Abstract
Objective
To describe a patient presenting with the rare constellation of synchronous parathyroid carcinoma, parathyroid adenoma, and papillary thyroid carcinoma.
Methods
Review of clinical presentation, diagnostic workup, surgical management, and pathological findings, and review of pertinent literature.
Results
The patient was a 59-year old man who presented with severe clinical manifestations of longstanding primary hyperparathyroidism (HPT), serum calcium of 14.4 mg/dL, and parathyroid hormone level of 2,023 pg/ml. The patient was found to have a 3.4 cm parathyroid carcinoma on the left side and a 3.2 cm papillary thyroid carcinoma on the right side. In addition, a 917 mg parathyroid adenoma was found on the right side.
Conclusion
Synchronous parathyroid and thyroid carcinomas are extremely rare. Our patient is the first documented case with a parathyroid adenoma in addition to synchronous parathyroid and thyroid carcinomas. The presence of concurrent parathyroid carcinoma and adenoma can cause diagnostic confusion and should be considered in patients presenting with severe HPT. Any concomitant thyroid nodules need to be investigated to rule out thyroid carcinoma.
INTRODUCTION
Primary hyperparathyroidism (HPT) is caused by parathyroid carcinoma in less than 1% (1,2). Coexistent thyroid carcinoma in these patients is extremely rare, and to our knowledge, only 5 patients with documented synchronous parathyroid and thyroid carcinomas have been reported (3-7). Patients with parathyroid carcinoma may also have concomitant disease in other parathyroid glands, including adenoma, hyperplasia, or a second parathyroid carcinoma (1,2,8-10). Here we report a patient with the unusual coexistence of parathyroid carcinoma, parathyroid adenoma, and papillary thyroid carcinoma. To our knowledge, this constellation has not been reported previously.
CASE REPORT
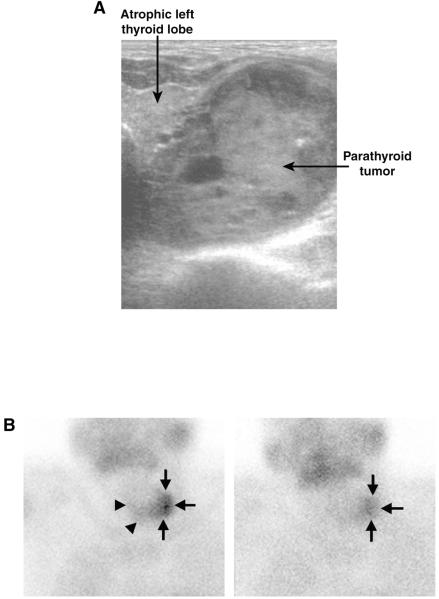
A 59-year old man was diagnosed approximately 8 years ago with primary HPT on the basis of hypercalcemia (12.6 mg/dL; reference range 8.4-10.2 mg/dL) and elevated serum parathyroid hormone (PTH) level (385 pg/mL; reference range 15-65 pg/mL). Neck ultrasound at that time showed a 3 cm mass believed to be an enlarged parathyroid gland at the posterior inferior aspect of the left thyroid lobe. He was scheduled to undergo parathyroidectomy but canceled the procedure because of pronounced fear of surgery. The patient refused continued medical care and was lost to follow-up. Approximately 6 years later, he presented with pathological fractures of the left femoral neck, the surgical neck of the right humerus, and the left superior and inferior pubic rami. Additional X-rays revealed osteitis fibrosa cystica, multiple lesions consistent with brown tumors, and a “salt and pepper appearance” of his skull bones. Peak serum calcium was 14.4 mg/dL and peak PTH level was 2,023 pg/mL. An orthopedic consultant recommended that the patient’s HPT be corrected before his fractures could be addressed. Neck ultrasonography demonstrated a 3.9 × 3.8 × 3.2 cm heterogeneous hyper-echoic mass with punctuate calcifications displacing an atrophic left thyroid lobe medially and considered likely to represent a parathyroid adenoma (Fig 1A). In addition, the patient had a hyper-echoic nodule with greatest diameter of 2.9 cm in the thyroid isthmus and a 3.4 cm hypo-echoic nodule in the right thyroid lobe. A sestamibi scan was consistent with a left-sided parathyroid adenoma corresponding to the mass seen on ultrasound (Fig 1B).
Figure 1.
(A) Ultrasonogram demonstrating a 3.9 × 3.8 × 3.2 cm parathyroid tumor adjacent to a seemingly atrophic left thyroid lobe. (B) Anterior images from a sestamibi study 20 min (left panel) and 2 h (right panel) after injection of the isotope. The arrowheads outline uptake in the right thyroid lobe. The arrows outline increased and retained activity in the parathyroid tumor.
The patient remained reluctant to undergo surgery but eventually agreed to neck exploration at which time an approximately 4 cm firm, white-gray mass was found lateral and partly posterior to the inferior pole of a seemingly atrophic left thyroid lobe. Because of the characteristics of the mass and the apparent lack of a plane between the mass and the left thyroid lobe, parathyroid cancer was suspected. An en bloc resection of the parathyroid mass, left thyroid lobe, and isthmus was performed (11). Of note, no additional parathyroid gland was identified on the left side. A PTH level determined immediately before surgery was 1,088 pg/ml and postoperative PTH level was 37 pg/ml.
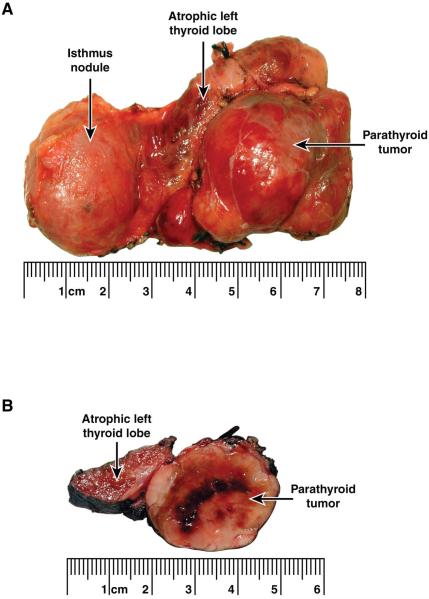
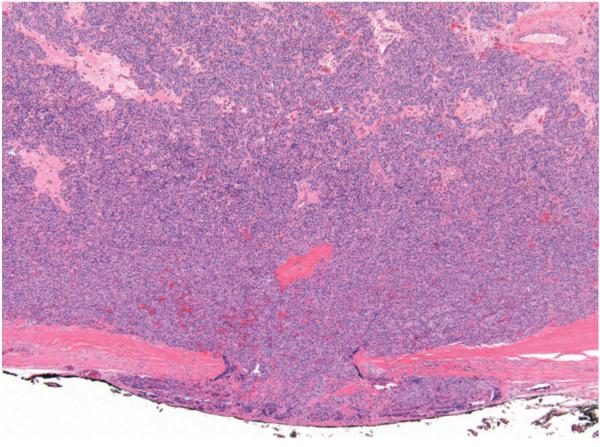
Gross examination of the surgical specimen revealed a 3.4 cm firm mass that seemed to be inseparable from the atrophic left thyroid lobe (Fig 2A and B). In addition, there was an approximately 3.5 cm, partly cystic, nodule in the thyroid isthmus. Histopathological examination showed that the mass adjacent to the left thyroid lobe was a parathyroid carcinoma with evidence of capsular invasion (Fig 3). An incidental 4 mm papillary microcarcinoma, follicular variant, was found in the left thyroid lobe. The partly cystic nodule in the isthmus was an adenomatous nodule without evidence of malignancy.
Figure 2.
(A) Surgical specimen from the patient’s original operation, excision of the parathyroid tumor, left thyroid lobe and isthmus en bloc. (B) Through-cut section of the surgical specimen demonstrating the parathyroid tumor and the adjacent left thyroid lobe.
Figure 3.
Histology of parathyroid carcinoma on the left side. Histopathological section demonstrating capsular invasion of the tumor. Original magnification x4. Hematoxylin & Eosin staining.
The patient became hypocalcemic on his first postoperative day (calcium 6.9 mg/dL) consistent with “hungry bone” syndrome. He was treated with intravenous and oral calcium, calcitriol and vitamin D3 and eventually was discharged on oral calcium and vitamin D3 supplementation. A bone density test performed 3 months postoperatively showed evidence of severe osteoporosis (T-score −7) in the forearm.
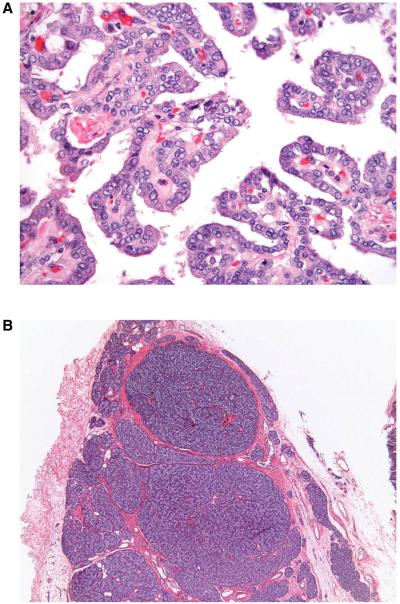
A subsequent ultrasound-guided fine needle aspiration biopsy of the right thyroid nodule yielded cytology suggestive of papillary carcinoma and right thyroid lobectomy was recommended. When presenting for right thyroid lobectomy, the patient was unexpectedly found to be hypercalcemic (13.7 mg/dL) with a PTH level within low normal range (23 pg/mL). We initially attributed the high calcium levels to the oral calcium and vitamin D supplementation and immediately discontinued these medications. The patient also received intravenous fluids and diuretics. With this treatment, serum calcium decreased to 10.7 mg/dL and PTH rose to 48 pg/ml. When the patient underwent right thyroid lobectomy, an approximately 3 cm nodule was identified in the right thyroid lobe. In addition, a 1.5 cm parathyroid adenoma-like lesion was found above the nerve-artery crossing and this lesion was excised separately. A normal-looking inferior parathyroid gland was identified and preserved in situ. An immediate preoperative PTH level was 57 pg/ml and PTH on postoperative day 1 was 12 pg/ml. Histopathological examination revealed a 3.2 cm papillary thyroid carcinoma in the right thyroid lobe (Fig 4A) and a hypercellular parathyroid gland weighing 917 mg (Fig 4B). In addition, a papillary microcarcinoma (2 mm) was found in the right thyroid lobe.
Figure 4.
(A) Histology of the 3.2 cm papillary carcinoma in the right thyroid lobe removed at the patient’s second operation. Histopathological section demonstrating typical features of a papillary carcinoma. Original magnification x40. Hematoxylin & Eosin. (B) Histology of a 917 mg parathyroid adenoma from the right side and removed at the patient’s second operation. Histopathological section demonstrating hypercellular parathyroid tissue without evidence of malignancy. Original magnification x4. Hematoxylin & Eosin staining.
Postoperatively, the patient became normo-calcemic with PTH levels ranging from 12-28 pg/mL. The patient was prescribed oral calcium and vitamin D3 after surgery. His bone symptoms, energy level, appetite, overall well-being, and mental state improved rapidly. X-rays (8 months after his first surgery) showed resorption of most of the brown tumors, resolution of the “salt and pepper” appearance of the skull bones, and interval healing of the pathological fractures (except the left femur neck fracture). The patient remains normo-calcemic with the most recent calcium level being 8.7 mg/dL 6 months after his second operation (1 year and 2 months after his first procedure).
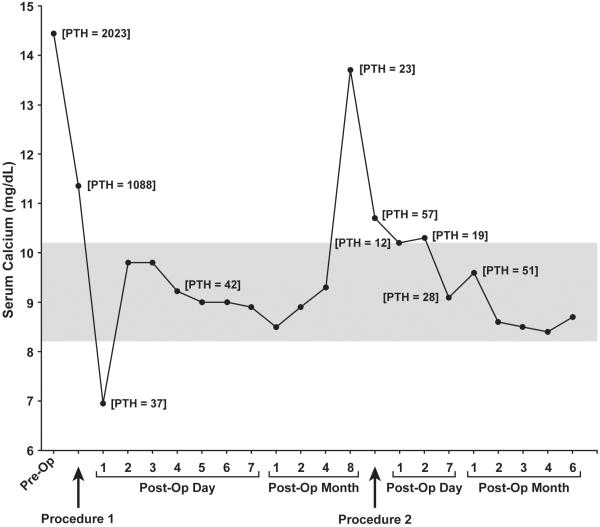
Changes in calcium and PTH levels during the course of the patient’s care are summarized in Fig 5.
Figure 5.
Calcium (solid line) and PTH levels during the course of the patient’s care. PTH levels are shown in brackets and are given as pg/ml. The gray area represents normal calcium levels.
DISCUSSION
Only 5 patients with documented synchronous parathyroid and thyroid carcinomas have been reported previously (3-7). The clinical characteristics of those patients and the case reported here are summarized in Table 1. Four of the patients (67%) were female and the patients were relatively young (mean age 55 years). In previous reports of patients with parathyroid carcinoma (not associated with thyroid carcinoma), the distribution between men and women was relatively even with an age range similar to that observed in the patients reviewed here (1,2). This differs from patients with primary HPT caused by adenoma or hyperplasia who are typically older and in whom the disease occurs predominantly in women.
Table 1.
Clinical features of 6 patients with coexistence of parathyroid and thyroid carcinoma
| Reference | Sex | Age | Calcium (mg/dL) |
PTH (pg/mL) |
Parathyroid Size (cm) |
Carcinoma Location |
Thyroid Carcinoma |
Associated Parathyroid Disease |
Surgical Treatment |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Kurita et al, 1979 |
F | 68 | 12.2 | 6,300 | 4.2×3.2×2.4 | Left Lower |
Papillary | None | En bloc resection |
Postop normocalcemia |
| Christmas et al, 1988 |
F | 62 | Hypercalcemia | Unknown | Unknown | Unknown | Follicular | None | Unknown | Died from metastatic para- thyroid carcinoma |
| Savli et al, 2001 |
F | 47 | Normal | N.D. | Unknown | Unknown | Papillary | Hyperplasia | Total thyroidectomy Parathyroidectomy (Excision of 2 hyperplastic glands) |
Normocalcemia (1 year) |
| Schoretsanitis et al, 2002 |
F | 55 | 14.2 | >1,000 | 3×3 | Left Lower |
Papillary (Follicular variant) |
None | En bloc resection |
Normocaclemia (2 years) |
| Lin et al, 2005 |
M | 38 | 16.5 | 351 | 4×3×3 | Left Lower |
Papillary | Two enlarged parathyroid glands on contralateral side (?) |
Total thyroidectomy Left Parathyroidectomy |
Normocalcemia (6 years) |
| Present Case |
M | 58 | 14.4 | 2,023 | 3.4×3.3×2.2 | Left Lower |
Papillary | Contralateral parathyroid adenoma |
En bloc resection |
Persistent hyper- calcemia after resection of para- thyroid carcinoma. Normocalcemia after excision of contralateral para- thyroid adenoma (1 year) |
The parathyroid carcinoma was functioning in 5 of the 6 patients described in Table 1 (including the patient reported here), and all patients with functioning parathyroid tumor for whom calcium and PTH levels were reported had severe hypercalcemia and substantially elevated PTH levels ranging from 15- to almost 100-fold increase above normal level. These observations are in line with previous reports of parathyroid carcinoma unrelated to thyroid carcinoma. Thus, in a large series, only 2% of patients with parathyroid carcinoma were nonfunctioning (1) and, interestingly, those patients had decreased survival, most likely reflecting delayed presentation (due to absence of symptoms from hypercalcemia) and possibly more aggressive tumor biology.
Trabecular architecture of the parenchymal cells, a thick capsule, fibrous trabeculae traversing the gland, nuclear atypia and high mitotic rates are commonly seen in parathyroid carcinomas but none of these changes is diagnostic (10,12-14). The most definitive diagnostic criteria for parathyroid carcinoma are capsular and vascular invasion, invasion of adjacent tissues, regional lymph node and distant metastases. Locally recurrent disease is not diagnostic since rupture of the capsule with seeding of cells in the operative field can occur also when a benign hyperfunctioning gland is resected. In our patient, the parathyroid tumor removed at the first operation showed capsular invasion, making the diagnosis of carcinoma unequivocal.
Previous reports suggest that history of neck irradiation, long-standing secondary HPT, end-stage renal disease, hereditary hyperparathyroidism-jaw tumor syndrome, and mutations of the HRPT2 gene, are associated with increased risk for parathyroid carcinoma (1,2,15). It is not known if specific risk factors are associated with synchronous parathyroid and thyroid carcinoma but, notably, neck irradiation is a risk factor for both thyroid and parathyroid carcinoma (as well as parathyroid adenoma). The patient reported by Christmas et al (4) developed synchronous parathyroid and thyroid carcinomas 25 years after external beam therapy for cervical tuberculosis. Our patient did not have a previous history of neck irradiation or any other known risk factor for parathyroid or thyroid carcinoma; he has not been tested for mutations of the HRPT2 gene.
The surgical management of other parathyroid glands in patients with synchronous parathyroid and thyroid carcinoma (Table 1) varied and was not always clearly described. For example, in the patient reported by Lin et al (7), preoperative ultrasound demonstrated 2 enlarged parathyroid glands adjacent to the right thyroid lobe in addition to a 4×3×3 cm parathyroid tumor at the inferior pole of the left thyroid lobe. It is unclear from the report whether the parathyroid glands on the right side were identified during surgery (described as total thyroidectomy and left parathyroidectomy). If the 2 right parathyroid glands were indeed enlarged and if they were not removed at the time of surgery, they were most likely non-functioning since the patient remained normocalcemic with normal PTH levels during follow-up for more than 6 years. In the patient reported by Savli et al (5) and who was normocalcemic, 2 hyperplastic parathyroid glands were removed during total thyroidectomy and one of the glands contained a carcinoma. The authors did not describe whether additional parathyroid glands were identified or searched for during the procedure. Our patient is the first documented case with a parathyroid adenoma occurring in a patient with synchronous thyroid and parathyroid carcinomas. Of note, in our patient, only 3 parathyroid glands were indentified, but because the patient has remained normocalcemic with normal PTH levels a year after his second operation, we assume he does not have any remaining hyperfunctioning gland.
The present report illustrates several important points that deserve to be emphasized. First, in patients with severe hypercalcemia, parathyroid carcinoma should be considered a possible underlying cause, and if the operative finding supports the suspicion of parathyroid carcinoma, an en bloc resection of the parathyroid tumor and the adjacent thyroid lobe should be performed. Second, although uncommon, patients with parathyroid carcinoma may have additional hyperfunctioning glands, and persistent or recurrent HPT after resection of a parathyroid carcinoma does not necessarily indicate residual or recurrent parathyroid carcinoma but can also be caused by a parathyroid adenoma. Indeed, this point is illustrated by the parathyroid adenoma found at the second procedure in our patient, explaining why PTH was not suppressed below normal range despite hypercalcemia when the patient presented for his second surgery. Finally, patients with HPT can have concomitant thyroid disease, including carcinoma, emphasizing the importance of thyroid imaging before neck exploration for HPT.
ACKNOWLEDGMENTS
We are grateful to Drs. Yuka Sumi and Kiyoshi Itagaki for translating references #3 into English and to Mr. Michael Cichanowski, Media Services, Beth Israel Deaconess Medical Center, for preparing Fig 5.
REFERENCES
- 1.Koea JB, Shaw JHF. Parathyroid cancer: biology and management. Surg Oncol. 1999;8:155–165. doi: 10.1016/s0960-7404(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 2.Shane E. Clinical review 122. Parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86:485–493. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 3.Kurita S, Mihashi S, Hirano M, Nakashima T, Tanimura A. Hyperfunctioning parathyroid carcinoma combined with papillary carcinoma of the thyroid gland. J Jap Soc Cancer Ther. 1979;14:1127–1135. [PubMed] [Google Scholar]
- 4.Christmas TJ, Chapple CR, Noble JG, Milroy EJG, Cowie AGA. Hyperparathyroidism after neck irradiation. Br J Surg. 1988;75:873–874. doi: 10.1002/bjs.1800750914. [DOI] [PubMed] [Google Scholar]
- 5.Savli H, Sevinc A, Sari R, Ozen S, Buyukberber S, Ertas E. Occult parathyroid carcinoma in a patient with papillary thyroid carcinoma and Hashimoto’s thyroiditis. J Endocrinol Invest. 2001;24:42–44. doi: 10.1007/BF03343807. [DOI] [PubMed] [Google Scholar]
- 6.Schoretsanitis G, Melissas J, Kafousi M, Karkavitsas N, Tsiftsis DD. Synchronous parathyroid and papillary thyroid carcinoma: a case report. Am J Otolaryngol. 2002;23:382–385. doi: 10.1053/ajot.2002.126317. [DOI] [PubMed] [Google Scholar]
- 7.Lin SD, Tu ST, Hsu SR, Chang JH, Yang KT, Yang LH. Synchronous parathyroid and papillary thyroid carcinoma. J Chin Med Assoc. 2005;68:87–91. doi: 10.1016/S1726-4901(09)70141-8. [DOI] [PubMed] [Google Scholar]
- 8.Dionisi S, Minisola S, Pepe J, et al. Concurrent parathyroid adenomas and carcinoma in the setting of multiple endocrine neoplasia type 1: presentation ad hypercalcemic crisis. Mayo Clin Proc. 2002;77:866–869. doi: 10.4065/77.8.866. [DOI] [PubMed] [Google Scholar]
- 9.Brown JJ, Mohamed H, Williams-Smith L, Osborne R, Coher J, Yee B. Primary hyperparathyroidism secondary to simultaneous bilateral parathyroid carcinoma. ENT J. 2002;81:395–401. [PubMed] [Google Scholar]
- 10.Fernandez-Ranvier GG, Khanafshar E, Jensen K, et al. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? Cancer. 2007;110:255–264. doi: 10.1002/cncr.22790. [DOI] [PubMed] [Google Scholar]
- 11.Ippolito G, Palazzo FF, Sebag F, De Micco C, Henry JF. Intraoperative diagnosis and treatment of parathyroid cancer and atypical parathyroid adenoma. Br J Surg. 2007;94:566–570. doi: 10.1002/bjs.5570. [DOI] [PubMed] [Google Scholar]
- 12.Schantz A, Catsleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–605. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Stojadinovic A, Hoos A, Nissan A, et al. Parathyroid neoplasms: clinical, histopathological, and tissue microarray-based molecular analysis. Human Pathol. 2003;34:54–64. doi: 10.1053/hupa.2003.55. [DOI] [PubMed] [Google Scholar]
- 14.Rawat N, Khetan N, Williams DW, Baxter JN. Parathyroid carcinoma. Br J Surg. 2005;92:1345–1353. doi: 10.1002/bjs.5182. [DOI] [PubMed] [Google Scholar]
- 15.Trisha M, Shattuck BS, Valimaki S, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–1729. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]