Abstract
Purpose
The trend toward personalized medicine will involve cancer treatment increasingly being tailored to the genetic characteristics of individuals. However, the availability of genetic information does not imply this information is desired or would impact treatment decision making.
Methods
One hundred sixty breast cancer survivors (BC group) and 205 healthy controls (HC group) were randomly assigned to respond to two different clinical scenarios varying in genetic-related risk of cognitive impairment (CI; little v very likely) and severity of CI (little v moderate problem) after chemotherapy. Ratings of the importance of being told this genetic information (information importance) and the likelihood this information would affect their decision to receive chemotherapy (information impact) were obtained.
Results
Results indicated the importance ascribed to genetic information was greatest when CI likelihood and severity were both high or low (P < .05). Information impact ratings were not sensitive to differences in CI likelihood or severity; the BC group was less likely to indicate genetic information would affect their decision to receive chemotherapy than the HC group (P < .001).
Conclusion
Results suggest lessened enthusiasm for genetic information that maintains or increases uncertainty about a specific course of action and highlight the importance of including clinically relevant groups in treatment decision-making research that employs hypothetical scenarios. Although women generally believe it is important to receive genetic information, they might benefit from assistance (eg, decision aid) in the difficult task of integrating information about survival and risk for adverse late effects from cancer treatment.
INTRODUCTION
Personalized medicine means treatments will be tailored increasingly to the genetic characteristics of individuals.1–2 In oncology, some important clinical outcomes, such as risk for breast and ovarian cancer3–5 or recurrence risk in women with early-stage breast cancer (BC),6–7 are linked to genetic factors. Consequently, decision making regarding cancer treatment, screening, and/or prophylaxis is increasingly likely to include consideration of genetic information.8
The availability of genetic information does not imply this information will impact decision making. Individuals often decline the opportunity to undergo genetic testing or decline disclosure of test results.9–10 Even when genetic test results are considered, their impact on treatment decision making is influenced by a host of factors. For example, the anticipated likelihood and severity of a clinical outcome would be expected to influence treatment decision making. People may be more willing to consider genetic information and to base treatment decisions on this information if test results indicate an important clinical outcome is likely and severe as opposed to unlikely and inconsequential.
Information regarding how genetic information might affect cancer treatment decision making is quite limited. Most research has considered women's interest in genetic information about risk for hereditary cancers and consequent decision making about prevention and control options11–14 or BC survivors' interest in chemotherapy after receipt of recurrence risk information on the basis of genomic test results.15–16 Although high interest in genetic information is evident,11,14,17 whether genetic testing to establish risk for hereditary cancer or cancer recurrence influences treatment decisions has not been closely evaluated.8
In addition to identifying risk for cancer diagnosis or recurrence, genetic information might also be linked to risk for treatment late effects. Research suggests the apolipoprotein E gene confers some risk for cognitive impairment (CI) in cancer survivors treated with chemotherapy.18–19 As CI is a fairly common and troubling late effect,20–22 genetic information regarding risk for CI might be important to consider when deciding whether to receive chemotherapy. However, no research has investigated survivors' interest in genetic information regarding CI risk after chemotherapy.
This study examines women's interest in receiving genetic information about risk for CI when considering whether to receive chemotherapy for BC and opinions of whether such information would affect their treatment decisions. We hypothesize interest in genetic information and the degree to which this information affects decisions to receive chemotherapy will be positively associated with the likelihood and severity of potential CI. To examine whether responses vary as a function of experience with BC, responses of BC survivors are compared with those of women without a BC history.
METHODS
Participants
All participants were previously enrolled onto a longitudinal study of the physical and psychosocial impact of adjuvant therapy in early-stage BC. BC participants in this parent study were initially treated at the University of Kentucky Markey Cancer Center or the Moffitt Cancer Center and were recruited after BC surgery but before beginning adjuvant treatment. Healthy control (HC) participants in the parent study were recruited from a commercially available listing of US residents and were matched with women in the BC group on age and zip code of residence. Additional information regarding eligibility criteria and procedures for this separate parent study have been described.23–24 Eligibility criteria for the present cross-sectional study consisted of participation in the parent study.
Procedures and Measures
All procedures were approved by the Institutional Review Boards at both study sites. All participants in the parent study (both BC and HC groups) were contacted by telephone or mail, informed of the current study, and invited to participate. Study materials were mailed, and participants returned a signed copy of the consent form and completed questionnaire via mail. The study questionnaire consisted of five questions.
Genetic knowledge.
Women rated their “knowledge and understanding of genetics and how different characteristics can be inherited from one's parents” on a 5-point Likert scale. The Likert scale ranged from 0 (poor) to 4 (excellent).
Clinical scenarios.
Four questions related to two hypothetical clinical scenarios. In responding to these written scenarios, women were instructed as follows: “Imagine you are a woman who has recently been diagnosed with breast cancer. You are now working with your doctor to decide whether or not you should receive chemotherapy as part of your treatment for breast cancer. There is some evidence to suggest chemotherapy will improve by a little bit your chances of cure and long-term survival. However, chemotherapy causes some adverse effects that can last even after you have finished treatment.”
After this written introduction, women were given one of four versions of scenario one, and each version contained a different combination of two factors: likelihood of CI risk given possession of a particular gene (ie, CI risk factor with two levels: a little likely v very likely) and anticipated CI severity (ie, CI severity factor with two levels: a little problem v moderate problem). The wording of scenario one was as follows: “Based on a blood test, your doctor knows you have a gene that makes it (a little or very) likely you will develop a (little or moderate) problem with your memory or thinking after you have finished chemotherapy. This (little or moderate) problem with your memory and thinking is likely to last for some time.” Women then rated the question, “How important would it be for your doctor to tell you this before you begin chemotherapy?” on a 5-point Likert scale from 1 (not important at all) to 5 (extremely important; ie, information importance).
Respondents were then give one of four versions of scenario two. The four versions of scenario two also differed in likelihood (CI risk: a little likely v very likely) and severity (CI severity: a little problem v moderate problem) of CI given possession of a particular gene. The wording of scenario two was as follows: “Now imagine your doctor told you that you had a gene that makes it (a little or very) likely that you will develop a (little or moderate) problem with your memory or thinking after you have finished chemotherapy. This (little or moderate) problem with your memory and thinking is likely to last for some time.” Women then rated the question, “How likely is it this new information would affect your decision about whether or not you would choose to receive chemotherapy?” on a 4-point Likert scale from 1 (would not affect my decision at all) to 4 (would completely determine my decision; ie, information impact).
Women were randomly assigned to one of the four combinations of CI risk and CI severity. This combination was used in both scenarios one and two for that woman.
Two final questions referred to the clinical scenarios. Women rated the questions, “How confident are you that you understood what you were asked in these last two questions?” on a 4-point Likert scale from 1 (not confident at all) to 4 (extremely confident; ie, scenario confidence) and “How hard was it for you to imagine yourself in the position of a woman with breast cancer using information about her genes to make a decision about whether to undergo chemotherapy?” on a 4-point Likert scale from 1 (not hard at all) to 4 (very hard; ie, scenario difficulty).
Self-reported CI.
Self reports of CI for women in the BC group were obtained using the Multiple Abilities Self-Report Questionnaire (MASQ).25 The MASQ assesses the subjective evaluation of cognitive function in routine daily activities. A total score is calculated (MASQ-Total), and higher scores reflect greater CI. For the BC group, self reports of CI were obtained in the parent study as part of an assessment 6 months after concluding all adjuvant therapy. This assessment occurred a mean of 1.0 year after BC diagnosis (standard deviation [SD], 0.2) and a mean of 4.0 years (SD, 1.4) from completion of the current study questionnaire.
Data Analysis
Analyses were performed by using SPSS, version 17.0 (SPSS, Chicago, IL). The criterion for statistical significance was set at P < .05. For our primary 2 × 2 analysis of variance (ANOVA) analyses, a priori power analysis suggested a sample size of 171 was sufficient to detect a moderate effect size (SD, 0.25) with one covariate and power set at 0.90.26
RESULTS
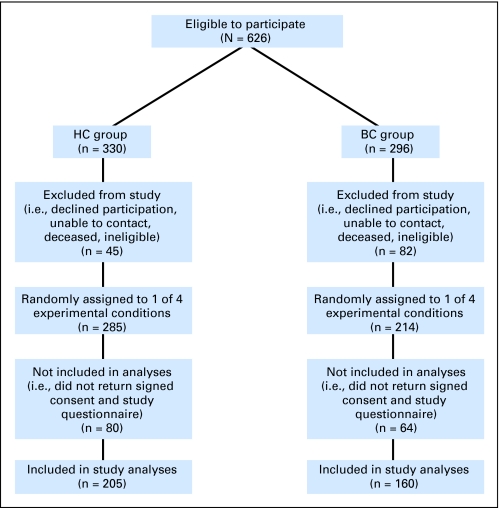
Invitations to participate in this study were issued to 296 and 330 women from the BC and HC groups in the parent study, respectively. Three hundred sixty-five women completed this study: 160 (54.1%) of 296 in the BC group and 205 (62.1%) of 330 in the HC group (Fig 1). Clinical characteristics of the BC group are listed in Table 1. Comparison of the BC and HC groups by using independent samples t test and χ2 analyses revealed no significant differences regarding age, education, study site, or ethnicity (Table 2).
Fig 1.
Flow chart showing study participation. HC, healthy control group; BC, breast cancer group.
Table 1.
Clinical Characteristics of the Breast Cancer Group
| Variable | % of Patients |
|---|---|
| Disease stage | |
| 0 | 11 |
| I | 55 |
| II | 34 |
| Breast surgery | |
| Lumpectomy | 87 |
| Single mastectomy | 8 |
| Lumpectomy plus mastectomy | 3 |
| Double mastectomy | 2 |
| Adjuvant treatment | |
| Radiotherapy only | 47 |
| Chemotherapy only | 7 |
| Radiotherapy and chemotherapy | 46 |
NOTE. Total number of patients = 160.
Table 2.
Comparison of Breast Cancer and Healthy Control Groups
| Variable | Breast Cancer Group (n = 160) |
Healthy Control Group (n = 205) |
P* | ||||
|---|---|---|---|---|---|---|---|
| % of Patients | Mean | SD | % of Patients | Mean | SD | ||
| White, non-Hispanic | 93 | 95 | .205 | ||||
| University of Kentucky study site† | 36 | 45 | .085 | ||||
| Education | .816 | ||||||
| < High school degree | 4 | 5 | |||||
| High school degree/ some college | 50 | 46 | |||||
| ≥ College degree | 46 | 49 | |||||
| Age, years | 58.8 | 9.5 | 59.0 | 9.4 | .850 | ||
| Genetic knowledge | 1.8 | 1.0 | 2.1 | 1.0 | .009 | ||
| Scenario difficulty | 1.5 | 0.9 | 2.2 | 1.1 | .000 | ||
| Scenario confidence | 3.6 | 0.5 | 3.5 | 0.6 | .158 | ||
Abbreviation: SD, standard deviation.
P value for percentages is associated with χ2 test for categoric variables. P value for mean and SD data is associated with independent-samples t-test for continuous variables.
Proportion of participants in breast cancer and healthy control groups enrolled at University of Kentucky study site.
Differences between the BC and HC groups in genetic knowledge, scenario confidence, and scenario difficulty were analyzed by using independent sample t tests (Table 2). The HC group reported greater genetic knowledge (t(363) = 2.61; P < .01) and scenario difficulty (t(363) = 6.29; P < .001). Success of our random assignment was examined by using a series of 2 × 2 × 2 (Group × CI risk × CI severity) ANOVA. Age, education, and genetic knowledge were dependent variables. Results indicated no significant main or interaction effects for education or age. Results indicated a Group × CI risk × CI severity interaction for genetic knowledge (F(1,357) = 5.83; P < .05).
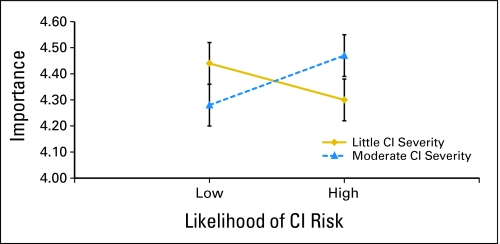
Our primary analyses consisted of two 2 × 2 × 2 (Group × CI risk × CI severity) analyses of covariance (ANCOVA) with genetic knowledge as covariate. The dependent variables were information importance and information impact (Tables 3, 4, 5, and 6). For information importance, results indicated a CI risk × CI severity interaction (F(1,356) = 3.98; P < .05), portrayed in Figure 2. Women in both the BC and HC groups rated the importance of being told information about genetic risk for CI higher when CI risk and CI severity were both low or both high. For information impact, results indicated a main effect for group (F(1,365) = 17.45; P < .001). Women in the BC group were less likely to report information about genetic risk for CI would affect their BC treatment decisions (BC mean, 2.17 [SD, 0.83] v HC mean, 2.54 [SD, 0.80]).
Table 3.
Summary of Analysis of Covariance for Information Importance Ratings
| Cell Means of CI Risk and Severity by Treatment Group | Analysis |
||
|---|---|---|---|
| Mean* | SE | No. of Patients | |
| Little likely and a little | |||
| Breast cancer | 4.40 | 0.12 | 43 |
| Healthy control | 4.51 | 0.11 | 51 |
| Little likely and moderate | |||
| Breast cancer | 4.21 | 0.13 | 38 |
| Healthy control | 4.35 | 0.11 | 48 |
| Very likely and a little | |||
| Breast cancer | 4.23 | 0.12 | 40 |
| Healthy control | 4.37 | 0.10 | 56 |
| Very likely and moderate | |||
| Breast cancer | 4.40 | 0.13 | 39 |
| Healthy control | 4.50 | 0.11 | 50 |
NOTE. Information Importance scores obtained on a 5-point Likert scale from 1 (not important at all) to 5 (extremely).
Abbreviations: CI, cognitive impairment; SE standard error.
Means shown are adjusted for Genetic Knowledge scores.
Table 4.
Analysis of Covariance Summary Table for Information Importance Ratings
| Variable Effect | Analysis |
||
|---|---|---|---|
| F | df | P | |
| CI risk | 0.01 | 1, 356 | .914 |
| CI severity | 0.01 | 1, 356 | .905 |
| Group | 2.40 | 1, 356 | .123 |
| CI risk × CI severity | 3.98 | 1, 356 | .047 |
| CI risk × group | 0.00 | 1, 356 | .970 |
| CI severity × group | 0.01 | 1, 356 | .933 |
| CI risk × CI severity × group | 0.06 | 1, 356 | .812 |
NOTE. Information importance scores were obtained on a 5-point Likert scale from 1 (not important at all) to 5 (extremely).
Abbreviation: CI, cognitive impairment.
Table 5.
Summary of Analysis of Covariance for Information Impact Ratings
| Cell Means of CI Risk and Severity by Treatment Group | Analysis |
||
|---|---|---|---|
| Mean* | SE | No. of Patients | |
| Little likely and a little | |||
| Breast cancer | 2.19 | 0.13 | 43 |
| Healthy control | 2.47 | 0.12 | 51 |
| Little likely and moderate | |||
| Breast cancer | 2.08 | 0.13 | 38 |
| Healthy control | 2.63 | 0.12 | 48 |
| Very likely and a little | |||
| Breast cancer | 2.23 | 0.12 | 40 |
| Healthy control | 2.48 | 0.11 | 56 |
| Very likely and moderate | |||
| Breast cancer | 2.20 | 0.13 | 39 |
| Healthy control | 3.57 | 0.12 | 50 |
NOTE. Information Impact scores were obtained on a 4-point Likert scale from 1 (would not affect my decision at all) to 4 (would completely determine my decision).
Abbreviations: CI, cognitive impairment; SE, standard error.
Means shown are adjusted for Genetic Knowledge scores.
Table 6.
Analysis of Covariance Summary Table for Information Impact Ratings
| Variable | Analysis |
||
|---|---|---|---|
| F | df | P | |
| CI risk | 0.12 | 1, 356 | .730 |
| CI severity | 0.13 | 1, 356 | .724 |
| Group | 17.45 | 1, 356 | .000 |
| CI risk × CI severity | 0.00 | 1, 356 | .988 |
| CI risk × group | 0.30 | 1, 356 | .586 |
| CI severity × group | 1.31 | 1, 356 | .253 |
| CI risk × CI severity × group | 0.18 | 1, 356 | .673 |
NOTE. Information Impact scores were obtained on a 4-point Likert scale from 1 (would not affect my decision at all) to 4 (would completely determine my decision).
Abbreviation: CI, cognitive impairment.
Fig 2.
Cognitive impairment (CI) risk by CI severity interaction for information importance ratings.
To examine whether results varied as a function of whether a woman in the BC group actually received chemotherapy as treatment for BC, we conducted a pair of 2 × 2 × 2 (CI risk × CI severity × chemotherapy) ANCOVAs with genetic knowledge as covariate. BC participants who received chemotherapy only or chemotherapy plus radiation therapy were grouped together (n = 85) and were contrasted with those who received radiation therapy only (n = 75). Dependent variables were information importance and information impact. No significant main or interaction effects for information importance were obtained. A main effect for chemotherapy was evident for information impact (F(1,151) = 5.29; P < .05). Women who received chemotherapy were less likely to report information about genetic risk for CI would affect their BC treatment decisions (chemotherapy mean, 2.03 [SD, 0.82] v no chemotherapy mean, 2.33 [SD, 0.81]).
Finally, within the BC group, the relationships between self-reported CI and ratings of information interest and information impact were examined using Pearson product-moment correlations. MASQ-Total scores were unrelated to information impact ratings (r = 0.05; nonsignificant) but were associated with information importance ratings (r = 0.22; P < .01). Women in the BC group reporting more CI 6 months after completing adjuvant treatment were more likely to indicate it was important to be given information about genetic risk for CI associated with chemotherapy.
DISCUSSION
The perceived importance of genetic information about CI risk at the time of treatment decision making was determined by both the likelihood and severity of any potential CI. As hypothesized, women expressed greater interest in genetic information about risk for CI when information suggested the likelihood and severity of CI were both high. Contrary to expectations, however, women expressed equally high interest in this information when the likelihood and severity of CI were both low. In both BC and HC groups, women expressed the least interest in genetic information when estimates of the likelihood and severity of CI were discordant (eg, little likelihood but moderate severity or high likelihood but low severity).
Why was this? Individuals often report interest in genetic testing because they anticipate test results will provide relief from uncertainty.8,11,14 Not surprisingly, BC survivors express regret about having undergone genetic testing if they received indeterminate test results.16 Furthermore, uncertainty about cancer treatment decisions has been associated with anxiety, depression, and cancer-related distress.13 Thus, it is understandable that women in this study would embrace information pointing to a clearer treatment decision, as when CI severity and CI risk were both high or both low, and express less interest in discordant information that increases decisional conflict,27 as when CI severity is low and CI risk is high, or vice versa. If genetic information adds to uncertainty and does not point clearly to a specific course of action, then women are less interested in this information. In contrast, women are more interested in genetic information that points to a clearer course of action, even when this information suggests inconsequential risks for adverse late effects.
Although preference for genetic information about CI risk was sensitive to our manipulation of the likelihood and severity of impairment, this was not the case for the anticipated impact of this information on treatment decision making. Differences in CI likelihood and severity were irrelevant to women's reports of whether this information would affect their decisions to undergo chemotherapy. This was surprising, as we had hypothesized that provision of genetic information indicating greater risk for adverse CI outcomes would be positively associated with the extent to which this information affected a treatment decision. Our finding that women's anticipated treatment decisions were not dependent on receipt of genetic test results is not without precedent, though. Previously, treatment intentions regarding a choice between prophylactic surgery and BC screening did not change on learning BRCA1/2 mutation status.13 Therefore, although contrary to hypothesis, our results are consistent with research that suggests receipt of genetic information may not influence anticipated treatment decisions.11
The anticipated impact of information regarding genetic risk for CI on treatment decisions was not affected by the nature of this information. Rather, it was affected by the women's history of BC. Relative to the HC group, the BC group indicated genetic information of any kind regarding risk for CI would be less likely to affect their decision to undergo chemotherapy. A couple of explanations are possible. First, this finding may reflect the emphasis patients with cancer place on survival and, often, their willingness to pursue any means of increasing the likelihood of cure even at the potential expense of quality of life.28 Healthy women that have not faced a cancer diagnosis cannot truly appreciate this choice and, thus, are more likely to indicate quality of life considerations might impact their treatment decisions. Second, the difference in impact of genetic information between the BC and HC groups could reflect cancer survivors' desires to reaffirm their treatment decisions. In other words, cancer survivors, because of their desires to believe previously made decisions are correct, judge treatment decisions consistent with their past behavior more favorably than those inconsistent with past behavior.29 In support of this, we found women in the BC group who had received chemotherapy were most likely to indicate genetic information about CI risk associated with chemotherapy would not have affected their decision to undergo chemotherapy.
There are limitations to this study. Our sample consisted primarily of white, reasonably well-educated women. Although this reflects the population of women most likely to undergo genetic testing in the oncology setting,17 it precluded determination of whether findings might vary across ethnicity and/or socioeconomic status. Additionally, although hypothetical scenarios are a well-established methodology for examining cancer treatment decision making in general15,30 and decisions regarding uptake and use of genetic information in particular16,31 there is an obvious limitation to this approach. The in vivo responses of women confronted with the circumstances described in our hypothetical scenarios may differ from those reported in response to the scenarios used here. Ideally, information regarding how women respond to genetic information about risk for late effects associated with cancer treatment should be collected in vivo. Prospective studies that observe women after cancer diagnosis through treatment initiation are best suited to increase understanding of the complex interaction of variables involved in determining interest in and use of information regarding genetic risk for treatment-related late effects.
In conclusion, this study has methodological and clinical import. Methodologically, by inclusion of an HC group, this study reveals a limitation of using hypothetical scenarios for examining uptake and impact of genetic information in treatment decision making. Differences between the responses of the BC and HC groups regarding the impact of genetic information on treatment decisions underscore the importance of including clinically relevant groups in research employing hypothetical scenarios. Consequently, researchers should be cautious about use of hypothetical scenarios with little personal relevance to study respondents. Clinically, although the importance ascribed to genetic information regarding CI risk varied as a function of the nature of this information, the importance ascribed to genetic information was still high, even in groups expressing the least interest. Mean information importance ratings for our two information discordant groups were approximately 4.2; a rating of 4 indicated that the respondent felt this information was very important. Rather than suggesting women do not want to be provided with genetic information that does not point clearly to a specific course of action, our results suggest women are less enthusiastic about the importance of this information. This may be due to the increased uncertainty and greater decisional conflict associated with decision making when risk information is discordant.27 If so, decision aids might be helpful for women confronted with the task of integrating information about survival and risk for adverse late effects, particularly when the latter information adds to uncertainty already present in the treatment decision making context. 32
Footnotes
Supported by National Institutes of Health Grants No. R03 CA121750 and R01 CA82822.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Michael A. Andrykowski, Brent J. Small, Paul B. Jacobsen
Financial support: Michael A. Andrykowski, Brent J. Small, Paul B. Jacobsen
Administrative support: Michael A. Andrykowski
Provision of study materials or patients: Michael A. Andrykowski, Brent J. Small, Paul B. Jacobsen
Collection and assembly of data: Michael A. Andrykowski, Erin Walsh, Brent J. Small
Data analysis and interpretation: Michael A. Andrykowski
Manuscript writing: Michael A. Andrykowski, Jessica L. Burris, Erin Walsh, Brent J. Small, Paul B. Jacobsen
Final approval of manuscript: Michael A. Andrykowski, Jessica L. Burris, Erin Walsh, Brent J. Small, Paul B. Jacobsen
REFERENCES
- 1.Guttmacher AE, Collins FS. Genomic medicine: A primer. N Engl J Med. 2002;347:1512–1520. doi: 10.1056/NEJMra012240. [DOI] [PubMed] [Google Scholar]
- 2.Khoury MJ, McCabe LL, McCabe ER. Population screening in the age of genomic medicine. N Engl J Med. 2003;348:50–58. doi: 10.1056/NEJMra013182. [DOI] [PubMed] [Google Scholar]
- 3.Blackwood MA, Weber BL. BRCA1 and BRCA2: From molecular genetics to clinical medicine. J Clin Oncol. 1998;16:1969–1977. doi: 10.1200/JCO.1998.16.5.1969. [DOI] [PubMed] [Google Scholar]
- 4.Fasouliotis SJ, Schenker JG. BRCA1 and BRCA2 gene mutations: Decision making dilemmas concerning testing and management. Obstet Gynecol Surv. 2000;55:373–384. doi: 10.1097/00006254-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Nathanson KL, Wooster R, Weber BL. Breast cancer genetics: What we know and what we need. Nat Med. 2001;7:552–556. doi: 10.1038/87876. [DOI] [PubMed] [Google Scholar]
- 6.Buyse M, Loi S, van't Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 7.Foekens JA, Atkins D, Zhang Y, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol. 2006;24:1665–1671. doi: 10.1200/JCO.2005.03.9115. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz MD, Peshkin BN, Tercyak KP, et al. Decision making and decision support for hereditary breast-ovarian cancer susceptibility. Health Psychol. 2005;24:S78–S84. doi: 10.1037/0278-6133.24.4.S78. [DOI] [PubMed] [Google Scholar]
- 9.Lerman C, Hughes C, Lemon SJ, et al. What you don't know can hurt you: Adverse psychologic effects in members of BRCA1-linked and BRCA2-linked families who decline genetic testing. J Clin Oncol. 1998;16:1650–1654. doi: 10.1200/JCO.1998.16.5.1650. [DOI] [PubMed] [Google Scholar]
- 10.Lerman C, Narod S, Schulman K, et al. BRCA1 testing in families with hereditary breast-ovarian cancer: A prospective study of patient decision making and outcomes. JAMA. 1996;275:1885–1892. [PubMed] [Google Scholar]
- 11.Pasacreta JV. Psychosocial issues associated with genetic testing for breast and ovarian cancer risk: An integrative review. Cancer Invest. 2003;21:588–623. doi: 10.1081/cnv-120022380. [DOI] [PubMed] [Google Scholar]
- 12.Rini C, O'Neill SC, Valdimarsdottir H, et al. Cognitive and emotional factors predicting decisional conflict among high-risk breast cancer survivors who receive uninformative BRCA1/2 results. Health Psychol. 2009;28:569–578. doi: 10.1037/a0015205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Roosmalen MS, Stalmeier PF, Verhoef LC, et al. Impact of BRCA1/2 testing and disclosure of a positive test result on women affected and unaffected with breast or ovarian cancer. Am J Med Genet. 2004;124A:346–355. doi: 10.1002/ajmg.a.20374. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Miller SM. Psychological issues in genetic testing. In: Miller SM, Bowen DJ, Croyle RT, Rowland JH, editors. Handbook of Cancer Control and Behavioral Science: A Resource for Researchers, Practitioners, and Policy Makers. Washington, DC: American Psychological Association; 2008. pp. 303–321. [Google Scholar]
- 15.Brewer NT, Edwards AS, O'Neill SC, et al. When genomic and standard test results diverge: Implications for breast cancer patients' preference for chemotherapy. Breast Cancer Res Treat. 2009;117:25–29. doi: 10.1007/s10549-008-0175-2. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill SC, Brewer NT, Rimer BK, et al. Women's interest in gene expression analysis for breast cancer recurrence risk. J Clin Oncol. 2007;25:4628–4634. doi: 10.1200/JCO.2006.09.6255. [DOI] [PubMed] [Google Scholar]
- 17.Bowen DJ, Patenaude A, Vernon SW, et al. Psychosocial issues in cancer genetics: From the laboratory to the public. Cancer Epidemiol Biomarkers Prev. 1999;8:326–328. [PubMed] [Google Scholar]
- 18.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 20.Morse R, Rodgers J, Verrill M, et al. Neuropsychological functioning following systemic treatment in women treated for breast cancer: A review. Eur J Cancer. 2003;39:2288–2297. doi: 10.1016/s0959-8049(03)00600-2. [DOI] [PubMed] [Google Scholar]
- 21.Phillips KA, Bernhard J. Adjuvant breast cancer treatment and cognitive function: Current knowledge and research directions. J Natl Cancer Inst. 2003;95:190–197. doi: 10.1093/jnci/95.3.190. [DOI] [PubMed] [Google Scholar]
- 22.Tannock IF, Ahles TA, Ganz PA, et al. Cognitive impairment associated with chemotherapy for cancer: Report of a workshop. J Clin Oncol. 2004;22:2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen PB, Donovan KA, Small BJ, et al. Fatigue following treatment for early stage breast cancer: A controlled comparison. Cancer. 2007;110:1851–1859. doi: 10.1002/cncr.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jim HS, Donovan KA, Small BJ, et al. Cognitive functioning in breast cancer survivors: A controlled comparison. Cancer. 2009;115:1776–1783. doi: 10.1002/cncr.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidenberg M, Haltiner A, Taylor MA, et al. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol. 1994;16:93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 27.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 28.Duric VM, Butow PN, Sharpe L, et al. Psychosocial factors and patients' preferences for adjuvant chemotherapy in early breast cancer. Psychooncology. 2007;16:48–59. doi: 10.1002/pon.1045. [DOI] [PubMed] [Google Scholar]
- 29.Jansen SJ, Otten W, Baas-Thijssen MC. Explaining differences in attitude toward adjuvant chemotherapy between experienced and inexperienced breast cancer patients. J Clin Oncol. 2005;23:6623–6630. doi: 10.1200/JCO.2005.07.171. [DOI] [PubMed] [Google Scholar]
- 30.McQuellon RP, Muss HB, Hoffman SL, et al. Patient preferences for treatment of metastatic breast cancer: A study of women with early-stage breast cancer. J Clin Oncol. 1995;13:858–868. doi: 10.1200/JCO.1995.13.4.858. [DOI] [PubMed] [Google Scholar]
- 31.Struewing JP, Lerman C, Kase RG, et al. Anticipated uptake and impact of genetic testing in hereditary breast and ovarian cancer families. Cancer Epidemiol Biomarkers Prev. 1995;4:169–173. [PubMed] [Google Scholar]
- 32.Kaufman EM, Peshkin BN, Lawrence WF, et al. Development of an interactive decision aid for female BRCA1/BRCA2 carriers. J Genet Counsel. 2003;12:109–129. doi: 10.1023/A:1022698112236. [DOI] [PubMed] [Google Scholar]