Abstract
Background
Timing abilities are critical to the successful management of everyday activities and personal safety, and timing abnormalities have been argued to be fundamental to impulsiveness, a core symptom of attention-deficit/hyperactivity disorder (ADHD). Despite substantial evidence of timing deficits in ADHD youth, only two studies have explicitly examined timing in ADHD adults, and only at the supra-second time-scale. Also, the neural substrates of these deficits are largely unknown for both youth and adults with ADHD. The present study examined sub-second sensorimotor timing and its neural substrates in ADHD adults.
Methods
Using fMRI, we examined paced and unpaced finger tapping in a sample of 20 unmedicated adults with ADHD and 19 controls comparable on age, sex and estimated-IQ. The blood oxygenation level-dependent contrast response was used to estimate task-related neural activity.
Results
Behavioral data showed no between-group differences in mean tapping rates but greater within-subject variability in tap-to-tap intervals for ADHD adults relative to controls. Importantly, ADHD adults had greater clock rather than motor variability, consistent with a central timing locus for the atypical movements. The imaging results demonstrated that, relative to controls, ADHD adults showed less activity in a number of regions associated with sensorimotor timing, including prefrontal and precentral gyri, basal ganglia, cerebellum, inferior parietal lobule, superior temporal gyri and insula.
Conclusions
Our findings show that sub-second timing abnormalities in ADHD youth persist into adulthood and suggest that abnormalities in the temporal structure of behavior observed in ADHD adults result from atypical function of cortico-cerebellar and cortico-striatal timing systems.
Keywords: ADHD, fMRI, timing, cerebellum, frontal cortex, basal ganglia
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurobehavioral disorder estimated to affect up to 10% of children and 5% of adults worldwide (1), and is associated with morbidity and disability across the lifecycle (2). Its core features include symptoms of inattention, impulsivity and hyperactivity.
One source of morbidity in ADHD has been attributed to deficits in the capacity to accurately and consistently process and act on temporal information, namely deficits in timing abilities. Such a capacity is critical to personal safety and to successful management of everyday activities such as social interactions, driving and performing cognitive tasks. It has also been argued that abnormalities in timing functions are fundamental to impulsiveness (3), a core feature of ADHD.
Observational and experimental data on ADHD support the hypothesis that individuals with ADHD have widespread timing deficits. For example, some impulsive behavior in ADHD individuals could indicate an inability to adjust to environmental timing demands. Also, difficulties arriving at appointments on time, poor planning and tendencies to forgo delayed larger rewards for smaller immediate rewards (4) could suggest an altered sense of time. Experimental data of ADHD youth have shown either less accurate or more variable performance than matched controls on a number of timing tasks including paced finger tapping (5), duration reproduction (6-8), duration discrimination (9), verbal time estimation (10), and temporal anticipation (5, for review see 11). In fact, one of the most consistent findings in ADHD youth is increased within-subject variability on a range of tasks (as reviewed in 12-13), indicating an inability to register responses at evenly-timed intervals. This variability has been shown to be related to attentional ratings and to decrease in response to methylphenidate (5,14). Thus, an increased understanding of this variability, as well as timing more generally, has potential implications for treatment of and understanding core features of ADHD.
Although most support for timing deficits have been shown in ADHD youth, abnormalities in time estimation, discrimination and reproduction have been shown in ADHD adults (7, 15). However, these data are limited, and restricted to the supra-second level.
In healthy adults, brain regions most commonly associated with timing processes are the frontal cortex, basal ganglia (BG), and cerebellum (16-18), with support for differential functional localization of timing involving short versus long time intervals. Timing of supra-second intervals, as used in the previous adult ADHD studies, is thought to occur in BG and frontal cortex regions with task-related neural activity often being attributed to attention and working memory demands (11,19-21). Timing of sub-second intervals is associated with the cerebellum which has been described as a millisecond-range timekeeper or the “internal clock” (19, 20). Despite these general distinctions, there is also evidence of BG and frontal involvement in sub-second timing as well as cerebellar involvement in supra-second timing (for reviews see 20,22-23).
Interestingly, although numerous brain regions have shown structural or functional neuroimaging abnormalities in ADHD, some of the most consistent findings have been in areas associated with timing including frontal, BG and cerebellar regions (24-30). There have been no studies using fMRI to examine timing functions in ADHD adults. However, Rubia and colleagues (31-32) showed that ADHD adolescents had less activation than controls in cingulate regions during 5s-interval synchronized tapping, although no differences were observed with a 600ms interval. Smith et al. (33) found less activation in several frontal regions for ADHD adolescents relative to controls during performance on a duration discrimination task. Also, ADHD youth showed less cerebellar activation than controls while performing a temporal expectancy task (34).
The neural signature of ADHD has been conceptualized in terms of abnormalities in networks of response inhibition (35), attention (36) and reward (37), among others. However, despite demonstrating behavioral impairments across a range of timing-related functions, the idea that the neural underpinnings of ADHD result from abnormalities in timing networks is not a common model. The goal of this study was to elucidate the neural substrates of sensorimotor timing in ADHD using a simple timing task to test the hypothesis that abnormalities in timing networks contribute to the pathophysiology of ADHD.
Thus, we used fMRI and a 500ms tapping task in ADHD adults. This tapping interval was chosen both to examine sub-second timing in ADHD adults, and also to decrease potentially confounding influences from other cognitive processes such as working memory. Additionally, for stimulus rates less than 2-3 seconds, participants tend to anticipate when the next tone will occur and tap synchronously with, or prior to the tone, rather than waiting to hear it (38). Thus, at this interval length, timing is expected to rely more strongly on the “internal clock” despite the presence of pacing tones. We also mitigated the potential effects of delay aversion (39) by using a temporal production, rather than an anticipation or reproduction task. As variability was our primary measure of timing control of the internal clock (40), we included unpaced tapping and used the Wing and Kristofferson (41) variance decomposition analysis of response variability. In this model, elevated central, “clock”, variance suggests problems with the internal timing processes, whereas elevated motor variance suggests problems with execution of motor plans. We predicted that, relative to controls, ADHD adults would show timing abnormalities as reflected by greater within-subject variability on the tapping task for both paced and unpaced trials, and that, for the unpaced trials, greater variability would be due to elevations in clock rather than motor variance. We also predicted that, relative to controls, ADHD adults would show less activity in the frontal, BG and cerebellar regions which are commonly engaged in timing and have been shown to be abnormal in ADHD.
Methods and Materials
Participants
Participants were 21 adults with ADHD and 19 controls comparable on age, sex, and estimated IQ. Participants were recruited from ongoing studies of ADHD conducted at Massachusetts General Hospital. Written informed consent was obtained for all participants, and the study was approved by the Partners Human Research IRB committee.
Participants were excluded if they: 1) were younger than 18 or older than 55; 2) had an estimated Full Scale IQ < 80; 3) had a current psychotic, depressive or generalized anxiety disorder; 4) had current alcohol or substance abuse or dependence (SCID; 42; 5); had an inadequate command of the English language; 6) had sensorimotor handicaps or neurological disorders; 7) had contraindications to MRI; or 8) were currently taking psychotropic medications (other than short-acting psychostimulants). One ADHD subject was left handed and all performed the tasks with their right hand after training. Fourteen of the 21 ADHD participants (67%) had taken medications for ADHD in their lifetime: five had taken them in the past (not currently), and nine were taking short-acting psychostimulants close to the time of scan but underwent a 24-hour washout period before the fMRI visit. (See the Supplement for more details.)
ADHD and control adults received identical assessments as in previous reports (43). To assess psychopathology we administered the SCID (42). To assess ADHD, we used a module derived from the Schedule of Affective Disorders and Schizophrenia for School Age Children (Kiddie-SADS E; (44). Two ADHD participants had an age of onset of 8 and one of age 12. However, given prior studies failing to support the validity of using the age cutoff of 7 years (45-46), these participants were considered ADHD. A committee of board-certified child and adult psychiatrists and licensed psychologists blind to referral source resolved diagnostic uncertainties.
Cognitive testing included subtests from the Wechsler Adult Intelligence Scale-III (WAIS-3; 47). Vocabulary and either block design or matrix reasoning were used to calculate an IQ estimate (48-49)
Task and Design
Functional imaging runs contained 15s blocks of: fixation (F; central crosshair), paced tapping to a 50ms tone at a rate of 500ms (P), unpaced tapping (U), and listening to the pacing tone without tapping (L). The F-P-U-L order repeated 3x/run (Fig. 1). Tones were presented through a pair of pneumatic headphones. The L blocks were included as a control task for which we did not anticipate between-group differences.
Figure 1.
Diagram for paced, unpaced and listen tasks. During the paced phase participants made key-presses with the right index finger on a button box in time with a series of 50ms computer-generated tones presented binaurally. Participants were instructed that they were to tap (press the key on the button box) to the beat of the tones (P), that the tones would stop, but that the participants were to continue tapping (U) while trying to maintain the original tapping rate until they saw the word “Stop” on the screen. For the L blocks, participants were instructed to simply listen to the tones without tapping when they saw the words “Listen Only” on the screen. The L blocks were included as a control task for which we did not anticipate differences between groups. (Instruction screens for starting the paced blocks “Ready? Tap along…”, stopping the unpaced blocks “Stop”, and beginning the listen blocks “Listen Only” are not shown in the diagram.)
Behavioral Analysis
Behavioral measures were the mean and standard deviation (SD) of the intertap interval. Intertap intervals outside 50% of the goal interval were excluded from analyses (e.g., 50-51). Using a linear regression analysis, data were detrended to remove variability associated with linear drift in tapping rate over time and possible effects of fatigue (52-53). Variance associated with timing versus motor implementation were partitioned using Wing and Kristofferson's (41) model. (See the Supplement for more details.) We also conducted correlational analyses between ADHD symptoms and within-subject variability of the unpaced data.
MRI Parameters
Data were acquired using a Siemens Trio 3T scanner, 12-channel head coil (42 axial slices, 3mm thick, 0.6mm interslice gap, gradient-echo EPI, TR=2500ms, TE=30ms, 3.1mm × 3.1mm in-plane resolution, 85 total images/run).
fMRI Analyses
Image analysis
fMRI data were analyzed using SPM2 for preprocessing and SPM5 for statistical modeling (Welcome Department of Cognitive Neurology, London). Preprocessing included correction for head motion, spatial normalization, and spatial smoothing with a Gaussian filter (8mm full-width half-maximum). Individual runs exhibiting a spike of more than 3mm of scan-to-scan head motion were dropped. As a result, 3 runs were dropped from the control group and 1 from the ADHD group.
First Level Analyses
First level analyses used a general linear model, with task blocks modeled as epochs convolved with a canonical hemodynamic response function. Contrasts of task against the implicit baseline were created for the paced, unpaced and listen blocks. The resulting parameter estimates derived for each subject were used in the second level analysis.
Second Level Analyses
A mixed-effects second level analysis was performed using a voxel-wise factorial ANOVA, with Subject, Group and Task factors. For our planned contrasts, the critical threshold for voxel-wise estimates of task-related activity was p < .05, FWE-corrected, with an extent threshold of 20 contiguous voxels. We computed statistical parametric maps of: (1) simple effects for the paced, unpaced, and listen activity within and between groups, and (2) a group by task interaction for paced vs. unpaced tapping. We also conducted regression analyses to look at the effect of ADHD symptoms on unpaced task-related neural activation. (See the Supplement for details and additional analyses.)
Results
Demographics
There were no differences between ADHD and control adults on age (t(38) = −.40, p > .05) or estimated IQ (t(38) = .24, p > .05; Table 1). The distribution of ADHD subtypes based on current symptoms was: combined, N=5; inattentive, N=12; and not otherwise specified (NOS), N=4. Participants were categorized as NOS if, as is typical of many ADHD adults (54), current symptoms were below the DSM-IV symptom threshold. (See the Supplement for more details.)
Table 1.
Participant demographics.
| ADHD (N = 21) | Control (N = 19) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 34.0 | 10.1 | 32.7 | 10.6 | |
| IQ estimate | 114.5 | 15.6 | 115.5 | 9.9 | |
|
|
|||||
| Ns | Ns | ||||
|
|
|||||
| Sex (F/M) | 6/15 | 7/12 | |||
There were no significant differences between ADHD and controls for age, IQ estimate or sex ratio. F = female; M = male.
Behavioral results
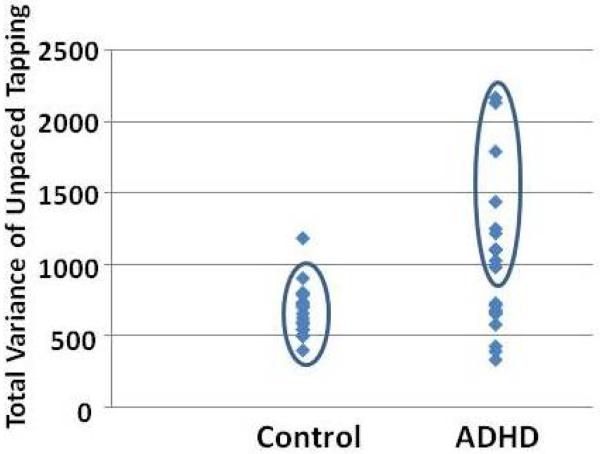
There were no differences in mean tapping rate for either paced (t(38) = −.08, p > .05) or unpaced (t(38) = −1.36, p > .05) tapping between ADHD and control adults. However, consistent with previous reports (13), ADHD participants exhibited higher within-subject variability for both paced (t(38) = −2.11, p = .042) and unpaced (t(38) = −2.89, p = .008) tapping (Table 2). The distribution of the unpaced tapping variability (Fig. 2) demonstrates that over half of the ADHD adults had higher variability than all but one control subject.
Table 2.
Kinematic results for paced and unpaced tapping tasks.
|
ADHD M (SD) |
Control M (SD) |
|
|---|---|---|
| Tapping Phase | ||
| Paced | 490 (14.6) | 490 (12.3) |
| Unpaced | 504 (27.6) | 493 (21.3) |
| Unpaced Variance | ||
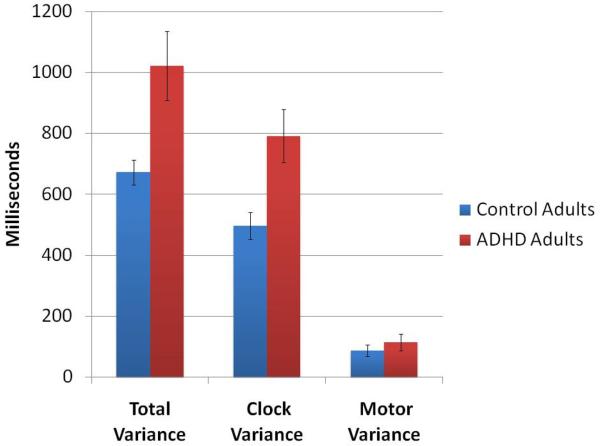
| Total | 1021.3 (520.3)* | 672.2 (179.5) |
| Clock | 791.8 (402.6)* | 497.2 (190.8) |
| Motor | 114.7 (126.1) | 87.5 (83.7) |
Indicates a significant difference between ADHD and control adults, p < .01. Between-group differences were assessed using independent sample t-tests. Values are reported as mean and standard deviation - M (SD). Tapping times and variability are reported in milliseconds (ms). Variance for the unpaced phase of the tapping task was generated using the method of analysis introduced by Wing and Kristofferson (41) to dissociate the contribution of the central (clock) variance from variance associated with the motor implementation.
Figure 2.
Distribution of unpaced tapping variability for control and ADHD adults. Twelve of 21 (57 %) of the ADHD adults have higher variability scores (shown in the ellipse for the ADHD group) than all but one of the control subjects (shown in the ellipse for the control group). Among the high variability ADHD subjects, half were currently medicated and half were not, suggesting that differences in variability were not simply due to medication effects.
We applied the variance decomposition analysis (41) to the unpaced blocks. There were significant differences between ADHD and control groups for clock (t(38) = −3.00, p = .005) but not motor (t(38) = −.80, p > .05) variance of unpaced tapping (Table 2; Fig. 3).
Figure 3.
Total, clock and motor variance and their standard error for the unpaced tapping generated using the method of analysis introduced by Wing and Kristofferson (41) to dissociate the contribution of the central (clock) variance from variance associated with the motor implementation.
There were no significant relationships between number of ADHD symptoms and total within-subject variability (r's = −.00 and .19, p>.05 for inattention and hyperactivity correlations respectively).
Functional imaging results
Simple effects of paced tapping
Areas of activation for control participants included numerous regions consistent with previous tapping and timing studies (18, 55-56; Table S1A in the Supplement; Fig. 4).
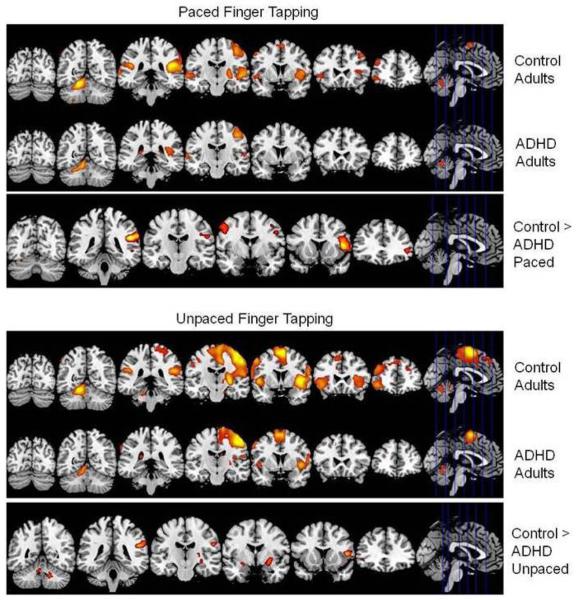
Figure 4.
Top panel. Activity associated with performance of paced tapping. We show t-values for signal change for the paced task greater than fixation baseline contrast in the ADHD and control groups as well as for the control group activation greater than the ADHD group activation. Coordinates are in MNI space. Height threshold: p < .05, FWE corrected. Extent threshold: k = 20 voxels. Bottom panel. Activity associated with performance of unpaced tapping. We show t-values for signal change for the unpaced task greater than fixation baseline contrast in the ADHD and control groups as well as for the control group activation greater than the ADHD group activation. Coordinates are in MNI space. Height threshold: p < .05, FWE corrected. Extent threshold: k = 20 voxels.
Relative to controls, ADHD adults showed significantly decreased activity in right cerebellum (declive), left inferior frontal gyrus (IFG) and middle frontal gyrus (MFG), bilateral precentral gyri and inferior parietal lobule (IPL), left middle and superior temporal gyri (STG), and bilateral insula (Table 3; Fig. 4). There were no regions for which ADHD adults showed significantly greater activation than controls.
Table 3.
Group differences for the paced and unpaced tasks. Contrast of control group greater than ADHD group for the paced and unpaced task. Local maxima of signal change are listed. Coordinates are in MNI space. Height threshold: p < .05, FWE corrected. Extent threshold: k = 20 voxels.
| Left | Right | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region label | BA | x | y | z | t | x | y | z | t |
| PACED TASK | |||||||||
| Cerebellum | |||||||||
| Declive | 30 | −69 | −18 | 5.3 | |||||
| 18 | −57 | −15 | 6.69 | ||||||
| Frontal | |||||||||
| Inferior frontal gyrus | 45 | −45 | 30 | 0 | 6.35 | ||||
| 45 | −51 | 36 | 3 | 6.64 | |||||
| 9 | −51 | 18 | 30 | 5.48 | |||||
| Middle frontal gyrus | 47 | −48 | 45 | −6 | 6.46 | ||||
| 6 | −42 | −3 | 48 | 6.01 | |||||
| 6 | −48 | 6 | 54 | 5.69 | |||||
| Precentral gyrus | 4 | −54 | −18 | 27 | 6.24 | 36 | −24 | 69 | 5.37 |
| 4 | 21 | −27 | 54 | 6.63 | |||||
| 4 | 15 | −30 | 66 | 5.81 | |||||
| 6 | −45 | −15 | 33 | 5.81 | 51 | −9 | 42 | 7.69 | |
| 6 | −48 | −6 | 36 | 6.62 | |||||
| Parietal | |||||||||
| Inferior parietal lobule | 40 | −63 | −42 | 30 | 8.18 | 54 | −36 | 24 | 6.33 |
| 40 | −66 | −30 | 33 | 5.89 | |||||
| Temporal | |||||||||
| Middle temporal gyrus | 22 | −54 | −42 | 0 | 5.65 | ||||
| Superior temporal gyrus | 22 | −57 | −54 | 6 | 6.01 | ||||
| 38 | −51 | 9 | −12 | 5.23 | |||||
| Subcortical | |||||||||
| Insula | 13 | −51 | −39 | 24 | 9.76 | 39 | −33 | 18 | 5.62 |
| 13 | −45 | 12 | 9 | 8.98 | |||||
| UNPACED TASK | |||||||||
| Cerebellum | |||||||||
| Culmen | −6 | −42 | −24 | 5.16 | 6 | −54 | −27 | 5.89 | |
| 6 | −48 | −21 | 6 | ||||||
| Dentate | −15 | −48 | −36 | 6.05 | |||||
| Frontal | |||||||||
| Inferior frontal gyrus | 47 | 48 | 27 | 0 | 5.03 | ||||
| 47 | 39 | 27 | −9 | 5.28 | |||||
| 45 | −60 | 15 | 3 | 5.57 | |||||
| Middle frontal gyrus | 47 | 42 | 33 | −3 | 5.43 | ||||
| Precentral gyrus | 4 | −54 | −18 | 27 | 6.27 | ||||
| 44 | −48 | 12 | 6 | 7.25 | |||||
| Limbic | |||||||||
| Parahippocampal gyrus | amygdala | 24 | −3 | −12 | 5.88 | ||||
| Subcortical | |||||||||
| Caudate | caudate tail | −36 | −27 | 0 | 5.64 | ||||
| Insula | 13 | −51 | −39 | 24 | 7.19 | ||||
| Lentiform nucleus | putamen | −24 | −15 | 12 | 5.38 | ||||
| −27 | −3 | −6 | 6.4 | ||||||
| −30 | −21 | 0 | 5.58 | ||||||
| −30 | −15 | −9 | 6.47 | ||||||
Simple effects of unpaced tapping
Areas of activation for controls included numerous regions consistent with unpaced tapping and timing observed in previous studies (18, 55-56; Table S1B in the Supplement; Fig. 4).
Relative to controls, ADHD adults showed relatively decreased activation in bilateral cerebellum (culmen and dentate nuclei), bilateral IFG, right MFG, as well as left precentral gyrus, caudate tail, putamen, insula and amygdala (Table 3; Fig. 4). There were no regions for which ADHD adults showed significantly greater activation than controls.
Simple effects of listening to the tones
Controls and ADHD adults showed activation patterns consistent with what would be expected from an auditory task with primary activation in bilateral auditory cortex (Brodmann area 41/42, STG). (Results available upon request.) As predicted, there were no differences between ADHD and control adults in any regions for the listen task.
Group by task interaction
The group by task interaction failed to show statistically significant differences in neural activity between paced vs. unpaced tapping for the two groups. This was not surprising since it is typical for participants to use their own internal timing rather than the tones to perform sub-second tapping tasks (38).
Symptom relationships to neural activity
There were no significant relationships between number of ADHD symptoms and BOLD response for the unpaced data.
Discussion
We used a tapping task with fMRI to examine motor timing abilities and their neural substrates in adults with ADHD. Behaviorally, we found that controls and ADHD adults showed similar mean tapping rates, but differences in within-subject variability, with ADHD adults showing greater variability than controls. Importantly, the ADHD adults showed increased clock rather than motor variability suggesting a deficit in timing rather than motor execution. These behavioral results were paralleled by atypical patterns of activity in a network of regions which are frequently associated with timing, namely the motor and premotor areas in frontal cortex, cerebellum, and BG.
The behavioral findings are notable in several ways. First, these data show that motor timing abnormalities (e.g., increased within-subject variability), previously observed in ADHD youth (57) can also be observed in ADHD adults. There have been no previous studies examining either tapping or sub-second time intervals in ADHD adults. Thus, this study extends what we know about timing irregularities in this population. Future studies designed to examine whether ADHD adults with high vs. low within-subject variability systematically differ in neural activity would be informative.
Second, the behavioral data show that, on average, ADHD adults have greater tapping variability than controls. Most interestingly, using a temporal variance decomposition method (41) we show for the first time that this variability is related to variability of the central clock rather than motor implementation. Given the ubiquitous nature of the higher performance variability for ADHD individuals, knowing more about the mechanism of this atypical variability is an important issue. Our data provide support for abnormalities in timing mechanisms rather than abnormalities in movement execution.
This result is consistent with conclusions drawn by Rommelse and colleagues (58) who had ADHD and control children conduct tapping tasks with and without a pacing stimulus. They found that ADHD children showed abnormal speed and variability for paced tapping, but typical performance while tapping as quickly as they could at their own rate. Rommelse et al. (58) interpreted this result as reflecting timing rather than motor function differences.
Neuroimaging results show that ADHD adults have abnormal patterns of activation while performing the tapping task. As predicted, ADHD adults showed relatively less activity than controls in frontal, cerebellar and BG regions. More specifically, regions of relative hypoactivity were observed in primary motor and premotor (IFG and MFG) frontal areas, and the cerebellum (culmen and declive lobules IV-VI and dentate nuclei), as well as the caudate and putamen.
Failure of the ADHD adults to activate frontal and cerebellar regions to the same degree as controls could indicate difficulties in engaging fronto-cerebellar circuits critical for optimal task performance. For example, lobules IV-VI in the right cerebellar hemisphere and the left primary motor cortex (BA 4) comprise nodes of a network associated with motor timing (16,59-60). Strick and colleagues (for review see 61) have shown that there are multiple cortico-cerebellar loops, one of which includes reciprocal projections between the motor cortex and cerebellar hemispheres IV-VI. These data suggest a fronto-cerebellar substrate for timing processes engaged for movement control (16, 60-62). It is possible that difficulties in optimally engaging this network could contribute to the relatively greater within-subject variability of the ADHD adults over controls while performing this task.
The BG and IFG have been noted to be key timing areas (63-64), shown to be active in tapping tasks similar to those used here (55) and also in paradigms for which motor confounds have been controlled (22, 65). The BG, in particular the putamen, have been shown to be preferentially involved in internally generated movements (66). The IFG may involve the internal timing of movements perhaps via subvocalization of the tones (55). Failure to activate the BG and IFG as strongly as controls may make it more difficult for the ADHD adults to internally generate movements at the appropriate rate.
Our neuroimaging findings contrast with the results of Rubia and colleagues (5,31) who reported no differences between ADHD and control youth for sub-second timing synchronization and differences in cingulate regions for a supra-second timing. However, these experiments differ from ours in that they were conducted with adolescents, relied on smaller samples (7 ADHD, 9 controls), and used a visual pacing stimulus. That we did not find between-group differences in cingulate regions could also result from our use of a sub-, rather than supra-, second timing pacing task. There is an abundance of evidence for differential functional neuroanatomy for sub- vs. supra-second timing. Our results show more consistency with the findings of Durston et al. (34) and Smith et al. (33) who found less activation in ADHD youth relative to controls in cerebellar and frontal regions (including the IFG) during time expectancy violations and duration discrimination tasks respectively. Notably, these studies were conducted with youth in contrast to our study using adults. Additional research is needed for all age groups to gain converging evidence on the neural substrates of timing in ADHD.
Functional abnormalities for non-timing-related tasks in the IFG and BG in ADHD samples have been well established (26,28). Also, our finding of decreased activation in the motor cortex is consistent with data of Mostofsky and colleagues (67) who found a smaller extent of activation in the motor cortex for ADHD children relative to controls during performance of a self-paced sequential finger tapping task.
In addition to predicted regions of abnormality during tapping task performance, ADHD adults showed less activation compared to controls in several other regions commonly associated with sensorimotor timing. The insula has been shown to be involved in sensorimotor synchronization (56,68). In conjunction with the IFG, the insula has been proposed to facilitate timing via subvocal rehearsal of the target interval and through multi-modal integration (69). Less activation for the ADHD adults relative to the controls in the IFG and insula could reflect more difficulty for the ADHD adults in integrating their internal rehearsal of the tones with the motor response.
The inferior parietal lobe's (IPL) involvement in time perception is well established (22,70-71). One hypothesized role for the IPL involves its making covert shifts of attention to temporal stimuli (72). Thus, abnormalities in this region could contribute to more difficulties shifting and maintaining attention to the tones.
The role of the STG in timing appears to vary depending on the location within the STG. Coull and colleagues (63) proposed an anterior-posterior timing gradient in the STG such that areas at ~y = −20 are linked with automatic motor timing, such as paced tapping to an auditory tone (55,73), and posterior regions are implicated in cognitive perceptual timing (74-75) showing activation in the absence of auditory cues (22). In our data, the location where the ADHD adults showed less activation than controls, ~y = −50, is consistent with the area that has been shown to be active during perceptual duration discrimination tasks. Thus, the failure of the ADHD adults to engage this region could contribute to difficulties perceiving the precise timing of the tones and subsequently maintaining the proper timing of the taps.
While performing the tapping task, ADHD adults consistently demonstrated less activation than the controls suggesting that the ADHD adults are not sufficiently recruiting regions associated with sensorimotor timing. Notably, the lack of activation differences between the ADHD adults and controls on the listen task and lack of differences in areas of the auditory cortex associated with hearing tones (~y = −20 in the STG) for the paced task, indicates that the hypoactivity relative to the controls was not a nonspecific global reduction, but rather more specific to the demands of the temporally demanding tapping task.
In all, abnormal neural activity was demonstrated in our predicted brain regions commonly associated with both ADHD and timing (frontal cortex, BG, cerebellum), as well as other regions often involved in timing (e.g., insula, IPL, STG). These data support our hypothesis that abnormalities in timing networks contribute to the pathophysiology of ADHD and may represent a core dysfunction of the disorder. The kinds of temporal processing deficits observed here are in keeping with the consistently reported increased within-subject response time variability in ADHD (12-13). We hypothesize that suboptimal engagement of this timing network (which overlaps with a cerebellar-prefrontal-striatal network long hypothesized to be abnormal in ADHD; 76) represents a core neurofunctional abnormality and contributes to basic timing impairments as well as possibly other behavioral features of ADHD (e.g., delay aversion, inability to wait one's turn). As such, testing hypotheses that these and other relevant behavioral manifestations of ADHD reflect abnormalities in timing-related neural networks should further our understanding of the disorder.
Finally, there were no relationships between ADHD symptoms and behavioral or imaging data for the unpaced tapping task. This suggests that this timing abnormality is not associated with number of ADHD symptoms and supports a hypothesis that timing abnormalities are core features of ADHD independently of inattentive and hyperactive symptoms. However, it could also be that other symptom measures which we did not use or perhaps a more precise measure of symptom severity (rather than symptom number) would have shown a relationship. Future studies designed to address such questions using dimensional ADHD rating scales could be revealing.
Our study and its conclusions need to be considered in light of methodological limitations. First, ADHD individuals have been shown to have abnormalities in both fine and gross motor abilities (77). The atypical activity associated with our tapping task could be associated with either timing or movement implementation problems rather than timing per se, or could be a result of the combination of the two. Our paradigm does not allow us to definitively resolve this question. However, analyses with the Wing and Kristofferson model (41), which show differences in clock, but not motor, variance, suggest a timing explanation for our results. Also, the lack of any between-group differences for the paced vs. listen group by task interaction (see the Supplement) suggests that ADHD and control adults do not differ in movement-related activation. Additionally, many of the regions showing atypical activation in the ADHD adults are consistent with results of a recent meta-analysis of timing which included a range of timing tasks that accounted for motor processes (18). Still, future studies which employ other timing paradigms without movement would be helpful in addressing this question. Second, some of our ADHD participants did not meet the symptom threshold at the time of scan. However, it is common for adults to fall below symptom threshold (54). If anything we would expect this to diminish the strength of ADHD vs. control effects rather than create spurious effects. Third, although matched, the mean IQ of our groups was higher than “average” and it is unclear how well these results would generalize to ADHD adults with average mean IQs. Additionally, although group-matched on sex, both males and females were included in our sample and it is possible that between-group differences may not be the same for males and females. Future studies should employ larger groups balanced by sex to address potential sex differences. ADHD subjects who were taking psychostimulants underwent a 24-hour wash-out period. However, it is still possible that long- or short-term effects of psychostimulants could have affected our results. If this were the case, we would expect potential effects of psychostimulants to diminish our between-group effects (78). Finally, employing a parametric characterization of the tapping task or supra-second timing would be useful in evaluating the effects of timing load and/or time scale on neural abnormalities in ADHD adults and should be used in future studies.
Overall, these findings significantly increase our understanding of the behavioral and neurofunctional aspects of sensorimotor timing abnormalities in ADHD adults. These data demonstrate that timing abnormalities at the sub-second level persist into adulthood and also provide the first evidence of the underlying neural substrates for the observed differences in movement rate control in adult ADHD.
Supplementary Material
Acknowledgements
This work was supported in part by: grants from the National Institutes of Health (MH 071535 to EMV, MH 57934 to SVF, MH 62152 to LJS, MH 064019 to TJS, AG29710 to RMCS); the National Alliance for Research on Schizophrenia and Depression to JB; Research Grants from Janssen and McNeil Pharmaceuticals to JB; and the philanthropic support from the Research Council for Pediatric Psychopharmacology at the Massachusetts General Hospital; The National Center for Research Resources (P41RR14075); and the Commonwealth Research Center, Massachusetts Department of Mental Health to LJS. These funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We thank the individuals who served as research participants as well as Denise Boriel, Stacey Bilicki, Alysa Doyle, Ronna Fried, Brittany LeBlanc, Michael C. Monuteaux, and Timothy Wilens for their contributions to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Eve M. Valera has received travel support and honoraria from Shire Pharmaceuticals and the McNeil and Janssen divisions of Ortho-McNeil-Janssen Pharmaceuticals. Dr. Joseph Biederman is currently receiving research support from the following sources: Alza, AstraZeneca, Bristol Myers Squibb, Eli Lilly and Co., Janssen Pharmaceuticals Inc., McNeil, Merck, Organon, Otsuka, Shire, NIMH, and NICHD. In 2009, Dr. Joseph Biederman received a speaker's fee from the following sources: Fundacion Areces, Medice Pharmaceuticals, and the Spanish Child Psychiatry Association. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker's fees for/from the following additional sources: Abbott, AstraZeneca, Celltech, Cephalon, Eli Lilly and Co., Esai, Forest, Glaxo, Gliatech, Janssen, McNeil, NARSAD, NIDA, New River, Novartis, Noven, Neurosearch, Pfizer, Pharmacia, The Prechter Foundation, Shire, The Stanley Foundation, UCB Pharma, Inc. and Wyeth. Dr. Thomas Spencer has received research support from, has been a speaker on aspeaker bureau or has been on an Advisory Board of the following sources: Shire Laboratories, Inc, Eli Lilly & Company, Glaxo-Smith Kline, Janssen Pharmaceutical, McNeil Pharmaceutical, Novartis Pharmaceuticals, Cephalon,Pfizer and the National Institute of Mental Health. In the past year, Dr. Stephen Faraone has received consulting fees and has been on Advisory Boards for Eli Lilly, Ortho-McNeil and Shire Development and has received research support from Eli Lilly, Pfizer, Shire and the National Institutes of Health. In previous years, Dr. Faraone has received consulting fees or has been on Advisory Boards or has been a speaker for the following sources: Shire, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. In previous years he has received research support from Eli Lilly, Shire, Pfizer and the National Institutes of Health. Dr. Larry J. Seidman reports no financial disclosures or conflicts of interest for the past 2 years. He has been a speaker for Shire Pharmaceuticals and received an unrestricted educational grant from Janssen Pharmaceuticals in the past 5 years. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32:631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- 3.Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sci. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--I. The effect of delay on choice. J Child Psychol Psychiatry. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 5.Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperactive behavior: the effect of methylphenidate on motor timing. J Abnorm Child Psychol. 2003;31:301–313. doi: 10.1023/a:1023233630774. [DOI] [PubMed] [Google Scholar]
- 6.Barkley RA, Koplowitz S, Anderson T, McMurray MB. Sense of time in children with ADHD: effects of duration, distraction, and stimulant medication. J Int Neuropsychol Soc. 1997;3:359–369. [PubMed] [Google Scholar]
- 7.Barkley RA, Murphy KR, Bush T. Time perception and reproduction in young adults with attention deficit hyperactivity disorder. Neuropsychology. 2001;15:351–360. doi: 10.1037//0894-4105.15.3.351. [DOI] [PubMed] [Google Scholar]
- 8.Meaux JB, Chelonis JJ. Time perception differences in children with and without ADHD. J Pediatr Health Care. 2003;17:64–71. doi: 10.1067/mph.2003.26. [DOI] [PubMed] [Google Scholar]
- 9.Toplak ME, Rucklidge JJ, Hetherington R, John SC, Tannock R. Time perception deficits in attention-deficit/ hyperactivity disorder and comorbid reading difficulties in child and adolescent samples. J Child Psychol Psychiatry. 2003;44:888–903. doi: 10.1111/1469-7610.00173. [DOI] [PubMed] [Google Scholar]
- 10.Smith A, Taylor E, Rogers JW, Newman S, Rubia K. Evidence for a pure time perception deficit in children with ADHD. J Child Psychol Psychiatry. 2002;43:529–542. doi: 10.1111/1469-7610.00043. [DOI] [PubMed] [Google Scholar]
- 11.Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: findings to date and new methods. J Neurosci Methods. 2006;151:15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin RL, Chelonis JJ, Flake RA, Edwards MC, Feild CR, Meaux JB, et al. Effect of methylphenidate on time perception in children with attention-deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2004;12:57–64. doi: 10.1037/1064-1297.12.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Marx I, Hubner T, Herpertz SC, Berger C, Reuter E, Kircher T, et al. Cross-sectional evaluation of cognitive functioning in children, adolescents and young adults with ADHD. J Neural Transm. 2009 doi: 10.1007/s00702-009-0345-3. [DOI] [PubMed] [Google Scholar]
- 16.Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Meck WH, Benson AM. Dissecting the brain's internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- 18.Wiener M, Turkeltaub P, Coslett HB. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- 19.Ivry RB. The representation of temporal information in perception and motor control. Curr Opin Neurobiol. 1996;6:851–857. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- 20.Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Brain Res Cogn Brain Res. 1998;7:15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 21.Nenadic I, Gaser C, Volz HP, Rammsayer T, Hager F, Sauer H. Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp Brain Res. 2003;148:238–246. doi: 10.1007/s00221-002-1188-4. [DOI] [PubMed] [Google Scholar]
- 22.Lewis PA, Miall RC. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003;41:1583–1592. doi: 10.1016/s0028-3932(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 23.Nichelli P, Clark K, Hollnagel C, Grafman J. Duration processing after frontal lobe lesions. Ann N Y Acad Sci. 1995;769:183–190. doi: 10.1111/j.1749-6632.1995.tb38139.x. [DOI] [PubMed] [Google Scholar]
- 24.Amat JA, Bronen RA, Saluja S, Sato N, Zhu H, Gorman DA, et al. Increased number of subcortical hyperintensities on MRI in children and adolescents with Tourette's syndrome, obsessive-compulsive disorder, and attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:1106–1108. doi: 10.1176/appi.ajp.163.6.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biederman J, Makris N, Valera EM, Monuteaux MC, Goldstein JM, Buka S, et al. Towards further understanding of the co-morbidity between attention deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychol Med. 2008;38:1045–1056. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- 26.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 27.Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother. 2007;7:1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 32.Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. Br J Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- 33.Smith AB, Taylor E, Brammer M, Halari R, Rubia K. Reduced activation in right lateral prefrontal cortex and anterior cingulate gyrus in medication-naive adolescents with attention deficit hyperactivity disorder during time discrimination. J Child Psychol Psychiatry. 2008;49:977–985. doi: 10.1111/j.1469-7610.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 34.Durston S, Davidson MC, Mulder MJ, Spicer JA, Galvan A, Tottenham N, et al. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 35.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. Jama. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mates J, Radil T, Muller R, Poppel E. Temporal integration in sensorimotor synchronization. J Cogn Neurosci. 1994;6:332–340. doi: 10.1162/jocn.1994.6.4.332. [DOI] [PubMed] [Google Scholar]
- 39.Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neurodevelopmental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- 41.Wing AM, Kristofferson AB. Response delays and the timing of discrete motor responses. Percept Psychophys. 1973;14:5–12. [Google Scholar]
- 42.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 43.Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 44.Orvaschel H. Schedule for Affective Disorder and Schizophrenia for School-Age Children Epidemiologic Version. 5th Edition Nova Southeastern University, Center for Psychological Studies; Ft. Lauderdale: 1994. [Google Scholar]
- 45.Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criterion for attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:1204–1210. doi: 10.1097/00004583-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Barkley RA, Murphey KR, Fischer M. ADHD in Adults: What the Science Says. Guilford; New York: 2008. [Google Scholar]
- 47.Wechsler D. Wechsler Adult Intelligence Scale III [manual] 3rd edn. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 48.Wechsler D. Manual for the Wechsler Adult Intelligence Scale - Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- 49.Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 50.Krampe RT, Mayr U, Kliegl R. Timing, sequencing, and executive control in repetitive movement production. J Exp Psychol Hum Percept Perform. 2005;31:379–397. doi: 10.1037/0096-1523.31.3.379. [DOI] [PubMed] [Google Scholar]
- 51.Terry P, Doumas M, Desai RI, Wing AM. Dissociations between motor timing, motor coordination, and time perception after the administration of alcohol or caffeine. Psychopharmacology (Berl) 2009;202:719–729. doi: 10.1007/s00213-008-1352-z. [DOI] [PubMed] [Google Scholar]
- 52.Keele SW, Pokorny RA, Corcos DM, Ivry R. Do perception and motor production share common timing mechanisms: a correctional analysis. Acta Psychol (Amst) 1985;60:173–191. doi: 10.1016/0001-6918(85)90054-x. [DOI] [PubMed] [Google Scholar]
- 53.Vorberg D, Wing A. Modeling variability and dependence in timing. In: Prinz W, Heuer H, Bridgeman B, Keele S, editors. Handbook of Perception and Action. Academic Press; San Diego: 1996. pp. 181–262. [Google Scholar]
- 54.Faraone SV, Biederman J, Spencer T, Mick E, Murray K, Petty C, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006;163:1720–1729. doi: 10.1176/ajp.2006.163.10.1720. quiz 1859. [DOI] [PubMed] [Google Scholar]
- 55.Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42:343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toplak ME, Tannock R. Tapping and anticipation performance in attention deficit hyperactivity disorder. Percept Mot Skills. 2005;100:659–675. doi: 10.2466/pms.100.3.659-675. [DOI] [PubMed] [Google Scholar]
- 58.Rommelse NN, Altink ME, Oosterlaan J, Beem L, Buschgens CJ, Buitelaar J, et al. Speed, variability, and timing of motor output in ADHD: which measures are useful for endophenotypic research? Behav Genet. 2008;38:121–132. doi: 10.1007/s10519-007-9186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrington DL, Lee RR, Boyd LA, Rapcsak SZ, Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain. 2004;127:561–574. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- 60.Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- 61.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 62.Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, et al. Neural representation of interval encoding and decision making. Brain Res Cogn Brain Res. 2004;21:193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Coull J, Nobre A. Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol. 2008;18:137–144. doi: 10.1016/j.conb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 64.Livesey AC, Wall MB, Smith AT. Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45:321–331. doi: 10.1016/j.neuropsychologia.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 65.Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Hum Brain Mapp. 2007;28:394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taniwaki T, Okayama A, Yoshiura T, Nakamura Y, Goto Y, Kira J, et al. Reappraisal of the motor role of basal ganglia: a functional magnetic resonance image study. J Neurosci. 2003;23:3432–3438. doi: 10.1523/JNEUROSCI.23-08-03432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, et al. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 69.Cerasa A, Hagberg GE, Peppe A, Bianciardi M, Gioia MC, Costa A, et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res Bull. 2006;71:259–269. doi: 10.1016/j.brainresbull.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 70.Bueti D, Walsh V, Frith C, Rees G. Different brain circuits underlie motor and perceptual representations of temporal intervals. J Cogn Neurosci. 2008;20:204–214. doi: 10.1162/jocn.2008.20017. [DOI] [PubMed] [Google Scholar]
- 71.Harrington DL, Haaland KY, Knight RT. Cortical networks underlying mechanisms of time perception. J Neurosci. 1998;18:1085–1095. doi: 10.1523/JNEUROSCI.18-03-01085.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp (Wars) 2004;64:329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- 73.Jantzen KJ, Steinberg FL, Kelso JA. Functional MRI reveals the existence of modality and coordination-dependent timing networks. Neuroimage. 2005;25:1031–1042. doi: 10.1016/j.neuroimage.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 74.Coull JT, Vidal F, Nazarian B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- 75.Ferrandez AM, Hugueville L, Lehericy S, Poline JB, Marsault C, Pouthas V. Basal ganglia and supplementary motor area subtend duration perception: an fMRI study. Neuroimage. 2003;19:1532–1544. doi: 10.1016/s1053-8119(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 76.Giedd JN, Blumenthal J, Molloy E, Castellanos FX. Brain imaging of attention deficit/hyperactivity disorder. In: Wasserstein J, Wolf L, editors. Adult Attention Deficit Disorder: Brain Mechanisms and Life Outcomes. Annals of the New York Academy Sciences. Vol. 931. New York Academy of Sciences; New York: 2001. [DOI] [PubMed] [Google Scholar]
- 77.Pitcher TM, Piek JP, Hay DA. Fine and gross motor ability in males with ADHD. Dev Med Child Neurol. 2003;45:525–535. doi: 10.1017/s0012162203000975. [DOI] [PubMed] [Google Scholar]
- 78.Bush G, Spencer T, Holmes J, Shin LM, Surman C, Valera EM, et al. Functional Magnetic Resonance Imaging of Methylphenidate and Placebo in Adults with Attention-Deficit/Hyperactivity Disorder during the Multi-Source Interference Task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.