Introduction
Cytomegalovirus (CMV) is a beta herpes virus which infects 60–100% of people (1). After a self-limited flu-like illness during primary infection, the virus enters a state of latency characterized by presence of viral DNA without transcription of late genes or viral DNA replication, thus persisting for the life of the host. Latent infections are generally thought to be of minimal consequence in immunocompetent individuals, but in immunosuppressed transplant patients, reactivation from latency can cause significant morbidity and mortality (2).
Although the relationship between allotransplantation and CMV reactivation is well described, the mechanism triggering productive virus is less clear. This mechanism has been somewhat obfuscated by concurrent immunosuppression, which has been shown in and of itself capable of triggering CMV reactivation (reviewed in (3)). Allogeneic stimulation was first proposed to cause CMV reactivation by Lang in 1972, either by transfusion or transplantation (4), and recent work suggests that allogeneic stimulation may indeed play an important role in reactivation after transplantation. Several investigators have shown that in-vitro allogeneic stimulation of latently infected cells can cause reactivation (5–7). Recent in-vivo work by others has lent further support to this hypothesis, but both studies have shortcomings (8, 9). One demonstrated early signs of viral reactivation after transplantation of latently infected allogeneic kidneys into mice inherently resistant to MCMV (8), while the other demonstrated reactivation using adoptively transferred latently infected donor allogeneic splenocytes into immunosuppressed recipients (9). To date, in-vivo testing of allogeneic stimulation alone as a trigger of CMV reactivation in a latently infected allograft recipient remains notably absent from the literature.
Testing the role of allogeneic stimulation in CMV reactivation is not suited to study in humans because of their inherent requirement of immunosuppression after transplantation. Therefore to test our hypothesis, we combined well described models of latent murine CMV (MCMV) infection and MHC-mismatched murine skin grafting without immunosuppression. Our chosen model of MCMV utilizes a susceptible mouse (BALB/c, H2d), and has many similarities to human CMV, developing latency after primary infection that can be reactivated by a variety of triggers (reviewed in (3)). Skin grafting with C57/BL6 (H2b) donors provides a harsh antigenic challenge that leads to vigorous rejection. Thus by combining these models, we sought to determine if allogeneic stimulation alone is sufficient to trigger reactivation of MCMV in latently infected recipients without the confounding effects of immunosuppression.
Methods
Mice
C57Bl/6 (H-2b) and BALB/c (H-2d) mice were obtained from Jackson Labs (Bar Harbor, MN). All mice were housed and treated in accordance with Animal Care Guidelines established by the National Institute of Health and The Ohio State University.
Virus infection
BALB/c mice received 1 × 105 pfu of Smith strain MCMV (ATCC) by intraperitoneal injection and allowed to become latent (>16 weeks) as previously published (10). To confirm successful MCMV infection, mice were tested for CMV reactive antibody titers prior to skin grafting (11), and their salivary glands were tested at experiments end for MCMV DNA as previously described using PCR (12). Mice that did not develop normal antibody responses to their initial infection were not utilized.
To detect viral reactivation, we evaluated recipient salivary glands using the most sensitive assay of infectivity in tissue (focused expansion assay or FEA) as previously described by Reddehase et al (13). Briefly, this technique identifies MCMV RNA from cell culture lysates by nested RT-PCR after viral inoculation. MCMV mRNA detection was performed as previously described using TRIzol Reagent (GIBCO BRL, Carlsbad CA) for mRNA isolation and 3U DNase I Amplification Grade (Invitrogen, Carlsbad, CA) for purification (12). Reverse transcription (RT) reactions were performed after DNase I treatment (GIBCO BRL) using 3U Super-transcriptase (GIBCO BRL). To control for DNA contamination, every sample had concomitant parallel experiments with no RT reaction. If the first reaction yielded no visible product, a second (nested) PCR was performed using 1μl of this first PCR reaction product. Primers for MCMV glycoprotein B (GB) and β-actin were as previously described (12).
Skin Grafting
Following confirmation of salivary gland latency in a cohort by focused expansion assay mice underwent skin grafting. Skin allografts were performed using abdominal skin from donor mice (C57/BL6). Square full-thickness grafts (approximately 8×10 mm) were placed on graft beds prepared on latently infected BALB/c recipient flanks. Grafts were covered with protective bandages for 7 days. Isografts were performed identically, using BALB/c mice for donor skin. Allograft recipients receiving cortisol and cortisol-only controls received i.p. cortisone acetate (Sigma) injections (100mg/kg) every other day for three weeks after grafting. Rejection was considered to occur when grafts exhibited dark discoloration, scabbing and necrotic degeneration.
Results
Allogeneic skin transplant/rejection causes MCMV reactivation
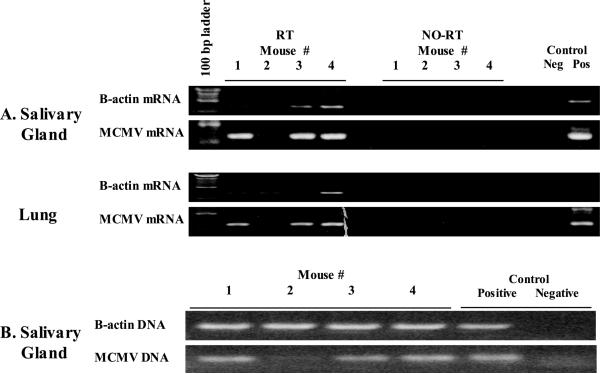
To determine if allogeneic skin grafts can trigger reactivation of latent CMV, skin from fully MHC mismatched mice (H2b) was grafted onto latently infected H2d mice. Viral latency was confirmed in a cohort of mice by presence of MCMV DNA in mouse salivary glands and absence of MCMV mRNA in salivary glands (not shown). As a pilot study, n=4 of this latently infected cohort of BALB/c mice received allogeneic skin grafts from MCMV naïve C57/BL6 donors. Seven to 12 days after grafting, all allogeneic skin grafts were rejected, evidenced by visible necrosis and sloughing of the skin (not shown). Our previous work has shown that reactivation occurs 14–21 days after stimulation (10), so we evaluated skin recipients 21 days after grafting for viral reactivation. Salivary glands, lungs, and blood were examined by RT-PCR for MCMV mRNA transcripts. Allogeneic skin graft recipients (3/4) showed transcriptional reactivation in both salivary glands and lungs (Figure 1A). There was no concomitant MCMV RNA detected in peripheral blood (not shown). These mice were tested for MCMV DNA, and mouse #2 showed very low levels of DNA, suggesting a problem with the initial infection (Figure 1B). This mouse was subsequently confirmed to have nominal MCMV reactive antibody (data not shown), and thus we felt it had a poor initial infection. Because of this we subsequently tested all of the remaining cohort of mice for MCMV reactive antibody, and all other poor responders were identified and excluded from skin grafting (n=1, data not shown).
Figure 1. Allograft transplantation activates viral transcription.
Salivary gland and lung tissue lysates were evaluated by RT-PCR for viral transcriptional reactivation after allogeneic (H2b) skin grafting onto H2d mice latently infected with murine cytomegalovirus (MCMV) 21 days after transplantation. Both salivary glands and lungs showed transcriptional reactivation of late gene MCMV glycoprotein B (GB) in 3/4 mice. B. PCR analysis for MCMV DNA shows that the mouse “negative” for reactivation (lane 2) did not have detectable MCMV DNA, and subsequent antibody analysis showed that same mouse to have MCMV reactive antibody comparable to naïve mice (data not shown). B-Actin rows confirm adequate recovery of RNA or DNA respectively, and no-RT lanes confirm that RNA lack DNA contamination. Pos and Neg controls are technique controls.
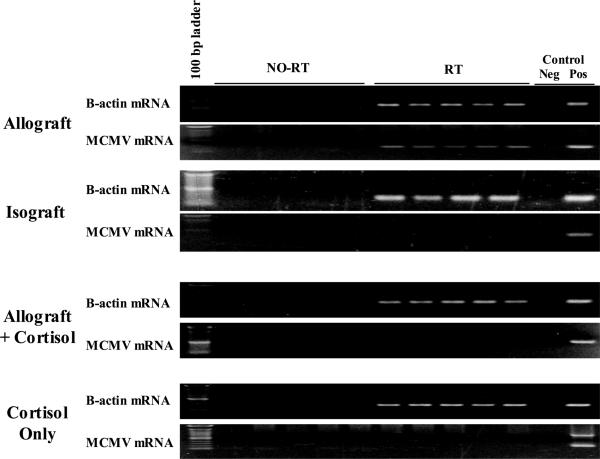
To confirm that reactivation could be triggered by allogeneic stimulation, larger cohorts (n=5) of latently infected BALB/c mice received either allogeneic H2b or syngeneic H2d skin grafts. All allografts were rejected, while 4/5 isografts were accepted and healed without complication. One isograft recipient developed a local infection 6 days after grafting, and because we have previously shown that bacterial infection can cause MCMV reactivation (10), this mouse was euthanized and excluded from analysis. Twenty-one days after grafting, recipient salivary glands were examined for evidence of viral reactivation. Allograft recipients showed transcriptional reactivation in the salivary glands (5/5), while salivary glands from syngeneic graft recipients showed no viral reactivation (0/4) (Figure 2). Thus we conclude that allogeneic skin graft rejection is capable of triggering viral reactivation in our model.
Figure 2. Influence of cortisol on allograft triggered viral transcription.
Mice latently infected with murine cytomegalovirus (MCMV) received allograft (n=10) or isograft (n=4) skin. One half of allograft recipients (n=5) received concomitant cortisol, and another cohort (n=5) received cortisol alone (no skin graft). Twenty one days after grafting, RT-PCR analyses of salivary gland lysates show all allograft recipients reactivated latent MCMV (5/5), and that cortisol treatment abrogates allograft induced reactivation (0/5). Isograft skin or cortisol alone were insufficient to trigger latent MCMV. All graft recipients had MCMV DNA in their tissues, and all tested positive for MCMV reactive antibodies (not shown), confirming adequate MCMV infection. B-Actin rows confirm successful RNA recovery, and no-RT lanes confirm that RNA lack DNA contamination. Pos and Neg controls are technique controls.
Immunosuppression with cortisol prevents allogeneic reactivation of MCMV
Deficiencies in immunity have been shown to be permissive to CMV reactivation, and we therefore hypothesized that immunosuppression induced by cortisol would lead to enhanced MCMV reactivation after allogeneic skin graft. Thus, latently infected BALB/c recipients (n=5 each) received allogeneic skin grafts or were non-grafted. Both cohorts subsequently received cortisol until tissue evaluation on day 21. As before, all skin grafts (5/5) were rejected within 10 days. Salivary glands from allograft + cortisol or cortisol only recipients showed no evidence of transcriptional reactivation by RT-PCR (Figure 2). Thus, administration of cortisol seems to impair MCMV reactivation in our model rather than being permissive.
Discussion
This study demonstrates that allogeneic stimulation can trigger transcriptional reactivation of MCMV in latently infected recipients. Although the association between CMV reactivation and transplantation are well described, previously it has been unclear whether allogeneic stimulation, concomitant immunosuppression, or the two combined contributes to reactivation. Use of a murine model allows transplantation without immunosuppression to distinguish the influence of allogeneic stimulation in reactivation of latent CMV. Interestingly, our data suggest not only that immunosuppression is not required for reactivation, but that some immunosuppressive agents might actually impair CMV reactivation.
Our results confirm previous work by several investigators suggesting that allogeneic stimulation can lead to reactivation of latent CMV in transplant recipients. In-vitro work by Olding et al first supported this hypothesis, showing that allogeneic stimulation of latently infected splenocytes could trigger MCMV reactivation (5). Later work by Soderberg-Naucler et al further supports this hypothesis, showing that allogeneic stimulation of MHC mismatched PBMCs causes HCMV reactivation (6), and more recent work by Guedes et al shows similar results using a porcine model (7). In-vivo work by Hummel et al using MCMV demonstrated that transplantation of latently infected renal allografts into naïve recipients causes intragraft activation of MCMV immediate early gene transcription (8). Finally, work by Gosselin et al shows that latently infected allogeneic splenocytes adoptively transferred to naïve recipients causes reactivation and transmission of virus, although the recipients were immunosuppressed (9). Results from the current study expand upon these findings, showing that fully MHC mismatched skin rejection can trigger transcriptional MCMV reactivation that is detectable distant from the transplant site, suggesting that viral reactivation occurs systemically in response to localized allo-stimulation.
The influence of cortisol in MCMV reactivation in our model was completely counterintuitive. We had originally included this treatment in an attempt to maximize reactivation in our model, and were surprised to find prevention of reactivation in cortisol treated mice. Recently, cortisol has been shown to inhibit cellular transcription activator NF-κB. NF-κB has been postulated to be important to CMV reactivation due to numerous NF-κB consensus sequences located in the MCMV major immediate-early promoter region. (14). Previous work by Hummel et al supports this mechanism, associating NF-κB activation with MCMV stimulation in allograft recipients (8). We therefore suspect that cortisol might block MCMV reactivation in our model by inhibiting NF-κB activation in allograft recipients. Inflammatory cytokines can trigger reactivation (12), so it is equally possible that cortisol blocks reactivation by tempering the inflammatory response to skin rejection. Whatever the mechanism, it is important to note that reactivation can be prevented independent of rejection.
Because human transplantation requires immunosuppression, our findings are presently somewhat more academic than clinically useful. Our data show that allograft rejection is capable of triggering reactivation, and that reactivation does not require concurrent immunosuppression. What is not directly addressed by the current study is the question of whether CMV reactivation contributes to rejection. In our model, allograft survival (~10–11 days, not shown) was not shorter than historical controls (15), but it is important to note that this model is biased toward rapid rejection, so CMV reactivation may have inadequate time to contribute to this process, particularly because reactivation appears to occur after the rejection episode. Interestingly, using a model of induced cardiac allograft tolerance (without ongoing immunosuppression), we have recently shown that cardiac allograft acceptance is disrupted when recipients are latently infected with CMV, and this disruption is associated with MCMV reactivation (16). Thus, when tolerance induction becomes clinical reality, reactivation of latent CMV may pose a significant barrier even if those tolerance induction strategies do not require transient immunosuppression.
Another unrelated but clinically relevant issue raised by this study is use of cadaveric skin grafts as temporary dressings in burn patients. In badly burned patients, there is often insufficient host skin to allow autografting to completely cover their wounds, and this has led many centers to utilize cadaveric allografts or xenografts to provide temporary burn wound coverage. Previous work has shown that latently infected donor skin allografts can serve as vectors for CMV transmission to naïve recipients (17). Our current study suggests that in addition, recipients of cadaveric skin grafts for burn wound coverage are at risk to reactivate CMV from latency from allogeneic skin. Given the immunosuppressive consequences of burn injuries, it is perhaps not surprising that CMV reactivation occurs frequently in these patients (18). Because CMV reactivation has recently been associated with significant morbidity and mortality in this population (18), our current results suggest that a re-evaluation of non-“self” grafting for temporary wound closure may be warranted.
In conclusion, our study shows that in-vivo allogeneic stimulation can trigger reactivation of latent CMV. This reactivation can occur without concurrent immunosuppression, and in fact some immunosuppressive agents may actually limit reactivation. Our data suggest that activation of NF-κB or inflammation after allogeneic grafting may be a trigger, and this could perhaps be a target to prevent CMV reactivation. Further study is needed to determine the clinical importance of these findings in tolerance induction strategies and in burn patients receiving cadaveric skin grafts for temporary wound coverage.
Acknowledgements
The authors would like to thank Nicholas Dipaola for his technical assistance with cytokine super-arrays. These studies were supported by NIAID grant # R01 AI053094. This work was presented in part during the First Annual Academic Surgical Congress, February 2006, San Diego California.
Supported by NIH RO1 AI053094 (CHC)
References
- 1.Staras SAS, Dollard SC, Radford KW, Dana Flanders W, Pass RF, Cannon MJ. Seroprevalence of Cytomegalovirus Infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 2.Rubin RH. Impact of cytomegalovirus infection on organ transplant recipients. Rev Infect Dis. 1990;12(Suppl 7):S754–766. doi: 10.1093/clinids/12.supplement_7.s754. [DOI] [PubMed] [Google Scholar]
- 3.Hummel M, Abecassis MM. A model for reactivation of CMV from latency. J Clin Virol. 2002;25(Suppl 2):S123–136. doi: 10.1016/s1386-6532(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 4.Lang DJ. Cytomegalovirus infections in organ transplantation and post transfusion. An hypothesis. Archiv fur die Gesamte Virusforschung. 1972;37:365–377. doi: 10.1007/BF01241460. [DOI] [PubMed] [Google Scholar]
- 5.Olding LB, Jensen FC, Oldstone MB. Pathogenesis of of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J Exp Med. 1975;141:561–572. doi: 10.1084/jem.141.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 7.Guedes MIMC, Risdahl JM, Wiseman B, Molitor TW. Reactivation of Porcine Cytomegalovirus through Allogeneic Stimulation. J. Clin. Microbiol. 2004;42:1756–1758. doi: 10.1128/JCM.42.4.1756-1758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hummel M, Zhang Z, Yan S, DePlaen I, Golia P, Varghese T, Thomas G, Abecassis MI. Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: a model for reactivation from latency. J Virol. 2001;75:4814–4822. doi: 10.1128/JVI.75.10.4814-4822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gosselin J, Borgeat P, Flamand L. Leukotriene B4 Protects Latently Infected Mice against Murine Cytomegalovirus Reactivation following Allogeneic Transplantation. J Immunol. 2005;174:1587–1593. doi: 10.4049/jimmunol.174.3.1587. [DOI] [PubMed] [Google Scholar]
- 10.Cook C, Zhang X, McGuinness B, Lahm M, Sedmak D, Ferguson R. Intra-abdominal Bacterial Infection Reactivates Latent Pulmonary Cytomegalovirus in Immunocompetent Mice. J Infect Dis. 2002;185:1395–1400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- 11.Bickerstaff AA, Zimmerman PD, Wing BA, Taylor F, Trgovcich J, Cook CH. A flow cytometry-based method for detecting antibody responses to murine cytomegalovirus infection. J Virol Methods. 2007;142:50–58. doi: 10.1016/j.jviromet.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, Tumor Necrosis Factor Alpha, or Interleukin-1{beta} Triggers Reactivation of Latent Cytomegalovirus in Immunocompetent Mice. J. Virol. 2006;80:9151–9158. doi: 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurz SK, Rapp M, Steffens HP, Grzimek NK, Schmalz S, Reddehase MJ. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J Virol. 1999;73:482–494. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bantel H, Schmitz ML, Raible A, Gregor M, Schulze-Osthoff K. Critical role of nuclear factor-kB and stress-activated protein kinases in steroid unresponsiveness. FASEB J. 2002:02–0223fje. doi: 10.1096/fj.02-0223fje. [DOI] [PubMed] [Google Scholar]
- 15.Bickerstaff A, Pelletier R, Wang J, Nadasdy G, DiPaola N, Orosz C, Satoskar A, Hadley G, Nadasdy T. An Experimental Model of Acute Humoral Rejection of Renal Allografts Associated with Concomitant Cellular Rejection. The American Journal of Pathology. 2008;173:347–357. doi: 10.2353/ajpath.2008.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook CH, Bickerstaff AA, Wang JJ, Zimmerman PD, Forster MR, Nadasdy T, Colvin RB, Hadley GA, Orosz CG. Disruption of Murine Cardiac Allograft Acceptance by Latent Cytomegalovirus. American Journal of Transplantation. 2009;9:42–53. doi: 10.1111/j.1600-6143.2008.02457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kealey GP, Aguiar J, Lewis RW, 2nd, Rosenquist MD, Strauss RG, Bale JF., Jr. Cadaver skin allografts and transmission of human cytomegalovirus to burn patients. J Am Coll Surg. 1996;182:201–205. [PubMed] [Google Scholar]
- 18.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang M-L, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus Reactivation in Critically Ill Immunocompetent Patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]