays to understanding in vivo processes in the human body. Immobilized cells on artificial substrates and enclosed in artificial environments often provide different mechanical responses in the absence of appropriate biochemical signals. Furthermore, different biophysical assays probe different components of the subcellular structures under different stress states to different levels of precision. It is therefore not surprising that conflicting information on the cell mechanical responses are extracted for the same cellular disease processes from different biomechanical probes. This is especially pronounced when large variations and experimental scatter occur in response characteristics and underscores the need for a large sample set to extract statistically significant results. There is also a critical need to develop more detailed computational simulations of cell and molecular mechanics that accurately capture interatomic and intermolecular interactions and cytoskeletal dynamics as the cytoskeletal network is altered by the disease state. Establishing comprehensive connections between cell mechanics on the one hand and chemical and biological cell functions in human health and disease on the other promises to provide novel and powerful developments for cancer diagnostics, prophylactics and therapeutics.
Table 3.
Characteristics of intermediate filaments
| Characteristics | Description | |
|---|---|---|
| Diameter | 10 nm | |
| Bending stiffness, KB | 4–12×10−27N m2 | |
| KB = EI = Eπa4/4 for a circular rod of radius a, E is elastic modulus and I is the moment of inertia | ||
| Young’s modulus, E | 1–5 GPa | |
| Persistence length, Lp | 1–3 µm | |
| Basic characteristics |
|
|
| Types of intermediate filaments | Type I |
|
| Type II | ||
| Type III | ||
| Type IV | ||
| Type V | ||
| Type VI | ||
Acknowledgements
Preparation of this article and related work in the area of cell mechanics received support from the National Institutes of Health/National Institute of General Medical Sciences Grant 1-R01-GM076689-01 and the Advanced Materials for Micro and NanoSystems Program and the Computational Systems Biology Program of the Singapore-MIT Alliance, and support from the National University of Singapore through the Tan Chin Tuan Centennial Overseas Chair, the Global Enterprise for Micromechanics and Molecular Medicine (GEM4), and an Inter-University Research Project. The author thanks members of his research group and collaborators for many insightful discussions on the topics covered in this paper. Preparations of this article also benefited from partial support from the Nano/Bio Mechanics Program of the Engineering Directorate of the US National Science Foundation for the GEM4 2007 Summer School on Cell and Molecular Mechanics in Biomedicine.
Biography
Subra Suresh is the Ford Professor of Engineering at the Massachusetts Institute of Technology (MIT), where he holds joint faculty appointments in the Departments of Materials Science and Engineering, Mechanical Engineering, and Biological Engineering. He is also an affiliated faculty member in the Harvard-MIT Division of Health Sciences and Technology, and the Tan Chin Tuan Centennial Professor (Overseas) at the National University of Singapore. He received a Bachelor of Technology degree in first class with distinction from Indian Institute of Technology in Madras, a Master of Science degree from Iowa State University and a Doctor of Science degree from MIT. He holds an honorary doctorate degree in engineering from Sweden’s Royal Institute of Technology. He was the Head of the Department of Materials Science and Engineering at MIT from 2000 to 2006. Prior to joining MIT, he was on the faculty of the Division of Engineering at Brown University from 1983 to 1993. Professor Suresh’s research has focused on the mechanical properties of materials from the atomistic to macroscopic length scales, with the particular aim of establishing connections among the material nano/microstructure, mechanical properties at multiple length scales, and function. His work has pioneered a wide variety of innovative experimental, analytical and computational methods to study different classes of materials in both bulk and thin-film form. His recent work has provided unique new perspectives on how the mechanics of biological cells and molecules in concert with chemical and biological factors influence, and are influenced by, a variety of human diseases. Professor Suresh has been elected to the United States National Academy of Engineering, American Academy of Arts and Sciences, Indian National Academy of Engineering, Indian Academy of Sciences, Academy of Sciences of the Developing World (Trieste, Italy), Royal Spanish Academy of Sciences, and the German Academy of Sciences Leopoldina.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The first Acta Materialia Gold Medal Lecture was presented by Professor Subra Suresh at the 2006 Fall Meeting of the Materials Research Society in Boston, MA, USA, on November 27, 2006.
CC531 rat colon carcinoma cells and HT-29 human colon carcinoma cells with comparable tumor characteristics are found to exhibit similar cytoadherence and invasive behavior in liver microcirculation [134]. It is, however, possible that human and rat adhesion molecules are not 100% compatible.
Cells exposed to stresses usually exhibit morphological changes such as geometrical contraction, flowering, etc. Such changes are often referred to as cell blebbing.
Mutations that cause EGFR overexpression (so-called upregulation) have been linked to different types of cancer, including lung cancer and glioblastoma multiforme. The identification of EGFR as an oncogene has spurred the development of anticancer drugs that target EGFR; such therapeutics include gefitinib (originally coded 2D1839) and erlotinib for lung cancer, cetuximab for colon cancer and trastuzumab for breast cancer. These drugs use monoclonal antibodies against EGFR or involve protein kinase inhibitors.
The protein Rho belongs to the Ras superfamily of G-proteins, which relay intracellular signals and regulate cell processes. Rho also controls actin’s association with cell membrane. Many human cancers can be triggered by oncogenic mutations in Ras genes.
References
- 1.Fung YC. Biomechanics: mechanical properties of living tissues. New York: Springer-Verlag; 1993. [Google Scholar]
- 2.Fung YC. Biomechanics: motion, flow, stress, and growth. New York: Springer-Verlag; 1990. [Google Scholar]
- 3.Fung YC. Biomechanics: circulation. 2nd ed. New York: Springer-Verlag; 1997. [Google Scholar]
- 4.Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol. 1989;67:2408–2419. doi: 10.1152/jappl.1989.67.6.2408. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey JD. Cardiovascular solid mechanics: cells, tissues, organs. New York: Springer-Verlag; 2002. [Google Scholar]
- 6.Martin RB, Burr D, Sharkey N. Skeletal tissue mechanics. New York: Springer-Verlag; 1998. [Google Scholar]
- 7.Bray D. Cell movements: from molecules to motility. 2nd ed. New York: Garland Publishing; 2000. [Google Scholar]
- 8.Evans EA. New membrane concept applied to analysis of fluid shear-deformed and micropipet-deformed red blood cells. Biophys J. 1973;13:941–954. doi: 10.1016/S0006-3495(73)86036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans EA, Skalak R. Mechanics and thermal dynamics of biomembranes. Boca Raton, FL: CRC Press; 1980. [Google Scholar]
- 10.Chien S, Sung KLP, Skalak R, Usami S. Theoretical and experimental studies on viscoelastic properties of erythrocyte membrane. Biophys J. 1978;24:463–487. doi: 10.1016/S0006-3495(78)85395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boal D. Mechanics of the cell. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 12.Weiss L, Schmid-Scho¨nbein G. Biomechanical interactions of cancer cells with the microvasculature during metastasis. Cell Biophysics. 1989;14:187–215. doi: 10.1007/BF02797133. [DOI] [PubMed] [Google Scholar]
- 13.Bao G, Suresh S. Cell and molecular mechanics of biological materials. Nature Mater. 2003;2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- 14.Dao M, Lim CT, Suresh S. Mechanics of the human red blood cell deformed by optical tweezers. J Mech Phys Solids. 2003;51:2259–2280. [Google Scholar]
- 15.Mills JP, Qie L, Dao M, Lim CT, Suresh S. Nonlinear elastic and viscoelastic deformation of the red blood cell induced by optical tweezers. Mech Chem Biosyst. 2004;1:169–180. [PubMed] [Google Scholar]
- 16.Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater. 2005;4:557–561. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- 17.Cokelet GR, Meiselman HJ. Rheological comparison of hemoglobin solutions and erythrocyte suspensions. Science. 1968;162:275–277. doi: 10.1126/science.162.3850.275. [DOI] [PubMed] [Google Scholar]
- 18.Engelhardt H, Sackmann E. On the measurement of shear elastic moduli and viscosities of erythrocyte plasma membranes by transient deformation in high frequency electric fields. Biophys J. 1988;54:495–508. doi: 10.1016/S0006-3495(88)82982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dao M, Li J, Suresh S. Molecularly based analysis of deformation of spectrin network and human erythrocyte. Mater Sci Eng C. 2006;26:1232–1244. [Google Scholar]
- 20.Discher DE, Boal DH, Boey SK. Simulations of the erythrocyte cytoskeleton at large deformation. II. Micropipette aspiration. Biophys J. 1998;75:1584–1597. doi: 10.1016/S0006-3495(98)74076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Dao M, Lim CT, Suresh S. Spectrin-level modeling of the cytoskeleton and optical tweezers stretching of the erythrocyte. Biophys J. 2005;88:3707–3719. doi: 10.1529/biophysj.104.047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gittes F, Schnurr B, Olmsted PD, MacKintosh FC, Schmidt CF. Microscopic viscoelasticity: shear moduli of soft materials determined from thermal fluctuations. Phys Rev Lett. 1997;79:3286–3289. [Google Scholar]
- 23.Gov N. Membrane undulations driven by force fluctuations of active proteins. Phys Rev Lett. 2004;93:268104–268108. doi: 10.1103/PhysRevLett.93.268104. [DOI] [PubMed] [Google Scholar]
- 24.Gov N, Zilman AG, Safran S. Cytoskeleton confinement and tension of red blood cell membranes. Phys Rev Lett. 2003;90:228101–228109. doi: 10.1103/PhysRevLett.90.228101. [DOI] [PubMed] [Google Scholar]
- 25.Gov NS, Safran SA. Red blood cell membrane fluctuations and shape controlled by ATP-induced cytoskeletal defects. Biophys J. 2005;88:1859–1874. doi: 10.1529/biophysj.104.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Lykotrafitis G, Dao M, Suresh S. Cytoskeletal dynamics of human erythrocyte. Proc Natl Acad Sci. 2007;104:4937–4942. doi: 10.1073/pnas.0700257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldmann WH, Tempel M, Sprenger I, Isenberg G, Ezzell RM. Viscoelasticity of actin-gelsolin networks in the presence of filamin. Eur J Biochem. 1997;246:373–379. doi: 10.1111/j.1432-1033.1997.00373.x. [DOI] [PubMed] [Google Scholar]
- 28.Desprat N, Richert A, Simeon J, Asnacios A. Creep function of a single living cell. Biophys J. 2005;88:2224–2233. doi: 10.1529/biophysj.104.050278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drochon A. Rheology of dilute suspensions of red blood cells: experimental and theoretical approaches. Eur Phys J – Appl Phys. 2003;22:155–162. [Google Scholar]
- 30.Evans EA, Hochmuth RM. Membrane viscoelasticity. Biophys J. 1976;16:1–11. doi: 10.1016/S0006-3495(76)85658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans EA, Hochmuth RM. Membrane viscoplastic flow. Biophys J. 1976;16:13–26. doi: 10.1016/S0006-3495(76)85659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochmuth RM, Worthy PR, Evans EA. Red-cell extensional recovery and the determination membrane viscosity. Biophys J. 1986;26:101–114. doi: 10.1016/S0006-3495(79)85238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hochmuth RM, Waugh RE. Erythrocyte membrane elasticity and viscosity. Annu Rev Physiol. 1987;49:209–219. doi: 10.1146/annurev.ph.49.030187.001233. [DOI] [PubMed] [Google Scholar]
- 34.Puig-de-Morales-Marinkovic M, Turner KT, Butler JP, Fredberg JJ, Suresh S. Viscoelasticity of the human red blood cell. Am J Physiol: Cell Phys. 2007 doi: 10.1152/ajpcell.00562.2006. in press. [DOI] [PubMed] [Google Scholar]
- 35.Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell matrix interactions in articular cartilage. J Biomech. 2000;33:1663–1673. [PubMed] [Google Scholar]
- 36.Mofrad MRK, Kamm RD. editorsCytoskeletal mechanics: models and measurements. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 37.Gibson LC, Ashby MF. Cellular solids: structure and properties. 2nd ed. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 38.Ingber DE. Cellular tensegrity and mechanochemical transduction. Ann Rev Phys. 1997;19:329–339. [Google Scholar]
- 39.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Ann Rev Phys. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 40.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 41.Johnson KL. Contact mechanics. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- 42.Radmacher M, Fritz M, Kacher CM, Cleveland JP, Hansma PK. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys J. 1996;70:556–567. doi: 10.1016/S0006-3495(96)79602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson KL, Kendall K, Roberts AD. Surface energy and the contact of elastic solids. Proc Roy Soc London. 1971;A324:301–313. [Google Scholar]
- 44.Chu Y-S, Dufour S, Thiery JP, Perez E, Pincet F. Johnson–Kendall–Roberts theory applied to living cells. Phys Rev Lett. 2005;94:028102. doi: 10.1103/PhysRevLett.94.028102. [DOI] [PubMed] [Google Scholar]
- 45.Moy VT, Jiao Y, Hillmann T, Lehmann H, Sano T. Adhesion energy of receptor-mediated interaction measured by elastic deformation. Biophys J. 1999;76:1632–1638. doi: 10.1016/S0006-3495(99)77322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arzt E, Gorb S, Spolenak R. From micro to nano contacts in biological attachment devices. Proc Natl Acad Sci. 2003;100:10603–10606. doi: 10.1073/pnas.1534701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hessler JA, et al. Atomic force microscopy study of early morphological changes during apoptosis. Langmuir. 2005;21:9280–9286. doi: 10.1021/la051837g. [DOI] [PubMed] [Google Scholar]
- 48.Van Vliet KJ, Bao G, Suresh S. The biomechanics toolbox: experimental approaches to living cells and biomolecules. Acta Mater. 2003;51:5881–5905. [Google Scholar]
- 49.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 50.Haupt BJ, Pelling AE, Horton MA. Integrated confocal and scanning probe microscopy for biomedical research. Sci World J. 2006;6:1609–1618. doi: 10.1100/tsw.2006.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tai K, Dao M, Suresh S, Palazoglu A, Ortiz C. Nanoscale heterogeneity promotes energy dissipation in bone. Nat Mater. 2007 doi: 10.1038/nmat1911. doi:10.1038/nmat1911. Available online 21 May, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Bustamante C, Marko JF, Siggia ED, Smith S. Entropic elasticity of lambda-phage DNA. Science. 1994;265:1599–1600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]
- 53.Marko JF, Siggia ED. Stretching DNA. Macromolecules. 1995;28:8759–8770. [Google Scholar]
- 54.Bustamante C, Bryant Z, Smith SB. Ten years of tension: single-molecule DNA mechanics. Nature. 2003;421:423–426. doi: 10.1038/nature01405. [DOI] [PubMed] [Google Scholar]
- 55.Howard J. Mechanics of motor proteins and the cytoskeleton. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 56.Rief M, Gautel M, Oesterhelt F, Fernandez JM, Gaub H. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 1997;276:1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- 57.Kellermayer MSZ, Smith SB, Granzier HL, Bustamante C. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276:1112–1116. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- 58.Tskhovrebova L, Trinnick J, Sleep JA, Simmons RM. Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature. 1997;387:308–312. doi: 10.1038/387308a0. [DOI] [PubMed] [Google Scholar]
- 59.Oberhauser AF, Marszalek PE, Erickson HP, Fernandez JM. The molecular elasticity of the extracellular matrix protein tenascin. Nature. 1998;393:181–185. doi: 10.1038/30270. [DOI] [PubMed] [Google Scholar]
- 60.Vogel V, Thomas WE, Craig DW, Krammer A, Baneyx G. Structural insights into the mechanical regulation of molecular recognition sites. Trends Biotechnol. 2001;19:416–423. doi: 10.1016/S0167-7799(01)01737-1. [DOI] [PubMed] [Google Scholar]
- 61.Bao G. Mechanics of biomolecules. J Mech Phys Solids. 2002;50:2237–2274. [Google Scholar]
- 62.Humphrey W, Dalke A, Schulten K. VMD - visual molecular dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 63.Li J. Atom eye - an efficient atomistic configuration viewer. Mol Simul Mater Sci Eng. 2003;11:173–177. [Google Scholar]
- 64.Goetz R, Gompper G, Lipowsky R. Mobility and elasticity of self-assembled membranes. Phys Rev Lett. 1999;82:221–224. [Google Scholar]
- 65.Lim HWG, Wortis M, Mukhopadhyay R. Stomatocyte, discocyte, echinocyte sequence of human red blood cell: evidence for the bilayer-couple hypothesis from membrane mechanics. Proc Natl Acad Sci USA. 2002;99:16766–16769. doi: 10.1073/pnas.202617299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartl DL, Jones EW. Genetics: analysis of genes and genomes. 5th ed. Boston, MA: Jones & Bartlet Publishers; 2005. [Google Scholar]
- 67.Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood. 2002;99:1060–1063. doi: 10.1182/blood.v99.3.1060. [DOI] [PubMed] [Google Scholar]
- 68.Mills JP, et al. Effect of plasmodial RESA protein on deformability of human red blood cells harboring Plasmodium falciparum. Proc Natl Acad Sci USA. 2007;104:9213–9217. doi: 10.1073/pnas.0703433104. doi:10.1073/pnas.0703433104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu C, Bao G, Wang N. Cell mechanics: mechanical response, cell adhesion and molecular deformation. Ann Rev Biomed Eng. 2000;2:189–226. doi: 10.1146/annurev.bioeng.2.1.189. [DOI] [PubMed] [Google Scholar]
- 70.Alberts B, et al. Molecular biology of the cell. 4th ed. New York: Garland; 2002. [Google Scholar]
- 71.Suresh S, et al. Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater. 2005;1:15–30. doi: 10.1016/j.actbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Shelby JP, White J, Ganesan K, Rathod PK, Chiu DT. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci. 2003;100:14618–14622. doi: 10.1073/pnas.2433968100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suresh S. Mechanical response of human red blood cells in health and disease: some structure-property-function relationships. J Mater Res. 2006;21:1871–1878. [Google Scholar]
- 74.The World Health Organization (WHO) website: ( http://www.who.int/cancer/en/) and The National Institutes of Health website: http://cancercontrolplanet.cancer.gov/atlas/timeall.jsp?c=ACC&o=f&wm=true&wf=true&bm=true&bf=true&fc=timeall&chart=timeall&ac=1&ss=US.
- 75.Service RF. Nanotechnology takes aim at cancer. Science. 2005;310:1132–1134. doi: 10.1126/science.310.5751.1132. [DOI] [PubMed] [Google Scholar]
- 76.Jamal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics. CA: Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 77.Weinberg RA. The biology of cancer. New York, NY: Garland Science; 2006. [Google Scholar]
- 78.Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976;260:170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- 79.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function and disease. Ann Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 80.Ingber DE. Mechanical signalling and cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 81.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 82.Cooke BM, Mohandas N, Coppell RL. The malaria-infected red blood cell: structural and functional changes. Adv Parasitol. 2001;50:1–86. doi: 10.1016/S0065-308X(01)50029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamm RD, Mofrad MRK. Cytoskeletal mechanics: models and measurements. Cambridge: Cambridge University Press; 2006. Introduction, with biological basis for cell mechanics; pp. 1–17. [Google Scholar]
- 84.Yasuda R, et al. Direct measurement of the torsional rigidity of single actin filaments. J Mol Biol. 1996;263:227–236. doi: 10.1006/jmbi.1996.0571. [DOI] [PubMed] [Google Scholar]
- 85.Tsuda Y, et al. Torsional rigidity of single actin filaments and actin-actin bond breaking force under torsion measured directly by in vitro micromanipulation. Proc Natl Acad Sci USA. 1996;93:12937–12942. doi: 10.1073/pnas.93.23.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 87.Gardel M, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;28:1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 88.Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Janmey PA, Hvidt S, Lamb J, Stossel TP. Resemblance of actin-binding protein/actin gels to covalently crosslinked networks. Nature. 1990;345:89–92. doi: 10.1038/345089a0. [DOI] [PubMed] [Google Scholar]
- 90.Nelson DL, Cox MM. Principles of biochemistry. 4th ed. New York: W.H. Freeman & Co; 2005. [Google Scholar]
- 91.Elson EL. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu Rev Biophys Chem. 1988;17:397–430. doi: 10.1146/annurev.bb.17.060188.002145. [DOI] [PubMed] [Google Scholar]
- 92.Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization and extracellular matrix mechanics. Biophys J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hendrix MJC, Seftor EA, Chu Y-W, Trevor KT, Seftor REB. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metast Rev. 1996;15:507–525. doi: 10.1007/BF00054016. [DOI] [PubMed] [Google Scholar]
- 94.Chu Y-W, Runyan RB, Oshima RG, Hendrix MJC. Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc Natl Acad Sci USA. 1993;90:4261–4265. doi: 10.1073/pnas.90.9.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang N, Stamenovic D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol. 2000;279:C188–C194. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- 96.Chu YW, Seftor EA, Romer LH, Hendrix MJC. Experimental coexpression of vimentin and keratin intermediate filaments in human melanoma cells augments motility. Am J Pathol. 1996;148:63–69. [PMC free article] [PubMed] [Google Scholar]
- 97.Bausch AR, Kroy K. A bottom-up approach to cell mechanics. Nature Phys. 2006;2:231–238. [Google Scholar]
- 98.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu Z, Cork LC, Griffin JW, Cleveland DW. Increased expression of neurofilament subunit NF-L produces morphological alterations. J Cell Sci. 1993;153:65–69. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]
- 100.Trask DK, Band V, Zajchowski DA, Yaswen P, Suh T, Sager R. Keratins as markers that distinguish normal and tumor-derived mammary epithelial cells. Proc Natl Acad Sci USA. 1990;87:2319–2323. doi: 10.1073/pnas.87.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grzanka A, Grzanka D, Orlikowska M. Cytoskeletal organization during process of apoptosis induced by cytostatic drugs in K-562 and HL-60 leukemia cell lines. Biochem Pharmacol. 2003;66:1611–1617. doi: 10.1016/s0006-2952(03)00532-x. [DOI] [PubMed] [Google Scholar]
- 102.Manelli-Oliveira R, Machado-Santelli GM. Cytoskeletal and nuclear alterations in human lung tumor cells: a confocal microscopy study. Histochem Cell Biol. 2001;115:403–411. doi: 10.1007/s004180100262. [DOI] [PubMed] [Google Scholar]
- 103.Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, et al. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol. 2006;173:733–741. doi: 10.1083/jcb.200601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Janmey P, Schmidt C. Experimental measurements of intracellular mechanics. In: Mofrad MRK, Kamm RD, editors. Cytoskeletal mechanics: models and measurements. Cambridge: Cambridge University Press; 2006. pp. 18–49. [Google Scholar]
- 105.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, et al. Time scale and other invariants of integrative mechanical behavior in living cells. Phys Rev E: Stat Nonlin Soft Matter Phys. 2003;68:041914. doi: 10.1103/PhysRevE.68.041914. [DOI] [PubMed] [Google Scholar]
- 106.Fabry B, Maksym GN, Shore SA, Moore PE, Panettieri RA, Jr, Butler JP, et al. Selected contribution: time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J Appl Physiol. 2001;91:986–994. doi: 10.1152/jappl.2001.91.2.986. [DOI] [PubMed] [Google Scholar]
- 107.Sokolov I. Atomic force microscopy in cancer cell research. [Chapter 17] In: Nalwa HS, Webster T, editors. Cancer Nanotechnology. 2007. in press. [Google Scholar]
- 108.Binning G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56:930–934. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 109.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–1583. [Google Scholar]
- 110.Svoboda K, Block SM. Biological applications of optical forces. Annu Rev Biophys Biomol Struct. 1994;23:247–285. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- 111.He´non S, Lenormand G, Richert A, Gallet F. A new determination of the shear modulus of the human erythrocyte membrane using optical tweezers. Biophys J. 1999;76:1145–1151. doi: 10.1016/S0006-3495(99)77279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sleep J, Wilson D, Simmons R, Gratzer W. Elasticity of red cell membrane and its relation to hemolytic disorders: an optical tweezers study. Biophys J. 1999;77:3085–3095. doi: 10.1016/S0006-3495(99)77139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thoumine O, Ott A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J Cell Sci. 1997;110:2109–2116. doi: 10.1242/jcs.110.17.2109. [DOI] [PubMed] [Google Scholar]
- 114.Thoumine O, Ott A, Cardoso O, Meister JJ. Microplates: a new tool for manipulation and mechanical perturbation of individual cells. J Biochem Biophys Methods. 1999;39:47–62. doi: 10.1016/s0165-022x(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 115.Beil M, et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nature Cell Biol. 2003;5:803–811. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]
- 116.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipette aspiration. Biophys J. 1989;56:151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hochmuth R. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 119.Usami S, Chen HH, Zhao Y, Chien S, Skalak R. Design and construction of a linear shear stress flow chamber. Ann Biomed Eng. 1993;21:77–83. doi: 10.1007/BF02368167. [DOI] [PubMed] [Google Scholar]
- 120.Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma. 1995;12:325–339. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- 121.Wang JH, Goldschmidt-Clermont P, Yin FC. Contractility affects stress fiber remodeling and reorientation of endothelial cells subjected to cyclic mechanical stretching. Ann Biomed Eng. 2001;28:1165–1171. doi: 10.1114/1.1317528. [DOI] [PubMed] [Google Scholar]
- 122.Erkell LJ, Ryd W, Hagmar B. Comments on the filter test for tumor cell deformability. Invasion Metast. 1982;2:260–267. [Google Scholar]
- 123.Quake SR, Scherer A. From micro- to nanofabrication with soft materials. Science. 2000;290:1536–1540. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- 124.Kenis PJA, Stroock AD. Materials for micro- and nanofluidics. Mater Res Soc Bull. 2006;31:87–90. [Google Scholar]
- 125.Manalis S, Suresh S, Berg T, Babcock K. Method and apparatus for high throughput diagnosis of diseased cells with microchannel devices. US Provisional Patent Application. 2007 [Google Scholar]
- 126.Lekka M, Laidler P, Gil D, Lekki J, Stachura Z, Hrynkiewicz AZ. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur Biophys J. 1999;28:312–316. doi: 10.1007/s002490050213. [DOI] [PubMed] [Google Scholar]
- 127.Lekka M, Lekki J, Marszalek M, Golonka P, Stachura P, Cleff B, et al. Local elastic properties of cells studied by SFM. Appl Surf Sci. 1999;141:345–350. [Google Scholar]
- 128.Zhang G, Long M, Wu Z-Z, Yu W-Q. Mechanical properties of hepatocellular carcinoma cells. World J Gastroenterol. 2002;8:243–246. doi: 10.3748/wjg.v8.i2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guck J, et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88:3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen, et al. Influence of expressed TRAIL on biophysical properties of human leukemic cell line JURKAT. Cell Res. 2004;14:161–168. doi: 10.1038/sj.cr.7290216. [DOI] [PubMed] [Google Scholar]
- 131.Hendrix MJC, Seftor EA, Seftor REB, Trevor KT. Experimental coexpression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am J Pathol. 1997;150:483–495. [PMC free article] [PubMed] [Google Scholar]
- 132.Rosenbluth MJ, Lam WA, Fletcher DA. Force microscopy of nonadherent cells: a comparison of leukemia cell deformability. Biophys J. 2006;90:2994–3003. doi: 10.1529/biophysj.105.067496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lam WA, Rosenbluth MJ, Fletcher DA. Chemotherapy exposure increases leukemia cell stiffness. Blood. 2007;109:3503–3508. doi: 10.1182/blood-2006-08-043570. doi:10.1182/blood-2006-08-043570, @ the American Society of Hematology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Korb T, Schluter K, Enns A, Spiegel H-U, Seninger N, Nicolson GL, et al. Integrity of actin stress fibers and microtubules influences metastatic tumor cell adhesion. Exp Cell Res. 2004;299:236–247. doi: 10.1016/j.yexcr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 135.Sato H, Suzuki M. Deformability and viability of tumor cells by transcapillary passage, with reference to organ affinity of metastasis in cancer. In: Weiss L, editor. Fundamental aspects of metastasis. Amsterdam: North-Holland Publishing Company; 1976. pp. 311–317. [Google Scholar]
- 136.Ward KA, Li W-I, Zimmer S, Davis T. Viscoelastic properties of transformed cells: role of tumor cell progression and metastasis formation. Biorheology. 1991;28:301–313. doi: 10.3233/bir-1991-283-419. [DOI] [PubMed] [Google Scholar]
- 137.Igawa S, et al. Nitric oxide generated by iNOS reduces deformability of Lewis lung carcinoma cells. Cancer Sci. 2004;95:342–347. doi: 10.1111/j.1349-7006.2004.tb03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ochalek T, Nordt FJ, Tullberg K, Burger MM. Correlation between cell deformability and metastatic potential in B16-F1 melanoma cell variants. Cancer Res. 1988;48:5124–5128. [PubMed] [Google Scholar]
- 139.Yao W, Gu L, Sun D, Ka W, Wen Z, Chien S. Wild type p53 gene causes reorganization of cytoskeleton and therefore the impaired deformability and difficult migration of murine erythroleukemia cells. Cell Motil Cytoskeleton. 2003;56:1–12. doi: 10.1002/cm.10129. [DOI] [PubMed] [Google Scholar]
- 140.Park S, Koch D, Cardenas R, Kas J, Shih CK. Cell motility and local viscoelasticity of fibroblast. Biophys J. 2005;89:4330–4342. doi: 10.1529/biophysj.104.053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang H-B, Dembo M, Wang Y-L. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 142.Paszek MJ, Zahir N, Johnson KR, Gefen A, Reinhart-King CA, Boettiger SS, et al. Tensional phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 143.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 144.Katsantonis J, Tosca A, Koukouritaki SB, Theodoropoulos PA, Gravanis A, Stournaras C. Differences in the G/total actin ratio and microfilament stability between normal and malignant human keratinocytes. Cell Biochem Funct. 1994;12:267–274. doi: 10.1002/cbf.290120407. [DOI] [PubMed] [Google Scholar]
- 145.Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers R, et al. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;235:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- 146.Maugis D. Adhesion of spheres: the JKR-DMT transition using a Dugdale model. J Colloid Interface Sci. 1992;150:243–269. [Google Scholar]
- 147.Ischraelishvili JN. Intermolecular and surface forces. 2nd ed. Oxford: Elsevier Science; 1992. [Google Scholar]
- 148.Johnson KL, Kendall K, Roberts AD. Surface energy and the contact of elastic solids. Proc R Soc Lond Ser A. 1971;324:301–313. [Google Scholar]
- 149.Evans E, Needham Attraction between lipid bilayer membranes in concentrated solutions of nonadsorbing polymers: comparison of mean field theory with measurement of adhesion energy D. Macromolecules. 1988;21:1822–1831. [Google Scholar]
- 150.de Gennes PG. Scaling concept in polymer physics. 2nd ed. Ithaca, NY: Cornell University Press; 1985. [Google Scholar]
- 151.Brochard-Wyart F, de Gennes PG. Unbinding of adhesive vesicles. CR Phys. 2003;4:281–287. [Google Scholar]
- 152.Ingber DE, Madri JA, Jamieson JD. Role of basal lamina in the neoplastic disorganization of tissue architecture. Proc Natl Acad Sci USA. 1981;78:3901–3905. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bischoff F, Bryson G. Malignant fibrous histiocytomas induced in rats by polymers. Prog Exp Tumor Res. 1964;14:85–133. doi: 10.1159/000385997. [DOI] [PubMed] [Google Scholar]
- 154.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;27:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 155.Wittelsberger SC, Kleene K, Penman S. Progressive loss of shape-responsive metabolic controls in cells with increasingly transformed phenotype. Cell. 1981;24:859–866. doi: 10.1016/0092-8674(81)90111-2. [DOI] [PubMed] [Google Scholar]
- 156.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 158.Holmes FA, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst. 1991;83:1797–1805. doi: 10.1093/jnci/83.24.1797-a. [DOI] [PubMed] [Google Scholar]
- 159.Kuffel MJ, Reid JM, Ames MM. Anthracyclines and their C-13 alcohol metabolites: growth inhibition and DNA damage following incubation with human tumor cells in culture. Cancer Chemother Pharmacol. 1992;30:51–57. doi: 10.1007/BF00686485. [DOI] [PubMed] [Google Scholar]
- 160.Reed E, Kohn EC, Sarosy G, Dabholkar M, Daivs P, Jacob J, et al. Paclitaxel, cisplatin and cyclophosphamide in human ovarian cancer: molecular rationale and early clinical results. Semin Oncol. 1995;22:90–96. [PubMed] [Google Scholar]
- 161.Rowinsky EK, Burke PJ, Karp JE, Tucker RW, Ettinger DS, Donehower RC. Phase I and pharmacodynamic study of taxol in refractory acute leukemias. Cancer Res. 1989;49:4640–4647. [PubMed] [Google Scholar]
- 162.Wiernik PH, Schwartz EL, Einzig A, Strauman JJ, Lipton RB, Dutcher JP. Phase I trial of taxol given as a 24-hour infusion every 21 days: responses observed in metastatic melanoma. J Clin Oncol. 1987;5:1232–1239. doi: 10.1200/JCO.1987.5.8.1232. [DOI] [PubMed] [Google Scholar]
- 163.Fritzer M, Barabas K, Szuts V, Berczi A, Szekeres T, Faulk WP, et al. Cytotoxicity of a transferrin-adriamycin conjugate to anthracycline-resistant cells. Int J Cancer. 1992;52:619–623. doi: 10.1002/ijc.2910520421. [DOI] [PubMed] [Google Scholar]
- 164.Novotny JR, Muller-Beissenhirtz H, Herget-Rosenthal S, Kribben A, Duhrsen U. Grading of symptoms in hyperleukocytic leukaemia: a clinical model for the role of different blast types and promyel-ocytes in the development of leukostasis syndrome. Eur J Haematol. 2005;74:501–510. doi: 10.1111/j.1600-0609.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- 165.Levee MG, Dabrowska MI, Lelli JL, Jr, Hinshaw DB. Actin polymerization and depolymerization during apoptosis in HL-60 cells. Am J Physiol. 1996;271:C1981–C1992. doi: 10.1152/ajpcell.1996.271.6.C1981. [DOI] [PubMed] [Google Scholar]
- 166.Grzanka A. Actin distribution patterns in HL-60 leukemia cells treated with etoposide. Acta Histochem. 2001;103:453–464. doi: 10.1078/0065-1281-00612. [DOI] [PubMed] [Google Scholar]
- 167.Grzanka A, Grzanka D, Orlikowska M. Fluorescence and ultra-structural localization of actin distribution patterns in the nucleus of HL-60 and K-562 cell lines treated with cytostatic drugs. Oncol Rep. 2004;11:765–770. [PubMed] [Google Scholar]
- 168.Grzanka A, Grzanka D, Zuryn A, Grzanka AA, Safiejko-Mroczka B. Reorganization of actin in K-562 and HL-60 cells treated with taxol. Neoplasma. 2006;53:56–61. [PubMed] [Google Scholar]
- 169.Stucki A, Rivier AS, Gikic M, Monai N, Schapira M, Spertini O. Endothelial cell activation by myeloblasts: molecular mechanisms of leukostasis and leukemic cell dissemination. Blood. 2001;97:2121–21229. doi: 10.1182/blood.v97.7.2121. [DOI] [PubMed] [Google Scholar]