Abstract
A direct evaluation of the in vivo release profile of drugs from carriers is a clinical demand in drug delivery systems, because drug release characterized in vitro correlates poorly with in vivo release. The purpose of this study is to demonstrate the in vivo applicability of the dual MR contrast technique as a useful tool for noninvasive monitoring of the stability and the release profile of drug carriers, by visualizing in vivo release of the encapsulated surrogate MR contrast agent from carriers and its subsequent intratumoral distribution profile. The important aspect of this technique is that it incorporates both positive and negative contrast agents within a single carrier. GdDTPA, superparamagnetic iron oxide nanoparticles, and 5-fluorouracil were encapsulated in nano- and microspheres composed of poly(d,l-lactide-co-glycolide), and which was used for a model carrier. In vivo studies were performed with orthotopic xenograft of human breast cancer. The MR-based technique demonstrated here has enabled visualization of the delivery of carriers, and release and intratumoral distribution of the encapsulated positive contrast agent. This study demonstrated proof-of-principle results for the noninvasive monitoring of in vivo release and distribution profiles of MR contrast agents, and thus, this technique will make a great contribution to the field.
Keywords: MRI (magnetic resonance imaging), Molecular imaging, Drug release, In vivo test, Nanoparticle
1. Introduction
Now, in the 21st century, attention is particularly focused on the clinical application of biomaterials in biomedical field, which includes biodegradable drug/gene carriers, implanted devices, and tissue engineering. Intrinsic properties of biomaterials used for the controlled release formulations, such as nano- or micro-sized drug carriers or implanted devices, play a crucial role in an optimal treatment regimen. Although it is known that a number of factors contribute to drug release, such as materials, size, and surface charge of carriers, as well as the molecular size, hydrophilicity, and net charge of the drug molecules, further investigations regarding drug release in vivo are in great demand. Current strategies to monitor the in vivo release profile of drugs include indirect measurements of drug concentrations in blood and urine samples or invasive measurements of biopsy tissues, and, to date, only limited studies of noninvasive in vivo release monitoring have been reported [1, 2]. Radionuclide imaging is not optimal for this purpose due to the innately short half-life of radioisotopes, and also because discrimination between encapsulated and released markers, as yet, is not possible. Activated optical imaging probes have been developed [3, 4], but, these agents have, as yet, limited applications in clinical settings due to low spatial resolution and depth penetration limits. To this end, a noninvasive MR-based multiparametric imaging technique has been developed to monitor the release of drug molecules either directly by MR spectroscopy or indirectly by MRI with gadolinium-based contrast agents [5]. Conventional contrast-enhanced MRI techniques that use a single contrast agent cannot distinguish between encapsulated and released molecules. The key advantage of the newly developed technique is that it can demarcate released cargo molecules from encapsulated molecules (Fig. 1). The principle of this technique is that the positive-enhancement T1 effects of encapsulated GdDTPA are cancelled out by loading the carrier or device with superparamagnetic iron oxide (SPIO) nanoparticles, whereas the T1 effect of GdDTPA released from the carrier/device becomes apparent once GdDTPA molecules diffuse beyond the range of the T2/T2* effects of SPIO. Similarly, encapsulated drug molecules that are co-encapsulated with SPIO have broad resonance lines in MRS due to the T2* effect of SPIO particles [6], while the released drug molecules give a narrow resonance line once drug molecules diffuse beyond the range of the T2* effects of SPIO. Initially, the concept behind this strategy was validated using an in vitro model system [5]. Due to their large molecular size, SPIO nanoparticles have a much shorter diffusion range compared to the small molecular contrast agent, GdDTPA. For instance, the intratumoral distribution of larger molecules is restricted due to the short diffusion distance from the vascular surface, compared to smaller molecules [7], which supports the feasibility of this technique in vivo, in this case in solid tumors. In this study, to demonstrate the potential use of this new MRI technique for noninvasive release monitoring in vivo, the release of a surrogate MR contrast agent, GdDTPA, from poly(d,l-lactide-co-glycolide) (PLGA) nano/microspheres was monitored in mice bearing orthotopic MCF-7 breast tumor xenograft.
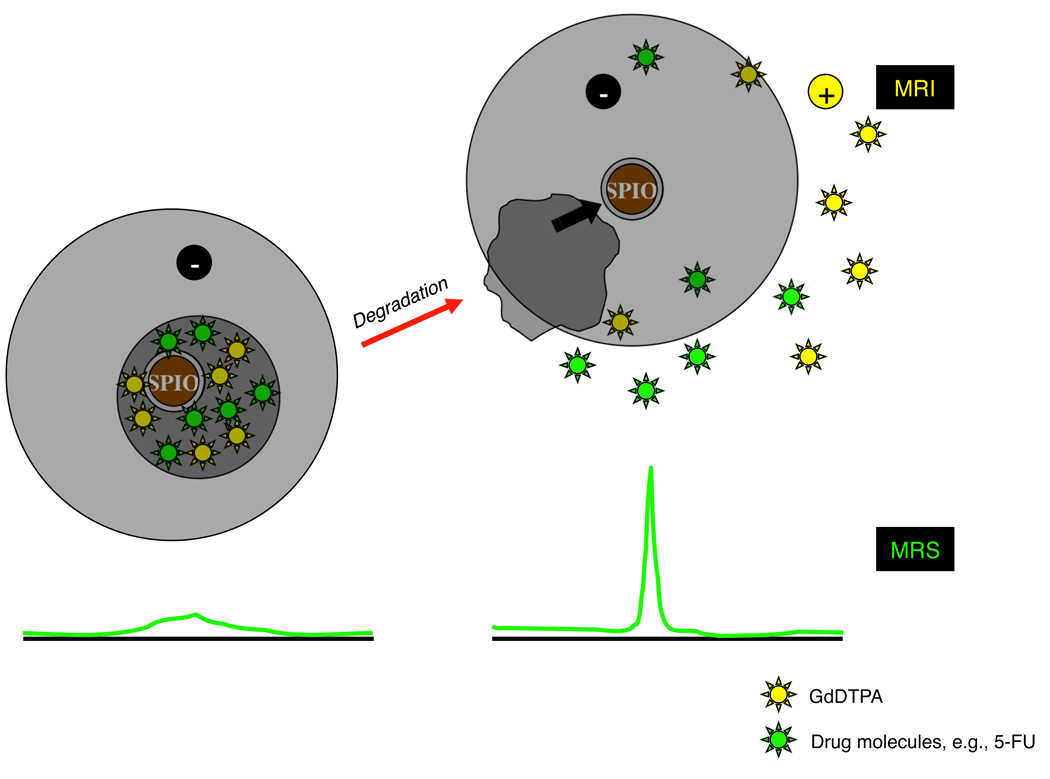
Fig. 1.
Schematic of the concept of the MR-based technique to monitor the release of cargo molecules by MRI/MRS. Intact GdDTPA/SPIO-loaded carriers generate strong negative contrast enhancement in MRI due to the prevailing T2/T2* effects of SPIO (left panel). For MRS, SPIO-loaded intact carriers broaden the resonance line of drug molecules; for example, anticancer agents, such as 5-FU, due to the T2* effects of SPIO (left panel). Upon release, the free GdDTPA generates positive contrast enhancement in areas beyond the T2/T2* effects of SPIO that has limited diffusion range, and the free drug molecules demonstrate a narrow resonance line (right panel). Therefore, we can (i) identify the location where carriers are delivered as a dark spot on MRI; (ii) detect a release of GdDTPA by a decrease in T1 values, which is caused only by the “released” GdDTPA in MRI, and detect a release of cargo drugs by the appearance of a narrow resonance line of the “released” drug molecules in MRS; and (iii) see the distribution of the “released” GdDTPA by a decrease in the T1 values in quantitative T1 maps. The concept of this technique was proven by in vitro phantom studies in our previous report [5].
2. Materials and methods
2.1. Chemicals, animals, and cell lines
Omniscan® (GdDTPA-BMA, 0.5 M) was purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, U.K.). Feridex® (SPIO, 11.2 mg/mL) was purchased from Bayer Healthcare Pharmaceuticals, AG (Leverkusen, Germany). Fluorouracil injection (5-FU, 50 mg/mL) was obtained from APP Pharmaceuticals, Inc. (Schaumburg, IL, USA). Poly(d,l-lactideco-glycolide) (PLGA, M.W. = 40–75 kDa), Span 80, poly(vinylalcohol) (PVA, M.W. = 8–10 kDa), and poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) copolymer (PEG-PPG-PEG, average Mn: 8.4 kDa) were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). Mouse plasma was obtained from Innovative Research (Novi, MI, U.S.A.). Nitric acid (TraceSELECT®) was obtained from Fluka (St. Louis, MO, U.S.A.). Water and methanol for the HPLC used were of HPLC grade. All of the other chemicals used were of analytical grade.
Severe combined immunodeficient (SCID) mice (female, 4–6 weeks old, 19–23 g) were purchased from NCI (Bethesda, MD, U.S.A.). Approval from the institutional animal care and use committee preceded all animal experiments in the present study. The culture of MCF-7 estrogen-dependent human breast carcinoma cells and the orthotopic inoculation of the cells into the mammary fat pad of a mouse were performed according to the previous study [8]. At least three animals were used for each in vivo study.
2.2. Preparation of contrast agent-loaded PLGA nano/microspheres
PLGA nano/microspheres (NS-GdDTPA/SPIO/5-FU and MS-GdDTPA/SPIO/5-FU, respectively) incorporating 5-FU, GdDTPA, and SPIO were prepared using a double-emulsion solvent evaporation method. Briefly, a mixture of Omniscan® (250 µL), a standard 5-FU solution (50 mg/mL, 250 µL) and Feridex® (50 µL) was added dropwise to 5 mL of dichloromethane containing 50 mg of PLGA and 20 mg of Span 80 as an emulsifier, to prepare NS-GdDTPA/SPIO/5-FU. For the preparation of MS-GdDTPA/SPIO/5-FU, 1 mL of dichloromethane containing 250 mg of PLGA, was used instead. The mixture was sonicated for 10 min on ice, which resulted in the formulation of a water-in-oil (w/o) emulsion. The w/o emulsion was poured onto the outer aqueous layer (PBS containing 1% PVA, 0.2% PEP-PPG-PEG, 1% NaCl), and sonicated again for 3 min on ice to obtain a water-in-oil-in-water (w/o/w) emulsion. To obtain nano- and micro-sized spheres, 7.5 and 5.0 mL of the outer aqueous phase were used, respectively (see Supplemental material, Fig. S1). After the evaporation for 1 hr at 37 °C to remove dichloromethane, NS-GdDTPA/SPIO/5-FU and MS-GdDTPA/SPIO/5-FU were washed with purified water several times by centrifugation (3 min, 4 °C) at 8,000 ×g and at 360 ×g, respectively, lyophilized, and stored in the refrigerator until use.
2.3. Characterization of PLGA nano/microspheres
To determine particle size, size distribution, and ζ-potential of the resultant PLGA nano/microspheres, dynamic laser-light scattering (DLS) measurements were performed using a Zetasizer Nano-ZS90 (Malvern Instruments Ltd., Worcestershire, UK) with a He-Ne laser system, which is operated at 633 nm and 25 ±1 °C, and the scattered light is measured at an angle of 90°. The morphology of PLGA nano/microspheres was characterized with a scanning electron microscope (SEM) (LEO1550, Carl Zeiss, Inc., Thornwood, NY, U.S.A.). For sample preparation, lyophilized PLGA nano/microspheres were dispersed in purified water (0.05 mg/mL), and the droplets were dried on the surface of a cover glass at room temperature. They were coated with gold sputter from the vapor on an auto fine coater.
Loading amounts of 5-FU and GdDTPA were determined by UV detection with reverse phase high-performance liquid chromatography (HPLC), and by T1 relaxation time measurements with MRI, respectively, after destruction of PLGA nano/microspheres with dichloromethane. The quantification analysis with HPLC for 5-FU was performed as in Coe et al. [9], with minor modifications. Briefly, 1 mg of lyophilized nano/microspheres was dissolved in 300 µL of dichloromethane, and then 150 µL of purified water was added to the sample to extract 5-FU. After vortexing, 20 µL of the sample solution was directly injected into a Waters 1525 binary HPLC pump equipped with a Waters Symmetry® C18 reversed phase column (4.6 mmφ × 150 mm) and a Waters Ultrahydrogel Guard Column (6.0 mmφ × 40 mm). A Waters 2487 dual λ absorbance detector was set at 266 nm. Potassium phosphate (10 mM, pH 5.5) and methanol were used as the mobile phase A and B, respectively, and the elution program was as follows: run mobile phase A for first 5 min, increase mobile phase B from 0% to 25% over next 1 min, and maintain mobile phase B at 25% for 3 min. The initial conditions were restored by decreasing mobile phase B to 0% over 1 min, and the column was equilibrated with 100% mobile phase A for 10 min. The flow rate was 0.8 mL/min, and HPLC analysis was performed at room temperature. The Breeze 3.30 program (Waters Corp., Milford, MA, USA) was used as acquisition and analysis software.
The quantification analysis with MRI for GdDTPA was performed in the same manner as in our previous report [5]. The T1 relaxation time of the sample solution was measured on a Bruker 9.4T horizontal bore spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany). The quantification of GdDTPA was also conducted with inductively-coupled plasma mass spectrometry (ICP-MS, ParkinElmer, Waltham, MA, U.S.A.). For the extraction of GdDTPA for ICP-MS measurement, 2% (v/v) nitric acid (TraceSELECT®) was used as an extraction medium, rather than purified water. The T1 relaxation time measurement was consistent with the results obtained by the ICP-MS measurement.
2.4. In vitro release characteristics
PLGA nano/microspheres (40 mg) were dispersed into 2 mL of 10% (v/v) mouse plasma in PBS. Each suspension was incubated with gentle shaking at 37 °C throughout the experiment. At designated intervals, an aliquot (500 µL) was withdrawn and the supernatant was separated from the PLGA nano/microspheres by centrifugation at 8,000 ×g for 3 min at 4 °C. After collecting the supernatant, the pellet of nano/microspheres was resuspended in the same volume of fresh release medium (500 µL), and then returned to the sample suspension. The amounts of 5-FU and GdDTPA in the supernatant were determined with HPLC and MRI, respectively, as described above.
2.5. Visualization of in vivo release of GdDTPA from PLGA nanospheres
MRI was carried out on a Bruker horizontal bore 9.4T spectrometer equipped with shielded gradients. The Paravision 3.0.2 program (Bruker Biospin, GmbH) was used as acquisition software. In vivo studies were performed with an orthotopic human breast carcinoma MCF-7 grown in female SCID mice. Each anesthetized mouse was immobilized in the probe, and the tumor was positioned in the 1H/19F RF coil. The tail vein of the mouse was catheterized before placing the animal in the magnet. During MRI experiments, animals were maintained under gas anesthesia (1% isoflurane in a mixture of 70% O2 and 30% N2 at 1 L/min), and animal body temperature was maintained at 37 °C by a circulating water thermo-pad, and respiration rate was monitored with a Bruker dedicated physiology monitoring system, using a pressure pad and a pressure transducer. After tuning the probe and shimming the magnet, tri-planar scout images were acquired to determine the position of the imaging slices. MRI was acquired before, 30 min, and 2.5 hr after administration of NS-GdDTPA/SPIO/5-FU. Mice received an intravenous administration of 200 µL of NS-GdDTPA/SPIO/5-FU suspension at a dose of 40 mg per mouse, which is equivalent to 25 and 8 µmol/kg of GdDTPA and 5-FU, respectively. To obtain both released GdDTPA and SPIO distribution images, a 3D fast spin echo (RARE: Rapid Acquisition with Relaxation Enhancement) sequence with an effective echo time (TE) of 50 ms and a repetition time (TR) of 1,000 ms was acquired. This technique provides fast acquisition of 3D volumes with a uniform spatial resolution of up to 100 µm in vivo within 30 min experimental time. For the quantitative T1 map, a 3D RARE pulse sequence with a TE of 50 ms, and five different TRs (250, 500, 1,000, 2,000, and 4,000 ms) was acquired with a spatial resolution of 0.125×0.125µ0.0625 mm (128×80×40 matrix zero-filled to 128×128×128, field of view = 16×16×8 mm). Quantification of T1 was carried out in the same manner as in the in vitro study. For T2* acquisition, a 3D fast low-angle shot (FLASH) pulse sequence was used with the following parameters: TE/TR = 7/100 ms; number of acquisitions = 2; and flip angle ≈32°. Final analysis was performed with the ImageJ® program (National Institutes of Health). T1 maps were reconstructed with in-house software written in the IDL programming environment (ITT Visual Information Solutions, Boulder, CO, U.S.A.), and 3D images were visualized with the Amira graphic package (TGS Inc., San Diego, CA, U.S.A.).
2.6. Visualization of in vivo release of GdDTPA from PLGA microspheres
Due to inefficient delivery of large particles into tumor tissue after intravenous administration [10], a MS-GdDTPA/SPIO/5-FU suspension (10 µL) was administered intratumorally into the MCF-7 tumor using a U-100 BD insulin syringe (28 gauge, Franklin Lakes, NJ, U.S.A.) at a dose of 550 µg per mouse, which is equivalent to 2.5 µmol/kg of GdDTPA. MRI was acquired pre- and 30 min, 2.5 hr, and 21 hr post-administration of MS-GdDTPA/SPIO/5-FU. The mouse was removed from the probe/magnet at the time of injection, and the tumor was placed back in the coil after injection. Acquisition parameters and image processing were the same as those used in the study on NS-GdDTPA/SPIO/5-FU.
2.7. Statistical analysis
Median T1 values in the selected region-of-interest (ROI) (N=5) were compared between each time point to determine if the T1 values in the region where the majority of nanospheres was delivered and accumulated in the tumor were significantly changed. The one-way Analysis of Variance (ANOVA) was used for omnibus F-test using StatPlus®:mac (AnalystSoft Inc., Alexandria, VA, U.S.A.) software, and the Scheffé’s test was undertaken for post hoc analysis. The T1 values were considered to be significantly different (P<0.05) when the F value is greater than the critical value of the F-distribution (Fcrit, α=0.05 = 3.89).
3. Results
3.1. In vitro characteristics of contrast agent-loaded PLGA nano/microspheres
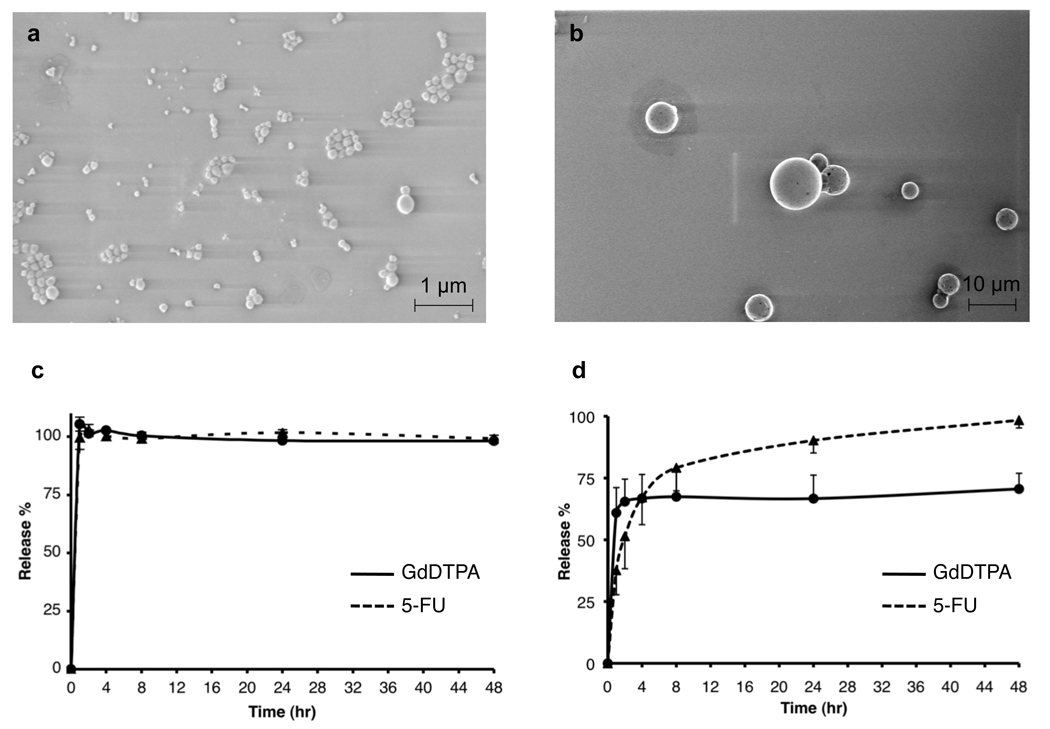
Scanning electron microscopy (SEM) confirmed that both carriers were spherical in shape (Fig. 2), and the sizes were approximately 194 nm for NS-GdDTPA/SPIO/5-FU and 2.6 µm for MS-GdDTPA/SPIO/5-FU (Table 1). The in vitro release profile of GdDTPA from NS-GdDTPA/ SPIO/5-FU was similar to that of 5-FU, indicating that GdDTPA can be used as an imaging tracer to visualize the release of 5-FU from the NS-GdDTPA/SPIO/5-FU. Both GdDTPA and 5-FU were released rapidly from NS-GdDTPA/SPIO/5-FU, i.e., releases were completed within 30 min. In contrast, the release profile of GdDTPA from MS-GdDTPA/SPIO/5-FU was significantly different from that of 5-FU, and both molecules were released slightly slower than from NS-GdDTPA/SPIO/5-FU. This result indicates that the GdDTPA used in this study is not a good surrogate for the release of 5-FU from MS-GdDTPA/SPIO/5-FU in vivo. Since a preliminary in vitro experiment demonstrated that the detection of the 5-FU peak in 19F-MRS requires high concentration of 5-FU in the tumor site (Fig. S2), we conducted in vivo release monitoring only by MRI. PLGA nanospheres and microspheres were used to delineate the feasibility of the dual MR contrast technique for noninvasive monitoring of release of GdDTPA from carriers with different release profiles.
Fig. 2.
In vitro characteristics of PLGA nano/microspheres. Scanning electron microscope (SEM) images of (a) PLGA nanospheres (NS-GdDTPA/SPIO/5-FU) and (b) PLGA microspheres (MS-GdDTPA/SPIO/5-FU). In vitro release profiles of GdDTPA and 5-FU from (c) NS-GdDTPA/SPIO/5-FU and (d) MS-GdDTPA/SPIO/5-FU. The quantification of GdDTPA was performed by T1 measurement of release medium with a calibration curve using standard Omniscan®, based on the linear relationship between GdDTPA concentration and 1/T1 (R2=0.999 for 0.5 µM – 50 mM on 9.4T MRI). The quantification of 5-FU was performed using reverse-phase HPLC. Please see supplemental materials for details.
Table 1.
In vitro characteristics of PLGA nano/microspheres
| Diameter (nm)1, 2 | Polydispersity Index ± S.D. |
ζ-potential (mV) ± ζ deviation1 |
GdDTPA contents (nmol/mg NPs) ± S.D. |
5-FU contents (nmol/mg NPs) ± S.D. |
|
|---|---|---|---|---|---|
| NS-GdDTPA/SPIO/5-FU | 194 ± 10 | 0.095 ± 0.042 | −2.2 ± 0.5 | 12.8 ± 2.7 | 4.3 ± 0.7 |
| MS-GdDTPA/SPIO/5-FU | 2612 ± 1137 | 0.801 ± 0.273 | −7.0 ± 1.6 | 90.6 ± 18.4 | 7.0 ± 1.7 |
Nanoparticles were dispersed in PBS (pH 7.4) for the size measurement, and dispersed in 10 mM of NaCl for the ζ-potential measurement.
The hydrodynamic diameter of nanoparticles was calculated by the Stokes-Einstein equation.
3.2. Visualization of in vivo release of GdDTPA from PLGA nanospheres
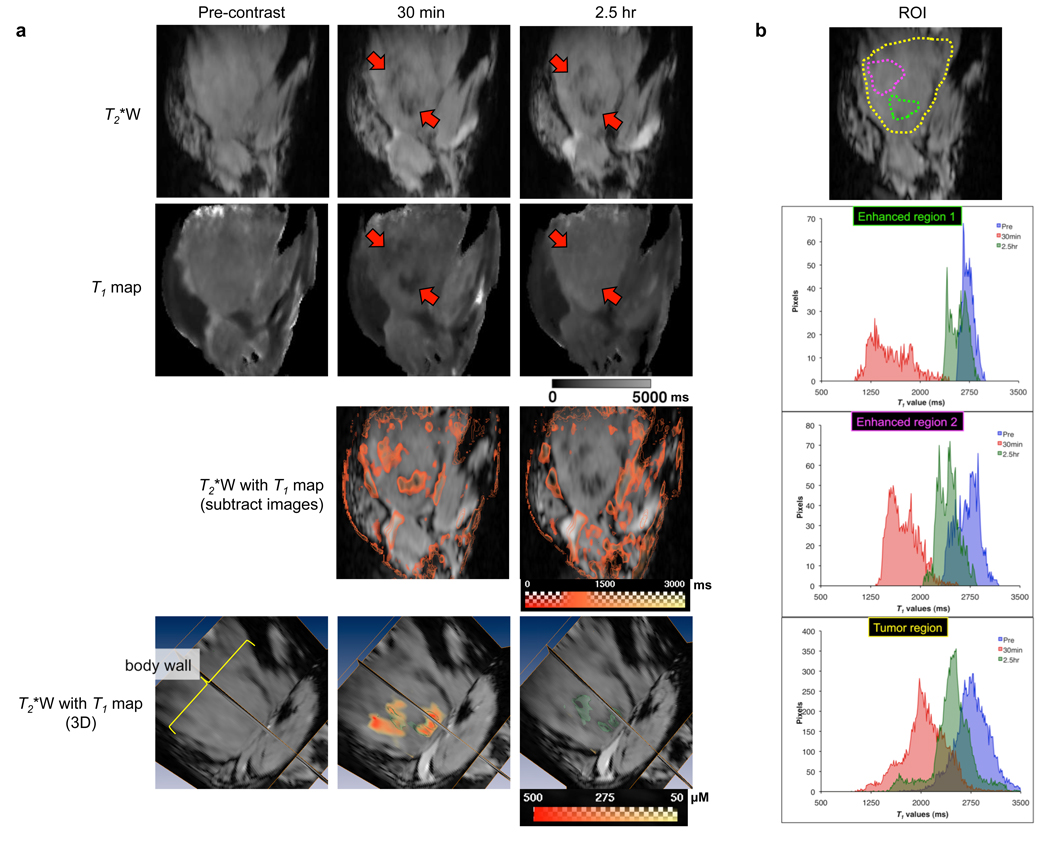
MR images of the MCF-7 xenografts acquired before and up to 2.5 hr post intravenous administration of NS-GdDTPA/SPIO/5-FU suspension are displayed in Fig. 3. Following intravenous administration of the NS-GdDTPA/SPIO/5-FU, SPIO particles generated strong T2* contrast, which corresponds to the dark spots in the T2* images (red arrow), indicating the region where the intact NS-GdDTPA/SPIO/5-FU were distributed in the tumor. In contrast, a T1 reduction was observed around the areas where the NS-GdDTPA/SPIO/5-FU were localized at 30 min post-contrast, while T1 values for the same area were restored at 2.5 hr post-contrast in the quantitative T1 map. This indicates that GdDTPA was released from NS-GdDTPA/SPIO/5-FU soon after the intravenous administration of NS-GdDTPA/SPIO/5-FU, and rapidly diffused away from the nanoparticles, so that T1 enhancement became visible. As reported earlier, the estimated spatial distance between SPIO and GdDTPA to enable the detection of the T1 effects of GdDTPA should be over 15 µm, when an echo time of 30 ms is used for breast cancer xenografts [5, 11]. The position of the large dark spot remained unchanged from its initial location, indicating that the diffusion of SPIO particles was limited. It is worth noting that, in our experiments performed at a high magnetic field (9.4T) and a high concentration of SPIO particles, the T2/T2* effects of SPIO prevailed over possible T1 effects. Quantitative T1 maps give absolute T1 values of the images on a pixel-to-pixel basis, and the concentration of GdDTPA in each pixel can be calculated using a calibration curve for a standard GdDTPA solution. Thus, quantitative T1 maps also provide quantitative information on GdDTPA distributed in the tumor (Fig. 3a, bottom panels). A T1 reduction was more clearly observed in the region where the majority of nanospheres was delivered and accumulated in the tumor compared to the whole tumor lobe (Fig. 3b). The regions where the release of GdDTPA occurs is clearly distinguished by the median T1 values (Table 2). It is worth noting that the in vivo release observed here was consistent with the in vitro release profile of GdDTPA from NS-GdDTPA/SPIO/5-FU. Taken together, this in vivo experiment has provided conclusive evidence that the dual MR contrast technique could monitor the delivery, release, and intratumoral distribution of GdDTPA, a surrogate tracer of an anticancer agent, after intravenous administration of nanocarriers noninvasively.
Fig. 3.
Visualization of the in vivo release of GdDTPA from NS-GdDTPA/SPIO/5-FU by the dual MR contrast technique. (a) MR images of the representative tumor over time. T2*-weighted images (top panels) and T1 maps (second row of panels) were from 10 stacked slices (≈1.25 mm) out of 128 slices. From the top panels, we could identify the location of NS-GdDTPA/SPIO/5-FU as a dark spot (a red arrow) on T2*-weighted MRI. We could observe a decrease in T1 values in the tumor around the area where the NS-GdDTPA/SPIO/5-FU were delivered (a red arrow) on the T1 map only 30 min after administration (second row of panels). The panels in the third row represent the subtraction images of the T1 map (pre-contrast minus post-contrast) overlaid on T2*-weighted images. We could clearly see the changes in T1 values around the area where the nanospheres were located. In the bottom panels (T2*-weighted images overlaid with quantitative T1 values), the green channel represents SPIO-loaded PLGA nanospheres, and the red-yellow channel indicates the distribution of GdDTPA released from the NS-GdDTPA/SPIO/5-FU in the tumor. We were able to determine the spatial distribution of GdDTPA released from the NS-GdDTPA/SPIO/5-FU quantitatively. Scale bars represent the range of T1s in milliseconds for the panels of the middle two rows, and the range of the concentration of GdDTPA in micromolar is depicted in the bottom row panels. (b) Selection of a region-of-interest (ROI) in the tumor, and histograms of the T1 values of the selected ROI. T1 values were calculated within selected ROIs encircled in the yellow (the whole tumor lobe), green (the area where the NS-GdDTPA/SPIO/5-FU were distributed), and pink (the area where the NS-GdDTPA/SPIO/5-FU were distributed) regions. The blue, red, and green channels represent pre-, 30 min, and 2.5 hr-post i.v. administration of the NS-GdDTPA/SPIO/5-FU, respectively. From the histograms, we could clearly distinguish whether nanospheres remained intact or released GdDTPA, especially around the area where nanospheres were delivered (also see Table 2).
Table 2.
Changes in T1 values of region-of-interests (ROIs) where T1 reduction was observed in the tumor over time
| median T1 value (ms) ± S.E. |
Statistical differences (Fcrit, α=0.05 = 3.89)1 |
|||
|---|---|---|---|---|
| Pre vs. 30 min | 30 min vs. 2.5 hr | 2.5 hr vs. Pre | ||
| Pre-injection | 2826 ± 88 | — | ||
| F = 68.55 SS | ||||
| 30 min post-injection | 1715 ± 53 | — | ||
| F = 40.55 SS | ||||
| 2.5 hr post-injection | 2570 ± 55 | — | F = 3.65 NS | |
T1 values of the enhanced regions in the tumor were significantly dropped at 30 min post-injection of NS-GdDTPA/ SPIO/5-FU, and T1 values were restored to pre-injection values at 2.5 hr after injection. SS: Statistically significant (P<0.05); NS: Not significant.
3.3. Visualization of in vivo release of GdDTPA from PLGA microspheres
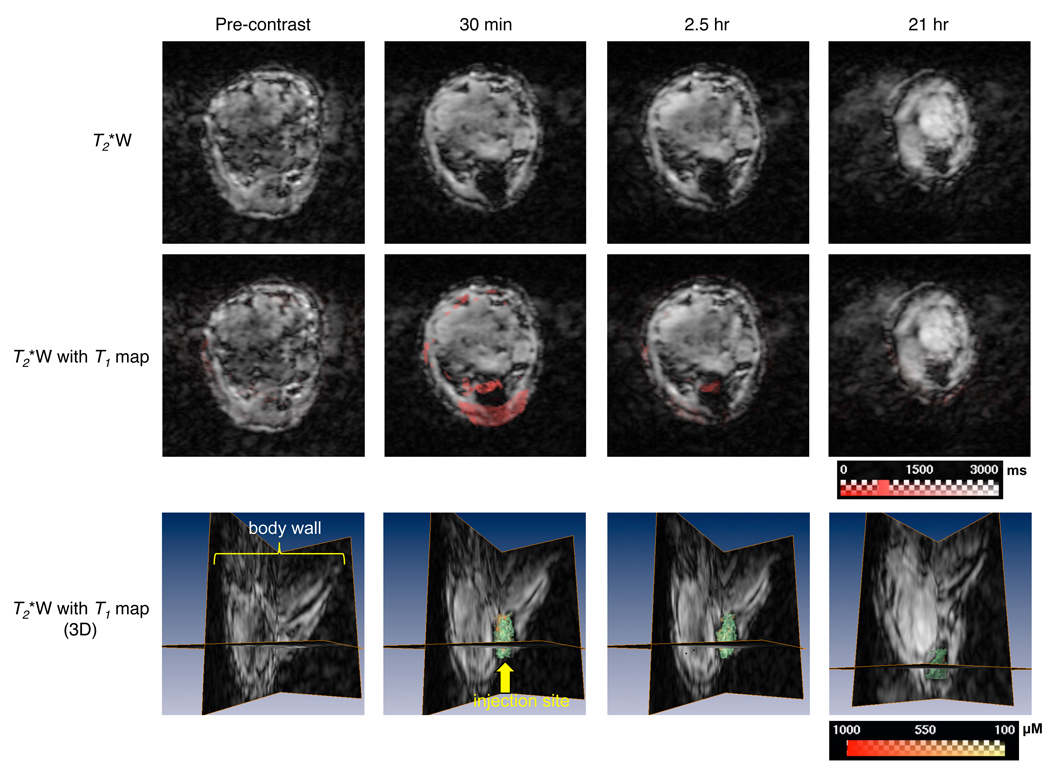
The release of GdDTPA from MS-GdDTPA/SPIO/5-FU delivered intratumorally is shown in Fig. 4. T2*-weighted images identified the location of the injection site as a dark spot. T1 maps revealed that the release of GdDTPA from MS-GdDTPA/SPIO/5-FU was predominantly observed about 30 min after the intratumoral administration of MS-GdDTPA/SPIO/5-FU. While the released GdDTPA was still detected at 2.5 hr post-administration of microspheres, no GdDTPA was detected at 21 hr post-administration. These results were also in good agreement with the in vitro release kinetics, which showed that the release of GdDTPA from the MS-GdDTPA/SPIO/5-FU was slower than that from the NS-GdDTPA/SPIO/5-FU, and plateaued after 4 hrs.
Fig. 4.
In vivo monitoring of the release of GdDTPA from MS-GdDTPA/SPIO/5-FU by dual MR contrast technique in MCF-7 breast cancer xenografts. Two-dimensional MR images were from one representative slice out of 128 slices in the z-direction. From the top row panels, we could identify the location of MS-GdDTPA/SPIO/5-FU as a dark spot on T2*-weighted MRI. We could observe, on the T1 map, a decrease in the T1 values of the tumor around the area where the PLGA microspheres were injected, notably at 30 min, and slightly, at 2.5 hr after intratumoral administration (panels of the middle row). In the bottom panels (T2*-weighted images overlaid with quantitative T1 values), the green channel represents SPIO-loaded PLGA microspheres, and the red-yellow channel indicates the distribution of GdDTPA released from the MS-GdDTPA/SPIO/5-FU in the tumor. Similar to the intravenous injection of NS-GdDTPA/SPIO/5-FU, we were able to determine the spatial distribution of GdDTPA released from the MS-GdDTPA/SPIO/5-FU quantitatively. Scale bars represent the range of T1s in milliseconds in the panels of the middle row, and the range of the concentration of GdDTPA in micromolars in the bottom row.
4. Discussion
The results presented here indicate that this technique can be used for variety of different nanocarriers, as well as for different tumor models (see Supplemental material, Fig. S3). MRI can double as a tool that can provide monitoring of the delivery, release, and distribution of cargo molecules in the target site, as well as for monitoring therapeutic progress with high-resolution anatomical and functional imaging. Therefore, MRI can be used to better understand drug delivery systems in combination with this technique, which will be conducive to more effective therapeutic strategies.
The technical issues that need to be addressed in this technique are sensitivity and the applicability of gadolinium chelates as a surrogate tracer for different drugs. MRI has inherently low sensitivity compared to radionuclide imaging or optical imaging, and MRS has even lower sensitivity. On the other hand, this unique dual-contrast mechanism enables us to distinguish between encapsulated and released molecules, and high-resolution imaging is also a significant advantage of MRI over other imaging modalities. MRI contrast agents are also well suited for longitudinal imaging, such as the monitoring of drug release for formulations or implanted devices that are intended for long-term treatment protocols. Significant efforts have been made to increase the sensitivity of MR contrast agents [12]. The conjugation of gadolinium chelates with macromolecules, such as albumin and dendrimers, helps to improve sensitivity by carrying multiple gadolinium chelates in a single conjugate and by slowing molecular tumbling. However, since conjugation increases the molecular size of gadolinium chelates, this option is only feasible, if encapsulated drug molecules have similar molecular sizes and properties to the gadolinium chelates, and the diffusion rate/distance of the gadolinium chelates still distinct from those of the T2/T2* agent. Another issue is long acquisition times, but this is not a critical hurdle in clinical settings. Although it currently takes about an hour to acquire three-dimensional images with five different repetition times in order to obtain a quantitative T1 map, quantitative T1 maps can easily be obtained with a variable flip angle (VFA), which will make acquisition time significantly shorter [13, 14]. With regard to gadolinium chelates, clinically-available gadolinium chelates are relatively small, water-soluble molecules. Although 5-FU is also a small, water-soluble molecule, the release profile of 5-FU was not similar to that of GdDTPA, as shown in Fig. 2d. Therefore, the similarity of the release and diffusion behavior between the drug and contrast agent loaded in the carriers must be confirmed prior to the in vivo use of this technique. It also should be noted that the possibility for the toxicity of gadolinium may increase due to an extended half-life in the body, given that gadolinium chelates are used with nanocarriers. From these standpoints, the direct detection of drug molecules by MRS is ideal because MRS does not require using a surrogate tracer, although concern remains about the sensitivity.
This technique can be applied to multiple drug carriers or implant devices by incorporating superparamagnetic iron particles into the carrier/reservoir and using gadolinium chelate as a surrogate diffusible tracer for drug molecules. Gadolinium agent must be similar to the intended drug with regard to factors such as molecular size, hydrophilicity (lipophilicity), and other physicochemical characteristics: more sophisticated comparisons between gadolinium agents and drugs may be needed in order to use them as surrogate tracers.
5. Conclusions
The intact carrier generates negative contrast enhancement due to the dominant T2/T2* effect of superparamagnetic iron oxide (SPIO) nanoparticles, while positive contrast enhancement is generated by GdDTPA that is released and diffused away outside the range of the T2/T2* effect of SPIO after the carrier has degraded. Although much work remains to be done about the use of MR contrast agents as surrogate tracers for drugs, we have here, for the first time, demonstrated the feasibility of a new MRI strategy to monitor the delivery, release, and intratumoral distribution of cargo molecules in vivo. This technique will also be of value for other purposes, particularly those that take advantage of the differences in the contrast mechanisms of positive and negative MRI contrast agents, which are currently under investigation.
Supplementary Material
Acknowledgement
We thank Ms. Nicole E. Benoit for maintaining the cell lines, Ms. Lan Wu for her technical assistance, and Ms. Mary McAllister for editing this manuscript. This work was supported by the National Institutes of Health (R21 EB008162) and The Nagai Foundation, Tokyo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix. Supplementary material
Supplementary data associated with this article can be found, in online version.
References
- 1.Mora L, Chumbimuni-Torres KY, Clawson C, Hernandez L, Zhang L, Wang J. Real-time electrochemical monitoring of drug release from therapeutic nanoparticles. J Control Release. 2009;140:69–73. doi: 10.1016/j.jconrel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Port RE, Schuster C, Port CR, Bachert P. Simultaneous sustained release of fludarabine monophosphate and Gd-DTPA from an interstitial liposome depot in rats: potential for indirect monitoring of drug release by magnetic resonance imaging. Cancer Chemother Pharmacol. 2006;58:607–617. doi: 10.1007/s00280-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 3.Tung CH, Bredow S, Mahmood U, Weissleder R. Preparation of a cathepsin D sensitive near-infrared fluorescence probe for imaging. Bioconjug Chem. 1999;10:892–896. doi: 10.1021/bc990052h. [DOI] [PubMed] [Google Scholar]
- 4.Weissleder R, Tung CH, Mahmood U, Bogdanov AJ. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 5.Kato Y, Artemov D. Monitoring of release of cargo from nanocarriers by MRI/MRSI: Significance of T2/T2* effect of iron particles. Magn Reson Med. 2009;61:1059–1065. doi: 10.1002/mrm.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walstedt RE, Walker LR. Nuclear-resonance line shapes due to magnetic impurities in metals. Phys Rev B. 1974;9:4857–4867. [Google Scholar]
- 7.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y, Okollie B, Raman V, Vesuna F, Zhao M, Baker SD, et al. Contributing factors of temozolomide resistance in MCF-7 tumor xenograft models. Cancer Biol Ther. 2007;6:891–897. doi: 10.4161/cbt.6.6.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coe RA, Earl RA, Johnson TC, Lee JW. Determination of 5-fluorouracil in human plasma by a simple and sensitive reversed-phase HPLC method. J Pharm Biomed Anal. 1996;14:1733–1741. doi: 10.1016/0731-7085(96)01832-8. [DOI] [PubMed] [Google Scholar]
- 10.Ishida O, Maruyama K, Sasaki K, Iwatsuru M. Size-dependent extravasation and interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing mice. Int J Pharm. 1999;190:49–56. doi: 10.1016/s0378-5173(99)00256-2. [DOI] [PubMed] [Google Scholar]
- 11.Paran Y, Bendel P, Margalit R, Degani H. Water diffusion in the different microenvironments of breast cancer. NMR Biomed. 2004;17:170–180. doi: 10.1002/nbm.882. [DOI] [PubMed] [Google Scholar]
- 12.Raymond KN, Pierre VC. Next generation, high relaxivity gadolinium MRI agents. Bioconjug Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Schweitzer ME, Padua A, Regatte RR. Rapid 3D-T1 mapping of cartilage with variable flip angle and parallel imaging at 3.0T. J Magn Reson Imaging. 2008;27:154–161. doi: 10.1002/jmri.21109. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HL, Wright GA. Rapid high-resolution T1 mapping by variable flip angles: accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn Reson Med. 2006;55:566–574. doi: 10.1002/mrm.20791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.