Abstract
Status epilepticus (SE) is a progressive and often lethal human disorder characterized by continuous or rapidly repeating seizures. Of major significance in the pathology of SE are deficits in the functional expression of GABAA receptors (GABAARs), the major sites of fast synaptic inhibition in the brain. We demonstrate that SE selectively decreases the phosphorylation of GABAARs on serine residues 408/9 (S408/9) in the β3 subunit by intimately associated protein kinase C isoforms. Dephosphorylation of S408/9 unmasks a basic patch-binding motif for the clathrin adaptor AP2, enhancing the endocytosis of selected GABAAR subtypes from the plasma membrane during SE. In agreement with this, enhancing S408/9 phosphorylation or selectively blocking the binding of the β3 subunit to AP2 increased GABAAR cell surface expression levels and restored the efficacy of synaptic inhibition in SE. Thus, enhancing phosphorylation of GABAARs or selectively blocking their interaction with AP2 may provide novel therapeutic strategies to ameliorate SE.
Keywords: synaptic inhibition, protein kinase C, endocytosis, GABAA receptor, phosphorylation, status epilepticus
Introduction
Status epilepticus (SE) is a frequently occurring medical emergency associated with high mortality and morbidity. It is defined by prolonged (>30 min) unremitting or rapidly repeating seizures (Lowenstein and Alldredge, 1998; Alldredge and Lowenstein, 1999). The fundamental pathophysiology of SE involves a failure of mechanisms that normally abort an isolated seizure, resulting in neuronal hyperexcitability and compromised inhibitory GABAergic synaptic transmission (Treiman, 1990; Lowenstein and Alldredge, 1998; Staley, 2004). Mechanisms underlying these deficits are at least in part postsynaptic and include reduced potency and efficacy of GABA together with altered transmembrane Cl− gradients (Staley, 2004); accordingly, the frontline treatments for SE are benzodiazepines (Bzs) and barbiturates, which potentiate the activity of GABAA receptors (GABAARs), the principal sites of fast synaptic inhibition in the brain.
GABAARs are Cl−-selective ligand-gated ion channels that are assembled from seven subunit classes: α1–6, β1–3, γ1–3, δ, ε, and π, providing the structural basis for extensive heterogeneity of receptor structure (Sieghart and Sperk, 2002; Rudolph and Mohler, 2004, 2006). A combination of molecular, biochemical, and genetic approaches suggests that, in the brain, the majority of benzodiazepine receptor subtypes are composed of α, β, and γ2 subunits (Rudolph and Mohler, 2004). γ2-containing receptors are highly enriched at synaptic sites and are responsible for mediating phasic inhibition (Essrich et al., 1998; Kittler and Moss, 2003; Luscher and Keller, 2004). In contrast, receptors composed of α, β, and δ form populations of extrasynaptic receptors that mediate tonic inhibition but are insensitive to modulation by Bzs (Farrant and Nusser, 2005).
Although GABAAR receptor allosteric modulators are effective in blocking SE shortly after its induction, they are ineffective at later time periods (>1 h), and this development of pharmacoresistance is associated with significantly higher rates of mortality. Studies performed in culture have established that GABAARs undergo constitutive endocytosis and recycling, a process that can directly regulate the efficacy of synaptic inhibition (Kittler et al., 2000, 2004; Blanchet et al., 2003; van Rijnsoever et al., 2005). To date, the relevance of these putative regulatory processes in the pathology of SE remains poorly understood (Goodkin et al., 2005; Naylor et al., 2005).
Here we demonstrate that SE selectively reduces the cell surface stability of distinct GABAAR subtypes and the protein kinase C (PKC)-dependent phosphorylation of 408/9 (S408/9) within the receptor β3 subunit. These residues lie within a basic patch-binding motif for the clathrin adaptor protein AP2, a critical regulator of endocytosis. Coincident with decreased phosphorylation enhanced association of GABAARs with AP2 was apparent in SE. Increasing the level of β3 subunit phosphorylation via the pharmacological activation of PKC or specifically blocking the binding of GABAARs to AP2 enhanced GABAA receptor cell surface expression levels and restored the efficacy of synaptic inhibition during SE. Thus, inhibiting endocytosis or enhancing the phosphorylation of GABAARs may provide novel therapeutic strategies to ameliorate SE.
Materials and Methods
Induction of status epilepticus and tissue preparation.
All procedures were performed in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Institutional Animal Care and by The Children's Hospital of Philadelphia Animal Care and Use Committee. Six- to eight-week-old male FVB/N mice (Jackson Laboratories, Bar Harbor, ME) were injected first with scopolamine methyl nitrate (1 mg/kg, i.p., Sigma, St. Louis, MO) to minimize the peripheral cholinergic effects of pilocarpine, and were injected 30 min later with pilocarpine hydrochloride (350 mg/kg, i.p., Sigma) or saline. Vehicle-treated animals were treated with scopolamine alone. After 1 h from the onset of stage 5 seizures, animals were deeply anesthetized with halothane and decapitated at the second cervical vertebrae. The hippocampus was then rapidly removed and rinsed with ice-cold artificial CSF (ACSF) containing 124 mm NaCl, 3 mm KCl, 2 mm CaCl2, 25 mm NaHCO3, 1.1 mm NaH2PO4, 2 mm MgSO4, and 10 mm d-glucose, equilibrated with 95% O2 and 5% CO2 to yield a pH of 7.4. The hippocampus was then cut into 350 μm slices using a tissue chopper for slice biotinylation or 400 μm slices using a vibratome (VT1000S; Leica, Deerfield, IL) for electrophysiology. Hippocampal slices were incubated in ASCF at 30°C for 1 h recovery before experimentation.
Surface biotinylation.
Kinase activators/inhibitors were applied for up to 15 min at 32°C, whereas myristoylated P4 and scrambled control peptides (Tocris, Bristol, UK) were used at a concentration of 90 μm in ASCF for 1 h. After treatment, slices were chilled to 4°C and incubated for 30 min with 1 mg/ml NHS-SS-biotin (Pierce, Rockford, IL). Excess biotin was removed by washing three times in cold ACSF and lysed as described previously (Terunuma et al., 2004). After correction for protein content, lysates were incubated with Streptavidin beads for 12 h at 4°C. Bound material was subjected to SDS-PAGE and then immunoblotted with antibodies and visualized by ECL (Pierce). Blots were then quantified using the CCD-based FujiFilm (Tokyo, Japan) LAS 3000 system.
GST protein “pull-down” assay.
GST-β3, which encodes residues 303–425 of the GABAAR β3 subunit major intracellular domain, was purified by affinity chromatography from Escherichia coli extracts and exposed to detergent-soluble extracts prepared from hippocampus as described previously (Brandon et al., 1999; Terunuma et al., 2004). Material was then immunoblotted or subjected to in vitro kinase assays in the presence of 10 μCi 32P-γATP (final ATP concentration 200 μm). Kinase assays were performed to 30 min in the presence and absence of 1 μm KT5720 (PKA inhibitor), 15 μm GF 109203X (PKC inhibitor), or 10 μm KN-62 (CamKII inhibitor). Phosphorylation was then assessed by SDS-PAGE and quantified using a phospho-imager.
Immunoprecipitation.
Hippocampal slices were lysed in 50 mm Tris-HCl, pH 7.5, 0.5 mm EDTA, 2 mm EGTA, and 1% Triton X-100. Soluble material was then immunoprecipitated with either anti-β3 antibody or control IgG coupled to protein A Sepharose (Brandon et al., 1999, 2000, 2002b, 2003). Bound material was then either subjected to immunoblotting or to an in vitro kinase assay as outlined above.
Protein phosphatase activity assays.
Protein phosphatase activity in hippocampal extracts was assayed by release of 32P-phosphate from phosphorylated GST-β3. GST-β3 immobilized on glutathione agarose beads was phosphorylated with 2 μCi [γ-32P]-ATP and 100 ng of PKC, followed by washing with dephosphorylation buffer containing 50 mm Tris-HCl, pH 7.0, 10 mm β-mercaptoethanol, 1 mm EDTA, 0.1 mm phenylsulfonyl fluoride, 2 mm benzamidine, 1 μg/ml leupeptin, and 1 μg/ml aprotinin. Hippocampal slices, homogenized in dephosphorylation buffer, were mixed with 32P-labeled GST-β3 and incubated at 30°C for 10 min followed by the addition of 200 μl of trichloroacetic acid. The mixture was kept for 10 min at room temperature and centrifuged at 13,000 rpm for 5 min, and the pellet was counted for radioactivity.
To analyze the phosphatase activity associated with GST-β3, bound material was washed twice in enzyme dilution assay containing 20 mm 3-morpholinopropane-1-sulfonic acid, pH 7.5, 150 mm NaCl, 60 mm β-mercaptoethanol, 1 mm MgCl2, 1 mm EGTA, 0.1 mm MnCl2, 1 mm DTT, 0.1 mg/ml bovine serum albumin, and 10% glycerol and was resuspended with 10 μl of enzyme dilution buffer with or without 10 nm okadaic acid. The resulting beads were incubated with 14.2 μm p-nitrophenyl phosphate (PNPP) for 15 min at 30°C, then terminated by 25 μl of 1 m NaOH, and the absorbance at 410 nm was measured (Terunuma et al., 2004).
Measuring basal PKA and PKC activity.
PKC activity in total lysates was measured using the SignaTECT (Promega, Madison, WI) kit in the presence and absence of 10 μm PKCI18–36 inhibitor peptide (Brandon et al., 1999). PKA activity was measured using Kemptide as a substrate (Terunuma et al., 2004).
Electrophysiology.
A fixed stage upright microscope (Leica DMLFSA) with a 63× water-immersion objective equipped with a CCD camera (#C5985; Hamamatsu, Shizuoka, Japan) was used. Slices were maintained at 25°C and gravity-superfused with ACSF solution containing 6,7-dinitroquinoxaline-2,3-dione (10 μm), and d-(−)-2-amino-5-phosphonopentanoic acid (50 μm; Tocris) to block glutamatergic responses and tetrodotoxin (400 nm; Sigma) to block action potentials. Whole-cell voltage-clamp recording of miniature IPSCs (mIPSCs) was conducted using a high-chloride internal pipette solution (EGABA = 0 mV), which resulted in an inward chloride current when cells were clamped at −70 mV (junction potential not corrected). The pipette solution consisted of the following (in mm): 140 CsCl, 1 CaCl2, 1 MgCl2, 10 EGTA, 10 HEPES, 2.5 phosphocreatine-2 Na, 2 Mg-ATP, and 1.0 GTP-Tris, titrated to pH 7.2–7.3 with 3 m CsOH (osmolarity, 280–290 mOsm). Internal solution was supplemented with Pepβ3 and Pepβ3P at 200 μg/ml, and phorbol-12,13-dibutyrate (PDBU) and calphostin were included at final concentrations of 100 nm PDBU and 10 μm calphostin, respectively. Synaptic currents were recorded using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 2 kHz, sampled at 5 kHz, digitized (Digidata 1320A; Molecular Devices), and stored for off-line analysis [using Minianalysis 9, Synaptosoft, Decatur, GA) and IGOR Pro (Wavemetrics, Lake Oswego, OR)]. Access resistance stability (10–18 MÙ; 80% compensation) was monitored using a −5 mV voltage, SE was applied every 120 s, and data from cells were discarded when >15% change occurred. In every experiment, a 2 min stabilization period was allowed before starting data collection at 0 min. Data are expressed as mean ± SEM, and p values are derived from student's paired t test with significance levels assessed at p < 0.01.
Results
SE selectively decreases GABAAR cell surface stability
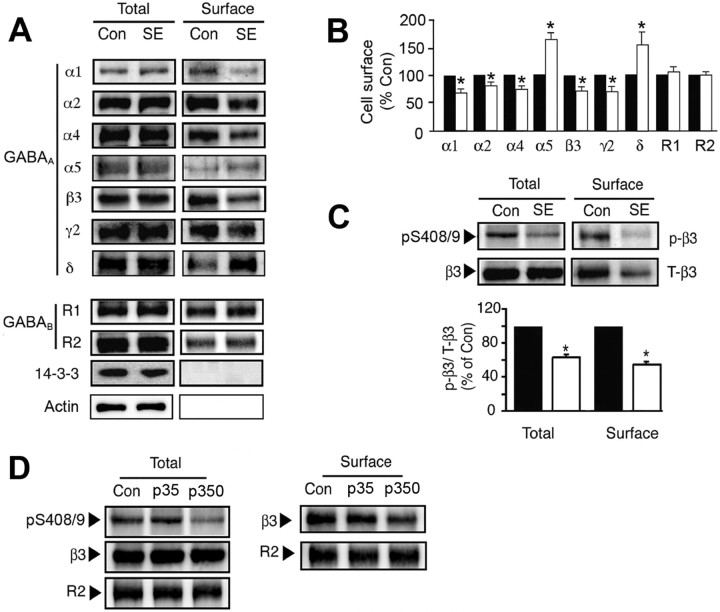
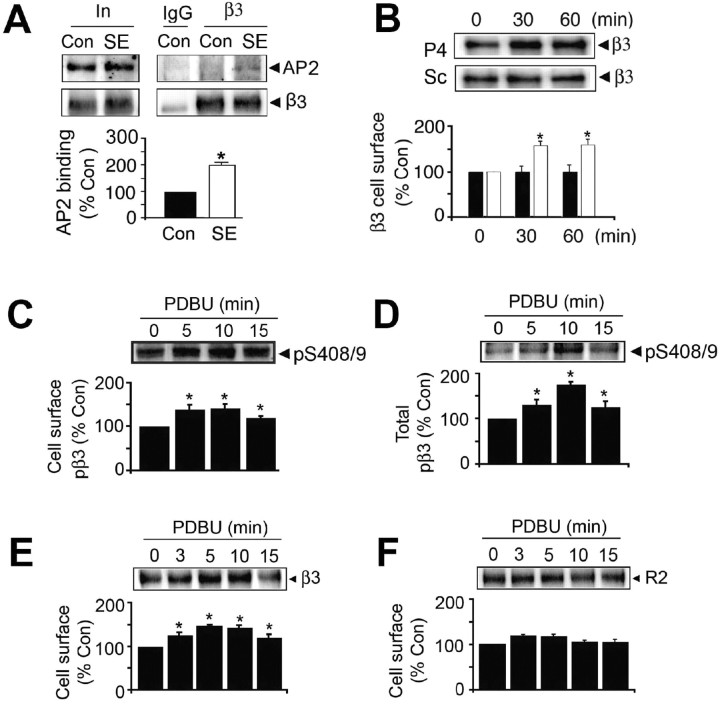
To induce SE, FVB/N mice were injected with scopolamine/pilocarpine, and animals with pronounced behavioral seizures were killed 1 h later. Hippocampal slices were prepared from these animals and control animals injected with scopolamine alone and allowed to recover at 30°C for 1 h before experimentation. Slices were labeled with NHS-biotin and lysed, and biotinylated proteins were purified on avidin. The levels of GABAAR α, β, γ, and δ subunit isoforms were then measured in the resulting cell surface and total fractions using immunoblotting. SE significantly decreased the cell surface expression levels of the GABAAR α1 (52 kDa), α2 (53 kDa), and α4 (67 kDa) subunits to 68.7 ± 4.3% (α1), 81.7 ± 4.1% (α2), and 77.5 ± 4.7% (α4) compared with control, but their total cellular levels were unaltered (Fig. 1A,B). Likewise reductions in the cell surface expression levels of the β3 (73.3 ± 7.7%) and γ2 (74.3 ± 7.8%) subunits were also apparent in SE (Fig. 1A,B). In contrast SE significantly increased the cell surface expression cellular levels of the GABAAR receptor α5 (62 kDa) and δ (48 kDa) subunits (180 ± 10.65 and 152.3 ± 16.0% of control, respectively). However, the cell surface expression levels of the GABABR R1 and R2 subunits remained unaltered in SE (Fig. 1A,B). To control for the integrity of our cell surface fractions, they were blotted with antibodies against 14-3-3ζ and actin, both of which are exclusively cytoplasmic proteins. Although 14-3-3ζ and actin could be detected in total extracts, they were absent from biotinylated cell surface fractions (Fig. 1A).
Figure 1.
The effects of SE on the cell surface stability and phosphorylation of GABAARs. A, B, SE modulates GABAA receptor surface expression levels. Hippocampal slices from control (Con) or SE were labeled with NHS-biotin and lysed, and biotinylated proteins were purified on avidin. A, Cell surface and total fractions (1/10 of input; In) were immunoblotted with antibodies against GABAA receptor α1–2, α4–5, β3, γ2, and δ subunits, GABAB R1 and R2 subunits, 14-3-3ζ, and actin. B, Data in SE (open bars) were normalized to the cell surface levels evident in control slices (filled bars; 100%). C, SE decreases the phosphorylation of S408/9 in the GABAAR β3 subunit. Top, Hippocampal slices from control and SE were subjected to biotinylation, and cell surface fractions were blotted with a phospho-specific antibody against S408/9 in the β3 subunit (p-β3) and anti-β3 antibodies (T-β3). Bottom, The ratio p-β3/T-β3 signals were then measured in control (filled bars) and SE (open bars) with values normalized to those in control (100%). In all panels, asterisks indicate significant difference from control (p < 0.01; Student's t test; n = 6–8). D, Hippocampal slices were prepared from Con, and animals were injected with 35 (p35) and 350 mg/kg (p350) pilocarpine, respectively. Slices were biotinylated, and cell surface and total fractions were isolated and immunoblotted with the respective antibodies as indicated.
Therefore, SE can differentially modulate the cell surface stability of individual receptor subunits, which suggests that the cell surface expression levels of receptor subtypes containing α1–4, β3, and γ2 subunits will be decreased, whereas those containing α5 and δ subunits will be increased in SE.
SE decreases phosphorylation of the β3 subunit
Phosphorylation of GABAARs is emerging as a critical process in regulating their accumulation on the neuronal plasma membrane. Central to this modulation are phosphorylation sites for a range of protein kinases that are conserved in the intracellular domains of β1–3 subunits; serines 408/9 (S408/9) in the case of β3 (Brandon et al., 2002a; Hinkle and Macdonald, 2003; Wang et al., 2003; Jovanovic et al., 2004). These serine residues lie within a “basic patch” binding motif for AP2, a critical regulator of protein entry into the endocytic pathway (Haucke et al., 2000, 2006). Phosphorylation of S408/9 decreases the affinity of AP2 for the receptor β3 subunit (Kittler et al., 2005). Therefore, we analyzed whether SE induces altered GABAARs phosphorylation by immunoblotting both cell surface and total fractions with a phospho-specific antibody against S408/9 and a phospho-independent epitope in the β3 subunit (Brandon et al., 2002b; Jovanovic et al., 2004). SE induced a significant decrease in the level of S408/9 phosphorylation for β3 subunits on the plasma membrane and in total extracts to 64.2 ± 4.5% and 54.8 ± 5.6% of control, respectively (Fig. 1C).
Pilocarpine is a muscarinic acetylcholine receptor (mAChR) agonist, and activation of these receptors has been established to modulate S408/9 phosphorylation in cultured cortical neurons (Brandon et al., 2002b). Therefore, we examined the effects of low doses of pilocarpine that do not induce SE on β3 subunit phosphorylation. Injection of animals with 35 mg/kg pilocarpine, which is sufficient to activate mAChRs (Nathanson, 1987; Wess, 2004), did not significantly modify 408/9 phosphorylation or GABAAR cell surface stability (Fig. 1D). Under the same conditions, 350 mg/kg pilocarpine decreased both 408/9 phosphorylation and GABAAR cell surface expression levels (Fig. 1D). Therefore, modified GABAAR phosphorylation in SE induced by pilocarpine does not simply result from the activation of mAChRs by this agent.
Thus, in addition to decreasing GABAAR cell surface stability, SE clearly also reduces the phosphorylation of S408/9 in the β3 subunit, key residues for regulating the endocytosis of these proteins (Kittler et al., 2005; Chen et al., 2006).
SE decreases PKC activity and its association with the receptor β3 subunit
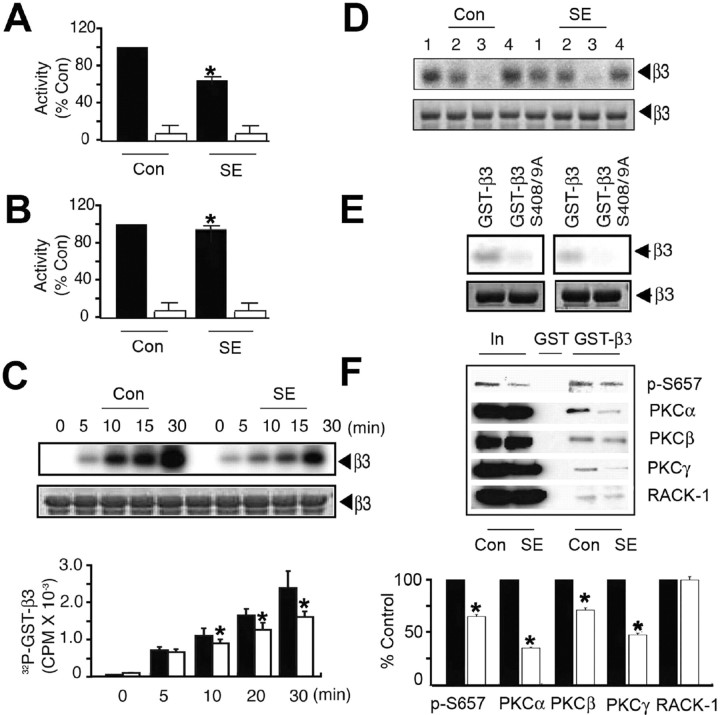
Basal phosphorylation of S408/9 in neurons is largely dependent on PKC activity; however, it is also dependent on substrates of cAMP-dependent protein kinase (PKA) (Brandon et al., 1999, 2000, 2002b, 2003). We thus compared the activity of these kinases in hippocampal extracts prepared from control and SE. As measured using the neurogranin peptide in the presence and absence of GF 10923, a specific PKC inhibitor, PKC activity was significantly reduced to 70.8 ± 2.5% of control in SE (Fig. 2A). However, the activity of PKA as measured by the phosphorylation of Kemptide was reduced by only 5% (Fig. 2B).
Figure 2.
SE decreases PKC activity and interaction with the GABAAR β3 subunit. A, B, SE decreases PKC activity in the hippocampus. A, Detergent-solubilized hippocampal extracts from control and SE were exposed to 50 μm neurogranin peptide in the absence (filled bars) and presence (open bars) of 100 nm PKCI19–36 inhibitor peptide in the presence of 10 μCi 32P-γATP. Data were normalized to level of activity seen with control extracts (100%). B, Similar experiments were also performed using 50 μm Kemptide, a PKA substrate, in the absence (filled bars) and presence (open bars) of 20 nm Walsh peptide, a specific PKA inhibitor, and data were normalized to the level of activity evident in control (100%). C, Phosphorylation of GST-β3 by hippocampal extracts. Top, Fusion proteins were incubated with hippocampal extracts, and bound material was incubated with 10 μCi of 32P-γATP at 30°C followed by SDS-PAGE. Bottom, The level of phosphorylation was then calculated, and data were normalized to levels seen with control extracts (100%). D, PKC activity mediates the phosphorylation of GST-β3 by hippocampal extracts. Material bound to GST-β3 was incubated for 30 min with 10 μCi 32P γATP at 30°C under control conditions (1) or in the presence of 1 μm KT5720 (2; PKA inhibitor), 15 μm GF 109203 (3; PKC inhibitor), or 10 μm KN-62 (4; Ca/calmodulin type 2-dependent protein kinase), and phosphorylation was examined by SDS-PAGE. E, Hippocampal extracts phosphorylated GST-β3 on S408/9. GST-β3 or a mutant in which S408/9 had been converted to alanine residues were incubated with hippocampal extracts, bound material was incubated for 30 min with 10 μCi 32P-γATP at 30°C, and phosphorylation was measured via SDS-PAGE. Top, Autoradiogram. Bottom, Coomassie stain of the same gel. Left, Control; right, SE. F, PKC binding to GST-β3 is reduced from SE extracts. Top, Material bound to GST-β3 was immunoblotted with antibodies against p-S657, antibodies specific to individual PKC isoforms, and against RACK-1. Bottom, The level of binding for each protein was then determined for control (filled bars) and SE (open bars) extracts, with data being normalized to the levels seen from control (100%). In all panels, asterisks indicate significant difference from control (p < 0.01; Student's t test; n = 7–10).
Previous studies have revealed that the intimate association of PKC isoforms with the intracellular domains of GABAAR β1–3 subunits is important in regulating GABAAR phosphorylation, and for the β3 subunit, amino acids 404–415 are critical in mediating PKC binding (Brandon et al., 2002a,b). Therefore, we examined whether the deficit in PKC activity evident in SE alters the targeting of this kinase to GABAARs. To initiate these studies, detergent-soluble hippocampal lysates were exposed to a glutathione S-transferase fusion protein encoding the intracellular domain of the β3 subunit (GST-β3) and bound material subjected to in vitro kinase assays (Brandon et al., 2002b). GST-β3 was phosphorylated under these conditions, but the level of phosphorylation by SE extracts was significantly lower than that seen in control (Fig. 2C) (67.5 ± 5.0% at 30 min). Phosphorylation by either extract was inhibited by GF 10923, but not by inhibitors of PKA or Ca/calmodulin type 2-dependent protein kinases (Fig. 2D). Moreover, consistent with published studies (Brandon et al., 2002b) phosphorylation of GST-β3 by both control and SE occurred principally on S408/9 (Fig. 2E), whereas this was not the case for GST alone (data not shown).
To further analyze PKC association with GABAARs, we used an antibody specific to phospho-S657, a residue conserved in all classical PKC isoforms (α, β, and γ), the phosphorylation of which reflects the catalytic activity of these enzymes (Parker, 2003). The level of phospho-S657 binding to GST-β3 from SE extracts was significantly reduced to 61.2 ± 1.8% of control (Fig. 2F). In agreement with this, the levels of the α, β, and γ isoforms of PKC bound to GST-β3 were also significantly reduced from those seen in SE (Fig. 2F) (33.1 ± 1%, 67.1 ± 2%, and 44.9 ± 1.3% of control, respectively). As a control for these experiments, we assessed binding of the receptor for activated-C kinase (RACK-1) to GST-β3. RACK-1 binds to GST-β3 but at a site distinct from that for PKC; they bind at residues 395–404, respectively (Brandon et al., 2002b). In contrast to our experiments with PKC, the level of RACK-1 binding to GST-β3 from SE extracts was unaltered relative to control (Fig. 2F) (101.6 ± 3% of control).
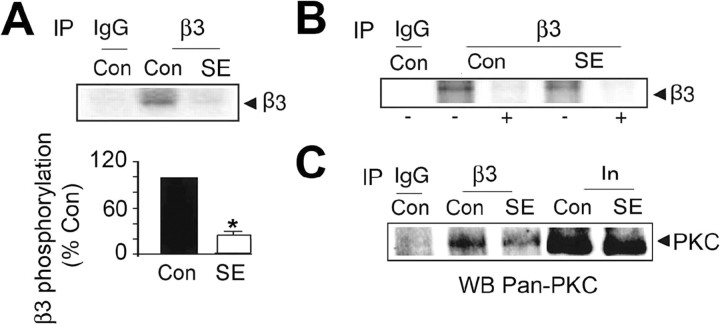
Immunoprecipitation was further used to examine the association of PKC with whole GABAARs. To do so, detergent-soluble hippocampal extracts were immunoprecipitated with antibodies against the β3 subunit, and precipitated material was subjected to kinase assays. Phosphorylation of a major band of 57 kDa corresponding to the receptor β3 subunit (Brandon et al., 1999) was seen with material immunoprecipitating with anti-β3 antibody, but not control IgG. However, phosphorylation of this band was significantly reduced to 15 ± 5% of control in SE (Fig. 3A). Significantly phosphorylation by both extracts was abolished in GF 10923 (Fig. 3B), and the level of the α, β, and γ PKC isoforms coimmunoprecipitating from SE extracts was reduced to 67.5 ± 8.9% of control (Fig. 3C). Together, our results demonstrate that the level and activity of PKC isoforms associated with the GABAARs in the hippocampus are reduced in SE.
Figure 3.
GABAAR phosphorylation by intimately associated PKC is decreased in SE. A, B, Phosphorylation of the β3 by intimately associated PKC activity. A, Top, Detergent-solubilized hippocampal extracts were immunoprecipitated with IgG or anti-β3 antibody, and precipitated material was incubated with 10 μCi 32P-γATP at 30°C for 15 min followed by SDS-PAGE. A, Bottom, The level of β3 subunit phosphorylation was then determined for control and SE extracts, and the level of β3 subunit phosphorylation was normalized to that seen with control extracts (100%). B, Phosphorylation was also assessed in the absence (−) or presence (+) of 15 μm GF 109203. C, The levels of the α, β, and γ PKC isoforms associated with GABAAR are reduced in SE. Material immunoprecipitating with IgG or anti-β3 from control and SE extracts was immunoblotted with an antibody specific to the PKC α, β, and γ isoforms. In, 10% of the input used for each immunoprecipitation.
SE increases the association of protein phosphatase 2A with the β3 subunit
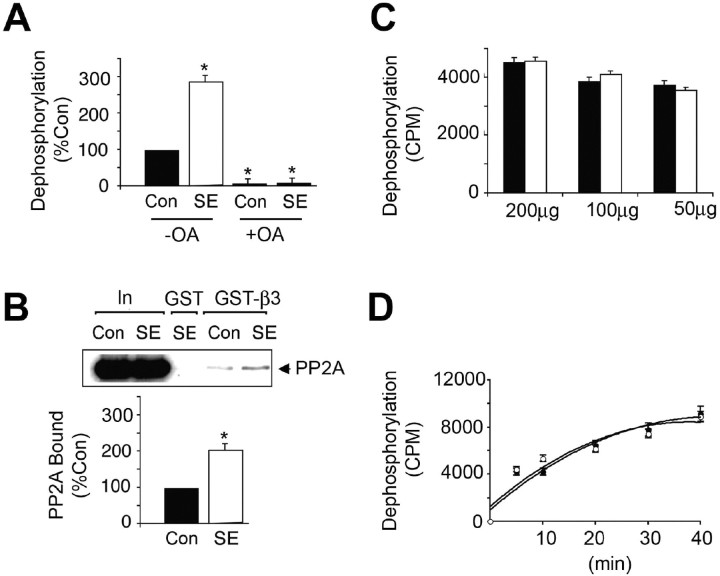
In addition to analyzing β3 subunit phosphorylation, we compared its dephosphorylation in SE, which is mediated largely by PP1 and PP2A (Jovanovic et al., 2004; Terunuma et al., 2004). For these experiments, the ability of material bound to GST-β3 to dephosphorylate exogenous PNPP was measured (Terunuma et al., 2004). A highly significant increase (p < 0.01) in dephosphorylation of PNPP by SE to 297.9 ± 8.9% of control was evident (Fig. 4A). This effect was completely blocked by 20 nm okadaic acid, a concentration that specifically inhibits PP2A. Consistent with this, increased binding of the PP2A catalytic subunit to GST-β3 was also observed (Fig. 4B). However, the level of total phosphatase activity in SE and control remained unaltered (Fig. 4C,D).
Figure 4.
Enhanced association of the GABAAR β3 subunit with PP2A in SE. A, Dephosphorylation of PNPP by hippocampal extracts. GST-β3 was exposed to lysates from control (filled bars) and SE (open bars) hippocampus, and the ability of bound material bound to dephosphorylated 14.2 μm PNPP in the absence (−) and presence (+) of 20 nm okadaic acid (OA) was measured. Data were then normalized to the level of dephosphorylation seen with control extracts (100%). B, Increased binding of PP2A to GST-β3 from SE extracts. Top, Material bound to GST-β3 or 10% of the input used for each immunoprecipitation (In) was immunoblotted with an antibody against the PP2A catalytic subunit. Bottom, The level of PP2A bound from control (filled bar) and SE (open bar) extracts was then determined, and values were normalized to those evident in control (100%). C, D, Dephosphorylation of 32P-GST-β3 by hippocampal extracts. Hippocampal lysates were prepared from control (filled bars and circles) and SE (open bars and circles). C, The ability of 200–50 μg of total detergent-soluble protein from these extracts to dephosphorylate 32P-GST-β3 was measured over a 10 min time course. D, The ability of 50 μg of total protein from control (filled circles) and SE (open circles) to dephosphorylate 32P-GST-β3 over a time course of 40 min was also examined. In all panels, asterisks indicate significant difference from control (p < 0.01; Student's t test; n = 3–4).
Blocking endocytosis or activating PKC enhances GABAAR cell surface stability in SE.
Our biochemical studies demonstrate that S408/9 phosphorylation is reduced during SE together with the level of PKC associated with GABAARs. The pool of PP2A associated with GABAARs is increased in SE, which may further promote dephosphorylation of S408/9. Residues 401–412 of the β3 subunit bind directly to the μ2 subunit of AP2, a critical regulator of endocytosis. This interaction is decreased by phosphorylation of S408/9 within this basic patch μ2-binding motif (Brandon et al., 2002a; Kittler et al., 2005; Haucke, 2006; Chen et al., 2006).
Therefore, we assessed whether deficits in S408/9 phosphorylation during SE led to enhanced association of GABAARs with AP2. Hippocampal extracts were immunoprecipitated with anti-β3 and then immunoblotted with antibodies against the receptor β3 subunit or the α subunit of AP2. Both proteins immunoprecipitated with anti-β3 but not control IgG. The ratios of AP2/β3 signals were then determined after controlling for the respective inputs. The ratio showed a significant increase of 217.2 ± 10.9% in SE compared with control (Fig. 5A). To examine the significance of this enhanced association with AP2, the effects of blocking clathrin-dependent endocytosis on GABAAR cell surface expression levels in SE were examined. Hippocampal slices were incubated at 30°C for 1 h and then for up to an additional 60 min in the presence of myristoylated P4 (P4) peptide, a potent inhibitor of GABAAR endocytosis (Kittler et al., 2000; Bogdanov et al., 2006). The cell surface expression levels of GABAARs incorporating β3 were then measured via immunoblotting after biotinylation. P4 peptide produced a significant increase in the cell surface expression levels of receptors incorporating β3 subunits to 142 ± 5.4% of control (p < 0.01) after 30 min, an effect that was sustained for up to 60 min. This effect was not replicated with the control scrambled (Sc) peptide (Fig. 5B). The effects of pharmacologically activating PKC on the phosphorylation and cell surface stability of receptors in SE were examined. Treatment of SE slices with PDBU increased the phosphorylation of S408/9 for both cell surface and total populations of GABAARs over 15 min (Fig. 5C) (141 ± 4.2% and 175 ± 5.2% of control at 10 min, respectively). Coincident with this, activation of PKC produced a sustained increase in the cell surface levels of GABAAR receptors over the same time course (145.9 ± 3.5% of control at 10 min). In contrast, under the same conditions the cell surface expression levels of GABAARs were not altered by exposure to PDBU.
Figure 5.
Blocking endocytosis and the activation of PKC increases GABAAR cell surface expression levels in SE. A, Increased association of GABAARs with AP2 in SE. Detergent-solubilized extracts were immunoprecipitated with IgG or anti-β3 antibodies, and precipitated material was immunoblotted with antibodies against the GABAAR β3 subunit or the α subunit of AP2. In represents 20% of the input used for each immunoprecipitation. After controlling for recovery of the β3 subunit, the level of AP2 binding was determined for control (filled bar) and SE (open bar), and data were normalized to levels evident in control (100%). B, Blocking endocytosis increases GABAAR cell surface stability in SE. Hippocampal slices from SE animals were incubated in the presence of 90 μm P4 or control Sc peptide. Top, Slices were then subjected to biotinylation with NHS-biotin, and cell surface fractions were immunoblotted with anti-β3 antibody. Bottom, The levels of cell surface β3 in slices exposed to either Sc (filled bars) or P4 (open bars) were then normalized to those evident at 0 time (100%). C, D, PKC activators increase GABAA receptor phosphorylation and cell surface expression levels in SE. Slices were incubated with 100 nm PDBU and labeled with NHS-biotin. Cell surface (C) and total fractions (D) were then immunoblotted with pS408/9 antibody. The level of phosphorylation of S408/9 was normalized to that evident at 0 time (100%). E, F, PKC activators increase GABAA receptor cell surface stability in SE. Cell surface fractions from SE slices were blotted with anti-β3 (E) and antibodies against the GABABR2 subunit (F), and levels of each protein were normalized to those seen at 0 time (100%). In all panels, asterisks indicate significant difference from control (p < 0.01; Student's t test; n = 5–7).
These results demonstrate that blocking endocytosis or increasing the phosphorylation of S408/9 in the β3 subunit enhances GABAAR cell surface stability during SE.
Phospho-dependent inhibition of GABAAR AP2 binding modifies mIPSCs in SE
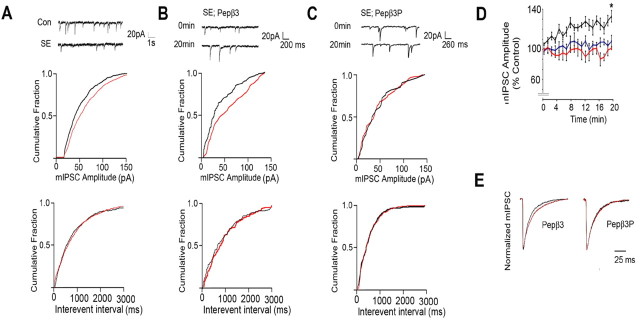
To analyze the functional significance of our biochemical studies, we compared the properties of mIPSCs in CA1 neurons from control and SE slices (Kapur and Coulter, 1995). mIPSCs were isolated using bath application of tetrodotoxin and glutamate receptor antagonists. Under these conditions, remaining events were blocked by the GABAAR antagonist bicuculline (data not shown), confirming their identity as mIPSCs. We first compared the properties of mIPSCs in CA1 pyramidal neurons from control slices and those from animals undergoing SE using standard patch pipette electrolyte. mIPSC peak amplitude was significantly reduced from 48.5 ± 3.8 pA in control compared with 39.8 ± 1.9 pA in SE (Fig. 6A, Table 1). In contrast, mIPSC frequency and rise and decay times were very similar in controls and SE (Fig. 6A, Table 1).
Figure 6.
Blockade of endocytosis increases the amplitude of mIPSCs and slows their decay in CA1 neurons undergoing SE. A, SE reduces mIPSC amplitude. Top, Representative sweeps. Middle, bottom, Cumulative probability analysis for mIPSC amplitude (middle) and frequency (bottom; interevent interval) recorded from CA1 neurons in control (black) and SE (red) slices. B, C, Pepβ3 selectively enhances mIPSC amplitude in SE neurons. Top, Representative sweeps at 0 and 20 min for CA1 neurons exposed to 100 μg/ml Pepβ3 (KTHLRRRSSQLK; B) or Pepβ3P (KTHLRRRSPSPQLK; C) via intracellular dialysis. Cumulative probability for mIPSC amplitude and frequency are also shown at 0 time (red) and 20 min (black), respectively. D, Time-dependent enhancement of mIPSCs by Pepβ3. mIPSC peak amplitude from CA1 neurons dialyzed with control (blue), Pepβ3 (black), and Pepβ3P (red) over a 20 min time course was normalized to that seen at 0 time (100%). E, Pepβ3 modulates mIPSC decay. Scaled mIPSCs are shown for CA1 neurons from SE slices dialyzed with Pepβ3 or Pepβ3P at 0 (black) and 20 min (red), respectively. In all panels, asterisks indicate significant difference from control (p < 0.01; Student's t test; n = 8–12).
Table 1.
The properties of mIPSC in CA1 neurons undergoing SE
| Parameter | Con | SE | SE + Pep_3 | SE + Pep_3P | PDBU | PDBU + CalC | CalC |
|---|---|---|---|---|---|---|---|
| Peak amplitude (pA) at 2 min | 46.6 ± 2.6 | 37.3 ± 1.8 | 36.2 ± 3.1 | 38.0 ± 3. | 3 35.3 ± 3.2 | 39.2 ± 3.5 | 36.0 ± 3.9 |
| Peak amplitude (pA) at 22 min | 48.5 ± 3.8 | 39.5 ± 1.9 | 53.9 ± 7.5* | 38.4 ± 3.1 | 51.5 ± 4.6* | 40.6 ± 3.9 | 37.1 ± 4.1 |
| 10–90% rise time (ms) | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.2 |
| τ decay (ms) at 2 min | 16.9 ± 1.1 | 17.4 ± 1.1 | 15.9 ± 0.9 | 16.3 ± 1.4 | 15.3 ± 0.9 | 14.6 ± 2.3 | 19.0 ± 0.5 |
| τ decay (ms) at 22 min | 17.9 ± 0.9 | 18.3 ± 1.1 | 21.7 ± 1.3* | 18.2 ± 1.7 | 21.3 ± 1.9* | 16.2 ± 2.5 | 21.9 ± 0.9 |
| Frequency | 2.1 ± 0.4 | 1.7 ± 0.2 | 2.1 ± 0.3 | 1.8 ± 0.4 | 1.2 ± 0.35 | 1.6 ± 0.3 | 1.2 ± 0.35 |
The kinetic parameters of mIPSCs of CA1 neurons in the absence and presence of peptides, PKC inhibitors, and activators were calculated at both 0 time and 20 min. In all panels, asterisks indicate significant difference from control (Con; p < 0.01; n = 8–12).
To examine the role that altered endocytosis plays in this phenomenon, we used intracellular dialysis with the patch pipette to introduce dominant peptides that have been documented to block the interaction of the AP2 adaptin with the GABAAR β3 subunit. For these experiments, we used Pepβ3 (KTHLRRRSSQLK) or its phosphorylated equivalent Pepβ3P (KTHLRRRSPSPQLK), which correspond to the basic patch AP2-binding motif residues 401–412 in the β3 subunit (Kittler et al., 2005; Haucke, 2006). Pepβ3 has an affinity of 300 nm for AP2, whereas that for Pepβ3P is reduced to 1300 nm (Kittler et al., 2005). In agreement with these differing affinities, Pepβ3P but not Pepβ3P blocks GABAAR endocytosis in cultured neurons (Kittler et al., 2005; Chen et al., 2006). Dialysis of neurons with Pepβ3 significantly increased mIPSC amplitude from 36.2 ± 3.1 at 0 to 53.9 ± 7.5 pA at 20 min in CA1 SE neurons, but their frequency was not altered (Fig. 6B, Table 1). Importantly, this effect was not replicated with Pepβ3P (Fig. 6C, Table 1).
To further evaluate this observation, we compared mIPSC amplitude at 2 min intervals over a 20 min recording period for cells dialyzed with these distinct agents. Pepβ3 produced a time-dependent increase in mIPSC amplitude that reached 139 ± 0.5% of that evident at 0 time after 20 min (Fig. 6D). In common with standard electrolyte Pepβ3P did not alter mIPSC amplitude over the same time course (Fig. 6D). The effects of Pepβ3 and Pepβ3P on mIPSC decay and rise times were measured. Data were fitted by single exponentials to determine τ decay at 0 time and after 20 min (Fig. 6E, Table 1). For Pepβ3, τ decay was significantly slowed from 15.9 ± 0.9 ms at 0 time to 21.7 ± 1.3 ms at 20 min, an effect not seen with Pepβ3P (16.3 ± 1.4 ms vs 18.2 ± 1.7 ms, respectively). In contrast, mIPSC rise time was not modulated by either peptide (Table 1). The effect of Pepβ3 on mIPSCs from CA1 neurons in control slices was also assessed. Intriguingly, and in contrast to our results using SE, Pepβ3 was without effect on mIPSC parameters in these neurons (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Together, our results suggest that SE reduces mIPSC amplitude in hippocampal CA1 neurons compared with control. It is also evident that blocking the interaction of GABAAR with the AP2 adaptin in SE can restore mIPSC amplitude to levels seen in control neurons in addition to slowing their decay.
Activation of PKC modifies modulates mIPSC in SE
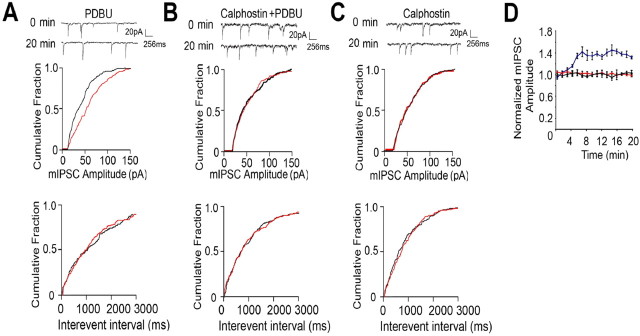
It is apparent that SE leads to reduced phosphorylation of S408/9 in the GABAAR β3 subunit (Fig. 1). We also illustrated that the activation of PKC in SE increases both S408/9 phosphorylation and the cell surface stability of GABAARs (Fig. 5). Therefore, we assessed the effects of PKC activation on mIPSC properties in SE. As illustrated in Figure 7A, 100 nm PDBU introduced into CA1 neurons via the patch pipette increased mIPSC amplitude from 35.5 ± 3.2 pA at 0 min to 51.5 ± 4.6 pA after 20 min, an effect that was blocked by coapplication of 10 μm calphostin C, a PKC inhibitor (Cal) (39.2 ± 3.5 vs 40.6 ± 3.9 pA at 0 and 20 min, respectively) (Fig. 7A,B, Table 1). Cal treatment alone was without effect on mIPSC amplitude (36.0 ± 3.9 vs 37.1 ± 4.1 pA at 0 and 20 min, respectively) (Fig. 7C, Table 1). In contrast to these results, none of these treatments modulated either mIPSC frequency or rise times (Fig. 7 A–C, Table 1).
Figure 7.
PKC-dependent activity modulates mIPSCs in SE. A–C, PKC activity enhances mIPSC amplitude in SE. Representative sweeps from CA1 neurons exposed to PKC activators/inhibitors and cumulative amplitude distributions for events at 0 time (red) and 20 min (black) are shown. D, PDBU enhances mIPSC amplitude in SE. Peak amplitudes were measured over a 20 min recording period for SE CA1 neurons treated with PDBU (100 nm; blue), PDBU/calphostin (100 nm and 10 μm, respectively; red), and calphostin alone (10 μm; black), and data were normalized to those seen at 0 time (100%).
To further evaluate the effects of PKC activation, we compared mIPSC amplitude at 2 min intervals over a 20 min recording period for neurons exposed to modulators of this kinase. This revealed that PDBU produced a time-dependent increase in mIPSC amplitude that reached 142 ± 5.4% of control after 20 min, an effect not replicated using PDBU/Cal or Cal alone (Fig. 7D). Finally we examined the effects of PKC activation on mIPSC decay. As illustrated in Table 1 PDBU slowed mIPSC decay (15.3 ± 0.9 vs 21.3 ± 1.9 ms at 0 and 20 min, respectively), an effect that was blocked by coapplication of Cal (14.6 ± 2.3 vs 16.2 ± 2.5 ms at 0 and 20 min, respectively).
Together, these results demonstrate that activation of PKC enhances the amplitude and slows the decay of mIPSCs in SE. This is consistent with the ability of this kinase activity to enhance the levels of S408/9 phosphorylation and the cell surface stability of GABAARs containing β3 subunits after this insult.
Discussion
SE arises from deficits in the efficacy of GABAergic inhibition that include altered GABAAR functional expression and pharmacology; however, the mechanisms underlying these deficits remain to be established (Lowenstein and Alldredge, 1998; Alldredge and Lowenstein, 1999). To address this critical question, we have examined the impact of SE on GABAAR functional expression together with the efficacy of synaptic inhibition in hippocampal slices derived from mice injected with pilocarpine. This animal model exhibits many similarities to patients undergoing SE including the development of pharmacoresistance (Kapur and Coulter, 1995; Coulter, 2001).
As measured using biotinylation, the cell surface stability of the GABAAR α1, 2, 4, β3, and γ2 subunits was significantly reduced in SE compared with controls, whereas those encoding the GABABR R1 and R2 subunits were unaltered. In contrast, the cell surface stability of the α5 and δ subunits was significantly increased. Thus, our results demonstrate that SE can induce alterations in the cell surface stability of individual GABAAR subunits. Although the precise subunit compositions of the GABAAR subtypes expressed on the cell surface of hippocampal neurons remains unknown, it is evident that heteromeric receptors that incorporate γ2 subunits (αβγ2) are sensitive to functional modulation by benzodiazepines, whereas those incorporating δ subunits (αβδ) are not (Farrant and Nusser, 2005; Rudolph and Mohler, 2006). The increase in cell surface expression levels of receptor subtypes containing δ subunits at the expense of those containing γ in SE may thus be a potential mechanism to explain altered GABAAR functional expression after this insult. In keeping with our results, deficits in the number of γ2-containing GABAARs clustered at inhibitory synapses have been reported in SE (Goodkin et al., 2005; Naylor et al., 2005).
In parallel with measurements on GABAAR cell surface stability, we examined the effects of SE on receptor phosphorylation, a key regulatory mechanism for modulating their functional properties and cell surface stability (Hinkle and Macdonald, 2003; Wang et al., 2003; Jovanovic et al., 2004). Compared with control, deficits in the phosphorylation of S408/9 were evident for cell surface populations of GABAARs containing β3 subunits in SE. Basal phosphorylation of S408/9 is largely dependent on the activity of PKC, whereas dephosphorylation is regulated principally by PP1 and PP2A (Brandon et al., 2000; Jovanovic et al., 2004; Terunuma et al., 2004). The specific association of these enzymes and their associated scaffolds with the GABAAR β3 subunit is also a critical determinant in regulating the stoichiometry of S408/9 (Brandon et al., 2000, 2002b, 2003; Jovanovic et al., 2004; Terunuma et al., 2004). Consistent with the deficits in both global PKC activity and the phosphorylation of S408/9 in SE, the levels and activity of the α, β, and γ2 isoform of PKC associated with GABAAR receptors were significantly reduced after this insult. In contrast to these results, the activity and level of PP2A associated with GABAAR were increased in SE. Therefore, the modified association of GABAAR with these signaling molecules provides a molecular mechanism to explain the decreased steady-state phosphorylation of S408/9 in SE.
S408/9 lie within a basic patch-binding motif for AP2 between residues 401–412 of the β3 subunit, and phosphorylation of S408/9 decreases the affinity of AP2 for the β3 subunit (Kittler et al., 2005; Chen et al., 2006). Concordant with these observations, deficits in S408/9 phosphorylation in SE were coincident with increased association of GABAARs with AP2. Activating PKC in SE enhanced the cell surface expression levels of receptors containing β3 subunits together with the level of 408/9 phosphorylation. Similar enhancements of GABAAR cell surface number were seen when blocking endocytosis by inhibiting dynamin activity. These experiments thus suggest that the decreased cell surface expression of GABAARs during SE results from their reduced phosphorylation, which acts to promote their endocytosis by facilitating their interaction with AP2.
To test the relevance of these biochemical observations for inhibitory synaptic transmission, the properties of mIPSCs were compared in control and SE slices. Consistent with our biochemical studies, mIPSC amplitudes were reduced in CA1 neurons in SE compared with control, whereas their frequencies and rise and decay times were unaltered. The effects of selectively blocking the interaction of GABAARs with AP2 were then examined using dominant negative peptides corresponding to residues 404–412 in the β3 subunit, Pepβ3 and its phosphorylated equivalent Pepβ3P, which have affinities of 300 and 1600 nm for AP2, respectively (Kittler et al., 2005; Chen et al., 2006). Pepβ3 increased mIPSC amplitude in SE to values similar to those in control CA1 neurons over a 20 min recording period. Although neither peptide modulated mIPSC frequency or rise time, Pepβ3 specifically slowed their decay.
Pepβ3 did not alter mIPSC properties in CA1 neurons from control slices. In contrast to this, Pep β3 has been shown to increase mIPSC amplitude in cultured hippocampal and cortical neurons in addition to medial spiny neurons from the nucleus accumbens (Kittler et al., 2000, 2005; Chen et al., 2006). This discrepancy may reflect lower rates of endocytosis or higher rates of basal β3 subunit phosphorylation in slices compared with culture. Finally, in addition to these experiments with dominant negative peptides, we also established that activation of PKC increased mIPSC amplitude during SE and slowed their decay but did not alter mIPSC frequency or rise time. In parallel with this functional modulation, we also established that PKC activation increased the phosphorylation of S408/9 together with the number of cell surface GABAARs containing β3 subunit. Together, our electrophysiological studies demonstrate that blocking the binding of GABAAR β3 subunits to the AP2 or increasing phosphorylation of S408/9 in this subunit can enhance the inhibitory synaptic transmission in SE.
In addition to a model of SE, termination of seizures induced by pilocarpine with benzodiazepines generates a spontaneous seizure phenotype, which is used as a model of temporal lobe epilepsy (TLE) (Coulter, 2001). It will therefore be of interest to examine whether altered levels of GABAA receptor phosphorylation persist in TLE, as has been reported for NMDA receptor phosphorylation (Niimura et al., 2005). Moreover, it will be of significance to examine the relationship between modified GABAA receptor phosphorylation and compromised transcriptional regulation of individual receptor subunit genes that are evident in TLE (Brooks-Kayal et al., 1998; Raol et al., 2006).
Collectively, our studies demonstrate that SE decreases PKC-dependent phosphorylation of conserved phosphorylation sites within the GABAAR β3 subunit. This deficit in phosphorylation enhances GABAAR binding to the AP2 adaptin, promoting their endocytosis and reducing their cell surface expression levels, thus compromising the efficacy of synaptic inhibition. Therefore, the development of agents that selectively decrease GABAAR endocytosis or enhance β subunit phosphorylation may be useful therapeutic agents to increase the strength of synaptic inhibition and thus alleviate SE and other forms of epilepsy.
Footnotes
M.T. was supported by a fellowship from the Uehara Memorial Foundation; S.J.M. by National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) Grants NS 046478, 048045, 051195, 056359, and P01NS054900, and the Medical Research Council (UK); D.A.C. by NIH/NINDS Grants NS 032403, 038572, and P01NS054900; and P.G.H. by NIH Grants NS054770 and MH071705. We thank Yolande Haydon for manuscript preparation.
References
- Alldredge BK, Lowenstein DH. Status epilepticus: new concepts. Curr Opin Neurol. 1999;12:183–190. doi: 10.1097/00019052-199904000-00009. [DOI] [PubMed] [Google Scholar]
- Blanchet C, Sollini M, Luscher C. Two distinct forms of desensitization of G-protein coupled inwardly rectifying potassium currents evoked by alkaloid and peptide mu-opioid receptor agonists. Mol Cell Neurosci. 2003;24:517–523. doi: 10.1016/s1044-7431(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Uren JM, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci. 1999;19:9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of gamma-aminobutyric acid(A) receptor function and cell surface expression. Pharmacol Ther. 2002a;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Smart TG, Moss SJ. Receptor for activated C kinase1 facilitates protein kinase C-dependent phosphorylation and functional modulation of GABAA receptors with the activation of G-protein-coupled receptors. J Neurosci. 2002b;22:6353–6361. doi: 10.1523/JNEUROSCI.22-15-06353.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Colledge M, Kittler JT, Brandon JM, Scott JD, Moss SJ. A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABA(A) receptors by cAMP-dependent protein kinase via selective interaction with receptor beta subunits. Mol Cell Neurosci. 2003;22:87–97. doi: 10.1016/s1044-7431(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med 1998. 1999;10:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Chen G, Kittler JT, Moss SJ, Yan Z. Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci. 2006;26:2513–2521. doi: 10.1523/JNEUROSCI.4712-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA. Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol. 2001;45:237–252. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V. Cargo takes control of endocytosis. Cell. 2006;127:35–37. doi: 10.1016/j.cell.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Haucke V, Wenk MR, Chapman ER, Farsad K, De Camilli P. Dual interaction of synaptotagmin with mu2- and alpha-adaptin facilitates clathrin-coated pit nucleation. EMBO J. 2000;19:6011–6019. doi: 10.1093/emboj/19.22.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle DJ, Macdonald RL. Beta subunit phosphorylation selectively increases fast desensitization and prolongs deactivation of α1β1γ2L and α1β3γ2L GABAA receptor currents. J Neurosci. 2003;23:11698–11710. doi: 10.1523/JNEUROSCI.23-37-11698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABAA receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Coulter DA. Experimental status epilepticus alters gamma-aminobutyric acid type A receptor function in CA1 pyramidal neurons. Ann Neurol. 1995;38:893–900. doi: 10.1002/ana.410380609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci USA. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Nathanson NM. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura M, Moussa R, Bissoon N, Ikeda-Douglas C, Milgram NW, Gurd JW. Changes in phosphorylation of the NMDA receptor in the rat hippocampus induced by status epilepticus. J Neurochem. 2005;92:1377–1385. doi: 10.1111/j.1471-4159.2005.02977.x. [DOI] [PubMed] [Google Scholar]
- Parker PJ. Protein kinase C phosphorylation: an introduction. Methods Mol Biol. 2003;233:159–162. doi: 10.1385/1-59259-397-6:159. [DOI] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Lund IV, Porter BE, Maronski MA, Brooks-Kayal AR. Increased GABA(A)-receptor alpha1-subunit expression in hippocampal dentate gyrus after early-life status epilepticus. Epilepsia 47. 2006;10:1665–1673. doi: 10.1111/j.1528-1167.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Staley KJ. Role of the depolarizing GABA response in epilepsy. Adv Exp Med Biol. 2004;548:104–109. doi: 10.1007/978-1-4757-6376-8_8. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phosphodependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J Neurosci. 2004;24:70747084. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiman DM. The role of benzodiazepines in the management of status epilepticus. Neurology. 1990;40:32–42. [PubMed] [Google Scholar]
- van Rijnsoever C, Sidler C, Fritschy JM. Internalized GABA-receptor subunits are transferred to an intracellular pool associated with the postsynaptic density. Eur J Neurosci. 2005;21:327–338. doi: 10.1111/j.1460-9568.2005.03884.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]