Abstract
Objectives
Conjugated equine estrogen (CEE) therapies when initiated among older women have been shown to produce small decrements in global cognitive function. We are interested whether these persist after cessation and extend to specific cognitive domains.
Design
Randomized controlled clinical trial
Setting
Fourteen clinical centers of the Women's Health Initiative
Participants
2,304 women aged 65-80 years and free of probable dementia at enrollment
Intervention
0.625 mg/day of CEE, with or without medroxyprogesterone acetate (MPA, 10 mg/day), and matching placebos
Measurements
Annual administrations of a battery of cognitive tests during and following the trial
Methods
General linear models were used to compare on-trial and post-trial mean standardized test scores between treatment groups, with adjustment for baseline risk factors for cognitive impairment.
Results
Assignment to CEE-based therapies was associated with small mean relative decrements in global and several domain-specific cognitive functions on-trial, which largely persisted through up to 4 years post-trial. The strongest statistical evidence was for global cognitive function: 0.07 standard deviation decrements both on-trial (p=0.007) and post-trial (p=0.01). Among domain specific scores, the mean relative decrements were slightly smaller, were less significant, and tended to be larger for CEE-alone therapy.
Conclusions
CEE-based therapies, when initiated after age 65 years, produce a small broad-based decrement in cognitive function that persists after their use is stopped. The differences in cognitive function however are small and would not be detectable or have clinical significance for an individual woman. Differences in effects among cognitive domains suggest that more than one mechanism may be involved.
Keywords: Postmenopausal hormone therapy, Cognitive function, Women's health
INTRODUCTION
The Women's Health Initiative Memory Study (WHIMS) randomized clinical trial found that assigning conjugated equine estrogen (CEE) therapy, alone or combined with medroxyprogesterone acetate (MPA), to women aged 65-80 years was associated with slightly worse mean scores in global cognitive function compared to assigning placebo therapy.1,2 These effects were observed within the first three to four years of the trial follow-up and remained fairly constant several years thereafter. The Women's Health Initiative Study of Cognitive Aging (WHISCA) examined whether these therapies influenced domain-specific cognitive function at initial assessment, an average of 3.0 years after randomization to CEE-based therapies or placebos, and subsequent age-related rates of change in eight cognitive domains over the remaining average 2.6 years of on-trial follow-up.3 Only small mean differences in cognitive test scores between treatment groups were found at women's initial WHISCA assessments and for the rates that scores changed over subsequent follow-up.4,5 Together, these findings suggest that if the use of these agents produces an initial decrement in at least some aspects of cognitive function during the first few years, this decrement does not markedly widen or diminish thereafter. Concerns whether this initial on-trial decrement continues after hormone therapy is discontinued arise from a recent report that the volumes of the frontal lobe and hippocampus were smaller among a subset of these women, 1.4-3.0 years after the prescription of WHIMS CEE-based therapies ended.6
In this paper, we re-analyze WHISCA data to estimate the average relative decrement in cognitive function test scores associated with CEE-based therapies over the course of the trial. We then examine the degree these relative decrements persist during up to 5 years of post-trial follow-up. Scaling treatment effects in standard deviation units, we compare on-trial and post-trial effect sizes across domains. Finally, we examine whether there are differences between CEE alone therapy and CEE plus progestin therapy on the size and duration of effects.
Methods
The WHIMS7 began enrolling its 7,145 participants from the parent Women's Health Initiative (WHI) hormone therapy trials8 May, 1996. These women were between 65-79 years of age at initial screening, were appropriate candidates for postmenopausal hormone therapy, and were free of dementia as assessed with a standard protocol. Women without a uterus were randomly assigned with equal probability to take one daily tablet that contained either 0.625 mg of CEE (Premarin™, Wyeth-Ayerst Philadelphia, PA) or a matching oral placebo in the WHI CEE-Alone trial. In a similar manner, women with a uterus were randomly assigned to take one daily tablet that contained either 0.625 mg of CEE with 2.5 mg medroxyprogesterone acetate (PremPro™, Wyeth-Ayerst Philadelphia, PA) or placebo in the WHI CEE+MPA trial.
The WHISCA enrolled 2,304 participants from 14 of the WHIMS clinical sites beginning in September, 1999. These women had been randomly assigned to WHI treatments an average (standard deviation) of 3.0 (0.7) years prior to enrollment in WHISCA. The National Institutes of Health and Institutional Review Boards for all participating institutions approved protocols and consent forms. Informed written consent was obtained from all participants.
The WHI CEE+MPA trial was terminated earlier than planned, in July, 2002, because significantly more non-cognitive adverse events occurred among women assigned to hormone therapy compared to placebo.9 In February, 2004, the WHI CEE-Alone trial was terminated earlier than planned because the excess risk of stroke in the hormone therapy group was judged to be unacceptable for healthy women in the absence of benefit for coronary heart disease, the primary outcome of the WHI hormone therapy trials.10 At the conclusion of each trial, study medications were ceased and women were informed of findings and encouraged to consult with their physicians.
Annual follow-up of the WHISCA women continued after the termination of the WHI trials. Women were re-consented for extended follow-up during 2004-5, which continued until September, 2007. Results from all cognitive assessments from initial WHISCA enrollment to the end of extended follow-up are presented in this report.
Cognitive assessments
A battery of cognitive measures was collected annually on WHISCA participants.3 It included the Primary Mental Abilities Vocabulary test to assess verbal knowledge;11 letter and semantic fluency tests to assess verbal fluency;12,13 the Benton Visual Retention Test (BVRT) to assess short-term figural memory;14 the California Verbal Learning Test (CVLT) to assess verbal memory;15 the Digit Span Forward and Backward Test to assess attention and working memory;16 the Card Rotations Test to measure spatial ability;17 the Finger Tapping Test to assess fine motor speed;18 and the Modified Mini Mental State (3MS) Exam to assess global cognitive function.19 Quality control was maintained through recertification of test administrators twice during the first year and annually thereafter.
Demographic and clinical cognitive risk factors
Baseline demographic, lifestyle, and clinical factors related to the risk of cognitive impairment were collected via self-report and standardized assessments.8
Statistical methods
The primary analysis defined in the WHISCA protocol compared the on-study rates of change in domain-specific cognitive function between treatment groups.4 In this re-analysis, we used a different approach. We examined mean differences between treatment groups in the cognitive function scores over time to estimate average on-trial and post-trial relative decrements associated with hormone therapy. We first standardized individual scores on each outcome measure by dividing their difference from the cohort mean at the initial WHISCA exam by the standard deviation (SD) of scores at this time. For cognitive domains assessed by more than one outcome measure, we averaged the standardized scores of these outcomes and renormalized these averages to have standard deviations of one. The means (SD) of cognitive tests used to create these standardized domain scores were as follows. Global cognitive function was based on the 3MS: 95.91 (3.70). Verbal knowledge was based on the Primary Mental Abilities Vocabulary test: 36.48 (9.80). Verbal fluency was a composite of letter and category tests: 39.64 (12.49) and 28.95 (6.25). Verbal memory was a composite of CVLT-A, CVLT-A Long Delay, and CVLT-A Short Delay tests: 28.63 (6.37), 9.12 (3.08), and 8.37 (3.11). Figural memory was based on BVRT: 7.14 (3.80). Attention and working memory was based on Digit Span forward and backward tests: 7.44 (2.05) and 6.66 (2.01). Spatial ability was based on the Card Rotations Test: 54.68 (27.70). Fine motor was an average of finger tapping tests from the dominant and non-dominant hands: 37.50 (6.69). Scores from the BVRT were subtracted from zero so that higher scores expressed better performance. Covariate-adjustment was used to control for differences in the two CVLT word lists that were alternated over time.
The balance of risk factors for cognitive impairment between treatment groups was assessed with logistic regression. Cognitive score means over time (as measured from randomization date) were developed from general linear models fitted by maximum likelihood estimation.20 To reduce the effects of rare extreme scores, those exceeding ±4 SDs were re-coded to be ±4. Estimates of on-trial and post-trial average relative treatment effects were developed from these models by including these markers as time-varying covariates. Because WHISCA did not begin until a year after the completion of WHI enrollment, the only baseline measure of cognitive function was the 3MS collected by WHIMS, which was included as a covariate in all analyses. Additional terms were included in models to adjust for the time on WHISCA (to control for learning effects). To contrast CEE-Alone and CEE+MPA treatment effects, we additionally included risk factors for cognitive impairment in models to account for differences in the trial cohorts. Women were analyzed according to treatment assignment (i.e. intention to treat).
RESULTS
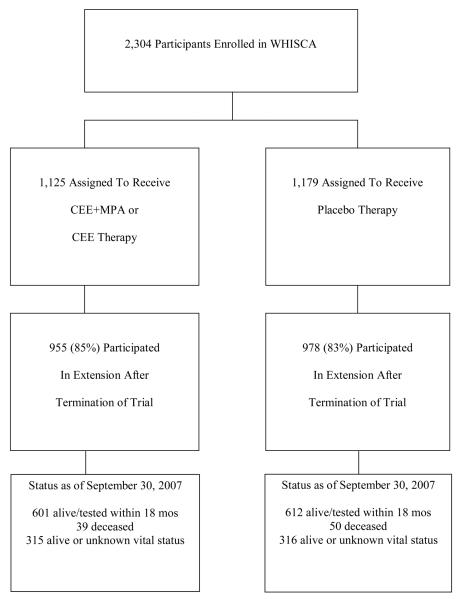
Table 1 presents a description of the 2,304 WHISCA participants at the time of their enrollment in the WHI. Although there were differences in the characteristics of women between trials with respect to many factors, within both trials randomization provided good balance between active intervention and placebo. Figure 1 describes follow-up. Of the original cohort, 1933 (84%) women consented to and attended at least one post-trial cognitive assessment. With respect to the factors in Table 1 and compared to those who did not participate in post-trial follow-up, these women at WHI enrollment tended to be younger, non-smokers, free of diabetes or cardiovascular disease, and prior users of oral contraceptives and to have higher 3MS scores (all p<0.05). Post-trial participation rates were similar between women assigned to active versus placebo therapy (p=0.21). Among women from the WHI CEE+MPA trial, the average (standard deviation) on-trial pre-WHISCA, on-trial WHISCA, and post-trial follow-ups were 3.0 (0.7), 2.6 (0.9), and 4.0 (1.3) years. Among women from the WHI CEE-Alone trial, these averages were 3.0 (0.7), 3.6 (0.4), and 2.4 (1.1) years. None of these averages differed significantly between women assigned to active versus placebo therapy (all p>0.10).
Table 1.
Characteristics of participants at the time of enrollment into the WHI by treatment assignment: frequency and (percent).
| Variable | WHISCA CEE+MPA Trial |
WHISCA CEE-Alone Trial |
Active vs Placebo p-value |
Between Trials p-value |
||
|---|---|---|---|---|---|---|
| CEE+ MPA |
Placebo | CEE- Alone |
Placebo | |||
| Age--yrs, No. (%) | 0.22 | 0.18 | ||||
| 65-69 | 337 (49) | 346 (48) | 193 (44) | 204 (45) | ||
| 70-74 | 258 (37) | 260 (36) | 169 (39) | 163 (36) | ||
| 75+ | 96 (14) | 121 (17) | 72 (17) | 85 (19) | ||
|
| ||||||
| Education, No. (%) | 0.92 | <0.0001 | ||||
| < High school | 31 (5) | 35 (5) | 26 (6) | 27 (6) | ||
| High school/GED | 119 (17) | 152 (21) | 116 (27) | 107 (24) | ||
| > High school < 4 yr college | 292 (42) | 288 (40) | 176 (41) | 191 (42) | ||
| ≥ 4 yr college | 247 (36) | 251 (35) | 116 (27) | 127 (28) | ||
|
| ||||||
| Ethnicity, No. (%) | 0.29 | <0.0001 | ||||
| American Ind/Alaskan Native | 2 (0) | 1 (0) | 3 (1) | 0 (0) | ||
| Asian/Pacific Islander | 8 (1) | 9 (1) | 7 (1) | 2 (0) | ||
| Black/African-American | 31 (4) | 32 (4) | 38 (9) | 44 (10) | ||
| Hispanic/Latino | 11 (2) | 5 (1) | 7 (1) | 6 (1) | ||
| White, non-Hispanic | 633 (92) | 676 (93) | 372 (86) | 393 (87) | ||
| Other | 6 (1) | 4 (1) | 7 (1) | 7 (1) | ||
|
| ||||||
| Smoking Status, No. (%) | 0.70 | 0.31 | ||||
| Never | 355 (52) | 401 (56) | 257 (60) | 244 (54) | ||
| Former | 283 (42) | 283 (39) | 157 (37) | 170 (38) | ||
| Current | 42 (6) | 36 (5) | 16 (4) | 34 (8) | ||
|
| ||||||
| Body mass index--kg/m2, No. (%) | 0.57 | <0.001 | ||||
| < 25 | 216 (31) | 243 (34) | 110 (25) | 110 (25) | ||
| 25-29 | 247 (36) | 252 (35) | 157 (36) | 164 (37) | ||
| 30-34 | 141 (20) | 152 (21) | 96 (22) | 109 (24) | ||
| 35+ | 86 (12) | 77 (11) | 70 (16) | 65 (15) | ||
|
| ||||||
| Hypertension status, No. (%) | 0.85 | <0.001 | ||||
| None | 384 (56) | 393 (54) | 201 (46) | 221 (49) | ||
| Current / controlled* | 93 (13) | 96 (13) | 83 (19) | 79 (17) | ||
| Current / uncontrolled | 214 (31) | 238 (33) | 150 (35) | 152 (34) | ||
|
| ||||||
| Alcohol intake—drinks, No. (%) | 0.88 | <0.001 | ||||
| None | 286 941) | 298 (41) | 211 (49) | 225 (50) | ||
| <1/day | 304 (44) | 330 (45) | 189 (44) | 173 (38) | ||
| 1-2/day | 88 (13) | 87 (12) | 30 (7) | 44 (10) | ||
| ≥3/day | 12 (2) | 12 (2) | 4 (1) | 8 (2) | ||
|
| ||||||
| Prior CVD, No. (%) | 0.54 | <0.001 | ||||
| No | 630 (91) | 679 (93) | 384 (88) | 395 (87) | ||
| History of stroke | 7 (1) | 7 (1) | 5 (1) | 8 (2) | ||
| History of other CVDǐ | 54 (8) | 41 (6) | 45 (10) | 49 (11) | ||
|
| ||||||
| Diabetes, No. (%) | 0.48 | <0.001 | ||||
| No | 653 (95) | 681 (94) | 390 (90) | 403 (89) | ||
| Yes | 37 (5) | 45 (6) | 44 (10) | 49 (11) | ||
|
| ||||||
| Prior use of hormone therapy | 0.85 | <0.001 | ||||
| No | 545 (79) | 562 (77) | 219 (50) | 243 (54) | ||
| Yes | 146 (21) | 165 (23) | 215 (50) | 209 (46) | ||
|
| ||||||
| Ever use of oral contraceptives | 0.55 | 0.02 | ||||
| No | 482 (70) | 515 (71) | 336 (77) | 329 (73) | ||
| Yes | 209 (30) | 212 (29) | 98 (23) | 123 (27) | ||
|
| ||||||
| 3MSE at WHI enrollment | 0.72 | <0.001 | ||||
| <90 | 39 (6) | 37 (5) | 39 (9) | 37 (8) | ||
| 90-94 | 120 (17) | 133 (18) | 98 (23) | 107 (24) | ||
| ≥95 | 532 (77) | 557 (77) | 297 (68) | 308 (68) | ||
Measured to be less than 140/90 mmHg.
Other CVD defined as myocardial infarction, angina, percutaneous transluminal coronary angioplasty, or coronary artery bypass graft
Figure 1.
Follow-up during WHISCA and the WHISCA Extension
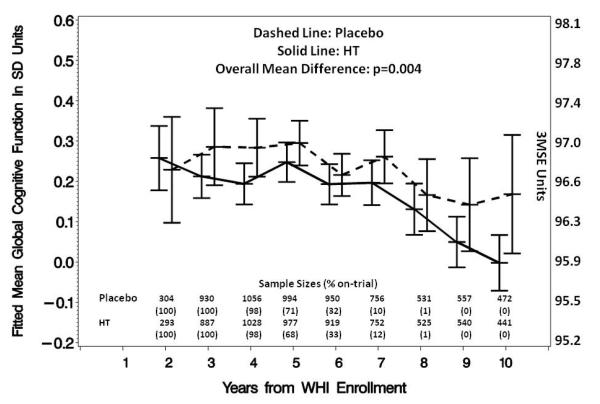
Table 2 provides a comparison of the on-trial and post-trial mean test scores and the estimated relative treatment effects for each standardized domain score, with mean effect sizes expressed in terms of standard deviation units. During the trials, CEE-based therapies were associated with a mean (standard error) relative decrement in global cognition of 0.073 (0.027) SD units; during post-trial follow-up, this relative decrement was 0.070 (0.025) SD units and the average relative decrement across the full span of follow-up was 0.071 (0.025) SD units. Each of these decrements in global cognitive function was statistically significant p≤0.01 and the on-trial and post-trial mean decrements were of similar magnitudes (p=0.89). Figure 2a portrays the fitted mean global cognitive function scores collected during WHISCA follow-up. In this cohort, relative decrements occurred, on average, 3-4 years from WHI enrollment and were maintained across time. Note that the termination of the CCE+MPA trial occurred an average of 5.6 (1.0) years from WHI enrollment and the termination of the CEE-Alone trial occurred an average of 6.6 (0.6) years from WHI enrollment for these women. As indicated in the figure, 6 years from randomization only 32% of the measurements occurred during the trial and in later years, almost all occurred after the termination of the trials.
Table 2.
Mean on-trial, post-trial, and overall test scores and, expressed in standard deviation units, relative domain-specific treatment effects ([unk]active minus placebo): adjustment for baseline 3MS score, trial (CEE+MPA vs CEE-Alone), time since WHISCA enrollment, age, education, race/ethnicity, smoking status, body mass index, hypertension, alcohol intake, prior cardiovascular disease, diabetes, prior use of hormone therapy, and prior use of oral contraceptives.
| Cognitive domain [Range] | On-trial | Post-trial | Overall | Consistency of on-trial and post-trial effects p-value |
|||
|---|---|---|---|---|---|---|---|
| Mean (SE) | p-value | Mean (SE) | p-value | Mean (SE) | p-value | ||
|
Global cognitive function 3MSE [0-100] |
|||||||
| Active | 96.58 (0.09) | 96.44 (0.09) | 96.51 (0.07) | ||||
| Placebo | 96.84 (0.09) | 96.68 (0.09) | 96.76 (0.07) | ||||
| Domain treatment effect, SD | −0.073 (0.027) | 0.007 | −0.070 (0.028) | 0.01 | −0.071 (0.025) | 0.004 | 0.89 |
|
| |||||||
|
Verbal knowledge PMA Vocabulary [0-50] |
|||||||
| Active | 37.21 (0.24) | 37.53 (0.24) | 37.37 (0.23) | ||||
| Placebo | 37.93 (0.24) | 38.04 (0.24) | 37.99 (0.23) | ||||
| Domain treatment effect, SD | −0.066 (0.032) | 0.04 | −0.053 (0.033) | 0.11 | −0.060 (0.031) | 0.05 | 0.53 |
|
| |||||||
|
Verbal fluency Letter Fluency [≥0] |
|||||||
| Active | 41.20 (0.32) | 41.56 (0.33) | 41.36 (0.29) | ||||
| Placebo | 41.76 (0.32) | 41.49 (0.32) | 41.64 (0.29) | ||||
| Category Fluency [≥0] | |||||||
| Active | 28.05 (0.16) | 28.18 (0.16) | 28.11 (0.13) | ||||
| Placebo | 28.60 (0.15) | 28.32 (0.16) | 28.47 (0.13) | ||||
| Domain treatment effect, SD | −0.083 (0.034) | 0.02 | −0.006 (0.035) | 0.86 | −0.048 (0.032) | 0.13 | 0.009 |
|
| |||||||
|
Verbal memory CVLT-A [0-48] |
|||||||
| Active | 27.33 (0.16) | 27.38 (0.16) | 27.35 (0.13) | ||||
| Placebo | 27.77 (0.16) | 27.68 (0.16) | 27.73 (0.13) | ||||
| CVLT-A Long Delay [0-16] | |||||||
| Active | 9.16 (0.08) | 9.10 (0.08) | 9.13 (0.06) | ||||
| Placebo | 9.27 (0.08) | 9.08 (0.08) | 9.17 (0.06) | ||||
| CVLT-A Short Delay [0-16] | |||||||
| Active | 8.29 (0.08) | 8.19 (0.08) | 8.24 (0.06) | ||||
| Placebo | 8.32 (0.08) | 8.21 (0.08) | 8.27 (0.06) | ||||
| Domain treatment effect, SD | −0.039 (0.034) | 0.24 | −0.013 (0.035) | 0.70 | −0.027 (0.030) | 0.37 | 0.43 |
|
| |||||||
| Figural memory | BVRT [0-26] | ||||||
| Active | −6.93 (0.10) | −6.97 (0.10) | −6.95 (0.09) | ||||
| Placebo | −6.73 (0.10) | −6.88 (0.10) | −6.80 (0.09) | ||||
| Domain treatment effect | −0.062 (0.031) | 0.05 | −0.026 (0.032) | 0.42 | −0.045 (0.027) | 0.09 | 0.28 |
|
| |||||||
|
Attention and working memory |
|||||||
| Digit Span Forward [0-14] | |||||||
| Active | 7.45 (0.06) | 7.51 (0.06) | 7.48 (0.05) | ||||
| Placebo | 7.58 (0.06) | 7.62 (0.06) | 7.60 (0.05) | ||||
| Digit Span Backward [0-14] | |||||||
| Active | 6.56 (0.05) | 6.60 (0.05) | 6.58 (0.04) | ||||
| Placebo | 6.66 (0.05) | 6.67 (0.05) | 6.67 (0.04) | ||||
| Domain treatment effect, SD | −0.064 (0.037) | 0.08 | −0.039 (0.037) | 0.29 | −0.053 (0.036) | 0.14 | 0.21 |
|
| |||||||
| Spatial ability | |||||||
| Card Rotations Test [0-160] | |||||||
| Active | 60.30 (0.75) | 59.08 (0.75) | 59.70 (0.67) | ||||
| Placebo | 61.55 (0.73) | 60.52 (0.74) | 61.04 (0.66) | ||||
| Domain treatment effect | −0.050 (0.039) | 0.20 | −0.040 (0.039) | 0.31 | −0.045 (0.038) | 0.23 | 0.59 |
|
| |||||||
| Fine motor speed | |||||||
| Finger Tapping (dominant hand) [≥0] |
|||||||
| Active | 39.37 (0.19) | 39.02 (0.19) | 39.20 (0.16) | ||||
| Placebo | 39.73 (0.18) | 39.37 (0.19) | 39.55 (0.16) | ||||
| Finger Tapping (non- dominant hand) [≥0] |
|||||||
| Active | 36.63 (0.17) | 36.51 (0.17) | 36.57 (0.16) | ||||
| Placebo | 36.92 (0.17) | 36.71 (0.17) | 36.82 (0.15) | ||||
| Domain treatment effect, SD | −0.068 (0.034) | 0.05 | −0.045 (0.035) | 0.20 | −0.057 (0.030) | 0.05 | 0.54 |
Figure 2a.
Fitted mean (with 95% confidence intervals) standardized global cognition test scores over time from women grouped by WHI treatment assignment with adjustment for baseline 3MS score, trial (CEE+MPA vs CEE-Alone), age, education, race/ethnicity, smoking status, body mass index, hypertension, alcohol intake, prior cardiovascular disease, diabetes, prior use of hormone therapy, and prior use of oral contraceptives. The left-hand axis provides units in the original scale. The lower panel provides the number of women who contributed to each data point and the percentage of measurements that were collected during on-trial.
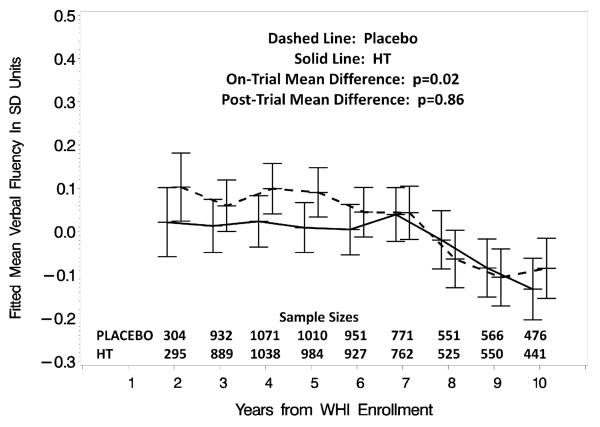
There was a mean on-trial relative decrement for each domain-specific cognitive function; these reached statistical significance (p≤0.05) for verbal knowledge, verbal fluency, figural memory, and fine motor speed. The magnitudes of effect sizes were uniformly smaller during the post-trial and none were significant on their own, however on-trial and post-trial decrements differ significantly only for verbal fluency (p=0.009). Figure 2b portrays the pattern over time of the means for verbal fluency, for which the initial relative decrement, which was apparent as early as Year 1, dissipated from 6 years on during follow-up.
Figure 2b.
Fitted mean (with 95% confidence intervals) standardized verbal fluency test scores from women grouped by WHI treatment assignment with adjustment for baseline 3MS score, trial (CEE+MPA vs CEE-Alone), age, education, race/ethnicity, smoking status, body mass index, hypertension, alcohol intake, prior cardiovascular disease, diabetes, prior use of hormone therapy, and prior use of oral contraceptives.
There was little difference in the relative effects of CEE+MPA versus CEE on global cognitive function, both during on-trial and post-trial follow-up (Table 3). For three domains, however, there was some evidence of differences between the two drug regimens. For figural memory (p=0.05), spatial ability (p=0.04), and fine motor speed (p=0.01), the on-trial decrements appeared to be limited to CEE therapy and were not apparent for CEE+MPA therapy. For the spatial ability, the differences in the effect sizes between regimens persisted into the post-trial follow-up (p=0.01).
Table 3.
Mean on-trial, post-trial, and overall relative treatment effects: adjustment for baseline 3MS score, trial (CEE+MPA vs CEE-Alone), time since WHISCA enrollment, age, education, race/ethnicity, smoking status, body mass index, hypertension, alcohol intake, prior cardiovascular disease, diabetes, prior use of hormone therapy, and prior use of oral contraceptives.
| Cognitive Domain | Mean Decrement in SD Units CEE-based Versus Placebo Therapy |
|||||
|---|---|---|---|---|---|---|
| On-trial | Post-trial | Overall | ||||
| Mean (SE) |
p-value | Mean (SE) | p-value | Mean (SE) | p-value | |
| Global cognitive function | ||||||
| CEE-Alone | −0.092 (0.039) |
0.02 | −0.081 (0.047) |
0.09 | −0.086 (0.035) |
0.01 |
| CEE+MPA | −0.080 (0.034) |
0.02 | −0.059 (0.032) |
0.06 | −0.069 (0.027) |
0.01 |
| CEE-Alone vs CEE+MPA | 0.83 | 0.70 | 0.68 | |||
|
| ||||||
| Verbal knowledge | ||||||
| CEE-Alone | −0.100 (0.051) |
0.05 | −0.071 (0.054) |
0.19 | −0.089 (0.050) |
0.07 |
| CEE+MPA | −0.044 (0.041) |
0.29 | −0.040 (0.041) |
0.33 | −0.042 (0.039) |
0.28 |
| CEE-Alone vs CEE+MPA | 0.39 | 0.65 | 0.46 | |||
|
| ||||||
| Verbal fluency | ||||||
| CEE-Alone | −0.118 (0.054) |
0.03 | −0.092 (0.060) |
0.13 | −0.099 (0.058) |
0.09 |
| CEE+MPA | −0.062 (0.044) |
0.16 | 0.039 (0.044) |
0.38 | −0.004 (0.046) |
0.94 |
| CEE-Alone vs CEE+MPA | 0.43 | 0.08 | 0.20 | |||
|
| ||||||
| Verbal memory | ||||||
| CEE-Alone | −0.040 (0.057) |
0.49 | −0.049 (0.062) |
0.43 | −0.043 (0.056) |
0.44 |
| CEE+MPA | −0.009 (0.047) |
0.84 | −0.020 (0.046) |
0.67 | −0.015 (0.044) |
0.74 |
| CEE-Alone vs CEE+MPA | 0.68 | 0.71 | 0.69 | |||
|
| ||||||
| Figural memory | ||||||
| CEE-Alone | −0.132 (0.048) |
0.006 | −0.009 (0.057) |
0.88 | −0.086 (0.044) |
0.05 |
| CEE+MPA | −0.008 (0.041) |
0.85 | −0.030 (0.040) |
0.45 | −0.020 (0.034) |
0.57 |
| CEE-Alone vs CEE+MPA | 0.05 | 0.76 | 0.24 | |||
|
| ||||||
| Attention and working memory |
||||||
| CEE-Alone | −0.063 (0.054) |
0.24 | −0.088 (0.060) |
0.14 | −0.073 (0.051) |
0.15 |
| CEE+MPA | −0.077 (0.044) |
0.08 | −0.052 (0.044) |
0.24 | −0.064 (0.040) |
0.11 |
| CEE-Alone vs CEE+MPA | 0.85 | 0.62 | 0.89 | |||
|
| ||||||
| Spatial ability | ||||||
| CEE-Alone | −0.137 (0.057) |
0.02 | −0.179 (0.063) |
0.004 | −0.153 (0.055) |
0.005 |
| CEE+MPA | 0.013 (0.047) |
0.78 | 0.019 (0.047) |
0.68 | 0.016 (0.043) |
0.70 |
| CEE-Alone vs CEE+MPA | 0.04 | 0.01 | 0.02 | |||
|
| ||||||
| Fine motor speed | ||||||
| CEE-Alone | −0.171 (0.053) |
0.001 | −0.120 (0.062) |
0.06 | −0.153 (0.048) |
0.002 |
| CEE+MPA | 0.006 (0.045) |
0.90 | −0.002 (0.043) |
0.97 | 0.002 (0.038) |
0.96 |
| CEE-Alone vs CEE+MPA | 0.01 | 0.12 | 0.01 | |||
DISCUSSION
To investigate whether CEE therapies affected age-related cognitive decline, the WHISCA investigators predefined rates of changes in annual cognitive test scores from the time of WHISCA enrollment to be the primary outcomes of the trial. For women who had been prescribed CEE-based therapies an average of 3 years earlier, they found that no significant improvement in the trajectories of these slopes related to hormone therapy. Instead, CEE-Alone appeared to have produced a small average relative decrement in spatial ability that waned during WHISCA follow-up and CEE+MPA therapy was associated with a continued small deleterious relative effect on age-related declines in verbal memory.4,6 Overall, however, there was little evidence that continued prescription of the CEE-based therapies produced widening differences in cognitive functions across multiple domains.
The analyses reported here differ from those pre-defined in the WHISCA protocol3 in four important ways. First, they are focused on estimating differences in the average cognitive scores over time rather than the slopes of these differences. Second, visits are ordered according to time from initial prescription of WHI treatments, rather than time from WHISCA enrollment. Third, rather than analyzing scores from individual tests, we have combined some to form domain scores. Finally, both on-trial and post-trial data are considered.
We derive three principal points, and take up each in turn. First, CEE-based therapies were associated with small, but detectable, mean on-trial decrements in global cognitive function that were largely maintained for several years after the conclusion of the trials. In pooled analyses, CEE-based therapies were associated with on-trial decrements among the individual domains whose magnitudes spanned those for global cognitive function and some of which reached statistical significance on-trial, but none post-trial. Finally, while the magnitude of the CEE-Alone and CEE+MPA effects on global cognition were fairly comparable throughout follow-up, CEE-Alone therapy appeared to be associated with greater adverse domain-specific effects.
Enduring Deficits in Global Cognitive Function
For the full WHIMS cohort, the pooled on-trial mean decrement in 3MS scores associated with assignment to CEE-based therapies was about 0.05 SD units,1,2 which was slightly lower than seen during follow-up of the WHISCA cohort. The reason for this is likely that the first few years following randomization were included in the WHIMS estimates, when differences were just beginning to emerge; the on-trial estimates from WHISCA more heavily reflect the later years of the WHIMS trial, when differences were more marked. These average differences in cognitive function are small and would not be detectable or have clinical significance for an individual woman. Of some concern is that these appear to persist long after cessation of hormone therapy and likely are linked to the smaller regional brain volumes as evident in MRI scans taken up to three years following the termination of these trials. It appears that these smaller volumes are an expression of how CEE-based therapies affect cognitive function: women who developed cognitive impairment after assignment to these therapies have smaller brain volumes than non-impaired women while women who developed cognitive impairment after assignment to placebo did not.21
The potential mechanisms for this broad-based effect are presently not known. Candidates include disruption of balance between neurogenesis and apoptosis, a loss of estrogen sensitivity and reversal of neuroprotective effects following prolonged hypoestrogenicity, or actions of equine estrogens on glial cells.22-26 Despite the increase risk of (predominantly ischemic) stroke associated with CEE-based therapies,27 data from the WHIMS-MRI study suggest that the principal pathway for its adverse effect on cognition is not through an increase of ischemic lesions.28 Whether these adverse effects are limited to initiation of hormone therapy in women over the age of 65 and to only CEE-based therapies is unknown. The magnitudes of these effects are small, however they may translate to significant increases in the risk of dementia and mild cognitive impairment.29,30
Effects on Domain-Specific Cognitive Functions
Much has been written about the potential beneficial effects of hormone therapy on specific cognitive domains in younger women and laboratory animals.22,31-33 In general, beneficial effects have been most consistently found for verbal memory and attention, however evidence for positive effects have been found for many domains. In older women, the evidence for domain-specific benefit is less clear.22,32,34 In general, we found the on-trial adverse effects of hormone therapy on individual cognitive domains to be more-or-less comparable in magnitude to those on global cognitive function, but less statistically significant. The lower power to detect differences for individual domains was not attributable to measurement error (the within-subject longitudinal correlations of domain specific tests were at least as high as for global cognitive function), but was likely due to the lack of a domain-specific baseline test score for use as a covariate (using baseline global cognitive function for this purpose did not improve power uniformly across all domains). The smallest on-trial adverse effects were found for verbal memory, raising the possibility that there may have been some moderation of the general adverse effect by other mechanisms for this domain, however this is speculative.
Overall, the magnitudes of domain-specific post-trial adverse effects were smaller than on-trial effects, but not significantly so, with one exception. The on-trial effect on verbal fluency, which was the largest in magnitude of any domain, virtually disappeared during post-trial follow-up. Laughlin, et al. have reported that higher levels of endogenous hormones are associated with lower performance in verbal fluency,35 which may signal an acute affect linked to elevated androgen levels, and these have been reported to suppress verbal fluency.36,37 Thus, it is possible that this provides evidence a second mechanism is involved whereby CEE-based therapies adversely affect cognition.
Differences between CEE+MPA and CEE-Alone Therapies
While CEE-Alone and CEE+MPA therapy had fairly comparable on-trial and post-trial effects on global cognitive function, some differences appeared among domain-specific tests. CEE-Alone therapy was associated with on-trial decrements in verbal knowledge, verbal fluency, figural memory, spatial ability, and fine motor speed (all p≤0.05), while CEE+MPA therapy was not. Several possible explanations may exist. The longer duration of the CEE-Alone trial (an average of 4.0 versus 3.0 years) is one: the means of on-trial effects for the CEE-Alone women more heavily weight longer exposures, when treatment effects were largest. Also, the women from the CEE-Alone trial, tended to have greater pre-trial risk factors for cognitive impairment (including lower education, lower income, and more prevalent hypertension and cardiovascular disease) and lower levels of global cognitive function. These may identify women with greater levels of pre-existing disease cerebrovascular disease, for whom the adverse effect of CEE-based therapies may be greater.6 Finally, it may be that MPA offers some protection against domain-specific adverse effects. If this is so, the strongest evidence for this may be for spatial ability: the on-trial adverse effect of CEE-Alone therapy on spatial ability continued post-trial, however no on-trial or post-trial effects for CEE+MPA therapy were seen. If MPA has a differential effect among global and domain-specific functions, this may be evidence of additional mechanisms linking hormone therapy to cognition.
Limitations
The WHISCA study was limited to postmenopausal women aged 65 years or older and only 0.625 mg/day CEE-based therapies. While we cannot rule out imbalances among treatment groups among women who enrolled in WHISCA with respect to unmeasured characteristics, none were detected among measured characteristics and extensive covariate-adjustment was used to promote balanced comparisons. We have not included data on the use of hormone therapies among WHISCA women following the WHI trials. Overall, this has been reported to be quite low, e.g. only 4% of participants who had been assigned to CEE+MPA therapy reported using any sort of hormone therapy one year after the termination of the trial.38 We report the results from many inferences. Because of this, Type 1 error is inflated across the individual tests. The magnitude of the estimated mean effects, <10% of a standard deviation, are small and would be clinically undetectable for women.
Conclusions
CEE-based hormone therapies, when initiated among women aged 65 years or older and provided for an average of 5 to 7 years, produced small average decrements in global and several domain-specific cognitive functions that endured several years after cessation of use. No evidence of benefit for any cognitive domain was found, however differences in how CEE-Alone and CEE+MPA affect domain-specific cognitive test scores suggest that more than one mechanism may be involved.
NIA Program Office
National Institute of Aging, Baltimore, MD: Alan Zonderman, Susan M. Resnick
WHISCA Central Coordinating Center
Wake Forest University Health Sciences, Winston-Salem, NC: Sally Shumaker, Principal Investigator; Stephen Rapp, Mark Espeland, Laura Coker, Deborah Farmer, Anita Hege, Patricia Hogan, Darrin Harris, Cynthia McQuellon, Anne Safrit, Lee Ann Andrews, Candace Warren, Carolyn Bell, Linda Allred
WHISCA Clinical Sites
Women's Health Initiative, Durham, NC, Carol Murphy; Rush Presbyterian-St. Luke's Medical Center, Chicago, IL, Linda Powell; Ohio State University Medical Center, Columbus, OH, Rebecca Jackson; University of California at Davis, Sacramento, CA, John Robbins; University of Iowa College of Medicine, Des Moines, IA, Robert Wallace; University of Florida, Gainesville/Jacksonville, FL, Marian Limacher; University of California at Los Angeles, Los Angeles, CA, Howard Judd; Medical College of Wisconsin, Milwaukee, WI, Jane Kotchen; The Berman Center for Outcomes and Clinical Research, Minneapolis, MN, Karen Margolis; University of Nevada School of Medicine, Reno, NV, Robert Brunner; Albert Einstein College of Medicine, Bronx, NY, Sylvia Smoller; The Leland Stanford Junior University, San Jose, CA, Marcia Stefanick; The State University of New York, Stony Brook, NY, Dorothy Lane; University of Massachusetts/Fallon Clinic, Worcester, MA, Judith Ockene
* The following investigators were the original investigators for these sites: Mary Haan, Davis; Richard Grimm, Minneapolis; Sandra Daugherty (deceased), Nevada
WHI Program Office
National Heart, Lung, and Blood Institute, Bethesda, MD: Barbara Alving, Jacques Rossouw, Linda Pottern
WHI Central Coordinating Center
Fred Hutchinson Cancer Research Center, Seattle, WA: Deborah Bowen, Gretchen Van Lom, Carolyn Burns
ACKNOWLEDGMENTS
The Women's Health Initiative Study of Cognitive Aging was supported by the Department of Health and Human Services and the National Institute on Aging, NO1-AG-1-2106, National Institutes of Health, Bethesda, Maryland. The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute, U.S. Department of Health and Human Services. Wyeth Pharmaceuticals provided the study drug and the placebo to the WHI trial. The Women's Health Initiative Memory Study was funded by Wyeth Pharmaceuticals, Inc, St. Davids, PA, Wake Forest University, and the National Heart, Lung, and Blood Institute. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Sponsor's Role: Indicate sponsor's role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
The NIH is represented by Dr. Resnick on this publication, who played a central role in the conception and design of the WHISCA trial and who fully meets criteria for authorship of this manuscript.
Wyeth-Ayerst, an original sponsor of the WHIMS trial, had no role in design, methods, subject recruitment, data collections, analysis and preparation of this paper.
Footnotes
ClinicalTrials.gov Identifiers: NCT00000611 (WHIMS)
| Elements of Financial/Personal Conflicts |
*Author 1 | Author 2 | Author 3 | Etc. | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Grants/Funds | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Honoraria | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Speaker Forum | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Consultant | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Stocks | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Royalties | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Expert Testimony | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Board Member | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Patents | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
| Personal Relationship | ||||||||
| MAE | RLB | PAH | SRR | |||||
| LHC | ||||||||
| CL | ||||||||
| IG | ||||||||
| SMR | ||||||||
REFERENCES
- 1.Rapp S, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 2.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 3.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clinical Trials. 2004;1:440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 4.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect: The Women's Health Initiative Study of Cognitive Aging (WHISCA) J Clin Endocrinol Metabol. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 5.Resnick SM, Espeland MA, An Y, et al. Effects of conjugated equine estrogens on cognition and affect in surgically menopausal women. J Clin Endocrinol Metab. 2009;94:4152–61. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resnick SR, Espeland MA, Jaramillo SA, et al. Effects of postmenopausal hormone therapy on regional brain volumes in older women: The Women's Health Initiative Magnetic Resonance Imaging Study (WHIMS-MRI) Neurology. 2009;72:135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 8.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 9.Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.The Women's Health Initiative Steering Committee Effects of conjugated equine estrogens in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 11.Kuse AR. Familial resemblances for cognitive abilities from two test batteries in Hawaii. University of Colorado; Boulder: 1977. Unpublished doctoral dissertation. [Google Scholar]
- 12.Benton A. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 13.Newcombe F. A study of psychological deficits. Oxford University Press; London: 1969. Missile wounds of the brain. [Google Scholar]
- 14.Benton AL, Eslinger PJ, Damasio AR. Normative observations on neuropsychological test performances in old age. J Clin Neuropsych. 1981;3:33–42. doi: 10.1080/01688638108403111. [DOI] [PubMed] [Google Scholar]
- 15.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Research Edition. The Psychological Corporation; New York: 1987. [Google Scholar]
- 16.Wechsler D. Weschler Adult Intelligence Scale - Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- 17.Ekstrom RB, French JW, Harman HH. Manual for Kit of Factor-Referenced Cognitive Tests. Educational Testing Service; Princeton: 1976. [Google Scholar]
- 18.Halstead WC. Brain and intelligence. University of Chicago Press; Chicago: 1947. [Google Scholar]
- 19.Teng EL, Chui HC. The Modified Mini Mental State (3MS) Examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 20.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute, Inc.; Cary, NC: 1996. [Google Scholar]
- 21.Espeland MA, Tindle HA, Bushnell CA, et al. Brain volumes, cognitive impairment, and conjugated equine estrogens. J Gerontol Med Sci. 2009;64:1243–50. doi: 10.1093/gerona/glp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users, and men: a possible window of opportunity effect. Neurobiol Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Brown CM, Dela Cruz CD, Ynag E, Bridwell DA, Wise PH. Timing of estrogen therapy after oviarectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. PNAS. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer JL, Waters MW, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncoveringthe mechanisms of estrogen effects on hippocampal function. Front Neruoendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozovsky I, Hoving S, Anderson CP, et al. Equine estrogens induce apolipoprotein E and glial fibrillary acidic protein in mixed glial cultures. Neurosci Letters. 2002;323:191–194. doi: 10.1016/s0304-3940(02)00146-5. [DOI] [PubMed] [Google Scholar]
- 27.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 28.Coker LH, Hogan PE, Bryan NR, et al. The effects of postmenopausal hormone therapy on volumetric sub-clinical cerebrovascular disease: The Women's Health Initiative Memory Study - Magnetic Resonance Imaging Study (WHIMS-MRI) Neurology. 2009;72:125–134. doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shumaker S, Legault C, Rapp S, et al. The effects of estrogen plus progestin on the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women's Health Initiative Memory Study (WHIMS) JAMA. 2003;289:2663–2674. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 30.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogen alone, pooled hormone therapy, and incidence of probable dementia and mild cognitive impairment in postmenopausal women: results from the Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 31.Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function. Ann N.Y Acad Sci. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 32.Maki PM, Sundermann E. Hormone therapy and cognitive function. Human Reproduct Update. 2009;00:1–15. doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voytko ML, Tinkler GP, Browne C, Tobin JR. Neuroprotective effects of estrogen therapy for cognitive and neurobiological profiles of monkey models of menopause. Am J Primatol. 2009;71:794–801. doi: 10.1002/ajp.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lethaby A, Hogervorst E, Richards M, Yesafu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. The Cochrane Library. 4:2008. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laughlin GA, Kritz-Silverstein D, Barrett-Connor E. Higher endogenous levels predict four year decline in verbal fluency in postmenopausal women: the Rancho Bernardo Study. Clin Endocrinol. doi: 10.1111/j.1365-2265.2009.03599.x. (in press): doi: 10.1111/j.1365-2265.2009.03599.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shattmann L, Sherwin BB. Testosterone levels and cognitive functioning in women with polycycstic ovary syndrome and in healthy young women. Horm Behav. 2007;51:587–596. doi: 10.1016/j.yhbeh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–193. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]