Abstract
Objective
To determine the association of blood pressure (BP) level and longterm fluctuation in BP with cerebrovascular disease.
Design
Participants received structural MRI and BP measurements in 3, 24 month intervals prior to scanning. We derived the mean and standard deviation (SD) of the mean BP for each participant over the 3 intervals and divided them into four groups defined as above and below the group median (≤ 96.48 mmHg or >96.48mmHg) and further subdivided by the median standard deviation (below SD ≤ 7.21 mmHg or above SD > 7.21 mmHg). This scheme yielded four groups representing the full range of BP and fluctuations in BP. We examined differences in white matter hyperintensity (WMH) volume and brain infarctions across these groups.
Setting
The Washington Heights-Inwood Columbia Aging Project, a community-based epidemiological study of older adults from northern Manhattan.
Participants
686 non-demented older adults who received structural MRI and had BP measurements over three study visits.
Results
WMH volume increased across the four groups in a linear fashion with the lowest WMH volume in the lowest mean/lowest SD group and the highest in the highest mean/highest SD group (F(3,610)=27.43, p=0.0017). Frequency of infarction also increased monotonically across groups (from 22% to 41%; p-for-trend=0.004).
Conclusions
Compared to individuals with low BP with low fluctuations in BP, the risk of cerebrovascular disease increases with increasing BP and BP fluctuation. Given that cerebrovascular disease is associated with disability, findings suggest that interventions should focus on longterm fluctuating BP as well as elevated BP.
Keywords: blood pressure, cerebrovascular disease, white matter hyperintensities
Introduction
Large and small vessel cerebrovascular disease in the elderly, visualized by structural magnetic resonance imaging (MRI) as infarction and white matter hyperintensities (WMH), is associated with cognitive decline and disability, but its determinants have not been fully explicated. Elevated blood pressure (BP) can cause cerebrovascular disease through a number of pathways, including atherosclerotic changes1, 2, blood-brain barrier dysfunction3, lipohyalinosis4, carotid stenosis1, 2, 5, and hemorrhage6. Elevated BP is associated with poorer cognitive function among older adults and has been shown to be a risk factor for the development of Alzheimer’s disease7, 8. Increased BP in mid- and late-life is also related to stroke and, although inconsistently, WMH volume9, 10. Studies that examine the association between BP and cerebrovascular disease generally consider BP measurements at one time point, limiting the appreciation of the full impact of BP on cerebrovascular disease. In clinical settings, absolute BP level is used as a therapeutic target to prevent clinical stroke and heart disease, but BP fluctuation over long periods and its impact on cerebrovascular disease are typically not considered.
The interplay between peripheral vascular systems and cerebrovascular function is complex. In his seminal synthesis of studies utilizing the inert gas method to examine cerebral blood flow, Lassen11 applied the term ‘autoregulation’ to describe the phenomenon in which brain perfusion remains constant over a large range of BP. Through interacting myogenic, neurogenic, and metabolic mechanisms, the cerebral vascular system adapts to changes in BP in order to maintain the nutritional supply necessary to fuel its high metabolic activity12, 13. Thus, within a range of increasing and decreasing BP, brain perfusion remains steady. In the face of hypertension and advancing age, the lower boundary of this so-called autoregulatory ‘plateau stage’ adaptively shifts towards higher BP levels12. Although compensatory, this adjustment may enhance the brain’s vulnerability to hypoperfusion at lower BP levels13. Based on these effects, it is possible that persistently elevated BP and longterm fluctuations in BP can lead to cerebrovascular disease directly through ischemic changes (e.g., atherosclerosis) and indirectly, through compromised cerebral autoregulation, which may lead to longer periods or high levels of transient hypoperfusion.
We investigated the relation between BP and fluctuations in BP over a three-year period on cerebrovascular disease, using high resolution MRI, in a large community cohort of older adults. We hypothesized that as BP and longterm fluctuations in BP increased greater amounts of WMH and number of infarcts would be observed.
Methods
Subjects
Data were included from individuals participating in a prospective study of aging in Medicare-eligible northern Manhattan residents, age 65 years and older (Washington Heights/Hamilton Heights-Inwood Columbia Aging Project: WHICAP; n=2,776). The WHICAP cohort represents a combination of continuing members of a cohort originally recruited in 1992 (n=602) and members of a new cohort recruited between 1999 and 2001 (n=2,174). The sampling and recruitment outcomes of these two cohorts have been described elsewhere14. The population from which participants were drawn comprises individuals from three broadly defined ethnic categories (i.e., Hispanic, African-American, and White). Ethnic group was determined by self-report using the format of the 2000 US Census15. Participants have been followed at approximately 24-month intervals with similar assessments at each interval. Recruitment, informed consent and study procedures were approved by ethics committees at Columbia University and NY State Psychiatric Institute.
Beginning in 2003, those who did not meet criteria for dementia at previous evaluations and who did not have contraindications were invited to participate in an MRI study. Scan acquisition corresponded temporally with the third assessment wave of the cohort recruited in the 1999–2001 interval. That is, participants recruited at that time had their initial/baseline evaluations in between 1999 and 2001, their second evaluations in the 2002–2004 assessment wave, and their third evaluation, including MRI, in the 2005–2007 wave. Derivation of the WHICAP imaging sample and full sample characteristics are described elsewhere16. Briefly, MRI was performed on 769 participants. Participants who refused the MRI study but otherwise met inclusion criteria (n=407) were one year older, more likely to be women, less likely to be African-American, but similar in number of years of education compared with those who agreed to receive an MRI scan. Fifty-two of the 769 participants were determined to meet diagnostic criteria for dementia at the follow-up visit closest to the MRI scan and thus excluded from analyses16. Of the remaining 717 participants, 31 did not have complete fluid attenuated inverse recovery (FLAIR) MRI data or complete BP data for the three follow-up waves. Thus, the current study comprises the remaining 686 participants.
MRI acquisition and analysis
Detailed procedures and parameters for MRI scan acquisition are included elsewhere16. Briefly, standard T1-weighted, T2-weighted FLAIR images, and double echo images were acquired on a 1.5 T Philips Intera scanner at Columbia University. MR data were transferred to the University of California at Davis for morphometric analysis in the Imaging of Dementia and Aging Laboratory. White matter hyperintensity volumes were derived on the FLAIR images following established procedures17–19, divided by total cranial volume17, 19, and log transformed to establish a measure of normally-distributed relative WMH volume. Determination of presence, size, and location of cerebral infarct was carried out according to previously-reported protocols10. The presence of brain infarction was determined using all available imaging data. Only lesions 3mm or larger qualified for consideration as brain infarcts. Infarcts were coded for size (small>3mm and <1cm; large≥1cm), hemisphere, and location. Two raters determined the presence of cerebral infarction on MRI. Previously published kappa values for agreement among raters has been generally good, ranging from 0.73 to 0.9020.
Clinical evaluation
At each visit, participants received an in-person evaluation that included interviews regarding health and functional abilities, medical history, physical and neurological examination, and a neuropsychological battery21. As part of the study visit, highly standardized BP measurements were made with the Dinamap Pro 100 (Critikon Co., Tampa, FL) device. The BP cuff was placed on the right arm while the individual was seated, and a recording was obtained every 3 minutes over 9 minutes. The third measurement was recorded in the database and used in the current analyses. WHICAP study visits usually take place during typical business hours, but precise time-of-day was not controlled. Thus, we did explicitly consider circadian variability22, but did not expect systematic differences across subject groups.
Dementia diagnosis was made based on standard research criteria23 at a consensus conference attended by study physicians and neuropsychologists. Blood pressure and neuroimaging data were not considered for diagnosis. Consensus was reached after review of all available information gathered from initial and follow-up assessments. Participants were excluded from the current analyses if they met criteria for dementia.
Blood pressure variables and participant grouping
As noted, BP measurements were made at each of the three visit waves (i.e., 1999–2001, 2002–2004, 2005–2007), the third one corresponding to the time of MRI. We sought to examine the relation between BP levels and longterm fluctuation and cerebrovascular disease. Our exposures of interest were the mean BP and its standard deviation (SD) over the three visits. Each participant’s mean BP was computed for each of the three visits, using the following equation: Mean BP=1/3*systolic BP)+(2/3*diastolic BP)24–26. For each participant, we then calculated the arithmetic mean and SD of the mean BP across the three follow-up visits. We derived four groups based on the median split of the mean BP measurement (median=96.48) and the median split of the SD (median= 7.21) across the study: Group 1 (mean BP<96.48 mmHg and mean SD<7.21 mmHg), group 2 (mean BP<96.48 mmHg and mean SD>7.21 mmHg), group 3 (mean BP>96.48 mmHg and mean SD<7.21 mmHg), and group 4 (mean BP>96.48 mmHg and mean SD>7.21 mmHg). Thus, the four groups represented subjects whose BP was in the low normal range with little fluctuations through individuals with higher BP with greater degree of fluctuations over the three evaluations. Participants were considered treated for hypertension if they reported taking diuretics, calcium channel blocking agents, beta blockers, or ACE inhibitors at any point over the three-visit period. We also examined other medications or medication classes that might have a secondary effect on BP, including digoxin, nitrates, anti-arrhythnics/anginals, or thyroid supplements. Further, history of diabetes, hypertension, and heart disease was ascertained by self report27 and coded as present or absent. Heart disease history included arrhythmias, coronary artery disease, and congestive heart failure.
Statistical analysis
General linear models were constructed to examine whether WMH volume differed across the four BP groups. In addition to comparing each BP group to the low BP/low fluctuation group as reference, we tested the linear trend in WMH volume across the groups. Analyses included age, sex, and treatment status as additional covariates. The proportion of participants with cerebral infarcts was compared across BP groups using logistic regression analysis, in which presence or absence of infarct was the dependent variable and age, sex, and treatment status were additional covariates. This analysis was run first with large and small infarcts combined and then separated by infarct size. For both WMH and infarct analyses, we also examined whether the primary findings were modified by ethnicity by including it as an additional covariate or by stratification of analysis by ethnic group. We also re-ran analyses with history of diabetes hypertension, and stroke as covariates.
Results
The four BP groups were similar in age, sex distribution, ethnicity distribution, and number of years of education (Table 1). Participants with the highest BP and greatest amount of BP fluctuation were the most likely to have been treated with antihypertensive medication while those with the lowest BP and BP fluctuation were the least likely. The distribution of other medications that might affect blood pressure did not differ across groups. By definition, there were significant group differences in mean BP, mean systolic BP, and mean diastolic BP across groups, as well as the standard deviations of these measures. The mean (in years) intervals between the 1999–2001 and 2002–2004 assessment wave was 2.12 (SD=0.71), between the 2002–2004 and 2005–2007 wave was 2.45 (SD=0.65), and between the 1999–2001 and 2005–2007 wave was 4.47 (SD=0.80). These intervals did not vary significantly across BP groups.
Table 1.
Demographic, treatment, blood pressure, and fluctuation (SD) differences across blood pressure groups. Group 1 contains participants with lower mean BP (<96.48 mmHg) and lower fluctuation (SD < 7.21 mmHg); group 2 comprises participants with lower mean BP (<96.48 mmHg) and higher fluctuation (SD < 7.21 mmHg); group 3 comprises participants with higher mean BP (>96.48 mmHg) and lower fluctuation (SD < 7.21 mmHg); and group 4 comprises participants with higher mean BP (>96.48 mmHg) and higher fluctuation (SD < 7.21 mmHg).
| Blood Pressure Group | Statistic | |||||
|---|---|---|---|---|---|---|
| Mean BP <96.48 mmHg | Mean BP BP >96.48 mmHg | |||||
| Fluctuation (<7.21 or >7.21 mmHg) | Group 1 Low | Group 2 High | Group 3 Low | Group 4 High | ||
| N | 176 | 166 | 167 | 177 | ||
| Age (mean ± SD) | 79.25 ± 5.38 | 80.78 ± 5.61 | 79.97 ± 5.31 | 79.98 ± 5.62 | F (3, 685) = 2.20, p = 0.087 | |
| Sex (% women) | 73.3% | 69.3% | 61.7% | 65.5% | χ2(3) = 5.83, p = 0.120 | |
| % Caucasian | 28.4 | 28.9 | 26.3 | 29.9 | χ2(9) = 6.03, p = 0.736 | |
| Ethnicity | % African American | 30.7 | 36.7 | 38.3 | 31.1 | |
| % Hispanic | 38.6 | 33.7 | 32.9 | 36.2 | ||
| % Other | 2.3 | 0.6 | 2.4 | 2.8 | ||
| Education (mean ± SD years) | 10.51 ± 4.97 | 11.02 ± 4.55 | 10.90 ± 4.62 | 10.80 ± 4.78 | F (3, 683) = 0.366, p = 0.778 | |
| % Treated with antihypertensive | 57.9 | 68.4 | 65.5 | 73.2 | χ2(3) = 9.01, p = 0.029 | |
| % Treated with other medications that might affect BP | 23.9 | 23.8 | 23.0 | 21.5 | χ2(3) =0.37, p=0.947 | |
| Mean BP | Mean (Mean ± SD, range) | 88.85 ± 5.88 (68.31–96.43) | 89.44 ± 5.14 (73.10–96.32) | 103.75 ± 5.91 (96.53–121.86) | 105.86 ± 7.21 (96.75–134.83) | F (3, 682) = 382.40, p < 0.001 |
| SD (Mean ± SD, range) | 4.90 ± 2.10 (0.47–8.26) | 12.66 ± 3.53 (8.48–22.89) | 5.42 ± 2.14 (0.47–8.38) | 14.99 ± 6.60 (8.44–52.33) | F (3, 682) = 272.46, p < 0.001 | |
| Systolic BP | Mean (Mean ± SD, range) | 130.30 ± 11.02 (93.33–155.33) | 131.30 ± 10.15 (101.33–161.67) | 151.81 ± 12.18 (125.67–194.67) | 155.87 ± 14.72 (115.00–257.33) | F (3, 682) = 209.600, p < 0.001 |
| SD (Mean ± SD, range) | 9.69 ± 5.28 (1.41–31.34) | 18.85 ± 8.15 (2.12–41.80) | 10.47 ± 5.82 (0.71–34.00) | 24.12 ± 16.30 (1.00–172.99) | F (3, 682) = 83.74, p < 0.001 | |
| Diastolic BP | Mean (Mean ± SD, range) | 68.16 ± 5.92 (48.00–82.00) | 68.55 ± 5.49 (46.67–79.00) | 79.75 ± 5.57 (64.33–94.33) | 80.89 ± 6.64 (70.00–104.00) | F (3, 682) = 234.30, p < 0.001 |
| SD (Mean ± SD, range) | 4.90 ± 2.52 (0.00–12.74) | 10.56 ± 4.35 (0.71–33.62) | 5.51 ± 2.89 (0.00–14.11) | 11.60 ± 5390 (2.08–36.23) | F (3, 682) = 118.05, p < 0.001 | |
Clinically, those classified as lower BP (groups 1 and 2) had mean, diastolic, and systolic blood pressures that were approximately in what is considered the normotensive range28, whereas those in the higher group (groups 3 and 4) were in the prehypertensive or hypertensive range (Table 1). In terms of the degree of longterm BP fluctuation, those with the lowest BP and lowest SD (group1) fluctuated about 5.5% over the three year period (i.e., SD/Mean=4.90/88.85 mmHg); BP for those in group 2 with the low mean BP but with greater SD fluctuated about 14.2% (i.e., SD/Mean=12.66/89.44) over the three year period. Those in the higher BP group but with low SD (group 3) fluctuated about 5.2% (i.e., SD/Mean=5.42/103.75), whereas those in the highest BP with the greatest SD group fluctuated about 14.2% (SD/Mean=14.99/105.86) over the three year period (Table 1).
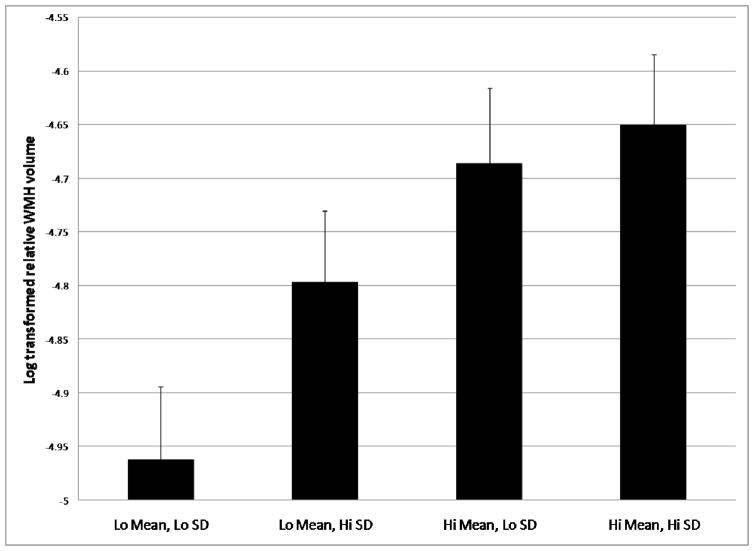
White matter hyperintensity volume increased across the four BP groups monotonically, with the lowest WMH volume in group 1 and highest WMH volume in group 4 (main effect of Group: F (3,610)=3.52, p=0.017; test of linear trend p=0.002 (Fig 1 and Table 2)). Increased age was associated with more severe WMH volume (main effect of Age: F(1,610)=27.43, p < 0.001); no other covariates were associated with WMH volume. Including ethnicity as a covariate or stratification of the analysis by ethnic group did not change the pattern of results. When history of diabetes, hypertension, and heart disease were included as additional covariates, the findings remained unchanged. Of these additional covariates, only history of hypertension entered into the model significantly (β=0.24, t=2.33, p=0.02), such that those with a self-reported history of hypertension had significantly more severe WMH.
Figure 1.
Differences in log transformed relative WMH volume across blood pressure groups. There was a significant monotonic trend across blood pressure groups (p=.015). Mean values are adjusted for age, sex, and treatment status. Group 1 contains participants with lower mean BP (<96.48 mmHg) and lower fluctuation (SD < 7.21 mmHg); group 2comprises participants with lower mean BP (<96.48 mmHg) and higher fluctuation (SD < 7.21 mmHg); group 3 comprises participants with higher mean BP (>96.48 mmHg) and lower fluctuation (SD < 7.21 mmHg); and group 4 comprises participants with higher mean BP (>96.48 mmHg) and higher fluctuation (SD < 7.21 mmHg).
Table 2.
Associations of BP level and longterm BP fluctuation with log transformed relative WMH volume.
| Covariate | β | Standard Error | t | p-value | |
|---|---|---|---|---|---|
| Age | 0.033 | 0.006 | 5.24 | <0.001 | |
| Sex (female = 1) | 0.123 | 0.072 | 1.70 | 0.090 | |
| Treated (yes = 1) | 0.130 | 0.072 | 1.81 | 0.071 | |
| Group 1 | Reference | Reference | Reference | Reference | |
| Group 2 | 0.095 | 0.096 | 0.98 | 0.324 | |
| Group 3 | 0.245 | 0.098 | 2.499 | 0.013 | |
| Group 4 | 0.267 | 0.094 | 2.828 | 0.005 | |
| p-value for trend | 0.015 |
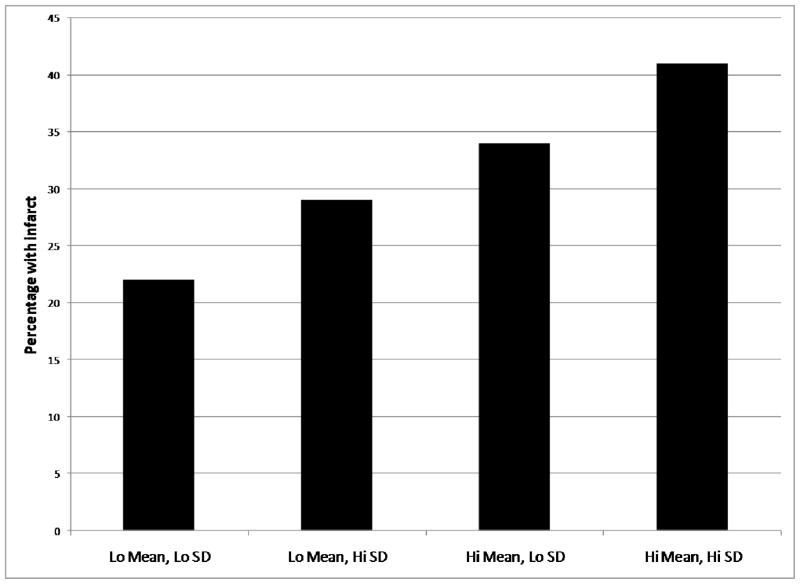
Two hundred fifteen (31.3%) of the 686 participants with available data had infarcts. Across the four groups, the proportion of subjects with infarcts also increased monotonically (test for linear trend: β=1.25, p=0.004 (Fig 2 and Table 3)). Women and those who had received treatment for hypertension were more likely to have infarcts. When stratified by infarct size, the pattern of results was similar. Including ethnicity or stratification by ethnic group yielded similar results, as did inclusion of history of diabetes, hypertension, and heart disease. In the latter case, only self reported history of heart disease was associated with increased stroke (β=0.37, p=0.045). When we repeated the analyses separately for diastolic or systolic BP the general pattern of results was similar.
Figure 2.
Percentage of participants with radiological infarct across blood pressure groups. There was a significant monotonic trend across groups (p=.004).
Table 3.
Associations of BP level and longterm BP fluctuation with presence of stroke.
| Covariate | N (%) with infarct | Odds ratio | 95% Confidence interval | P | |
|---|---|---|---|---|---|
| Age | -- | 1.001 | 0.969–1.034 | 0.948 | |
| Sex (female=1) | -- | 0.651 | 0.452–0.937 | 0.021 | |
| Treated (yes = 1) | -- | 1.747 | 1.192–2.560 | 0.004 | |
| Group 1 | 39/176 (22%) | 1.00 | Reference | Reference | |
| Group 2 | 48/166 (29%) | 1.255 | 0.750–2.098 | 0.387 | |
| Group 3 | 56/167 (34%) | 1.418 | 0.849–2.370 | 0.182 | |
| Group 4 | 72/177 (41%) | 2.020 | 1.238–3.294 | 0.005 | |
| p-value for trend | 0.004 |
Discussion
Through examination of BP characteristics over three prospective evaluations, separated by intervals of about 2 years, we demonstrated that both mean BP and longterm fluctuation in BP are associated with cerebrovascular disease in the form of WMH and infarction in a linear fashion. Importantly, the presence of either factor – elevated mean BP or elevated fluctuations in BP – alone was sufficient to incur a greater burden of WMH or frequency of infarct, although elevated mean BP was associated with more severe cerebrovascular disease. The two factors also appear to be additive; those with the higher mean BP and greater fluctuation had proportionately more cerebrovascular disease than those with either one alone. Cerebrovascular disease is associated with a constellation of conditions that lead to disability, including cognitive impairment, mood, and movement disorders29. Thus, our findings may have important clinical implications.
The association between BP and stroke is a well replicated finding. Elevated BP is associated with stroke through a number of avenues, such as atherosclerosis, lipohyalinosis, carotid stenosis, or hemorrhage. Investigators have become interested in identifying the etiology and determinants of WMH because of its relationship to cognitive dysfunction and disability. White matter hyperintensities are ubiquitous among older adults30, 31 and pathological studies suggest an ischemic etiology32, including incomplete and total infarction and demyelination33, although there is some evidence that WMH are heterogeneous in etiology34. Persistently elevated BP is likely to be related to increased WMH volume and stroke through similar mechanisms. However, elevated BP and increased age also result in a shift in the lower boundary of the autoregulatory curve12, 13, which may put areas with relatively low blood supply, including white matter35, 36, at risk for ischemic damage due to hypoperfusion37.
The terms BP ‘variability’ or ‘fluctuation’ typically refer to modulation in diastolic or systolic BP on the order of minutes to hours or in response to experimental manipulation38. In the current study, we were interested in determining whether longterm fluctuation in BP is associated with cerebrovascular disease. To this end, the study design paralleled routine clinical medical follow up. The BP information in our study is similar to what is available to any clinician using standard BP measurements and can be computed easily. Consistent with our hypothesis, longterm fluctuation in BP was associated with both small and larger vessel vascular disease, even when mean BP was low. Typically, clinicians examine absolute levels of BP but do not consider fluctuations across visits. The findings highlight the potential clinical importance of monitoring, and perhaps treating, persistent fluctuation in BP over months and years, although future studies need to establish more definitively a causal link between BP fluctuation and cerebrovascular disease. Further, cerebrovascular disease may lead to hippocampal dysfunction39, places individuals at increased risk of development of dementia34, and is associated with increased rate of decline among individuals with mild cognitive impairment or dementia40, 41. Thus, proper risk factor management is essential for reducing risk of cerebrovascular-related cognitive decline in aging and dementia. It is noteworthy that participants in the current study who had the highest BP and fluctuation levels were most likely to have been treated with anti-hypertensive agents, which suggests that intermittent lack of compliance may be one source of longterm BP fluctuation and further highlights the potential clinical importance of maintaining a consistently normotensive BP.
White matter is particularly susceptible to fluctuations, or inconsistent perfusion42. Consistent with this notion, our findings show that fluctuation in BP is related to a higher volume of WMH. We also found that fluctuation in BP was more informative in addition to BP status than each parameter alone. This finding is particularly important in a population with high prevalence of hypertension, relatively homogeneous in BP level, but heterogeneous in BP variability. Hypertension treatment has been shown to restore BP regulation in addition to treating BP levels in the elderly43. Thus, fluctuation in BP may be important as an exposure in epidemiologic research in the elderly and as a clinical parameter.
Whether longterm fluctuation in BP is a reflection of degree of BP variability over shorter intervals remains to be tested empirically. Nonetheless, much like with persistently elevated BP, the association between fluctuations in BP, over any interval, and cerebrovascular disease could reflect age-associated autoregulatory dysfunction. Even among normotensive individuals, high variability in BP could cause ischemic damage due to hypoperfusion during periods of particularly low BP. This possibility is particularly evident in the current study among individuals with low mean BP but greater fluctuation who had increased cerebrovascular disease compared to those with low BP and less variability.
The current study has several strengths. The WHICAP cohort is a large and ethnically diverse community-based elderly cohort with quantitative neuroimaging. The neuroimaging protocol and morphometric analysis was standardized across subjects. These strengths must be appreciated in the context of some weaknesses. Although we characterized participants based on reliable longitudinal BP data, MRI data were only acquired at one point in time and, thus, analyses were correlational. It is unlikely that structural brain changes caused hypertension or fluctuations in BP; however, the time course of the relationship between the two remains to be elucidated. Future studies should examine incidents and change in cerebrovascular disease as they relate to BP characteristics.
Our results demonstrate a strong association between BP and fluctuations in BP with measures of cerebrovascular disease. While the control of elevated BP or treatment of hypertension is an obvious and well-replicated conclusion, our findings suggest that management of BP fluctuations – even among normotensive older adults – may be beneficial in reducing the risk of cerebrovascular disease and maximizing healthy cognitive aging.
Acknowledgments
This work was supported by National Institutes of Health grants P01 AG007232 and K23 AG029949.
References
- 1.Lakka TA, Salonen R, Kaplan GA, Salonen JT. Blood pressure and the progression of carotid atherosclerosis in middle-aged men. Hypertension. 1999;34(1):51–56. doi: 10.1161/01.hyp.34.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Sander D, Kukla C, Klingelhofer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3-year follow-up study. Circulation. 2000;102(13):1536–1541. doi: 10.1161/01.cir.102.13.1536. [DOI] [PubMed] [Google Scholar]
- 3.Zumkeller M, Hollerhage HG, Reale E, Dietz H. Ultrastructural changes in the blood-brain barrier after nimodipine treatment and induced hypertension. Experimental neurology. 1991;113(3):315–321. doi: 10.1016/0014-4886(91)90021-4. [DOI] [PubMed] [Google Scholar]
- 4.Munoz DG. Small vessel disease: neuropathology. International psychogeriatrics/IPA. 2003;15 (Suppl 1):67–69. doi: 10.1017/S1041610203008986. [DOI] [PubMed] [Google Scholar]
- 5.Crouse JR, Goldbourt U, Evans G, Pinsky J, Sharrett AR, Sorlie P, Riley W, Heiss G. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke; a journal of cerebral circulation. 1996;27(1):69–75. doi: 10.1161/01.str.27.1.69. [DOI] [PubMed] [Google Scholar]
- 6.Zia E, Hedblad B, Pessah-Rasmussen H, Berglund G, Janzon L, Engstrom G. Blood pressure in relation to the incidence of cerebral infarction and intracerebral hemorrhage. Hypertensive hemorrhage: debated nomenclature is still relevant. Stroke; a journal of cerebral circulation. 2007;38(10):2681–2685. doi: 10.1161/STROKEAHA.106.479725. [DOI] [PubMed] [Google Scholar]
- 7.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Archives of neurology. 2007;64(12):1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skoog I. Vascular aspects in Alzheimer’s disease. J Neural Transm Suppl. 2000;59:37–43. doi: 10.1007/978-3-7091-6781-6_6. [DOI] [PubMed] [Google Scholar]
- 9.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 10.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke; a journal of cerebral circulation. 1999;30(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 11.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiological reviews. 1959;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- 12.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovascular and brain metabolism reviews. 1990;2(2):161–192. [PubMed] [Google Scholar]
- 13.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 14.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of Alzheimer’s disease in African-Americans, Caribbean Hispanics and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Census PaH. Census of Population and Housing Summary Tape File1, Technical Documentation. WAshington, DC: Bureau of the Census; 1991. [Google Scholar]
- 16.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Archives of neurology. 2008;65(8):1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. Journal of computer assisted tomography. 1992;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 18.DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging. 1996;6(3):519–528. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- 19.DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45(11):2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiology of aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, Mayeux R. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives of neurology. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 22.van Boxtel MP, Henskens LH, Kroon AA, Hofman PA, Gronenschild EH, Jolles J, de Leeuw PW. Ambulatory blood pressure, asymptomatic cerebrovascular damage and cognitive function in essential hypertension. Journal of human hypertension. 2006;20(1):5–13. doi: 10.1038/sj.jhh.1001934. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnosticand statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 24.Safar ME, London Gm. The arterial system in human hypertension. In: Swales JD, editor. Textbook of Hypertension. London: Blackwell Scientific; 1994. pp. 85–102. [Google Scholar]
- 25.Nichols WW, O’Rourke M. McDonald’s blood flow in arteries: Theorertical, experimental, and clinical principles. 4. London: Oxford University Press; 1998. [Google Scholar]
- 26.Milnor WR. Hemodynamics. Baltimore: Williams and Wilkins; 1989. [Google Scholar]
- 27.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 29.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiology of aging. 2002;23(3):421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 30.Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, van Harskamp F, Tanghe HL, de Jong PT, van Gijn J, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 31.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in elderly African Americans, Caribbean Hispanics, and Caucasians from northern Manhattan. Archives of neurology. doi: 10.1001/archneur.65.8.1053. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 33.Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, Ellis WG, Zarow C, Mungas D, Reed BR, Kramer JH, Schuff N, DeCarli C, Chui HC. Neuropathological basis of magnetic resonance images in aging and dementia. Annals of neurology. 2008;63(1):72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Altheimer’s disease: do white matter hyperintensities matter? Dialogues in clinical neuroscience. 2009;11(2):181–190. doi: 10.31887/DCNS.2009.11.2/ambrickman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial Distribution of White-Matter Hyperintensities in Alzheimer Disease, Cerebral Amyloid Angiopathy, and Healthy Aging. Stroke; a journalof cerebral circulation. 2008 doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, Borogovac A, Ramos MA, Brown TR, Asllani I, Stern Y. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2008.11.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. A follow-up study of blood pressure and cerebral white matter lesions. Annals of neurology. 1999;46(6):827–833. doi: 10.1002/1531-8249(199912)46:6<827::aid-ana4>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Parati G, Faini A, Valentini M. Blood pressure variability: its measurement and significance in hypertension. Current hypertension reports. 2006;8(3):199–204. doi: 10.1007/s11906-006-0051-6. [DOI] [PubMed] [Google Scholar]
- 39.Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, Brown T, DeCarli C, Barnes CA, Mayeux R, Vannucci SJ, Small SA. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Annals of neurology. 2008;64(6):698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, Brandt J, Blacker D, Stern Y. Cerebral atrophy and white matter hyperintensity burden predict rate of cognitive decline in Alzheimer’s disease. Archives of neurology. doi: 10.1001/archneur.65.9.1202. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, Tanzi RE, Albert MS, Greenberg SM, Guttmann CR. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Archives of neurology. 2008;65(1):94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- 42.Pantoni L. White matter ischemia: Time to begin integrating experimental and clinical data. European neurology. 2006;56(2):71–73. doi: 10.1159/000095542. [DOI] [PubMed] [Google Scholar]
- 43.Lipsitz LA, Gagnon M, Vyas M, Iloputaife I, Kiely DK, Sorond F, Serrador J, Cheng DM, Babikian V, Cupples LA. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45(2):216–221. doi: 10.1161/01.HYP.0000153094.09615.11. [DOI] [PubMed] [Google Scholar]