Abstract
Background
Adaptive villus growth following massive small bowel resection (SBR) is an important response to the loss of intestinal surface area and is regulated via epidermal growth factor receptor (EGFR) signaling. Increased levels of the pro-angiogenic chemokine (CXC) ligand 5 (CXCL5) have been found within the adapting bowel in which angiogenesis is increased. We sought to determine if CXCL5 was expressed specifically in the villus mesenchymal zone (area of increased blood vessel growth) and if this expression was affected by EGF.
Methods
C57BL/6J mice were subjected to sham operation (bowel transaction with reanastomosis) or 50% proximal SBR. The remnant intestine was harvested and the villus lamina propria isolated by laser capture microdissection. The expression of CXCL5 mRNA was analyzed using RT-PCR. Further, CXCL5 mRNA levels were determined in EGF stimulated human umbilical vein endothelial cells (HUVECs).
Results
A 2.39 fold increase (p<0.05) in CXCL5 mRNA occurred in the lamina propria after SBR. In addition, villus height was found to be directly related to the degree of CXCL5 mRNA (R2 = 0.97) expression. HUVECs treated with EGF demonstrated a 9-fold increase in CXCL5 mRNA expression.
Conclusions
The villus growth seen in resection-induced adaptation is associated with elevated expression of the chemokine CXCL5 within the lamina propria. Since EGF enhances CXCL5 expression directly in endothelial cells, EGFR-directed pro-angiogenic gene expression may be a critical mechanism for adaptive ileal villus growth.
Keywords: angiogenesis, adaptation, EGFR, CXCL5
Introduction
Adaptation is an important compensatory reaction to the acute loss of intestinal length. Previous studies have shown enhanced enterocyte proliferation that leads to taller villi, deeper crypts, and a greater content of DNA, RNA, and protein in the remnant intestinal wall 1,2. Increasing villus length is an effective response to augment absorptive and digestive capacity per unit length of the remnant bowel. While the mechanism of villus growth likely involves accelerated enterocyte production within the intestinal crypt, other factors are likely to be involved.
Angiogenesis is the formation of new capillaries from pre-existing capillary networks and involves the activation of endothelial cells. Endothelial cell proliferation is essential in several physiological conditions including endometrial proliferation, post-ischemic recovery, and wound healing 3-5. We recently discovered evidence for enhanced blood vessel growth within the central core of adapting villi following massive small bowel resection (SBR) 6. Simultaneous with this finding, we identified significant expression of a pro-angiogenic chemokine termed chemokine (CXC) ligand 5 (CXCL5) within the de-epithelialized segment of intestinal wall.
In this study, we sought to investigate the relationship between post-resection adaptation and expression of the pro-angiogenic chemokine CXCL5. More specifically, we sought to determine the specific site for CXCL5 expression within the region of new villus growth-the mesenchymal villus core. Further, we sought to test the hypothesis that EGFR stimulation affects CXCL5 expression within vascular endothelial cells.
Materials and Methods
Animals
A protocol for this study was approved by Washington University Institutional Animal Care and use committee (Protocol # 20070145; Washington University School of Medicine, St. Louis, MO). Male C57/BL6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Male mice aged 8-10 weeks were housed in a standard facility on a 12-hour light-dark schedule. Mice were allowed 7 days to acclimate to their environment prior to any experiments. Our laboratory has generated and maintains a transgenic mouse line that overexpresses EGF within small intestine enterocytes 7. Male EGF(tg) aged 8-10 weeks were compared to age matched wildtype (FVBN/J) mice. Male FVBN/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and treated as described above.
Operative procedure
A 50% proximal SBR or sham operation was performed as previously described 9. For SBR, the small bowel 12 cm proximal to the cecum and 1 cm distal to the ligament of Treitz was transected. The intervening (approximately 12 cm) segment was then removed and intestinal continuity was restored with an end-to-end, single layered, interrupted anastomosis using a 9-0 monofilament suture. The sham operation was performed by completely transecting the small bowel 12 cm proximal to the cecum. The transected bowel was then re-anastomosed. All mice were fed liquid diet (Micro-stabilized Rodent Liquid Diet LD101; Purina Mills, St. Louis, MO) preoperatively. In the immediate 24 hours postoperatively, the mice were given water ad libitum. The mice were then continued on the same liquid diet until harvest. Criteria for exclusion from the study included; (1) death prior to harvest, (2) signs of illness (lethargy, unkept fur), (3) or signs of intestinal obstruction (dilated proximal small bowel, lack of food in cecum, and distended stomach).
Small bowel harvest and enterocyte removal
On postoperative day three, the mice were anesthetized with a subcutaneous injection of ketamine, xylazine, and acepromazine (4:1:1 ratio). The 3-day time point was chosen as we have already established increased expression of CXCL5 in de-epithelialized intestinal segments at this time point 6. The abdominal cavity was opened, the ileocecal and gastroduodenal junctions were transected, and the intestine was flushed antegrade with ice-cold, phosphate-buffered saline (PBS) with the mesenteric blood flow intact. The anastomosis was then identified and transected and the remaining distal bowel was excised from the mesentery and placed into ice-cold PBS.
The first centimeter of ileum distal to the anastomosis was discarded and the next 1.5 cm was fixed for histology in 10% neutral-buffered formalin. The next 1.5 cm was opened longitudinally and transferred into a tube of ice-cold PBS. After sixty to eighty minutes, the tissue was transferred into a solution containing 1.5 mmol/L KCl, 96mmol/L NaCl, 27 mmol/L Na citrate, 8 mmol/L KH2PO4, 5.6 mmol/L Na2HPO4, 15 mmol/L EDTA, and 1 mmol/L DTT and vortexed at 1800 rpm for a total of 20 minutes at 4°C. This processing removes the epithelial layer, leaving the muscle and villus core behind. The tissue was then transferred into Optimal Cutting Temperature (OCT) medium and quickly frozen using dry ice and cryogen freeze (Triangle Biomedical Sciences, INC., Durham, NC). The tissue in OCT was kept at -80°C until slides were made for laser capture microdissection.
Laser Capture Microdissection (LCM)
Seven micrometer (μm) sections of the OCT blocks were cut on a cryostat and mounted on polyethylene napthalate (PEN) membrane glass slides (MDS Analytical Technologies, Mississauga, Ontario). Slides were kept at -80°C until used. Frozen sections were fixed in 70% ethanol, stained in hematoxylin, then rinsed in double-distilled H2O (ddH2O) and 1× PBS, and counterstained in eosin. Slides were then dehydrated with graded ethanol concentrations followed by xylene. A Veritas laser capture microdissection (LCM) instrument (Arcturus Engineering, Mountain View CA) was used according to the manufacturer's protocols. More than 500 villus cores per sample were collected on polymer film (CapSure HS LCM Caps; Arcturus Engineering, Mountain View, CA) by the LCM process of the instrument. After collection, the villus core samples were processed immediately.
RNA extraction and real-time PCR
Total RNA was extracted from the LCM cap polymer film using an RNAqueous-Micro kit (Ambion, Austin, TX) according to the manufacturer's protocol. The RNA samples were evaluated for quality and concentration using a Bio-Rad Experion System with RNA StdSens Chip and reagents (Bio-Rad laboratories, Richmond, CA). Samples that did not demonstrate adequate 18s and 28s ribosomal RNA peaks were discarded. The isolated mRNA was used to make complementary DNA (cDNA) using an RT2 First Strand Kit (SABioscience, Fredrick, MD). The cDNA was analyzed on a mouse angiogenesis RT2 Profiler PCR Array (SABioscience, Fredrick, MD) using an Applied Biosystems 7500 Fast Real-Time PCR system (Foster City, CA). Results were verified with individual primers for CXCL5 and vWF (SABioscience, Fredrick, MD) in triplicate.
Histology
Formalin-fixed specimens of ileum were embedded in paraffin and oriented in the longitudinal axis of the bowel. Five μm tissue slices were mounted and stained with hematoxylin and eosin. Villus height was measured with a video-assisted computer program (Metamorph, UIC, Dowington, PA). A minimum of twenty villi were counted per slide by an investigator who was blinded to the type of operation performed on the mouse. Only well oriented villi with intact crypts, a continuous epithelial layer from the crypt to the villus tip and with a central villus core visible were measured.
Immunohistochemistry
Five μm slices were mounted and incubated with primary antibodies for Factor 8 (Dako, Glostrup, Denmark) and LIX/CXCL5 (R&D systems, Minneapolis, MN). 4′, 6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA) was used for nuclear staining. Antibodies for Factor VIII and LIX/CXCL5 were used in a 1:500 dilution. Secondary labeling antibodies with TRITC and FITC were used for immunofluorescence. The slides were then examined using fluorescent microscopy (Carl Zeiss, Germany) and images were taken using computer software (Axiovision, Carl Zeiss, Germany).
Cell cultures
Human Umbilical Vein Endothelial Cells
Primary Human umbilical vein endothelial cells (HUVECs) cell cultures were graciously supplied by Dr. Jeffrey Arbeit (Department of Surgery, Washington University School of Medicine, St. Louis, MO). Cells were grown using EGM-2 Bulletkit (Lonza group LTD, Basel, Switzerland). Cells in passage 2-4 were used for all experiments. HUVECs were allowed to grow to 60-70% confluence and then placed in serum-free starvation media for 24 hours to allow all the cells to synchronize in the cell cycle. After 24 hours of starvation, the media was replaced with serum-free starvation media plus growth factor with or without inhibitors as described in the results. Control culture media was replaced with serum-free starvation media and baseline culture media was replaced with full growth media. The pharmacological EGFR inhibitors Tyrphostin AG1478 (A.G. Scientific, Inc., San Diego, CA) and ZD 1839 (Iressa/Gefitinib, Tocris Bioscience, Ellisville MO) were used to block EGFR signaling.
Statistics
Student's t-test and Excel software were used to analyze our data. Post-operative villus height is presented as the mean value ± standard error of the mean of the sham and SBR groups (n=5 per group). Relative mRNA expression is the average of three experiments and is presented as the mean value ± standard deviation. A p value of ≤ 0.05 was considered statistically significant.
Results
Ileal morphology
Villus height was significantly higher at 3 days following SBR when compared to sham operated animals (Figure 1) thus verifying a normal adaptation response.
Figure 1.
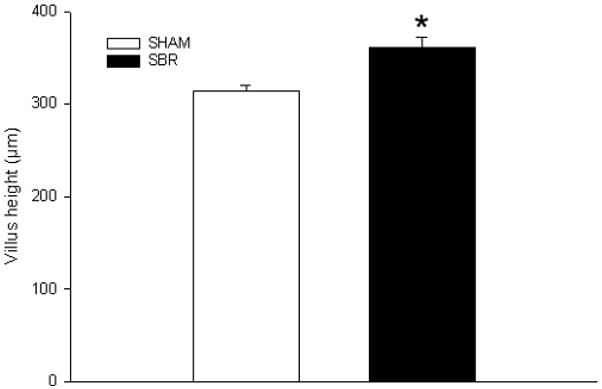
Average (± SEM) villus height in mice at 3 days following 50% proximal small bowel resection (SBR) or sham operation (transection of the bowel with reanastomosis alone). * denotes p<0.05 (n= 5 animals per group).
Angiogenesis mouse array
Intestinal villus mRNA was isolated and used to profile the expression of 84 genes involved in either pro- or anti-angiogenic pathways. We confirmed a significant (2.39 fold increase; p<0.05) in the expression of CXCL5 mRNA when comparing the SBR group (n=5) to sham-operated animals (n=5) (Figure 2). Further analysis revealed a direct (R2=0.91) and significant (p< 0.05) relationship between the magnitude of SBR-induced villus growth and increasing CXCL5 mRNA expression (Figure 3). These findings were verified with RT-PCR using individual primers for CXCL5.
Figure 2.
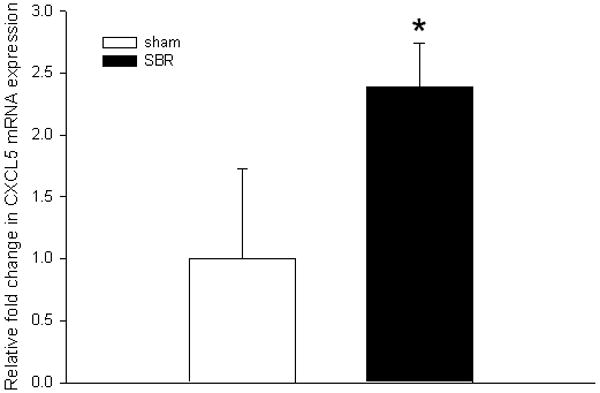
Average (± SEM) expression of the pro-angiogenic CXCL5 mRNA in ileal villus lamina propria at 3 days following 50% proximal small bowel resection (SBR) or sham operation (transection of the bowel with reanastomosis alone). mRNA was isolated from lamina propria using laser capture microdissection microscopy and quantified using real-time PCR. The fold difference in expression was compared with sham operated mice. All samples were run in triplicate; * denotes p<0.05; (n=5 animals per group).
Figure 3.
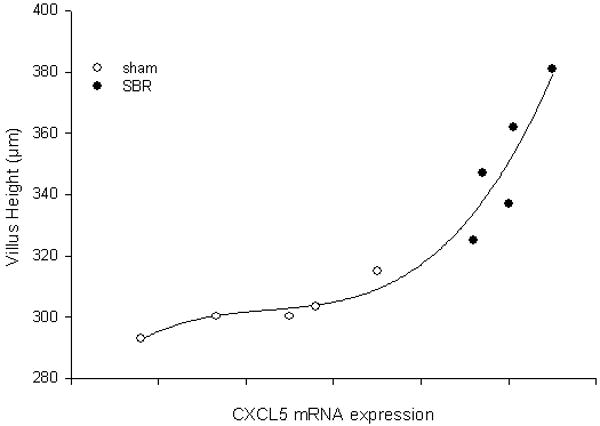
The relationship between average (± SEM) villus height and CXCL5 mRNA expression in ileal villus core at 3 days following 50% proximal small bowel resection (SBR) or sham operation (transection of the bowel with reanastomosis alone). As a marker of mRNA expression, the inverse relationship between the number of PCR cycles required for amplification for each sample were quantified and plotted along the y-axis.. R2=0.91; p<0.01.
We next performed RT-PCR using von Willebrand Factor (vWF) expression as a surrogate measure of endothelial cell quantity. We found that vWF mRNA expression was increased 3.42 fold in the SBR group (n=5) when compared to the sham group (n=5) (p<0.01) (Figure 4).
Figure 4.
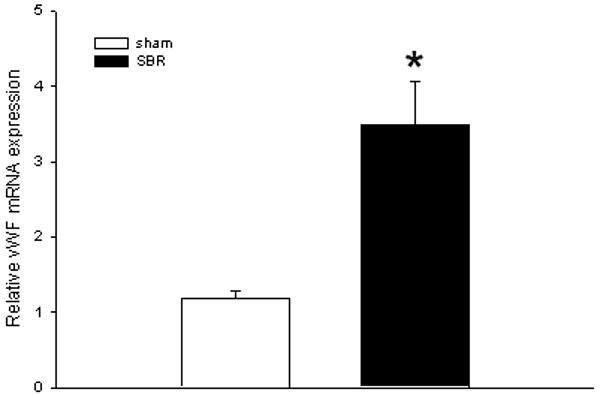
Average (± SEM) expression of VonWillebrand Factor (vWF) mRNA in ileal villus mesenchymal core of mice at 3 days following 50% proximal small bowel resection (SBR) or sham operation (transection of the bowel with reanastomosis alone). The expression of vWF was employed as a surrogate marker of increased vascular endothelial cells in the lamina propria. *p<0.01; (n=5 animals per group).
Using fluorescence microscopy, the expression of CXCL5 protein and the vascular endothelial-derived Factor VIII were co-localized (Figure 5). With DAPI counterstaining of the nuclei, we reveal that CXCL5 protein was not present in the intestinal epithelial cells but instead localized to the areas that stained with Factor VIII, in the villus core. This co-localization experiment confirms that CXCL5 protein is present in the vascular endothelium of the lamina propria after SBR.
Figure 5.
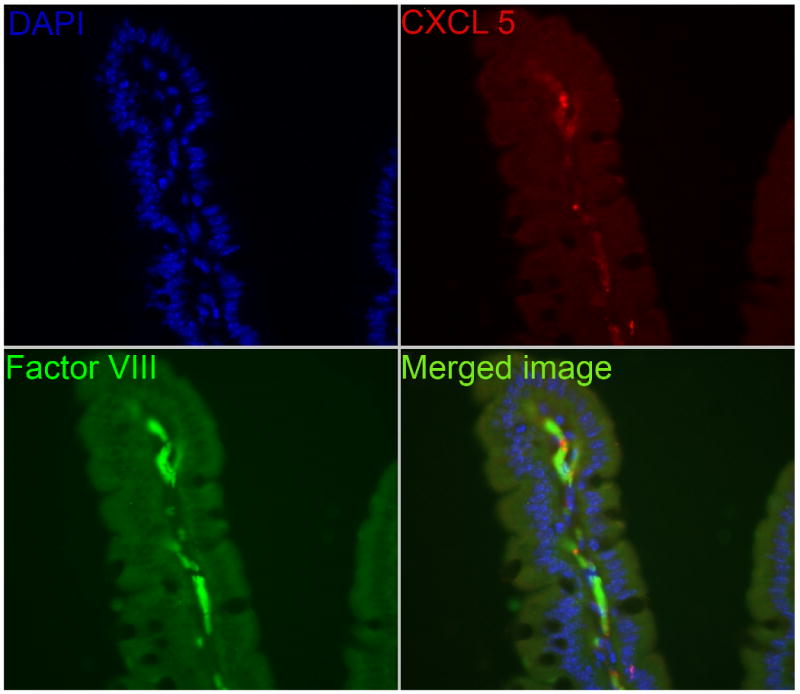
Immunofluorescent staining of mouse ileum after small bowel resection demonstrating co- localization of CXCL5 protein to the vascular endothelium (Factor VIII) of the lamina propria. 4′, 6-diamidino-2-phenylindole was used to counterstain nuclei.
HUVEC
When treated with EGF (50ng/mL), human umbilical vein cells increased CXCL5 mRNA expression by approximately 9-fold when compared to baseline and control treated cells (Figure 6). This effect on CXCL5 mRNA expression was abrogated by the pharmacologic EGFR inhibitors AG 1478 (10μM) and ZD 1839 (15 μM).
Figure 6.
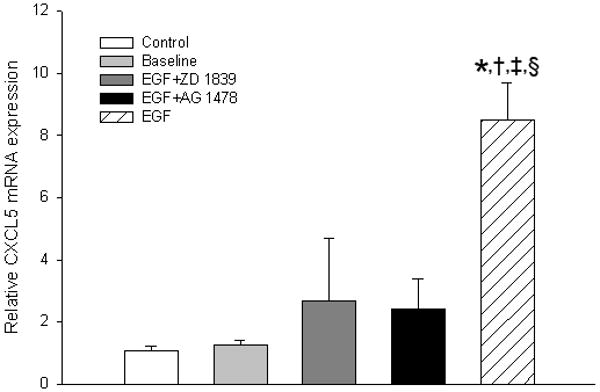
Expression of CXCL5 mRNA in human umbilical vein vascular endothelial cells (HUVEC) treated with epidermal growth factor (EGF). The effect of EGF stimulation is abrogated by the pharmacologic EGFR tyrosine kinase inhibitors ZD1839 and AG1478. *Control vs. EGF stimulated p <0.01, † Baseline vs EGF stimulated p<0.01, ‡ZD vs EGF stimulated p <0.05, § AG vs EGF stimulated p <0.01
EGF transgenic mice
Mice that overexpress EGF in the intestinal mucosa had significantly elevated levels of CXCL5 mRNA in the villus core when compared to their wildtype counterparts (Figure 7). This increase was found at baseline, without bowel resection.
Figure 7.
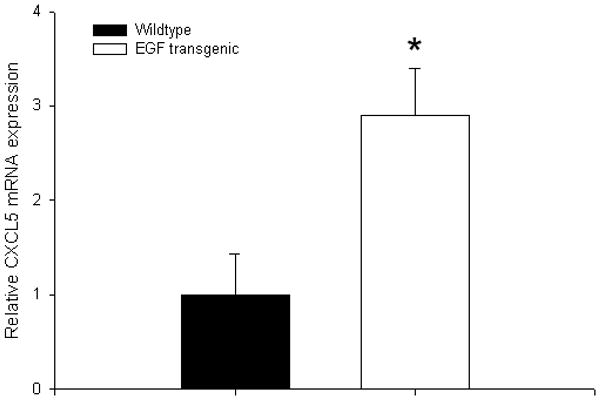
Average (± SEM) expression of CXCL5 mRNA at baseline in transgenic mice that express EGF in intestinal epithelial cells and their wildtype counterparts (FVBN/J). mRNA was isolated from lamina propria using laser capture microdissection microscopy and quantified using real-time PCR. The fold difference in expression was compared with wildtype mice. All samples were run in triplicate; * denotes p<0.05; (n=3 animals per group)
Discussion
In the present study, we have expanded upon prior observations of increased expression of a pro-angiogenic chemokine (CXCL5) within intestinal segments during the adaptation response to massive SBR. We have targeted increased CXCL5 expression to the villus mesenchymal core, specifically within newly formed capillaries. Further, we have demonstrated that CXCL5 expression within model endothelial cells in vitro is regulated at least in part, by EGFR signaling. This observation is strengthened by the evidence of increased CXCL5 expression in mice that have enhanced EGF production within the intestine at baseline. Taken together, these findings suggest that one possible mechanism for EGFR regulation of resection-induced villus growth involves CXCL5 induction of angiogenesis.
In the current experiments, we confirmed that CXCL5 expression was elevated after SBR (2.39-fold), but we were not able to demonstrate the same magnitude of change as in our prior study (14-18-fold). It is possible that the dissimilar intestinal wall components accounted for the differences since we previously measured CXCL5 expression within the entire bowel wall devoid of epithelial cells whereas CXCL5 expression has now been recorded in LCM-isolated lamina propria. As such, it is possible that a large amount of CXCL5 expression occurs within the intestinal smooth muscle cell layers and larger blood vessels. Since we had already shown increased capillary density in the villus core, we felt it was most logical to measure CXCL5 expression in this specific region. Another explanation for the disparate findings between the two studies may be the conditions for mRNA isolation.
Intestinal villus units are composed of an epithelial lining and underlying lamina propria. The capillaries of the lamina propria carry the digested nutrients from the intestine via the portal circulation to the liver. Since the entire villus unit grows during resection-induced adaptation, it is logical to assume that there is also growth of new capillaries. This was confirmed in a prior study by our laboratory whereby capillary villus density was elevated at 7 days following SBR 6. This 7-day time point was preceded by an increase in CXCL5 expression (3rd postoperative day) as well as two other pro-angiogenic peptides interleukin-1β and placental growth factor. We chose to focus the present study on CXCL5, since this was the pro-angiogenic gene whose expression increased to the greatest extent. For the first time, we were able to demonstrate that CXCL5 expression co-localizes with villus endothelial cells. This finding supports a local role for CXCL5 to act within the villus capillaries to induce new capillary growth during adaptation.
Our laboratory has previously established a critical role for EGFR signaling as a major mediator of resection-induced adaptation 9. In experiments whereby the EGFR is stimulated by either administration of various EGFR ligands or transgenic EGF overexpression, the magnitude of adaptation (increased villus growth) is augmented. Alternatively, adaptive responses are attenuated when the EGFR is inhibited by several different paradigms, which include removal of the major source of endogenous EGF (submandibular gland excision), administration of an EGFR inhibitor, or performing SBR procedures in mutant mice with perturbed EGFR signaling capacity. The mechanisms for EGFR regulation of adaptation have been assumed to be due to the effects of this receptor on the kinetics of enterocyte turnover via stimulation of proliferation and inhibition of apoptosis. In addition to this consideration, the findings of this study would suggest that EGFR signaling may have separate, but complementary effects, depending on the cell type.
A known ligand of the EGFR, heparin binding EGF-like factor (HB-EGF), has been shown to induce endothelial cell migration and vascular network formation in HUVECs and to be involved in the angiogenic response that occurs after ischemia/reperfusion injury 10-12. After ischemia/reperfusion injury, mice with a defective HB-EGF gene showed delayed wound healing and decreased angiogenesis 12. These studies further support a role for EGFR signaling in the angiogenic response to injury, and possibly adaptation, in the intestine.
Since EGFR stimulation has also been demonstrated to enhance angiogenesis in other tissues and organs 13,14 our finding of increased angiogenesis in the adapting intestine logically directed us to test the hypothesis that this receptor may also be a key player in this important response. The results of this study would support the concept that EGFR stimulation is associated with angiogenesis via regulation of CXCL5 within villus endothelial cells. It is possible that CXCL5 expression in human umbilical endothelial cells may not reflect the expression profile of intestinal endothelial cells. As such, a more specific intestinal endothelial cell line 15 may be a more suitable model to test the role of EGFR regulation of CXCL5 expression.
Although we have established increased CXCL5 expression in endothelial cells in response to EGFR stimulation, the effect of CXCL5 on angiogenesis in vitro and in vivo has not yet been established. Further, we recognize that the relationship that we have demonstrated between CXCL5 mRNA and post-operative villus height is only a correlation and does not imply that the increase in villus height is caused directly by CXCL5. While outside the scope of this study, one approach to interrogate this relationship would be to perform experiments with transgenic mice that have the receptor for CXCL5 knocked out. These mice would allow a more direct interrogation of CXCL5 signaling and resection induced angiogenesis and its importance in adaptation.
Acknowledgments
• National Institute of Health Surgical Oncology Training Grant #2T32CA009621-21 (MEM)
• National Institute of Health R01 DK 059288 (BWW)
• National Institute of Health Digestive Diseases Research Center Morphology Core, Washington University School of Medicine, Grant #P30 DK52574
• Children's Surgical Sciences Research Institute of St. Louis Children's Hospital
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williamson RC. Intestinal adaptation (second of two parts). Mechanisms of control. N Engl J Med. 1978 Jun 29;298(26):1444–50. doi: 10.1056/NEJM197806292982604. [DOI] [PubMed] [Google Scholar]
- 2.Williamson RC. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978 Jun 22;298(25):1393–402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]
- 3.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008 Nov;28(11):1928–36. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risau W. What, if anything, is an angiogenic factor? Cancer Metastasis Rev. 1996 Jun;15(2):149–51. doi: 10.1007/BF00437466. [DOI] [PubMed] [Google Scholar]
- 5.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000 Nov 1;165(9):5269–77. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 6.Martin CA, Perrone EE, Longshore SW, Toste P, Bitter K, Nair R, et al. Intestinal resection induces angiogenesis within adapting intestinal villi. J Pediatr Surg. 2009 Jun;44(6):1077–82. doi: 10.1016/j.jpedsurg.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erwin CR, Helmrath MA, Shin CE, Falcone RA, Stern LE, et al. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol. 1999 Sep;277(3 Pt 1):G533–40. doi: 10.1152/ajpgi.1999.277.3.G533. [DOI] [PubMed] [Google Scholar]
- 8.Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996 Nov;183(5):441–9. [PubMed] [Google Scholar]
- 9.Longshore SW, Wakeman D, McMellen M, Warner BW. Bowel resection induced intestinal adaptation: progress from bench to bedside. Minerva Pediatr. 2009 Jun;61(3):239–51. [PubMed] [Google Scholar]
- 10.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007 Aug;25(4):253–63. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 11.Mehta VB, Zhou Y, Radulescu A, Besner GE. HB-EGF stimulates eNOS expression and nitric oxide production and promotes eNOS dependent angiogenesis. Growth Factors. 2008 Dec;26(6):301–15. doi: 10.1080/08977190802393596. [DOI] [PubMed] [Google Scholar]
- 12.El-Assal ON, Paddock H, Marquez A, Besner GE. Heparin-binding epidermal growth factor-like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. J Pediatr Surg. 2008 Jun;43(6):1182–90. doi: 10.1016/j.jpedsurg.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelrahim M, Konduri S, Basha R, Philip PA, Baker CH. Angiogenesis: an update and potential drug approaches (review) Int J Oncol. 2010 Jan;36(1):5–18. [PubMed] [Google Scholar]
- 14.Faller BA, Burtness B. Treatment of pancreatic cancer with epidermal growth factor receptor-targeted therapy. Biologics. 2009;3:419–28. [PMC free article] [PubMed] [Google Scholar]
- 15.Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006 Jun;130(7):2060–73. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]


